Abstract
One of the prominent features of Alzheimer's disease is the excessive accumulation of the protein amyloid beta (Aβ) in certain areas of the brain leading to neurodegeneration. Aβ is cytotoxic and disrupts several cytoprotective pathways. Recent literature has demonstrated that certain cytochrome P450 (CYP) products are neuroprotective, including epoxide metabolites of arachidonic acid (AA), epoxyeicosatrienoic acids (EETs). The action of Aβ with respect to regionally produced EETs in the brain has yet to be defined. Epoxygenases metabolize AA into 4 regioisomers of EETs (14,15 -, 11,12-, 8,9- and 5,6-EET). EETs are rapidly degraded into dihydroxyeicosatrienoic acids (DiHETEs) by soluble epoxide hydrolase (sEH). To determine the effect of Aβ on the epoxygenase activity in different regions of the brain, microsomes were prepared from the cerebrum and cerebellum of adult Sprague-Dawley rats and incubated with 1 and 10 μM Aβ for 30 minutes after which epoxygenase activity assay was performed. Mass spectrometry indicated that incubation with Aβ reduced 14,15-EET production by 30% as compared to vehicle in the cerebrum, but not in the cerebellum. When we separated the cerebrum into cortex and hippocampus, significant decrease in the production of total EETs and DiHETEs were seen in presence of Aβ (81% and 74%) in the cortex. Moreover, 11, 12-EET production was decreased to ∼70% of vehicle in both cortex and hippocampus. Epoxygenase activity in the cultured astrocytes and neurons also showed reduction in total EET and DiHETE production (to 80% and ∼70% of vehicle respectively) in presence of Aβ. Altogether, our data suggest that Aβ reduces epoxygenase activity differentially in a region-specific and cell-specific manner. The reduction of cytoprotective EETs by Aβ in the cerebrum may make it more prone to degeneration than the cerebellum. Further understanding of these interactions will improve our ability to protect against the pathology of Alzheimer's disease.
Keywords: epoxygenase, epoxyeicosatrienoic acids, Alzheimer's disease, Amyloid Beta
Alzheimer's disease (AD) is a chronic neurodegenerative disease–marked by excessive deposition of the protein amyloid beta (Aβ) in the forms of plaques and neurofibrillary tangles leading to neurodegeneration, loss of memory and cognitive impairment (Selkoe and Schenk, 2003). An overproduction of amyloid beta in the brain leads to the accumulation and polymerization of this protein and which eventually precipitates as fibrils (Chartier-Harlin et al., 1991, Mullan and Crawford, 1994). Although the fibrils and associated plaques were initially thought to cause neurodegeneration (Pike et al., 1993), a recently discovered soluble, non-fibrillar, oligomeric form of Aβ was proven to be most potent in inducing toxicity (Dahlgren et al., 2002). Also, the level of the oligomers is well correlated with the severity of the disease in AD patients (Lue et al., 1999, McLean et al., 1999). The toxicity of Aβ is region-specific. The neurodegeneration starts in the hippocampus and prefrontal cortex and spreads through the whole cerebrum, while the cerebellum remains largely unaffected by Aβ (Braak and Braak, 1991). The cerebellum also shows remarkably less volume shrinkage (Barclay and Brady, 1992) and better cellular integrity than the cortical neurons in presence of Aβ (Li et al., 1994, Kim et al., 2003). The reason behind this region-specific difference is not known. The soluble oligomers of Aβ are known to affect a number of cell survival pathways in order to inflict the toxicity. Aβ has been shown to downregulate or inhibit pro-survival or anti-apoptotic molecules, like Akt/p-Akt and Bcl-2 (Paradis et al., 1996, Magrane et al., 2005). Aβ also upregulates the pro-apoptotic pathways like ERK/MAPK or Bax (Paradis et al., 1996, Kuperstein and Yavin, 2002, Serrano et al., 2009, Young et al., 2009). All of these studies indicate that Aβ mediated cytotoxicity may have multiple regulatory elements which are not yet fully understood. Furthermore, the effect of Aβ on the cytoprotective epoxygenase pathway is not known. The epoxygenases are a subgroup of enzymes in the CYP450 family (e.g. CYP2C, 2J, 4×1 etc.) that metabolize arachidonic acid into 4 regioisomers of epoxyeicosatrienoic acid (5,6-, 8,9-, 11,12- and 14,15 EETs) (Zeldin, 2001). They are quickly metabolized in the cell into their less active diols, dihydroxyeicosatrienoic acids (DiHETEs) by soluble epoxide hydrolase (sEH). EETs are potent vasodilators (Gebremedhin et al., 1992), pro-angiogenic (Medhora et al., 2003, Michaelis et al., 2005) and cytoprotective in endothelial cells, epithelial cells and cardiomyocytes against a number of insults (Wu et al., 1997, Chen et al., 2001, Dhanasekaran et al., 2006). In the brain, EETs are released by astrocytes (Alkayed et al., 1996b) in response to glutamate from neurons and help dilate blood vessels (Alkayed et al., 1996a) to meet the metabolic demand of active neurons (Alkayed et al., 1997). They also promote angiogenic pathways in brain microvascular endothelial cells in culture (Munzenmaier and Harder, 2000, Zhang and Harder, 2002). Recently it was shown that EETs also protect neurons from ischemia-reperfusion injury. When sEH-KO mice were subjected to stroke, they showed reduced infarct size than controls (Zhang et al., 2008). Pharmacological inhibition of sEH by 12-(3-Adamantan-1-yl-ureido)dodecanoic Acid (AUDA) was demonstrated to be protective against simulated stroke yielding lower infarct volume and higher levels of circulatory EETs in the brain (Simpkins et al., 2009). The goal of this study was to evaluate the effect of Aβ on the epoxygenase activity in different regions of the brain, the rationale being that if Aβ affected EETs production differently in various regions of the brain, it might provide an explanation for the region-specific cytotoxicity seen in AD. We report the effect of acute Aβ exposure in microsomes from adult rat brains and in cultured astrocytes and neurons from hippocampus of neonatal rats.
Experimental procedures
Microsome isolation
Enriched enzyme fractions (microsomes) were obtained from cerebrum, cerebellum, cortex and hippocampus of adult (2-4 month old) male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) by differential centrifugation (Gebremedhin et al., 1992). All procedures involving animals were performed according to protocols approved by the Institutional Animal Care and Use Committee at Medical College of Wisconsin and were in accordance with National Institute of Health guidelines. Briefly, rats were euthanized with CO2, brain was removed and the desired area was dissected under a light microscope. The tissue was homogenized in twice the volume of cold 10 mM phosphate buffer solution at pH 7.7 containing 250 mM sucrose, 1 mM EDTA and protease inhibitors using an electrical homogenizer. The homogenate was centrifuged for 30 minutes at 4°C at 10,000g to remove tissue debris. The supernatant was collected and centrifuged for 90 minutes at 4°C at 100,000g. At the end of the centrifugation, the supernatant was poured off and the pellet was suspended by sonication in 0.1 M potassium phosphate buffer containing 1 mM EDTA, 1mM Dithiothreitol (DTT), 30% v/v glycerol and protease inhibitors at pH 7.25. The protein content was measured with Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The microsomes were stored at -80°C until further use.
Hippocampal astrocyte culture
Primary astrocytes were cultured from 2-3 day old Sprague-Dawley rat pup brains as described previously (Alkayed et al., 1996b). Briefly, the hippocampus was removed and digested in 20 U/ml papain (Worthington Biochemical Corp., Freehold, NJ, USA) and L-cystine (1.5×10−4 g/ml; Sigma, St. Louis, MO, USA) for 45 minutes at 37°C in Earle's Balanced Salt Solution (Gibco BRL, Gaithersburg, MD, USA) with gentle shaking. Digestion was stopped by washing the pellet three times with growth media containing Dulbecco's Modified Eagle Medium (DMEM, Invitrogen Corporations, Carlsbad, CA, USA) supplemented with 10% FBS (Invitrogen), 1% Penicillin–Streptomycin and 0.1% Gentamicin (Invitrogen). The tissues were triturated and plated on 10 cm culture dishes (Sigma Chemicals, St. Louis, MO, USA) at a density of approximately 2×105 cells per square centimeter. The cells were incubated at 37°C in an atmosphere of 5% CO2 in air. The medium was replaced after 24-hours to gentamicin-free growth media and was subsequently changed thrice a week after that. Confluent monolayers of astrocytes were used between passages 2-4.
Hippocampal neuron culture
Primary cultures of hippocampal neurons were established according to protocols described by Stein and Chetkovich (2010) and Nunez (2008) with minor modifications. The hippocampus was removed from 2-3 day old Sprague-Dawley rat pups and digested in 0.05% Trypsin-EDTA (Invitrogen) for 20 minutes at 37°C with gentle shaking. Digestion was stopped by washing the pellet three times with neuronal growth media containing Neurobasal medium (Invitrogen) supplemented with 10% FBS (Invitrogen) and 0.5 mM Glutamine (Invitrogen). The tissue was then triturated with a sterile pipette and plated onto appropriate dishes precoated with poly-D-lysine (0.1 mg/mL in 0.1 M borate buffer, pH 8.5; Sigma) at a density of approximately 1×105 cells per square centimeter. The cells were incubated at 37°C in an atmosphere of 5% CO2 in air. Two hours after plating the neuronal media was changed to serum-free growth media (Neurobasal media supplemented with 2% B-27(Invitrogen) with 0.5 mM Glutamine) and 5 μM C-Arabinoside (Sigma) was added 24 hours later to the media to inhibit glial growth. Thereafter, the media was changed every third day. The cultures contained mostly pure population of neurons (∼95%) verified by staining with neuronal marker, NeuN and very minimal astrocyte contamination (∼5%), confirmed by staining with glial fibrillary acidic protein.
Amyloid β preparation
A soluble form of oligomeric Aβ 1-42 was prepared as described by Stine et al in 2003. The amorphous powder (Sigma) was suspended in 1 mM 1,1,1,3,3,3-hexafluoro-2-prpanol (HFIP, Sigma) and aliquoted. The HFIP was then evaporated to dryness in a speed-vac system and the resulting film was stored at -80°C until further use. 24 hour prior to use the peptide film was dissolved in 5 mM DMSO (Sigma), sonicated for 10 min and then diluted in 200 μM DMEM for oligomerization. The formation of the oligomers was detected by Western blot (Stine et al., 2003). The vehicle control was prepared by diluting equivalent amounts of DMSO in media.
CYP 450 epoxygenase assay
For the enzyme activity assay the microsomes were preincubated with two concentrations of Aβ (1 and 10 μM) or vehicle for 30 minutes followed by 50 μM of epoxygenase substrate arachidonic acid (AA) for 30 minutes. The microsomes (0.5 mg/ml) were added to an assay buffer containing 100 mM phosphate buffer pH 7.4 containing 5 mM MgCl2, 1 mM EDTA, and 1 mM nicotinamide adenine dinucleotide phosphate (NADP) in the presence of NADPH regenerating system (10 mM isocitrate and 0.4 U/mL isocitrate dehydrogenase) at 37°C with gentle shaking. The reaction was stopped by the addition of 1 M formic acid and the metabolites were extracted with ethyl acetate (Sigma) after addition of 5 ng of internal deuterated fatty acid standard (20-HETE-d6). For the cultured cells: The cultures were serum-starved at least 24 h prior to the assay and were incubated with 50 μM Arachidonic acid for 60 minutes at the start of the assay. After the incubation, the plates were washed with Dulbecco's Phosphate Buffer Solution (DPBS, Invitrogen) 3 times to get rid of excess unbound AA and were then incubated with 1 and 10 μM Aβ or the vehicle for 60 minutes in DMEM containing 0.1% BSA (Sigma). After 60 minutes the cells and the media were transferred to extraction tubes containing 5 ng of internal deuterated fatty acid standard (20-HETE-d6) and diethyl ether.
The lipid component of the reaction was extracted twice with 2 ml of ethyl acetate or diethyl ether, back-extracted with 1 ml of distilled water and then the organic layer was evaporated to dryness under nitrogen. Production of total EETs were measured by Liquid Chromatography–Mass Spectrometry (LC/MS) in the Biochemistry Core.
The samples were reconstituted with ethanol and metabolites of AA were separated by HPLC on a Zorbax Eclipse Plus C18 Column (100×2.1 mm, 3.5 μm; Agilent Technologies, New Castle, DE) at a flow rate of 0.3 mL/min. The metabolites were eluted using a mixture of acetonitrile: methanol: water: acetic acid over a step gradient from 17:3:80:0.01 to 68:12:20:0.01 for 15 minutes. The effluent was ionized using negative ion electrospray and peaks eluting with a mass/charge ratio (m/z) of 319>301 (HETEs and EETs), 337>319 (DiHETEs), or 323>270 (internal standard, 20-HETE-d6) were monitored in multiple reaction monitoring mode using a triple quadrapole mass spectrometer (API 3000, Applied Biosystems, Foster City, CA, USA). The ratio of ion abundance in the peaks of interest versus that seen in the internal standard were determined and compared with standard curves generated with authentic EETs and DiHETEs over a range from 0.2 to 10 ng. Individual EETs regioisomers were identified based on their m/z ratio and specific retention time (DiHETEs: 12-14 min; EETs: 18-20 minutes). The x-axis represents run time (minutes) and y-axis represents counts per second (cps)(Zagorac et al., 2008,Dunn et al., 2008).
Data analysis
Mean value ± s.e.m. are presented. EETs and DiHETE production in presence of Aβ are expressed as percentage of vehicle control. The differences between the mean values of the groups were assessed using One-way analysis of variance (ANOVA) followed by a post-hoc Dunnett's test (when comparing to control) or Holm-Sidak test (when comparing all pair-wise groups). P < 0.05 was considered to be significant. Each set of experiments were performed in duplicates and at least 3 replicable experiments were analyzed in each group.
Results
Regional distribution of epoxygenase activity in the brain
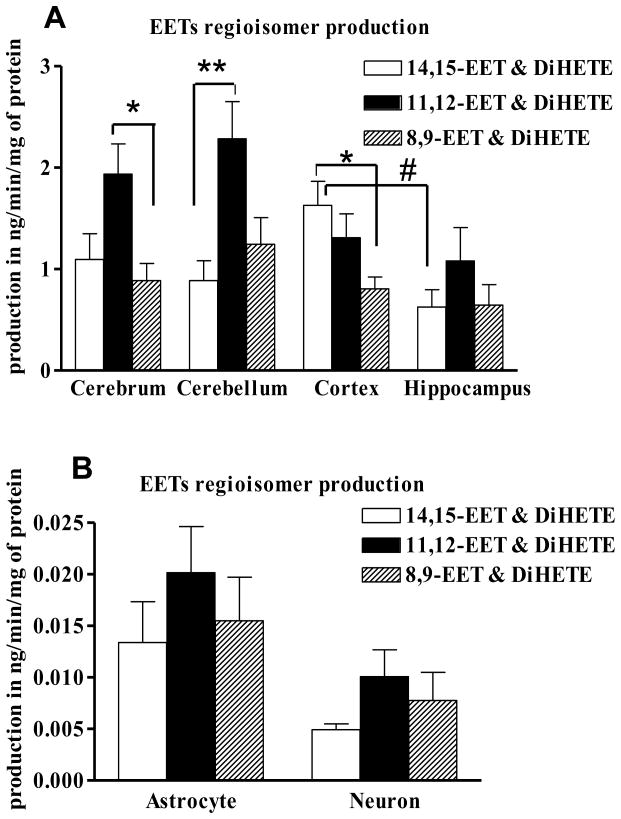
To define the profile of epoxygenase activity in different regions of the brain, we examined individual regioisomer production in the microsomes and cultured cells as described under methods section by Mass Spectrometry. Since EETs are rapidly degraded into the DiHETE form (Zeldin, 2001), epoxygenase activity is more accurately expressed as the summation of total EET and DiHETE production in nanograms per minute per milligram of microsomal proteins. Similarly, individual regioisomer production is measured by combining the EET and its corresponding DiHETE form. 11,12-EET is the more abundant regioisomer in cerebrum, cerebellum and hippocampus (50%, 52% and 44% of total EETs and DiHETEs production, respectively). In cortical microsomes, 14,15-EET (44%) was almost equal to that of 11,12-EET (34%). 8,9-EET (21-25%) did not appear to be the main product in any region (Fig. 1A). The total EETs and DiHETEs production did not vary significantly between cerebrum, cerebellum, cortex and hippocampus (3.9 ± 1.3, 4.4 ± 1.6, 3.7 ± 1.1 and 2.35 ± 1 ng/min/mg of protein, respectively).
Fig. 1.
Differential production of EETs regioisomers in brain microsomes (A) and cultured neonatal hippocampal astrocytes and neurons (B). Microsomes from different areas of the brain and cultured cells were incubated with arachidonic acid (50 μM) for 30 and 60 minutes respectively. The products of arachidonic acid metabolism by the epoxygenase pathway were detected and quantified by Mass Spectrometry. Enzyme activity is expressed as the total of EETs and the corresponding DiHETE produced/min/mg of protein. 11,12-EET is the abundant product in most of the areas, along with 14,15-EET in the cortex. Data are represented as mean ± S.E.M., n= 4-5 per group. One-way ANOVA was used to determine the difference between regioisomers of one specific area and the difference between the areas for one specific regioisomer. *P<0.05; **P<0.01 for intra-group variation and #P<0.05 for inter-group variation.
Cultured astrocytes and neurons were incubated with arachidonic acid (50 μM) for 60 minutes for uptake in the cell, after which the excess was washed off by replacing the media. After incubation with the vehicle, the cell and media were collected for quantification of EETs synthesis and release. Similar to hippocampal microsomes, 11,12-EET (42% and 43% for astrocytes and neurons, respectively) was the more abundant product in the cultured cells (Figure 1B). Total epoxygenase activity was calculated to be 0.03 ± 0.01 and 0.02 ± 0.01 ng of EETs and DiHETEs/ min/ mg of protein.
Aβ reduces 14,15-EET production in the cerebrum but not in the cerebellum
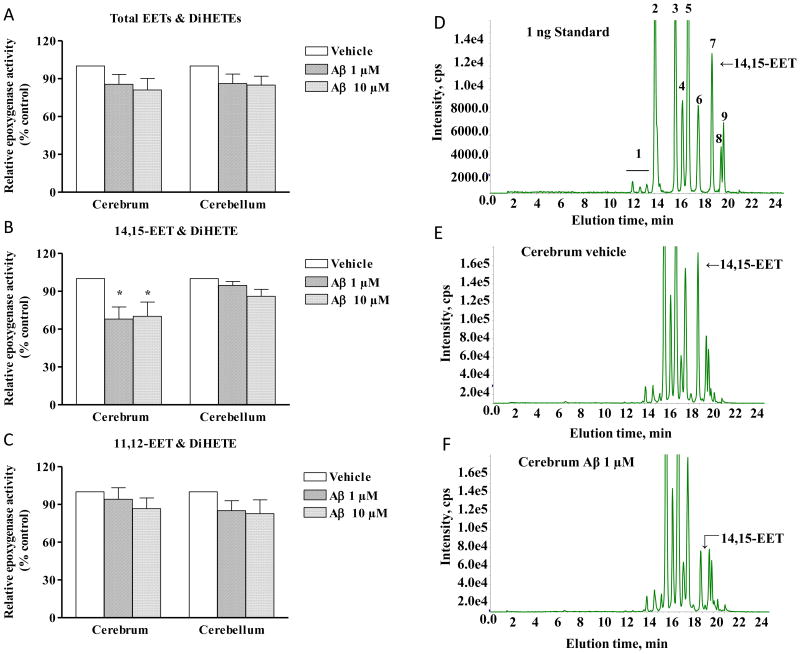
Microsomes obtained from cerebrum and cerebellum (n=4-5) were incubated with two concentrations of soluble oligomers of Aβ (1 and 10 μM) or vehicle for 30 minutes. Epoxygenase activity was measured as total EET & DiHETE production (Fig. 2A) and expressed as percentage of vehicle. Although there was a trend for reduced EET production in both cerebrum and cerebellum no significant difference was observed with or without the presence of Aβ. We therefore looked into the production of individual regioisomers and found that in presence of both concentrations of Aβ, total 14,15-EET production (expressed as a summation of 14,15-EET and its corresponding DiHETE product, Fig. 2B) was significantly decreased in the cerebrum (p<0.05), but not in the cerebellum. Aβ had no effect on 11,12-EET production (Fig. 2C) in both regions of the brain as compared to control.
Fig. 2.
Effect of Aβ on total EET & DiHETE (A), 14,15-EET & DiHETE (B) and 11,12-EET & DiHETE (C) production in cerebrum and cerebellum. Microsomes isolated from cerebrum and cerebellum were preincubated with Aβ or vehicle for 30 minutes, followed by incubation with arachidonic acid (50μM, 30 minutes) and the products were detected by Mass Spectrometry. Representative Liquid chromatography-mass spectrometry chromatograms illustrate the profile of CYP 450 eicosanoids in 1 ng standard (D), cerebral microsomal tissue incubated with vehicle (E) and 1 μM Aβ (F).Enzyme activity is expressed as the total of EETs and the corresponding DiHETE produced/min/mg of protein. Relative activity is shown by calculating the percentage change from vehicle set at 100%. Aβ causes a decrease in 14,15-EET & DiHETE production in cerebrum but not in cerebellum. Data are represented as mean ± S.E.M., n= 4-5 per group. One-way ANOVA was used to determine the difference between groups. *P<0.05 compared to vehicle. cps, counts per second. Peaks as denoted by numbers are: 1. 14,15-, 11,12- and 8,9 DiHETE, 2. 20-HETE, 3. 15-HETE, 4. 11-HETE, 5. 12/8 HETE, 6. 5-HETE, 7. 14,15-EET, 8. 11,12-EET & 9. 8,9 EET.
Cortical and hippocampal microsomes show a reduction in 11,12-EET & DiHETE production in presence of Aβ
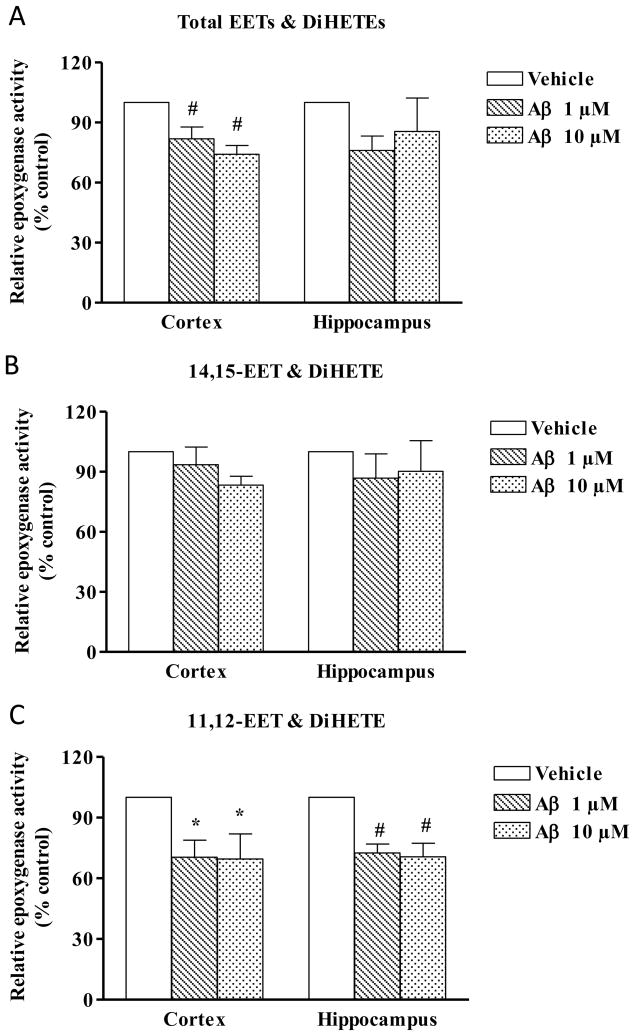
In this experiment, cerebrum was further divided into cortex and hippocampus and the CYP epoxygenase activity were determined in microsomes prepared from these two tissues. The total production of EETs (Fig. 3A) was significantly reduced in the cortex (n=5, p<0.01) when incubated with 1 μM and 10 μM Aβ, but not in the hippocampus (n=4). Upon further analysis of the regioisomers it was seen that 11,12- (Fig. 3C), not 14,15-EET (Fig.3B) was decreased in presence of Aβ in both cortex (p<0.05) and hippocampus (p<0.01).
Fig. 3.
Effect of Aβ on total EET & DiHETE (A), 14,15-EET & DiHETE (B) and 11,12-EET & DiHETE (C) production in cortex and hippocampus. Microsomes isolated from cortex and hippocampus was preincubated with Aβ or vehicle for 30 minutes, followed by incubation with arachidonic acid (50μM, 30 minutes) and the products were detected by Mass Spectrometry. Enzyme activity is expressed as the total of EETs and the corresponding DiHETE produced/min/mg of protein. Relative activity is shown by calculating the percentage change from vehicle set at 100%. Both cortex and hippocampus show a reduction in 11,12-EET & DiHETE production in presence of Aβ. Data are represented as mean ± S.E.M., n= 4-5 per group. One-way ANOVA was used to determine the difference between groups. *P<0.05, #P<0.01 compared to vehicle.
Astrocytes and neurons show a decrease in 14,15-EET & DiHETE production with Aβ but 11,12-EET & DIHETE are decreased only in astrocytes
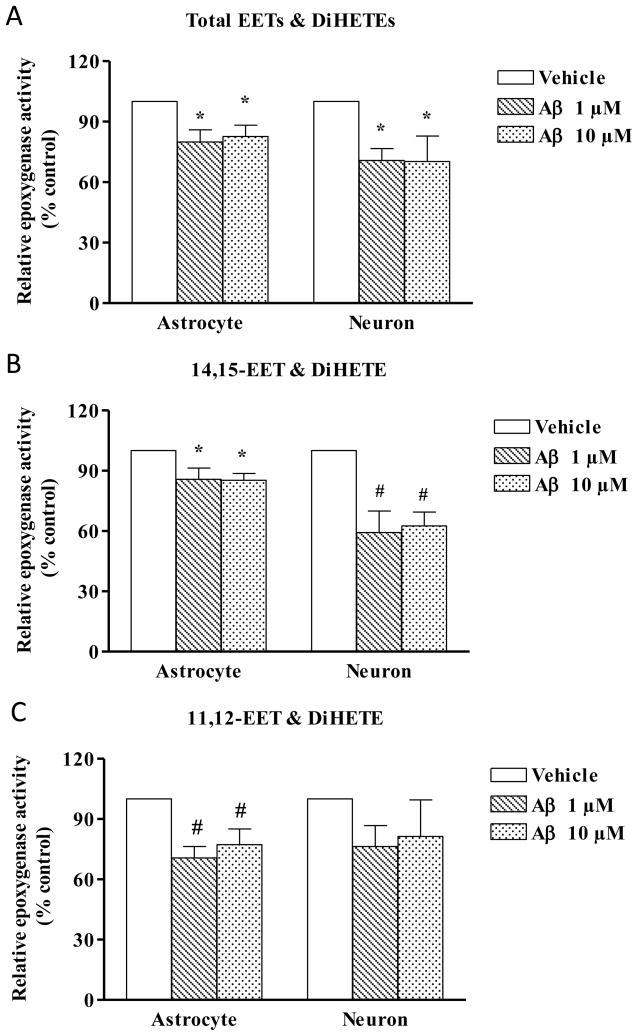
To further validate the findings of ex vivo experiments, we carried out studies in individual cell types such as cultured astrocytes and neurons (n=4-7) to determine if Aβ has any differential effects on these cell types. Total EETs and DiHETEs (Fig. 4A) production was decreased in astrocytes with both 1 μM (p<0.01) and 10 μM Aβ (p<0.05). In the neurons, the decrease was 70% for both doses (p<0.05). 14,15-EET (Fig. 4B) was decreased in astrocytes to 85% by both doses of Aβ, but only to 60% in neurons (p<0.01). When treated with Aβ, 11,12-EET (Fig. 4C) production was decreased in astrocytes (70-77%, p<0.01), but not in neurons.
Fig. 4.
Effect of Aβ on total EET & DiHETE (A), 14,15-EET & DiHETE (B) and 11,12-EET & DiHETE (C) production in neonatal hippocampal astrocyte and neuronal culture. Neonatal hippocampal cultured astrocytes and neurons were incubated first with arachidonic acid (50μM, 60 minutes) and then with Aβ or vehicle for 60 minutes and the cells and media were collected for epoxygenase assay. Enzyme activity is expressed as the total of EETs and the corresponding DiHETE produced/min/mg of protein. Relative activity is shown by calculating the percentage change from vehicle set at 100%. Astrocytes and neurons show a decrease in 14,15-EET & DiHETE production with Aβ but 11,12-EET & DiHETE are decreased only in astrocytes. Data are represented as mean ± S.E.M., n= 4-7 per group. One-way ANOVA was used to determine the difference between groups. *P<0.05, #P<0.01 compared to vehicle.
Discussion
Our study demonstrates that acute exposure to soluble, oligomeric Aβ reduced the epoxygenase activity in brain microsomes and cultured cells. This disruption of the enzyme activity is, importantly, area- and regioisomer-specific. We also demonstrate the first anatomic site-specific production of EETs regioisomers in the brain. In particular we report that: (i) 11,12-EET is the more abundant regioisomer produced in microsomes from cerebrum, cerebellum and hippocampus; (ii) 14,15- and 11,12-EET are the main products in cortical microsomes; (iii) microsomes from cerebrum showed a reduction in 14,15-EET production in the presence of Aβ, while the cerebellum is spared from this effect; (iv) Aβ decreased production of 11,12-EET in cortical and hippocampal microsomes; and (v) 14,15-EET production is reduced in both cultured astrocytes and neurons, while 11,12-EET production is only decreased in astrocytes.
While the conversion of AA into its eicosanoid derivatives EETs and DIHETEs by the brain epoxygenases have been well established (Amruthesh et al., 1992, Gebremedhin et al., 1992), we report for the first time the activity profile of these enzymes in various areas of the brain. Specifically, we observed that epoxygenases in the whole forebrain and cerebellum produced 11,12-EET more than other regioisomers. In the cerebrum however, cortical microsomes generated significantly more 14,15-EET than from the hippocampus. The CYP epoxygenase isoforms (mainly the 2C, 2J family and 4×1) have different catalytic activity and are regioselective and stereoselective. Depending on the availability and catalytic strength of the CYP isoform, EETs production can vary widely in tissues and may have different regioisomers as the main product (reviewed by Zeldin, 2001). Interestingly, in the rat brain only two CYP epoxygenases, namely, CYP2C11 (Alkayed et al., 1996b) and CYP4×1 (Bylund et al., 2002) have been well characterized. RT-PCR screens however, have identified the expression of at least five lesser known CYP isoforms (Liff et al., 2010). So it may be possible that the distribution of CYP epoxygenase isoform is different in distinct areas of cerebrum resulting in heterogeneous distribution of EETs profile seen in our study. In the cultured astrocytes and neurons the regioisomeric distribution of EETs were 8,9 – 32%; 11,12 – 42% and 14,15 – 25%, which is in agreement with previous reports of epoxygenase activity in cells (Liff et al., 2010). Although it is unclear how the site-specific distribution of regioisomers affects that area, regioisomer-specific actions of EETs have been reported (Abdu et al., 2010, Alkayed et al., 1996b). These studies demonstrated that one specific regioisomer may be more potent than the others in one particular function. These observations suggest the intriguing possibility that the variation in the cytoprotective, pro-angiogenic and vasodilatory EETs in different brain regions may define how that area responds to Aβ-induced toxicity.
The mechanism for Aβ-mediated cytotoxicity is still unclear. It is reported to be mediated via the generation of hydrogen peroxide (H2O2) (Behl et al., 1994) which leads to oxidation of proteins, lipids and DNA (Markesbery and Carney, 1999). The superoxide production also leads to vasoconstriction (Niwa et al., 2001) and reduction in angiogenesis, thus aggravating the AD phenotype. Genetic (Park et al., 2008) or pharmacological manipulation to block or scavenge the superoxide reverses the effects of Aβ (Bruce et al., 1996, Dietrich et al., 2010). EETs, on the other hand have been shown to rescue or protect organs (Nithipatikom et al., 2006, Seubert et al., 2006, Gross et al., 2008) or cells (Dhanasekaran et al., 2006) against oxidative damages of H2O2, ischemia-reperfusion and other insults. They also dilate cerebral vessel (Gebremedhin et al., 1992) and mediate angiogenesis in brain microvasculature (Munzenmaier and Harder, 2000) making them relevant to study in context of AD. Furthermore, the inhibition of CYP epoxygenases renders neuronal cells more susceptible to oxidative damage (Liu and Alkayed, 2005, Gross et al., 2009). Hence, our finding that the epoxygenase product 14,15-EET is decreased in microsomes from cerebrum by Aβ, but not in microsomes from the cerebellum, may provide an explanation as to why the cerebrum is more prone to neurodegeneration by Aβ compared to the cerebellum. Although the mechanism as to how Aβ affects epoxygenase activity is not known, we postulate that a difference in the EET-dependent downstream signaling pathway between these two areas may contribute to the region-specificity of Aβ.
The cerebral atrophy seen in AD patients also has a distinct temporal pattern as described by numerous histopathological and MRI-based studies (Braak and Braak, 1991, 1998, Whitwell et al., 2007). The neurodegeneration begins in the entorhinal cortex and hippocampus, slowly progresses toward limbic cortex and finally affects the association cortex (Braak stages 1-6 for classification of AD). A clinical diagnosis of AD is made at the last stages 5 and 6, when cortical destruction is severe. The hippocampus, on the other hand, shows progressive change throughout the disease (Rossler et al., 2002) and these changes are well correlated with disease progression and pathology (Fox et al., 1996, Jack et al., 1997). Based on these observations, we further attempted to dissect the cerebrum into cortex and hippocampus, to investigate if these two areas have any difference in epoxygenase activity. To our surprise, we found that 11,12-EET is decreased in both cortex and hippocampus, while 14,15-EET is reduced in the cerebrum. The fact that Aβ still affects epoxygenase activity in these regions, but the outcome is reduction of a different regioisomer suggests three plausible explanations: (i) the CYP isoform distribution in cortex and hippocampus can be different from the rest of the cerebrum; (ii) Several pharmacological agents act as secondary mediators to change the regiospecificity of the CYP epoxygenases (Karara et al., 1989). So the possibility of Aβ affecting CYP isoforms in such a way that it reduces regiospecificity for 11,12-EET in the cortex and hippocampus and for 14,15-EET in the rest of the cerebrum cannot be ruled out. (iii) The presence of cerebral resistance vessels in the whole cerebrum but not in the cortical or hippocampal tissue also points toward the fact that cerebral vessels may have an altogether different activity profile in presence of Aβ. The vascular endothelium produces a significant amount of EETs in the brain (Gebremedhin et al., 1992). Since these vessels undergo severe endothelial dysfunction in AD patients and mice (Niwa et al., 2001), future experiments should assess the effect of Aβ on the epoxygenase activity in these vessels.
The EET/DiHETE ratio (Table 1) remains almost unaffected in presence of Aβ, indicating that Aβ reduces EETs production but not the degradation. Hence, it is likely that Aβ has no short-term effect on soluble epoxide hydrolase (sEH). However, an effect from chronic Aβ exposure cannot be ruled out as it has been shown to upregulate the microsomal enzyme, mEH (Liu et al., 2006).
Table 1.
The effect of Aβ on the EET/DiHETE ratio in the tissues and in the cells. The total EET production from each experiment was divided by the total DiHETE production from the same experiment to obtain the EET/DiHETE ratio. No significant difference is seen between the vehicle and Aβ-treated groups. Data are represented as mean ± S.E.M., n= 4-7 per group. One-way ANOVA was used to determine the difference between groups.
| Vehicle | Aβ 1 μM | Aβ 10 μM | |
|---|---|---|---|
| Cerebrum | 8.243 ±(2.30) | 7.295 ±(1.94) | 7.099 ±(1.95) |
| Cerebellum | 8.725 ±(2.67) | 8.552 ±(2.5) | 8.241 ±(2.38) |
| Cortex | 8.348 ±(1.52) | 7.736 ±(1.73) | 6.520 ±(1.25) |
| Hippocampus | 17.027 ±(7.74) | 14.659 ±(7.7) | 13.290 ±(5.87) |
| Astrocyte | 2.821 ±(0.4) | 2.498 ±(0.35) | 2.599 ±(0.31) |
| Neuron | 2.747 ±(0.85) | 2.489 ±(0.63) | 2.087 ±(0.47) |
±(SEM)
The microsomes used in this study are enriched preparations of CYP enzymes in the endoplasmic reticulum and plasma membrane and are devoid of cytoplasmic or nuclear elements. While microsomal epoxygenase assays provide an idea as to how a pharmacological agent might affect the enzyme's activity, it is not possible to differentiate the response of individual cell types in the tissue. Thus we looked into the effect of Aβ on the epoxygenase activity of cultured neonatal hippocampal astrocytes and neurons and found that Aβ reduces total EETs and DiHETE production in both cell types. The regioisomers however were again found to be differentially affected in the presence of Aβ. Astrocytes showed reduction in 14,15- and 11,12-EET, while only 14,15-EET was affected in the neurons. This further strengthens the possibility of identifying an already existing difference in CYP epoxygenase isoform between cell types and/or the capability of Aβ to change the regiospecificity of an enzyme in future studies.
In the normal brain, increase in neuronal activity leads to glutamate release that is taken up by the astrocytes which in turn release EETs to the surrounding blood vessels signaling them to dilate, hence matching an increase in metabolic demand with heightened nutrient supply, a phenomenon widely known as functional hyperemia (Alkayed et al., 1997). Since blocking EETs synthesis can disrupt functional hyperemia (Peng et al., 2002), an Aβ-induced reduction in astrocytic EETs from this study fits perfectly with the severe neurovascular dysregulation found in both AD patients and mice (Park et al., 2004). Furthermore, reduction in neuronal EETs synthesis with Aβ might suggest a possible decrease in neurogenic control of blood vessels (Iliff et al., 2009) and stunted axonal growth (Abdu et al., 2010). These speculations are interesting and worth pursuing, but they need to be verified by in vivo studies with adult animals as onset of AD pathology is age-dependent (Gao et al., 1998) and these cells were derived from neonates as long-term survival of adult cells in vitro has been difficult to establish.
Although this is the first study to report that Aβ affects epoxygenase activity, a recent in vivo study has reported that endogenous 14,15-EET is elevated in AD mice in the cortex and the hippocampus(Sanchez-Mejia et al., 2008). Sanchez-Mejia et al (2008) observed that an increase in the group IVA-Phospholipase A2 (GIVA-PLA2) activity leads to an increase in AA release and a concomitant increase in AA products which exacerbate the AD pathology. Although the findings from this study differ from ours, a direct comparison cannot be made due to some fundamental differences in experimental design. Sanchez-Mejia et al. have investigated the long-term effect of Aβ with 2 months old animals and have measured endogenous EETs production while we examined short-term effect of Aβ on epoxygenase activity by adding a fixed concentration of AA to cells or microsomes. The elevated EETs seen in their study can be a compensatory response to chronic exposure to Aβ. Also, AA release can lead to increase in EETs as well as other products like 20-HETE and COX products which are pro-inflammatory and pro-apoptotic (Wyss-Coray and Mucke, 2002, Dunn et al., 2008, Renic et al., 2009) which might render the increase in EETs ineffective. Complete blockade of AA release on the other hand would block the beneficial EETs. Hence, it would be interesting to design future experiments where the beneficial effect of EETs can be explored by selectively upregulating the epoxygenase pathway (Zheng et al., 2010) or blocking EETs breakdown by sEH inhibition (Seubert et al., 2006) in AD models.
Conclusion
In summary, EETs production is regioisomer and anatomic site specific in microsomal preparations from cerebrum, cerebellum, cortex and hippocampus. Short-term incubation with soluble, oligomers of Aβ can decrease epoxygenase activity in rat brain microsomes and cultured hippocampal astrocytes and neurons. The regioisomers that are mostly affected are 14,15- and 11,12-EET, although the pattern varies between regions of the brain and cell types. Cerebellum shows no decrease in epoxygenase activity indicating that Aβ-induced reduction in synthesis of cytoprotective EETs may render the cerebrum more susceptible to neurodegeneration caused by Aβ.
Highlights.
Amyloid β reduces cytoprotective EETs production in brain microsomes
Cerebrum shows decreased 14,15 –EET production in presence of Aβ, not cerebellum
Total 11,12 –EET production is reduced in cortex and hippocampus in presence of Aβ
Astrocyte and neuronal cultures also show decreased epoxygenase activity with Aβ
Acknowledgments
We acknowledge Drs. Meetha Medhora and Maia Terashvili for their useful comments and feedback on the manuscript. This work was funded by NIH/NHLBI RO1 HL033833, RO1 HL092105 and PO1 HL059996.
Abbreviations
- Aβ
Amyloid Beta
- AD
Alzheimer's disease
- EET
epoxyeicosatrienoic acid
- DiHETE
dihydroxyeicosatrienoic acid
- CYP 450
Cytochrome P450
- sEH
soluble epoxide hydrolase
- AA
arachidonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdu E, Bruun DA, Yang D, Yang J, Inceoglu B, Hammock BD, Alkayed NJ, Lein PJ. Epoxyeicosatrienoic acids enhance axonal growth in primary sensory and cortical neuronal cell cultures. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.07139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkayed NJ, Birks EK, Hudetz AG, Roman RJ, Henderson L, Harder DR. Inhibition of brain P-450 arachidonic acid epoxygenase decreases baseline cerebral blood flow. Am J Physiol. 1996a;271:H1541–1546. doi: 10.1152/ajpheart.1996.271.4.H1541. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28:1066–1072. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996b;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Amruthesh SC, Falck JR, Ellis EF. Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem. 1992;58(2):503–10. doi: 10.1111/j.1471-4159.1992.tb09749.x. [DOI] [PubMed] [Google Scholar]
- Barclay LL, Brady PA. Cerebellar atrophy as a CT marker for mixed dementia. Biol Psychiatry. 1992;31:520–524. doi: 10.1016/0006-3223(92)90263-y. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Transm Suppl. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- Bruce AJ, Malfroy B, Baudry M. beta-Amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci U S A. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund J, Zhang C, Harder DR. Identification of a novel cytochrome P450, CYP4×1, with unique localization specific to the brain. Biochem Biophys Res Commun. 2002;296(3):677–84. doi: 10.1016/s0006-291x(02)00918-x. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Chen JK, Capdevila J, Harris RC. Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Mol Cell Biol. 2001;21:6322–6331. doi: 10.1128/MCB.21.18.6322-6331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291:H517–531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- Dietrich HH, Xiang C, Han BH, Zipfel GJ, Holtzman DM. Soluble amyloid-beta, effect on cerebral arteriolar regulation and vascular cells. Mol Neurodegener. 2010;5:15. doi: 10.1186/1750-1326-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KM, Renic M, Flasch AK, Harder DR, Falck J, Roman RJ. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H2455–2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119(Pt 6):2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The Relationships Between Age, Sex, and the Incidence of Dementia and Alzheimer Disease: A Meta-analysis. Arch Gen Psychiatry. 1998;55(9):809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263:H519–525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Gauthier KM, Moore J, Campbell WB, Falck JR, Nithipatikom K. Evidence for role of epoxyeicosatrienoic acids in mediating ischemic preconditioning and postconditioning in dog. Am J Physiol Heart Circ Physiol. 2009;297:H47–52. doi: 10.1152/ajpheart.01084.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol. 2008;294:H2838–2844. doi: 10.1152/ajpheart.00186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang R, Zeldin DC, Alkayed NJ. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol. 2009;296:H1352–1363. doi: 10.1152/ajpheart.00950.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Jia J, Nelson J, Goyagi T, Klaus J, Alkayed NJ. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010;91(3-4):68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, Xu YC, Waring SC, O'Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karara A, Dishman E, Blair I, Falck JR, Capdevila JH. Endogenous epoxyeicosatrienoic acids. Cytochrome P-450 controlled stereoselectivity of the hepatic arachidonic acid epoxygenase. J Biol Chem. 1989;264:19822–19827. [PubMed] [Google Scholar]
- Kim HJ, Chae SC, Lee DK, Chromy B, Lee SC, Park YC, Klein WL, Krafft GA, Hong ST. Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. Faseb J. 2003;17:118–120. doi: 10.1096/fj.01-0987fje. [DOI] [PubMed] [Google Scholar]
- Kuperstein F, Yavin E. ERK activation and nuclear translocation in amyloid-beta peptide- and iron-stressed neuronal cell cultures. Eur J Neurosci. 2002;16:44–54. doi: 10.1046/j.1460-9568.2002.02056.x. [DOI] [PubMed] [Google Scholar]
- Li YT, Woodruff-Pak DS, Trojanowski JQ. Amyloid plaques in cerebellar cortex and the integrity of Purkinje cell dendrites. Neurobiol Aging. 1994;15:1–9. doi: 10.1016/0197-4580(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25:939–948. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- Liu M, Sun A, Shin EJ, Liu X, Kim SG, Runyons CR, Markesbery W, Kim HC, Bing G. Expression of microsomal epoxide hydrolase is elevated in Alzheimer's hippocampus and induced by exogenous beta-amyloid and trimethyl-tin. Eur J Neurosci. 2006;23:2027–2034. doi: 10.1111/j.1460-9568.2006.04724.x. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J, Rosen KM, Smith RC, Walsh K, Gouras GK, Querfurth HW. Intraneuronal beta-amyloid expression downregulates the Akt survival pathway and blunts the stress response. J Neurosci. 2005;25:10960–10969. doi: 10.1523/JNEUROSCI.1723-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR, Carney JM. Oxidative alterations in Alzheimer's disease. Brain Pathol. 1999;9:133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, Harder DR. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2003;284:H215–224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- Michaelis UR, Fisslthaler B, Barbosa-Sicard E, Falck JR, Fleming I, Busse R. Cytochrome P450 epoxygenases 2C8 and 2C9 are implicated in hypoxia-induced endothelial cell migration and angiogenesis. J Cell Sci. 2005;118:5489–5498. doi: 10.1242/jcs.02674. [DOI] [PubMed] [Google Scholar]
- Mullan M, Crawford F. The molecular genetics of Alzheimer's disease. Mol Neurobiol. 1994;9:15–22. doi: 10.1007/BF02816100. [DOI] [PubMed] [Google Scholar]
- Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H537–542. doi: 10.1152/ajpheart.00071.2006. [DOI] [PubMed] [Google Scholar]
- Niwa K, Porter VA, Kazama K, Cornfield D, Carlson GA, Iadecola C. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001;281:H2417–2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- Nunez J. Primary Culture of Hippocampal Neurons from P0 Newborn Rats. JoVE. 2008;19 doi: 10.3791/895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis E, Douillard H, Koutroumanis M, Goodyer C, LeBlanc A. Amyloid beta peptide of Alzheimer's disease downregulates Bcl-2 and upregulates bax expression in human neurons. J Neurosci. 1996;16:7533–7539. doi: 10.1523/JNEUROSCI.16-23-07533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Forster C, Kazama K, Carlson GA, Iadecola C. Abeta-induced vascular oxidative stress and attenuation of functional hyperemia in mouse somatosensory cortex. J Cereb Blood Flow Metab. 2004;24:334–342. doi: 10.1097/01.WCB.0000105800.49957.1E. [DOI] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283:H2029–2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renic M, Klaus JA, Omura T, Kawashima N, Onishi M, Miyata N, Koehler RC, Harder DR, Roman RJ. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2009;29:629–639. doi: 10.1038/jcbfm.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler M, Zarski R, Bohl J, Ohm TG. Stage-dependent and sector-specific neuronal loss in hippocampus during Alzheimer's disease. Acta Neuropathol. 2002;103:363–369. doi: 10.1007/s00401-001-0475-7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou Y, Halabisky B, Cisse M, Scearce-Levie K, Cheng IH, Gan L, Palop JJ, Bonventre JV, Mucke L. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat Neurosci. 2008;11:1311–1318. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Schenk D. Alzheimer's disease: molecular understanding predicts amyloid-based therapeutics. Annu Rev Pharmacol Toxicol. 2003;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- Serrano F, Chang A, Hernandez C, Pautler RG, Sweatt JD, Klann E. NADPH oxidase mediates beta-amyloid peptide-induced activation of ERK in hippocampal organotypic cultures. Mol Brain. 2009;2:31. doi: 10.1186/1756-6606-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, Tsai HJ, Hammock BD, Imig JD. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174:2086–2095. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein ELA, Chetkovich DM. Regulation of stargazin synaptic trafficking by C-terminal PDZ ligand phosphorylation in bidirectional synaptic plasticity. J Neurochem. 2010;113:42–53. doi: 10.1111/j.1471-4159.2009.06529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Chen W, Murphy E, Gabel S, Tomer KB, Foley J, Steenbergen C, Falck JR, Moomaw CR, Zeldin DC. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J Biol Chem. 1997;272:12551–12559. doi: 10.1074/jbc.272.19.12551. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Young KF, Pasternak SH, Rylett RJ. Oligomeric aggregates of amyloid beta peptide 1-42 activate ERK/MAPK in SH-SY5Y cells via the alpha7 nicotinic receptor. Neurochem Int. 2009;55:796–801. doi: 10.1016/j.neuint.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Zagorac D, Jakovcevic D, Gebremedhin D, Harder DR. Antiangiogenic effect of inhibitors of cytochrome P450 on rats with glioblastoma multiforme. J Cereb Blood Flow Metab. 2008;28:1431–1439. doi: 10.1038/jcbfm.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–2078. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Wang L, Li R, Ma B, Tu L, Xu X, Dackor RT, Zeldin DC, Wang DW. Gene delivery of cytochrome p450 epoxygenase ameliorates monocrotaline-induced pulmonary artery hypertension in rats. Am J Respir Cell Mol Biol. 2010;43:740–749. doi: 10.1165/rcmb.2009-0161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]