Although psychosis (e.g., hallucinations and delusions) is the most striking clinical feature of schizophrenia, multiple lines of evidence indicate that impairments in cognition represent the core of the illness, as these deficits are present and progressive years before the onset of psychosis[1]. The cognitive deficits span multiple domains, suggesting that they reflect an overarching alteration in cognitive control, the ability to adjust thoughts or behaviors in order to achieve goals[2]. Cognitive control depends on the coordinated activity of a number of brain regions, including the dorsolateral prefrontal cortex (DLPFC). Subjects with schizophrenia exhibit altered activation of the DLPFC[3], and reduced frontal lobe gamma band (30–80 Hz) oscillations, while performing tasks that require cognitive control[4;5].
Because gamma oscillations require strong and fast inhibition from GABA neurons[6], alterations in DLPFC GABA neurotransmission have been hypothesized to contribute to altered gamma oscillations and impaired cognition in schizophrenia[7]. For example, administration of iomazenil, an inverse agonist at the benzodiazepine site of GABAA receptors that negatively modulates GABA neurotransmission, exacerbates symptoms in subjects with schizophrenia at doses that do not affect healthy controls[8]. Consistent with this interpretation, manipulations in animal models that reduce GABA-mediated inhibition diminish gamma oscillations[9] and impair cognitive function[10;11]. In contrast, treatment with a positive allosteric modulator of GABAA receptors was associated with increased frontal lobe gamma oscillations during a cognitive control task in subjects with schizophrenia[12].
The cellular basis for altered GABA neurotransmission in schizophrenia appears to include a presynaptic deficit in GABA synthesis. For example, although cortical expression of the 65 kD isoform of glutamic acid decarboxylase (GAD65) appears to be normal or only modestly altered in schizophrenia[13;14], transcript levels of GAD67, which is responsible for most GABA synthesis in the cortex[15], have been consistently found to be lower in the DLPFC of subjects with schizophrenia[16]. Lower levels of GAD67 mRNA are particularly prominent in the subclass of GABA neurons that express the calcium-binding protein parvalbumin (PV)[17]. Importantly, in vivo studies using optogenetic techniques have demonstrated that PV neuron activity is essential for driving cortical gamma oscillations[18;19].
Role of PV basket neurons in gamma oscillations
Cortical PV-containing GABA neurons consist of two major types: chandelier or axoaxonic cells which innervate the axon initial segment (AIS) of pyramidal neurons, and basket cells which innervate the cell body and proximal dendrites and spines of pyramidal neurons[20–22]. The location of these inputs onto the perisomatic membrane compartment indicates that both cell types are capable of activating relatively large GABAA receptor conductances near the site of action potential initiation in the AIS of pyramidal neurons. Because the strength of synaptic GABAA receptor conductance is determined by its proximity to the site of spike initiation[23], chandelier cells would be predicted to have the strongest inhibitory power.
However, synaptic inputs from neocortical chandelier cells actually appears to be excitatory, as stimulation of these cells can initiate spikes in postsynaptic pyramidal cells[24]. This excitatory effect may be due to much lower levels of the chloride transporter KCC2, which extrudes chloride, at the AIS relative to the soma or dendrites[24;25]. The resulting higher intracellular levels of chloride in the AIS would lead to a depolarizing postsynaptic GABAA receptor current[26]. Indeed, under experimental conditions that preserve the physiological intracellular chloride concentration, chandelier cell inputs depolarize postsynaptic pyramidal neurons, whereas inputs from PV-containing basket cells are hyperpolarizing[24;27], consistent with higher levels of KCC2 transporters in the somatic membrane[24;25]. In contrast, paired recording experiments in the hippocampus have demonstrated that inputs from both chandelier and basket neurons hyperpolarize pyramidal neurons[28], even though low levels of KCC2 are found at the AIS membrane of hippocampal pyramidal cells[25]. Understanding and reconciling these differences in the functional properties of chandelier cell inputs[29] is critical to determining how alterations in these neurons contribute to cortical circuit dysfunction in schizophrenia[30].
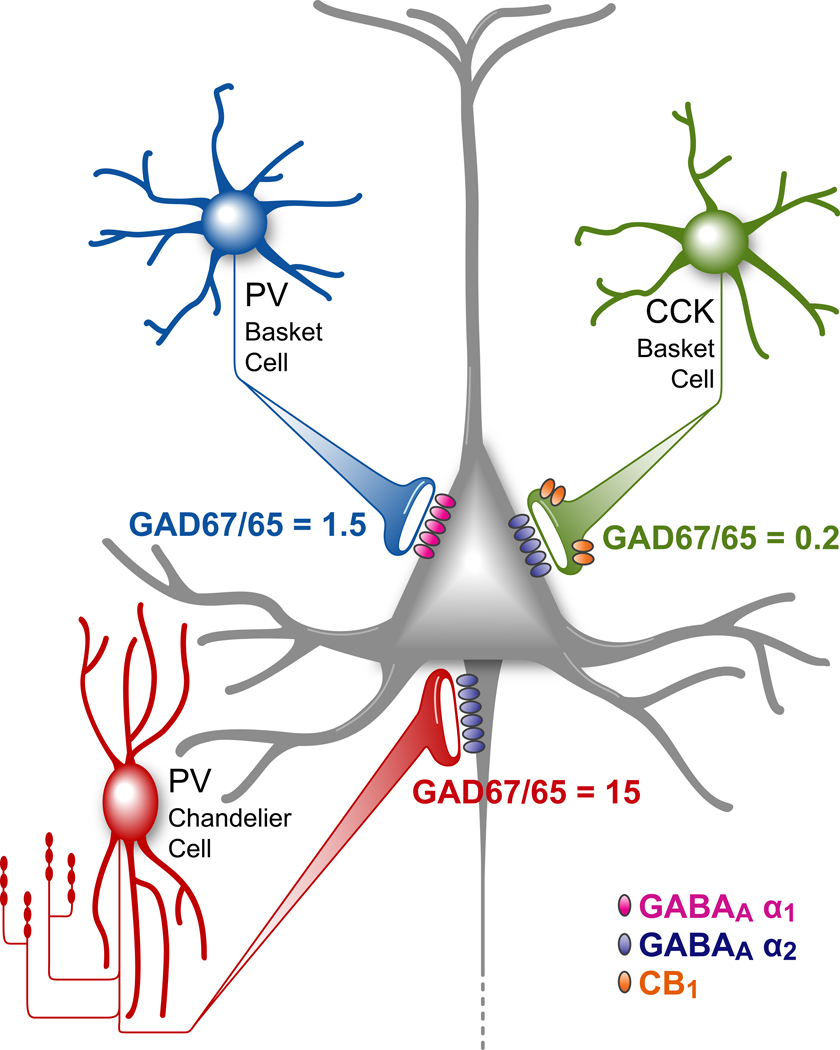
Important functional differences among PV-containing chandelier and basket neurons, and the class of basket neurons that express the cannabinoid receptor 1 (CB1R) and the neuropeptide cholecystokinin (CCK)[31], are suggested by findings that the relative levels of GAD67 and GAD65 proteins in axon terminals differ substantially across these cell types[32]. The GAD67/GAD65 ratio is ~1.5 in the axon terminals of PV-containing basket cells, but the ratio is very high (> 15) in chandelier cell axon terminals and very low (< 0.2) in the axon terminals of CB1R basket cells[32] (Figure 1). These cell type-specific differences are likely to have important functional implications given the existing data on the nature of the contributions of GAD67 and GAD65 to GABA synthesis. For example, deletion of the GAD67 gene in mice is lethal and is associated with ~90% reduction of brain GABA levels[33;34]. Consistent with these observations, mice with GAD67 deficiency restricted to the cerebellum have very weak GABAA receptor-mediated transmission even at low stimulus frequency[35]. In contrast, mice with deletion of the GAD65 gene survive into adulthood, have only a ~ 20% reduction in total brain GABA concentration[36], and exhibit normal GABAA receptor-mediated synaptic transmission during low frequency stimulation, but have markedly impaired transmission at stimulation frequencies >30 Hz[37;38]. Together, these findings suggest an essential role for GAD67 in baseline GABA synthesis and synaptic transmission, with GAD65 contributing to GABA synthesis principally under conditions of high frequency firing. Thus, at least in the primate DLPFC, the GAD67/65 ratio in PV basket cell terminals might equip these cells with a greater capacity, relative to chandelier neurons, for the type of repetitive neurotransmitter release required for gamma oscillations.
Figure 1.
Schematic summary of the three main types of GABA neurons providing perisomatic inputs to pyramidal neurons and the ratio of the two GABA synthesizing enzymes in the axon terminal levels of each cell type. Adapted from [32].
The kinetics of GABA neurotransmission, as determined by the type of post-synaptic GABAA receptor, may also favor an essential role for PV basket cells in gamma oscillations. That is, the GABAA receptor-mediated inputs involved in gamma synchronization must inhibit postsynaptic neurons for a length of time compatible with the gamma oscillation period. The GABAA receptor current duration depends on the subunit composition of the receptor[26;39], with GABAA receptors containing α1 subunits producing currents with a faster decay than those containing other α subunits[40]. The inputs from PV basket cells are principally mediated by α1-containing receptors, whereas slower α2-containing receptors predominate at both chandelier and CB1R/CCK basket cell inputs[41–45]. Thus, PV basket cells produce IPSCs with the faster current decay required for gamma oscillations. Furthermore, PV basket cell terminals produce a single synchronous GABA release event per presynaptic action potential[46;47] in contrast to the multiple asynchronous release events, which result in long-lasting inhibition, produced by CB1R/CCK basket cells.
The idea that PV basket cells have the unique properties required for gamma band synchrony is further supported by electrophysiological findings that PV basket cell firing is more strongly coupled to the gamma oscillation cycle than is the firing of chandelier neurons or CCK basket cells[48], and the firing of the latter two cell types is strongly coupled to the much slower theta oscillations[49–51]. Furthermore, gamma oscillations are significantly reduced by stimulation of presynaptic opioid receptors that suppress GABA release from PV basket cells but not from chandelier neurons or CCK basket cells[48].
Alterations in prefrontal GABA neurons and gamma oscillations in schizophrenia
The findings reviewed above converge on the conclusion that perisomatic inputs from PV basket cells are the main source of GABAA receptor-mediated synchronization at gamma frequencies. This interpretation then raises the question of whether and how alterations in PV basket cells in the DLPFC of subjects with schizophrenia could provide the pathological basis for altered frontal lobe gamma oscillations. Because phasic excitation of PV neurons is thought to be required for gamma oscillations in pyramidal-interneuron network models, and their tonic depolarization is implicated in interneuron network models[6,52], the impaired gamma oscillations in schizophrenia could be due to a deficit of glutamate transmission[53]. Indeed, hypofunction of NMDA receptors in PV cells has been suggested to be a core abnormality of the disorder, accounting for the deficits in both GAD67 and PV expression[54–56]. However, experimental data[57] indicate that glutamate synapses onto PV basket cells typically produce excitatory postsynaptic potentials (EPSCs) with a faster decay and weaker NMDA receptor contribution than do those onto pyramidal cells or other types of GABA neurons, suggesting that the rapid synaptic activation of PV basket cells is dependent, at least in part, on their fast AMPA receptor-mediated EPSCs[58]. Similarly, computational modeling studies demonstrated that fast AMPA-mediated excitation of PV basket cells is critical for gamma band synchrony and that including slower decaying NMDA-mediated EPSCs in basket cells disrupts gamma synchrony[57]. Consistent with these observations, NMDA receptor antagonists either enhance or do not affect gamma oscillations, whereas AMPA receptor antagonism completely abolish them[59–62]. Thus, although the effects of NMDA receptor antagonists on gamma oscillations might differ across cortical regions or layers, the observation that in most cases gamma synchrony is unaffected by NMDA receptor blockade[61;63] suggests that the disturbance in PV basket cell function contributing to impaired gamma oscillations in schizophrenia is independent of phasic NMDA receptor activation at synapses onto these cells.
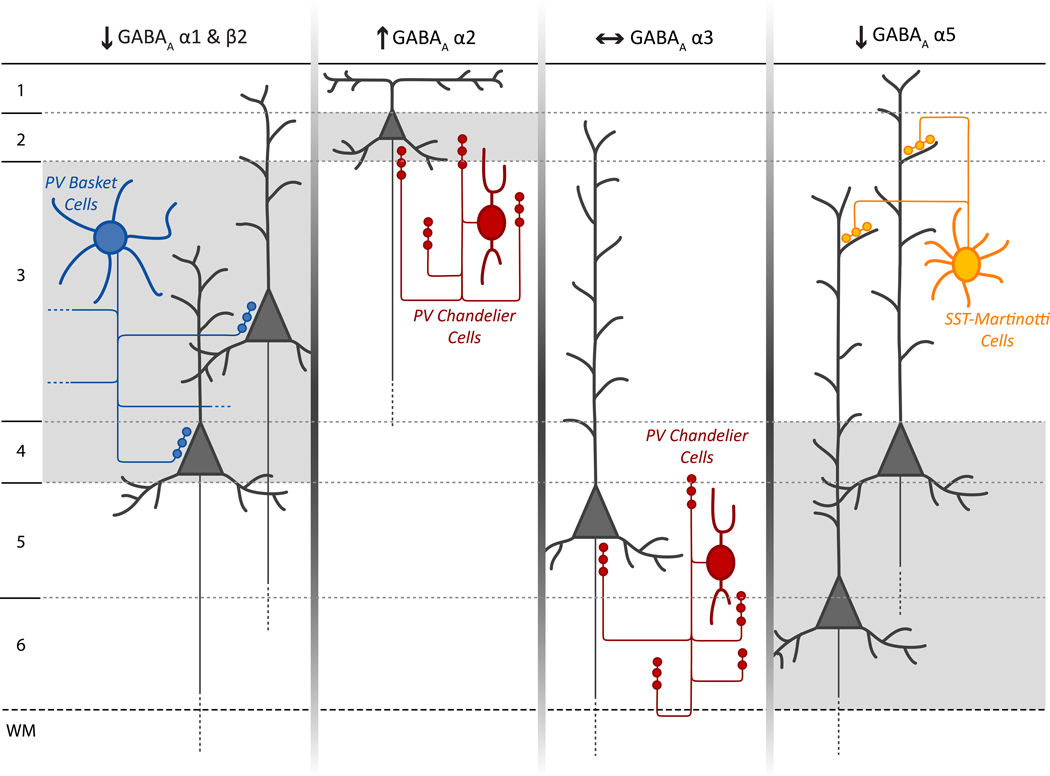
Alternatively, impaired PV basket cell function in schizophrenia might reflect altered GABA synthesis in these neurons. Levels of GAD67 mRNA are markedly lower in ~50% of PV neurons in schizophrenia[17], but in the absence of cell type specific markers, it has not been possible to determine whether this expression deficit is present in chandelier and/or basket cells. However, both the density of[64], and levels of GAD67 protein in[65], PV-labeled puncta, presumed to be basket cell terminals, appear to be lower in DLPFC layers 3 and 4 of subjects with schizophrenia. In addition, expression of the GABAA receptor α1 subunit (and of the β2 subunit with which it preferentially assembles) is also preferentially lower in this laminar location, whereas other GABAA receptor subunits are either unchanged or show a different laminar pattern of altered expression[66–68] (Figure 2). Thus, both pre- and post-synaptic markers of PV basket cell inputs appear to be lower in layers 3 and 4 of subjects with schizophrenia, suggesting that weaker synaptic transmission from these PV basket cells could contribute to impaired gamma oscillations in the illness.
Figure 2.
Schematic summary of layer-specific transcript alterations in postsynaptic GABAA receptor subunits in the DLPFC of subjects with schizophrenia and their hypothesized relationship to different classes of GABA neurons. For each GABAA subunit, the background shading marks the cortical layers where the indicated change in expression of that subunit was found. The lower expression of α1 and β2 mRNAs in layers 3 and 4 match that of the presynaptic alterations in PV basket cells. The increase in α2 expression in layer 2 is consistent with previous findings of pre- and post-synaptic alterations in chandelier cell inputs to the axon initial segment of pyramidal cells in this location[7]. In contrast, the absence of alterations in α3 subunit expression, which is present post-synaptic to chandelier cells in pyramidal neurons in layers 5–6, matches the failure to find significant changes in chandelier cell markers in these layers. The lower α5 subunit expression was observed in the deeper layers of the DLPFC where the somata of pyramidal neurons whose apical dendrites are known to be innervated by SST+ Martinotti cells, thought also to be affected in schizophrenia[72;73], are predominantly located. Adapted from [67].
The strength of the postsynaptic response to GABA depends on the driving force for the influx of chloride through GABAA receptor channels. Intracellular chloride levels are determined by the balance of activity between the chloride transporters NKCC1 and KCC2 which mediate chloride uptake and extrusion, respectively[26]. Although the expression of these transporters does not appear to be altered in the DLPFC of subjects with schizophrenia, two kinases (OXSR1 and WNK3) that phosphorylate both chloride transporters, decreasing the activity of KCC2 and increasing the activity of NKCC1, are markedly over-expressed in schizophrenia, including in layer 3 pyramidal neurons[69]. If the higher levels of OXSR1 and WNK3 mRNA represent increased kinase activity, then the intracellular chloride concentration would be expected to be greater than normal in schizophrenia (Figure 3), potentially resulting in less hyperpolarization of layer 3 pyramidal neurons when GABA is released from PV basket cells.
Figure 3.
Hypothesized model of the interactions between chloride (Cl−) transporters (NKCC1 and KCC2) and two kinases (OXSR1 and WNK3) that regulate their activity via phosphorylation. In both panels, the orange bar represents the cell membrane, with the extracellular domain above and the intracellular domain below the bar. The size and orientation of the green arrows indicates the magnitude and direction of Cl− ion flow mediated by NKCC1, KCC2 and GABAA receptor Cl− channels. (A) In normal adult neurons, intracellular Cl− concentration is low due to low levels of NKCC1 and high levels of KCC2. The binding of GABA to GABAA receptors triggers Cl− entry and membrane hyperpolarization. (B) In schizophrenia, increased OXSR1 and WNK3 kinase levels lead to increased phosphorylation (P) of both chloride transporters and consequently increased NKCC1 activity and decreased KCC2 activity, producing a greater intracellular Cl− concentration. Thus, when GABAA receptors are activated, Cl− influx is reduced and GABA neurotransmission is less hyperpolarizing. Adapted from [69].
Thus, the available data suggest that a number of factors (pre-synaptic reductions in GABA synthesis or the number of axon terminals; post-synaptic reductions in the number of, or chloride ion flow through, GABAA receptors) might contribute to a reduced capacity of PV basket cells to synchronize pyramidal neurons at gamma frequencies in schizophrenia, and thus might represent a neural substrate for the disease-related impairments in cognitive control. The tendency for at least some of these alterations to be most prominent in layer 3 is consistent with evidence that circuitry in this laminar location of the primate neocortex is critical for both gamma oscillations[70] and for delay-dependent cognitive control tasks[71]. Thus, interventions directed at enhancing the function of layer 3 PV basket cells might prove to be an effective means of improving cognitive control in individuals with schizophrenia.
Highlights.
Deficits of cognitive control in schizophrenia are associated with altered gamma oscillations in the prefrontal cortex.
Paralbumin basket interneurons appear to play a central role in generating cortical gamma oscillations.
Pre- and post-synaptic alterations in the strength of inhibitory inputs from parvalbumin basket neurons to pyramidal neurons in the middle layers of the prefrontal cortex may contribute to the neural substrate for impaired gamma oscillations in schizophrenia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1. Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, Poulton R, Moffitt TE. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. ** This study of the cognitive development of more than over 1,000 children over a 30-year period demonstrated that in individuals who later develop schizophrenia, some cognitive deficits are present at an early age and others are progressive before the onset of clinical symptoms.
- 2.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharm. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. * An important meta-analysis of a large body of literature documenting altered functional activation of the dorsolateral prefrontal cortex during cognitive tasks in individuals with schizophrenia.
- 4.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS. Gamma oscillatory power is impaired during cognitive control independent of medication status in first-episode schizophrenia. Neuropsychopharm. 2010;35:2590–2599. doi: 10.1038/npp.2010.150. * An important study showing that cognitive deficits and altered gamma oscillations are present in schizophrenia independent of medication effects or illness chronicity.
- 6. Whittington MA, Cunningham MO, LeBeau FE, Racca C, Traub RD. Multiple origins of the cortical gamma rhythm. Dev Neurobiol. 2011;71:92–106. doi: 10.1002/dneu.20814. * An informative review of the neural factors that underlie gamma band oscillations.
- 7.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 8.Ahn K, Gil R, Seibyl J, Sewell RA, D'Souza DC. Probing GABA receptor function in schizophrenia with iomazenil. Neuropsychopharm. 2011;36:677–683. doi: 10.1038/npp.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Enomoto T, Tse MT, Floresco SB. Reducing prefrontal gamma-aminobutyric Acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry. 2011;69:432–441. doi: 10.1016/j.biopsych.2010.09.038. * This study provides evidence from an animal model supporting the hypothesis that deficient GABA neurotransmission in the prefrontal cortex could contribute to the core cognitive features of schizophrenia.
- 11.Gruber AJ, Calhoon GG, Shusterman I, Schoenbaum G, Roesch MR, O'Donnell P. More is less: a disinhibited prefrontal cortex impairs cognitive flexibility. J Neurosci. 2010;30:17102–17110. doi: 10.1523/JNEUROSCI.4623-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry. 2008;165:1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidotti A, Auta J, Davis JM, Gerevini VD, Dwivedi Y, Grayson DR, Impagnatiello F, Pandey G, Pesold C, Sharma R, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, Rothman DL, Behar KL. Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD(67) protein. Brain Res. 2001;914:81–91. doi: 10.1016/s0006-8993(01)02778-0. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Burgos G, Hashimoto T, Lewis DA. Alterations of cortical GABA neurons and network oscillations in schizophrenia. Curr Psychiatry Rep. 2010;12:335–344. doi: 10.1007/s11920-010-0124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. ** Using optogenetic manipulations, this study demonstrated a direct link between PV interneuron activation and gamma oscillations.
- 19. Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. ** Using optogenetic manipulations, this study showed that PV interneuron activation could modulate gamma oscillation frequency and affect cortical synchronization.
- 20.Williams SM, Goldman-Rakic PS, Leranth C. The synaptology of parvalbumin-immunoreactive neurons in primate prefrontal cortex. J Comp Neurol. 1992;320:353–369. doi: 10.1002/cne.903200307. [DOI] [PubMed] [Google Scholar]
- 21.Zaitsev AV, Povysheva NV, Gonzalez-Burgos G, Rotaru D, Fish KN, Krimer LS, Lewis DA. Interneuron diversity in layers 2–3 of monkey prefrontal cortex. Cereb Cortex. 2009;19:1597–1615. doi: 10.1093/cercor/bhn198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;93:942–953. doi: 10.1152/jn.00787.2004. [DOI] [PubMed] [Google Scholar]
- 23.Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- 24.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 25.Baldi R, Varga C, Tamas G. Differential distribution of KCC2 along the axo-somatodendritic axis of hippocampal principal cells. Eur J Neurosci. 2010;32:1319–1325. doi: 10.1111/j.1460-9568.2010.07361.x. [DOI] [PubMed] [Google Scholar]
- 26.Farrant M, Kaila K. The cellular, molecular and ionic basis of GABA(A) receptor signalling. Prog Brain Res. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 27.Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- 29.Woodruff AR, Anderson SA, Yuste R. The enigmatic function of chandelier cells. Front Neurosci. 2010;4:201. doi: 10.3389/fnins.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis DA. The chandelier neuron in schizophrenia. Dev Neurobiol. 2011;71:118–127. doi: 10.1002/dneu.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169:1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fish KN, Sweet RA, Lewis DA. Differential distribution of proteins regulating GABA synthesis and reuptake in axon terminals of subpopulations of cortical interneurons. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr007. In Press. * This study demonstrated that the relative levels of GAD65 and GAD67 in axon terminals substantially differ across subclasses of interneurons in primate prefrontal cortex.
- 33.Asada H, Kawamura Y, Maruyama K, Kume H, Ding RG, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Cleft palate and decreased brain gamma-aminobutyric acid in mice lacking the 67-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1997;94:6496–6499. doi: 10.1073/pnas.94.12.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji F, Obata K. Development of the GABA system in organotypic culture of hippocampal and cerebellar slices from a 67-kDa isoform of glutamic acid decarboxylase (GAD67)-deficient mice. Neurosci Res. 1999;33:233–237. doi: 10.1016/s0168-0102(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 35.Obata K, Hirono M, Kume N, Kawaguchi Y, Itohara S, Yanagawa Y. GABA and synaptic inhibition of mouse cerebellum lacking glutamate decarboxylase 67. Biochem Biophys Res Commun. 2008;370:429–433. doi: 10.1016/j.bbrc.2008.03.110. [DOI] [PubMed] [Google Scholar]
- 36.Asada H, Kawamura Y, Maruyama K, Kume H, Ding R, Ji FY, Kanbara N, Kuzume H, Sanbo M, Yagi T, et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Comm. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 37.Tian N, Petersen C, Kash S, Baekkeskov S, Copenhagen D, Nicoll R. The role of the synthetic enzyme GAD65 in the control of neuronal gamma-aminobutyric acid release. Proc Natl Acad Sci U S A. 1999;96:12911–12916. doi: 10.1073/pnas.96.22.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi SY, Morales B, Lee HK, Kirkwood A. Absence of long-term depression in the visual cortex of glutamic Acid decarboxylase-65 knock-out mice. J Neurosci. 2002;22:5271–5276. doi: 10.1523/JNEUROSCI.22-13-05271.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capogna M, Pearce RA. GABA(A,slow): causes and consequences. Trends Neurosci. 2011;34:101–112. doi: 10.1016/j.tins.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szabadics J, Tamas G, Soltesz I. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci U S A. 2007;104:14831–14836. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABAA receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 47.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 48. Gulyas AI, Szabo GG, Ulbert I, Holderith N, Monyer H, Erdelyi F, Szabo G, Freund TF, Hajos N. Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci. 2010;30:15134–15145. doi: 10.1523/JNEUROSCI.4104-10.2010. * This study demonstrates that parvalbumin fast spiking interneurons activity is necessary to generate pyramidal network synchronization.
- 49.Klausberger T, Magill PJ, Marton LF, Roberts JDB, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 50.Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hajos N, Paulsen O. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 2009;22:1113–1119. doi: 10.1016/j.neunet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 53.Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 54.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 55.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behrens MM, Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rotaru DC, Yoshino H, Lewis DA, Ermentrout B, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31:142–126. doi: 10.1523/JNEUROSCI.1970-10.2011. ** A novel study of the biophysical properties of glutamate synapses onto PV basket cells in prefrontal cortex and their importance for the mechanisms of gamma oscillations and their alterations in schizophrenia.
- 58. Hu H, Martina M, Jonas P. Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science. 2010;327:52–58. doi: 10.1126/science.1177876. * This study shows that dendritic properties linked to specific K+ to Na+ conductance ratios contribute to the fast spiking phenotype of PV neurons.
- 59.Buhl EH, Tamas G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol. 1998;513(Pt 1):117–126. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, LeBeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 61.Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mann EO, Mody I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat Neurosci. 2010;13:205–212. doi: 10.1038/nn.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Traub RD, Whittington MA. Overview of normal and abnormal cortical oscillations Schizophrenia. In: Traub RD, Whittington MA, editors. Cortical oscillations in health and disease. New York: Oxford University Press; 2010. pp. 123–151. [Google Scholar]
- 64.Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: Evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- 65. Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of GAD 67 expression in Schizophrenia: Clinical, protein, and cell type-specific features. Am J Psychiatry. 2011 doi: 10.1176/appi.ajp.2011.11010052. * This study found that lower levels of GAD67 protein in schizophrenia were especially prominent in the axon terminals of PV neurons.
- 66.Akbarian S, Huntsman MS, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jr, Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: Comparison of schizophrenics and controls. Cereb Cortex. 1995;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- 67. Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-Specific Alterations in Cortical GABAA Receptor Subunit Expression in Schizophrenia. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq169. * This study demonstrated laminar-specific patterns of altered GABAA receptor subunit expression in the prefrontal cortex of subjects with schizophrenia.
- 68.Maldonado-Aviles JG, Curley AA, Hashimoto T, Morrow AL, Ramsey AJ, O'Donnell P, Volk DW, Lewis DA. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. doi: 10.1176/appi.ajp.2008.08101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Arion D, Lewis DA. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry. 2011;68:21–31. doi: 10.1001/archgenpsychiatry.2010.114. * First demonstration that regulation of intracellular chloride levels, and thus the nature of GABA neurotransmission, might be altered in schizophrenia.
- 70.Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in Gamma and Alpha coherence in the ventral stream. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1011284108. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 72.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beneyto M, Morris HM, Rovensky KC, Lewis DA. Lamina- and cell-specific alterations in cortical somatostatin receptor 2 mRNA expression in schizophrenia. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]