Abstract
Verticillium dahliae is the causal agent of vascular wilt in many economically important crops worldwide. Identification of genes that control pathogenicity or virulence may suggest targets for alternative control methods for this fungus. In this study, Agrobacterium tumefaciens-mediated transformation (ATMT) was applied for insertional mutagenesis of V. dahliae conidia. Southern blot analysis indicated that T-DNAs were inserted randomly into the V. dahliae genome and that 69% of the transformants were the result of single copy T-DNA insertion. DNA sequences flanking T-DNA insertion were isolated through inverse PCR (iPCR), and these sequences were aligned to the genome sequence to identify the genomic position of insertion. V. dahliae mutants of particular interest selected based on culture phenotypes included those that had lost the ability to form microsclerotia and subsequently used for virulence assay. Based on the virulence assay of 181 transformants, we identified several mutant strains of V. dahliae that did not cause symptoms on lettuce plants. Among these mutants, T-DNA was inserted in genes encoding an endoglucanase 1 (VdEg-1), a hydroxyl-methyl glutaryl-CoA synthase (VdHMGS), a major facilitator superfamily 1 (VdMFS1), and a glycosylphosphatidylinositol (GPI) mannosyltransferase 3 (VdGPIM3). These results suggest that ATMT can effectively be used to identify genes associated with pathogenicity and other functions in V. dahliae.
Keywords: Verticillium dahliae, Lactuca sativa, ATMT, Pathogenicity related genes
Introduction
Verticillium wilt disease, caused by the soilborne fungus Verticillium dahliae, is a devastating disease on many economically important crops worldwide [1, 2]. Verticillium wilt is particularly difficult to control because the fungus produces melanized structure known as microsclerotia that can survive for years in the soil [1]. Disease control has relied on soil fumigation that has economic and environmental costs inconsistent with sustainable agriculture or the deployment of Verticillium wilt-resistant cultivars that carry the major resistance (R) genes [2]. Effective fungicides have never been available for the control of Verticillium wilt [2]. Furthermore, two distinct races with differential virulence have been described in V. dahliae [3–5]. Resistance for race 1 has been identified in tomato and lettuce [3, 4, 6] but is currently not available for race 2 in either crop. Because of continual immigration of race 2 of V. dahliae from exotic sources in some agricultural production regions [5] and the selection for race 2 through deployment of race 1-resistant crops, additional control methods beyond resistance need to be developed.
Verticillium dahliae has evolved mechanisms to expand its host range and overcome various barriers encountered during the infection [2, 7, 8]. A greater understanding of the disease process caused by this fungus is necessary for the development of novel disease management methods against Verticillium wilts. Only a few genes required for pathogenicity have been characterized in Verticillium spp. [9–14]. The availability of novel molecular techniques has facilitated the identification and characterization of the genes involved in disease development. Forward genetic approaches such as random insertional mutagenesis provide a unique opportunity to generate genetic mutations in a manner that facilitates subsequent isolation of mutated genes [15, 16]. The recently released genome sequences of V. dahliae and V. albo-atrum (http://www.broadinstitute.org/annotation/genome/verticillium_dahliae) will be useful for easy identification of T-DNA insertion loci based on detailed genetic blueprints of these pathogens and the potential virulence factors.
Transformation has been particularly useful in the study of fungal genes associated with diverse phenotypes, including pathogenicity and pathogenesis-associated development. In the last three decades, methods such as polyethylene glycol (PEG), restriction enzyme-mediated integration (REMI), and Agrobacterium tumefaciens-mediated transformation (ATMT), have been deployed to transform many fungi [15, 17, 18] and to conduct large-scale genome-wide mutagenesis [16]. Among these methods, ATMT is the most versatile since A. tumefaciens can transform an array of starting materials including protoplasts, hyphae, spores, or blocks of the mycelial tissue [15, 19, 20]. In a recent study, Knight et al. [21] reported the successful application of ATMT to generate random insertional mutants of V. albo-atrum. Several studies have also utilized ATMT for targeted gene disruption in Verticillium species [9, 10, 13, 14], but to date, the applicability of random insertional mutagenesis via ATMT for investigating pathogenicity mechanisms in V. dahliae has not been evaluated.
The aim of this study was to generate random insertional mutants of V. dahliae that have defects in pathogenicity and/or affect the development and survival of this economically important fungus. The results of this study indicate that insertional mutagenesis by ATMT can be a powerful tool for identifying genes involved in these processes.
Materials and Methods
Fungal and Bacterial Strains
Verticillium dahliae strain VdLs17, for which the whole genome sequence is available (http://www.broadinstitute.org/annotation/genome/verticillium_dahliae), was grown on potato dextrose agar (PDA, Sigma-Aldrich, St. Louis, MO) at 25°C. Strain VdLs17 was initially tested for sensitivity to the antibiobtic hygromycin B at multiple concentrations (ranging from 25 to 125 μg/ml). A T-DNA binary vector (pSK2241) harboring the hygromycin B resistance gene (hph) under control of the Aspergillus nidulans trpC promoter and the ZsGreen fluorescent protein gene under control of the Fusarium verticilliodes translation elongation factor 1α gene promoter was used for ATMT. This vector was introduced to A. tumefaciens strain EHA105 to transform conidia of V. dahliae.
Transformation
Although some of the transformation procedures used in this study have already appeared in a previous publication [10], for the sake of completeness, all are described below. A. tumefaciens strain EHA105 was grown at 28°C for 48 h in minimal medium (MM: [22] supplemented with kanamycin (75 μg/ml). Two ml of this culture was centrifuged at 5,000 rpm for 1 min to pellet cells. After washing A. tumefaciens cells with induction media (IM: [23], they were resuspended in 5 ml of IM amended with 200 μM acetosyringone (AS) and cultured for an additional 6 h at 28°C at 200 rpm on an orbital shaker. Bacterial cells and the conidial suspension of VdLs17 (5 × 106) were mixed (1:1), and 200 μl was placed on each nitrocellulose filter (0.45 μm-pores and 45 mm diameter, Whatman) on co-cultivation medium (CM). CM contains the same constituents as IM except that it contains 5 mM of glucose instead of 10 mM of glucose.
Following three different co-cultivation times, including 24, 36, and 48 h at 28°C, the nitrocellulose membranes were transferred to selection medium containing hygromycin B (50 μg/ml) as a selection agent for transformants of V. dahliae and cefotoxime (200 μg/ml) to eliminate A. tumefaciens cells. Typically, transformants of V. dahliae appeared after 5–7 days of incubation. Putative transformants were transferred into 24 well plates (Costar, USA) containing 1.5 ml of PDA with hygromycin B (50 μg/ml) and incubated at 25°C for 4–5 days. After the second selection, conidia of the individual transformants were harvested and re-suspended in sterile distilled water (SDW) and plated on water agar media. To obtain mono-conidial cultures, one germinating conidium from each transformant was plucked and transferred to PDA plates. Transformants were maintained on PDA for routine work. A few transformants were subcultured at least five times on PDA without hygromycin and subsequently transferred to plates containing hygromycin to test the stability of integrated T-DNA. In addition, the fluorescence from ZsGreen was visualized by compound microscope (Olympus BX60), which is equipped with GFP filter (450–490 nm excitation, 500-nm longpass emission), and the system is linked to a CCD device. A Nikon compound microscope with filter blocks for GFP (450–490 nm excitation, 590-nm longpass emission), coupled to a MRC1024 Bio-Rad Confocal System (Bio-Rad, Hercules, CA) was used for all confocal laser scanning microscopy (CLSM), and all the settings followed were as previously described [24].
Molecular Analysis of Fungal Transformants
DNA Extraction and PCR Analysis
To extract fungal DNA for PCR and Southern blot analysis, transformants were grown in 75 ml of Potato Dextrose Broth (PDB) in 250 ml Erlenmeyer flasks at 25°C for 5 days. Genomic DNA of individual transformants was extracted from powdered mycelium according to the method of Al-Sammarai and Schmid [25] with the addition of an extra phenol chloroform extraction. The DNA pellet was dissolved in TE solution (pH 8.0) and quantified using a NanoDrop 1000 (NanoDrop Products, Wilmington, DE). DNA concentrations were adjusted to 10 ng with SDW for PCR assays. DNA samples were stored at 4°C for further use. To verify the integration of T-DNA in the fungal transformants, randomly selected transformants of V. dahliae were analyzed by PCR using the hph-specific primers Hy_F (5′-TCA GCT TCG ATG TAG GAG GG-3′) and Hy_R (5′-TTC TAC ACA GCC ATC GGT CC-3′). The PCR was carried out in 20 μl mixture, which contains 10 ng of genomic DNA, 2× GoTag master mix (Promega, Madison, WI), 5 pmol of each primer, and the reaction program included 5 min at 95°C; 35 cycles of 1 min at 95°C; 1 min at 60°C and 1 min at 72°C, and 10 min at 72°C for the final extension. PCR products were separated on a 0.8% agarose gel stained with ethidium bromide (EtBr) and photographed.
Total RNA of the selected mutants was extracted using the Qiagen RNeasy Plant Mini Kit (Qiagen, La Jolla, CA). For RT-PCR, 1 μg of total RNA was reverse-transcribed into first-strand cDNA with oligo dT primer using the SuperScript first-strand synthesis system (Invitrogen, Life Technologies, Carlsbad, CA) according to the manufacturer’s instruction. PCR was performed in 50 μl reaction mixture containing 5 μl of cDNA from each strain, 2 μl of dNTP mix, 5 μl of 10× PCR buffer, 6 μl of 20 mM MgCl2, 2 μl of 5 pmol each primer, 0.5 μl of Taq DNA polymerase (5 U/μl) and 27.5 µl of SDW. In all, 35 cycles of RT-PCR were run on a Bio-Rad DNA Engine thermocycler (Bio-Rad Laboratories, Hercules, CA).
For detecting transcripts of the five genes, the following primers were used: RTV47F and RTV47R (VDAG_07825.1), RT021F and RT021R (VDAG_10091.1), RT136F and RT136R (VDAG_09647.1), RT202F and RT202R (VDAG_04078.1), RT235F and RT235R (VDAG_02992.1) and actin VActF and VActR (VDAG_08445.1).
Southern Blot Analysis
Genomic DNA (approximately 8 μg) from more than 100 putative transformants was digested with EcoRI. The digested DNA (19 samples/run) was separated on a 0.7% agarose gel, and transferred to a nylon membrane (Roche) using the method described by Selden [26]. The probe was amplified from the hph-coding region using vector pCB1004 as a template [27]. The probe was labeled with digoxigenin (DIG) (Roche, Basel, Switzerland) according to the manufacturer’s instructions. Hybridization and detection was performed according to the manufacturer instructions (Roche). X-ray film (Kodak, Rochester, NY) was developed after a 30 min exposure.
Isolation and Identification of Sequences Flanking T-DNA Insertion Sites
Inverse PCR (iPCR) with modification of the original protocol [28] was used to isolate genomic sequences flanking inserted T-DNA. DNA samples (2 μg) from ~100 transformants were digested with either TaqI or PstI restriction enzyme (5 U) for 2 h at 65°C (TaqI) or overnight at 37°C (PstI) in 50 μl reactions. Digested DNA was purified by phenol–chloroform extraction and ethanol precipitated. The DNA pellet was dissolved in 40 μl SDW, and the digested DNA (100 ng) was ligated at 16°C for 5 h using T4 DNA ligase (Promega, Madison, WI).
iPCR was carried out with ligated DNA as a template. PCR was carried out in 20 μl reactions containing 3 μl of ligated genomic DNA, 2 μl of 10× PCR buffer, 2 μl of dNTP mixture, 1 μl of each RB3 (5′-CCCTTCCCAACAGTTGCGCA-3′) and RBn1 primers (5′-TTACAACGTCGTGACTGGGAAAAC-3′) and 0.2 μl of Taq DNA polymerase (Promega, Madison, WI) and 10.8 μl of SDW. EXOSAP-IT (Affymetrix/USB, Cleveland, OH) was applied to the PCR products to eliminate unincorporated primers and used for direct sequencing. Sequencing was carried out using the RB3 primer at the University of California-Davis CAES Genomics Facility (Davis, CA), using an automated ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA).
Resulting sequences were used as queries to search the Verticillium Group Database at the Broad Institute (http://www.broadinstitute.org/annotation/genome/verticillium_dahliae) and the National Center for Biotechnology Information (NCBI) websites (http://www.ncbi.nlm.nih.gov) via the Blastn and Blastx algorithms.
Phenotype Characterization
To study the colony morphology, the transformants were grown on PDA for 10 days and then growth rates (based on colony diameters measured once a week over a 2-week period) of individual transformants were measured and compared with wild-type strain VdLs17. Similarly, their microsclerotia production was compared with that of VdLs17 (the number of microsclerotia in three microscopic fields were counted and averaged). Based on these phenotypic characters, we selected those mutants that had varied phenotypes compared with wild-type strain VdLs17 and used for pathogenicity assay. Soilless virulence assays on lettuce (Lactuca sativa) cultivar PI 251246 were conducted as previously described [29]. For virulence assay, conidia of individual transformants were harvested from PDA plates and conidial density was adjusted to 1 × 107 conidia/ml. We screened 181 mutants (Table 1), inoculating three plants per mutant. Inoculated plants were incubated in a Conviron E15 growth chamber set to 16-h light/8-h dark cycles at 25°C. Three plants were inoculated with wild-type strain VdLs17 served as inoculated control plants, and three plants mock inoculated with SDW served as uninoculated control plants for virulence assay. Three weeks after inoculation, leaf and root symptoms were observed and recorded as described in Klosterman and Hayes [29].
Table 1.
Proportion of symptomatic leaves based on the first round of pathogenicity screening of transformants using soilless Verticillium wilt assay
| Experiment | Proportion of symptomatic leavesa | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wild type mean | Water mean | Population means | Number of isolates | |||||||
| 0.00 | 0.01–0.20 | 0.21–0.40 | 0.41–0.60 | 0.61–0.80 | 0.81–1.0 | Total | ||||
| Unreplicated screen | 0.41 | 0.13 | 0.35 | 20 | 31 | 54 | 63 | 13 | 0 | 181 |
aProportion of symptomatic leaves was calculated from a total of three plants for each isolate and the water control
The pathogenicity assay was repeated for those mutants that exhibited reduced virulence or were a putative nonpathogenic mutant (four mutants). Each mutant was tested in three replicate experiments with six plants per experiment per mutant (Table 2). Inoculated plants were spaced in rows of six plants in a foam container (Fig. 3a). The proportion of symptomatic leaves was recorded for each plant, and the percentage of plants with root discoloration was recorded for each mutant in each replicate experiment. Proportion symptomatic leaves data was totaled for each mutant in each experiment, arcsine-transformed, and analyzed in Proc Mixed of SAS (Version 9.2) with mutant and experiment as fixed effects. Least square means and 99.9% confidence interval were determined for each mutant, and means with non-overlapping confidence intervals were considered to be significantly different. Means and confidence intervals were backtransformed and reported as disease rating (DR) in the table. Root discoloration data were analyzed using analysis of variance (ANOVA)-type statistics of ranked data using the PROC Mixed procedure of SAS. The LD_CI macro was used to generate relative marginal effects (RME) for each mutant and 95% confidence intervals for detection of statistical differences between treatments [30, 31]. RME with non-overlapping confidence intervals were considered to be significantly different. Median root discoloration for each mutant was calculated.
Table 2.
Foliar disease rating and proportion of plants with root vascular discoloration of lettuce plants inoculated with four insertional mutants of Verticillium dahliae
| Treatment | Number of plants tested | Proportion root discoloration | Foliar disease ratingb | |||||
|---|---|---|---|---|---|---|---|---|
| Median | RMEa | 95% confidence interval | Mean | 99.9% confidence interval | ||||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||
| VdLs.17 (WT) | 18 | 0.83 | 0.80 | 0.55 | 0.89 | 0.64 | 0.35 | 0.91 |
| VdATMT-V47 | 18 | 0.17 | 0.36 | 0.24 | 0.52 | 0.07 | 0.04 | 0.12 |
| VdATMT-020 | 18 | 0.83 | 0.72 | 0.55 | 0.83 | 0.56 | 0.28 | 0.85 |
| VdATMT-021 | 18 | 0 | 0.19 | 0.14 | 0.29 | 0.05 | 0.03 | 0.09 |
| VdATMT-036 | 18 | 0.83 | 0.73 | 0.43 | 0.87 | 0.48 | 0.22 | 0.77 |
| Water control | 18 | 0 | 0.19 | 0.14 | 0.29 | 0.04 | 0.02 | 0.05 |
aThe relative marginal effect (RME) and 95% confidence intervals were calculated from the analysis of rank values of the root vascular discoloration data
bDisease ratings are the backtransformed values from analysis of arcsine-transformed proportion symptomatic leaves data. Higher values indicate higher percentage of symptomatic leaves
Fig. 3.
Application of a soilless Verticillium wilt assay using the early flowering lettuce cultivar, PI 251246, to assess the virulence of strains of Verticillium dahliae transformed by random insertional mutagenesis. a Falcon tubes containing lettuce seedlings were inoculated with 1 × 107 spore concentrations of individual transformants arranged in foam insulated trays and kept in a growth chamber for 3 weeks at 25°C. b Inoculated plant showing symptoms of vascular wilt disease 21 days after inoculation. Uninoculated plant (left) did not show Verticillium wilt symptoms. White arrow indicates brownish root discoloration and red arrows indicate symptomatic leaves showing necrosis and wilting. A representative healthy leaf (c), healthy root (d) and symptomatic leaf (e) and symptomatic root tissue (f)
Results
ATMT of V. dahliae
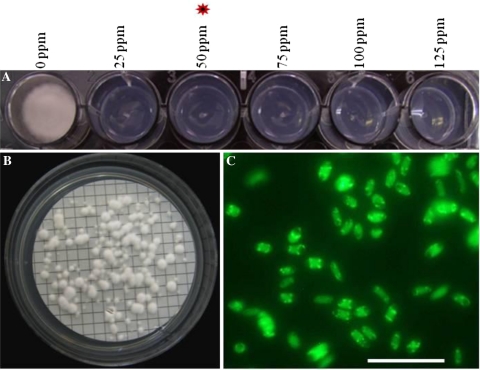
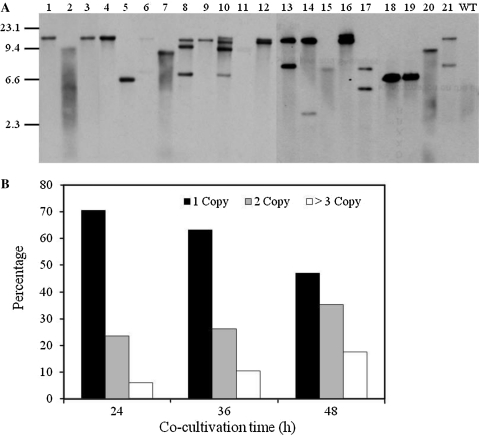
Hygromycin B sensitivity of VdLs17 was evaluated by growth on PDA amended with different concentrations of hygromycin B (up to 125 μg/ml). Growth was completely inhibited at 50 μg/ml hygromycin B. (Fig. 1a), and this concentration was used to select transformants. A. tumefaciens strain EHA 105 carrying binary vector pSK2241, which carries the hph and ZsGreen genes in the T-DNA, was used for the transformation of V. dahliae. First, co-cultivation time for ATMT was evaluated to optimize insertional mutagenesis. Specifically, the transformation efficiency and the copy number of T-DNA in individual transformants were examined in relation to the length of the co-cultivation period. Among the three different co-cultivation periods with 5 × 106 spore concentration, 36 h was found to be optimal. After 36 h of co-cultivation, 80–105 transformants per plate were obtained, and after 48 h, an additional 125–150 transformants per plate (Fig. 1b) were obtained, but the frequency of single copy integration of the T-DNA decreased. Prolonged co-cultivation (>48 h) led to excessive mycelial growth, confounding identification of individual transformants. No transformants were obtained using co-cultivation media without AS, confirming that AS is essential for A. tumefaciens transformation of V. dahliae (data not shown).
Fig. 1.
Agrobacterium tumefaciens-mediated transformation of Verticillium dahliae. a Hygromycin B sensitivity of V. dahliae strain VdLs17. b Individual colonies of transformants appearing on nylon membrane after 48-h co-cultivation and selection on a medium containing hygromycin B. c Expression of green fluorescent protein in conidia of V. dahliae transformant (VdATMT-316) was analyzed by compound microscope. c Scale bar = 100 μm
Five randomly selected V. dahliae transformants were subcultured for five successive generations in the absence of hygromycin B and screened for hygromycin B resistance and green fluorescence in the sixth generation. All the transformants remained resistant to hygromycin B (data not shown) and exhibited stable green florescence (Fig. 1c), confirming stability of the transformants.
Integration of T-DNA into Fungal Genomic DNA
The integration of the hph gene in the monoconidial colonies of the transformants was initially ascertained by PCR. PCR amplification of this gene from 100 putative transformants confirmed its presence in the genome of all transformants, whereas no product was amplified from the wild type (data not shown).
To further verify the integration of T-DNA and to assess the copy number of T-DNA in the genome, randomly selected transformants were analyzed by Southern hybridization using an hph probe (see “Materials and Methods” section). About 69% of transformants harbored a single insert of T-DNA (Fig. 2a). After 24 h of co-cultivation, 12 out of the 17 transformants had a single T-DNA insert with the site of insertion appearing to vary among transformants (Fig. 2a; Table 3). Four transformants had two T-DNA insertions, and one had three insertions (Fig. 2b).
Fig. 2.
T-DNA copy number analysis in Verticillium dahliae transformants. a Southern blot analysis of T-DNA integration for randomly selected V. dahliae transformants. Genomic DNA was digested with EcoRI, separated on 0.7% agarose gel, transferred to a nylon membrane (Roche), and hybridized with a digoxigenin-labeled 1.4 kb fragment of hph. Lanes 1–21 transformants of strain VdLs17, WT wild-type strain VdLs17. b Analysis of T-DNA copy number at differing co-cultivation times of 24, 36, and 48 h
Table 3.
Sequence analysis of T-DNA flanking regions of Verticillium dahliae transformants using Verticillium group and NCBI databases
| Transformant | T-DNA copy no | BLAST hit | Locus | E-value | Chromosome |
|---|---|---|---|---|---|
| VdATMT-005 | 2 | Predicted protein | VDAG_02684.1 | 1.20289E-20 | 3 |
| VdATMT-018 | 1 | Transmembrane and coiled-coil domain-containing protein | VDAG_03258.1 | 0.0 | 6 |
| VdATMT-021 | 2 | Hydroxymethylglutaryl-CoA synthase | VDAG_10091.1 | 0.0 | 1 |
| VdATMT-025 | 2 | Cytochrome oxidase subunit I_Mitochondria | VDAG_17012.1 | 1.43385E-7 | Mitochondria |
| VdATMT-030 | 1 | F-actin-capping protein subunit alpha | VDAG_01574.1 | 0.0 | 1 |
| VdATMT-V47 | 1 | Endoglucanase EG-1 | VDAG_07825.1 | 0.0 | 2 |
| VdATMT-048 | 2 | Choline/ethanolaminephosphotransferase | VDAG_02561.1 | 0.0 | 3 |
| VdATMT-070 | 4 | Aromatic amino acid aminotransferase | VDAG_01337.1 | 0.0 | 1 |
| VdATMT-084 | 1 | Conserved hypothetical protein | VDAG_01624.1 | 0.0 | 1 |
| VdATMT-094 | 2 | Conserved hypothetical protein | VDAG_03742.1 | 0.0 | 3 |
| VdATMT-100 | 4 | NAD(P)H-dependent d-xylose reductase | VDAG_01073.1 | 0.0 | 1 |
| VdATMT-111 | ND | Intergenic region | ND | 1.31368E-35 | ND |
| VdATMT-118 | 1 | C6 Zinc finger domain-containing protein | VDAG_08079.1 | 0.0 | 6 |
| VdATMT-136 | 1 | Major facilitator super family (MFS) transporter | VDAG_09647.1 | 0.0 | 7 |
| VdATMT-141 | ND | Cytochrome b5 | VDAG_01573.1 | 0.0 | 1 |
| VdATMT-145 | 2 | Intergenic region | ND | 0.0 | 2 |
| VdATMT-156 | ND | Conserved hypothetical protein | VDAG_02688.1 | 0.0 | 3 |
| VdATMT-184 | 2 | Conserved hypothetical protein | VDAG_01624.1 | 1 | |
| VdATMT-202 | 1 | Ras GTPase activator | VDAG_04078.1 | 2.98277E-14 | 3 |
| VdATMT-212 | 2 | Hypothetical protein | VDAG_00726.1 | 0.0 | 2 |
| VdATMT-220 | 1 | Transcriptional activator SPT7 | VDAG_00822.1 | 0.0 | 1 |
| VdATMT-223 | 1 | Intergenic region | ND | 3.21862E-29 | 3 |
| VdATMT-224 | 2 | Intergenic region | ND | 3.73693E-7 | 4 |
| VdATMT-235 | 1 | Prolyl-tRNA synthetase | VDAG_02992.1 | 0.0 | 6 |
| VdATMT-236 | 1 | Intergenic region | ND | 1.28399E-28 | 3 |
| VdATMT-239 | 1 | Predicted protein | VDAG_03441.1 | 1.91778E-20 | 6 |
| VdATMT-240 | 1 | Conserved hypothetical protein | VDAG_09053.1 | 0.0 | 5 |
| VdATMT-245 | ND | GPI mannosyltransferase | VDAG_05742.1 | 0.0 | 4 |
| VdATMT-255 | 1 | Choline dehydrogenase | VDAG_03611.1 | 6.21806E-32 | 3 |
| VdATMT-256 | 1 | Intergenic region | ND | 0.0 | 3 |
| VdATMT-264 | 1 | Intergenic region | ND | 0.0 | 2 |
| VdATMT-279 | 1 | Fungal-specific transcription factor domain-containing protein | VDAG_08521.1 | 0.0 | 2 |
| VdATMT-303 | 2 | Conserved hypothetical protein | VDAG_01611.1 | 1.60958E-29 | 1 |
| VdATMT-314 | ND | Type I inositol-1,4,5-triphosphate 5-phosphatase CVP2 | VDAG_01273.1 | 0.0 | 1 |
| VdATMT-316 | ND | Membrane protein | VDAG_05753.1 | 4.27732E-21 | 4 |
| VdATMT-320 | ND | Conserved hypothetical protein | VDAG_02295.1 | 3.58475E-34 | 7 |
ND not determined
Pathogenicity Assay
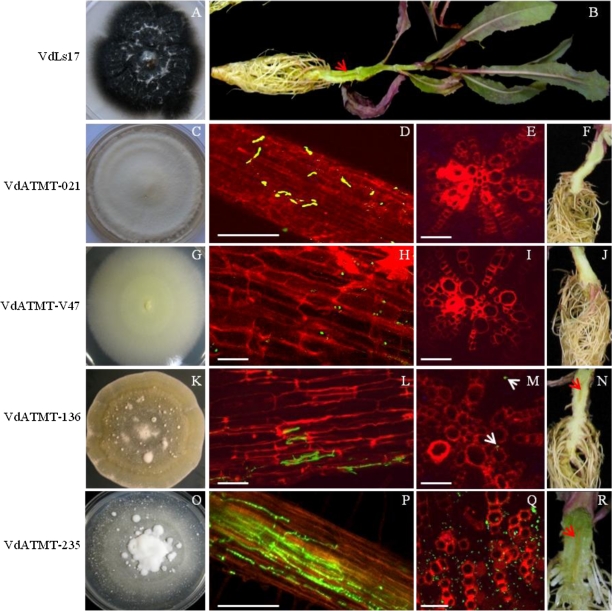
We screened 181 transformants for changes in virulence using a soilless Verticillium wilt assay [29] with lettuce cultivar PI 251246. This assay enabled the testing of 100 transformants in a single experiment (Fig. 3). These 181 transformants were selected for their interesting phenotypes, such as defects in growth, colony color, and reduced microsclerotia production (data not shown), and a few of the representative phenotypes are displayed in Fig. 4. Of the 181 transformants assayed, many caused severe necrosis, wilting of leaves, and root discoloration comparable to the symptoms associated with infection caused by the wild-type strain VdLs17 (Table 1). However, about 20 transformants showed no virulence, and 31 transformants exhibited reduced virulence relative to the wild-type strain. Among the 20 mutants potentially defective for pathogenicity identified in the first round of screening, we selected four mutants that caused no symptoms and tested these further using 18 plants (six plants/replication). Among these four, two (insertional mutants VdATMT-V47 and VdATMT-021) were nonpathogenic (Table 2) and interestingly, the mutant VdATMT-V47 did not produce microsclerotia on PDA plates (Fig. 5g). In addition, VdATMT-V47 did not colonize the xylem vessels (Fig. 5h–j). Similarly, no vascular colonization was observed in VdATMT-021 inoculated plant, and this mutant produced less microsclerotia on PDA plate (Fig. 5c–f). The mutants, VdATMT-21 and VdATMT-V47, showed neither necrosis (mean foliar DR, 0.05, 0.07) nor significant root discoloration (0 and 11%) on lettuce relative to water control (0.04 mean foliar DR and root discoloration 0%). The remaining two mutants (VdATMT-020 and VdATMT-036) appeared to be false positive, because they were comparable to the wild-type strain in foliar DR (Table 2).
Fig. 4.
Representative T-DNA insertional mutants of Verticillium dahliae examined for colony growth characteristics, and the ability to form microsclerotia on PDA medium
Fig. 5.
Pathogenicity of VdLs17, VdATMT-021, VdATMT-V47, VdATMT-136, and VdATMT-235. Lettuce cultivar PI 251246 was inoculated by soilless Verticillium assay using conidial suspension (5 × 106) of the mutants and wild-type strain VdLs17. Part of images was captured using CLSM. a, c, g, k, o The cultures phenotype during growth on potato dextrose agar. b Plant inoculated with VdLs17 showing symptoms of vascular wilt disease 21 days after inoculation. d Conidia of VdATMT-021 were showing delayed germination on the lateral roots. e Cross section of main root showing no colonization of VdATMT-021 mutant-inoculated plant, 3 weeks after inoculation. f Vascular discoloration was not observed in ATMT-021 mutant-inoculated plant root system. h Conidia of the VdATMT-V47 mutant were trying to germinate on the lateral roots, 2 weeks after inoculation. i Cross section of main root showing no colonization of VdATMT-V47 mutant-inoculated plant, 3 weeks after inoculation. j Vascular discoloration was not observed in VdATMT-V47 mutant-inoculated plant root system. l Mutant VdATMT-136 showing less colonization on lateral root, 2 weeks after inoculation. m Cross section of main root showing less colonization of VdATMT-136 in the xylem of the plant. n Less vascular discoloration was observed in ATMT-136 mutant-inoculated root system. p Intense colonization of VdATMT-235 on lateral root system, 2 weeks after inoculation. q Cross section of main root showing intense colonization of VdATMT-235 in the xylem of the plant. r Vascular discoloration of the taproot of the plant inoculated with VdATMT-235. d, h, l, p Scale bars = 100 μm; e, i, m, q Scale bars = 50 μm. Red and white arrowheads indicate the site of root colonization and vascular discoloration
The other two mutants, VdATMT-136 and VdATMT-235, used for confocal microscopic study to compare VdATMT-V47 and VdATMT-021 mutants virulence phenotypes, caused weak (VdATMT-136) or intense colonization (VdATMT-235) on inoculated plants (Fig. 5l–n, p–r).
Identification of the Genes Tagged by T-DNA Insertions Using iPCR
iPCR is a rapid and an efficient tool for isolating sequences adjacent to inserted T-DNA in the transformants of fungal species [32, 33]. For iPCR amplification of genomic DNA flanking the T-DNA insertion site, we used two primers, RB3 and RBn1, which are designed to amplify the right T-DNA border sequence and adjacent sequence from V. dahliae. Sequences of amplified iPCR products were used for searching the Verticillium group genome database, and the NCBI database using Blastn and Blastx algorithms [34] to identify T-DNA insertion site.
Analysis of 36 mutants by iPCR sequences revealed that the genes tagged by T-DNA may have roles in toxin efflux, plant cell wall degradation, and other processes that could contribute to virulence (Table 3). In one of the mutants, VdATMT-136, the T-DNA was inserted in a gene encoding a major facilitator superfamily (MFS) transporter (VDAG_09647.1). In mutant VdATMT-245, the T-DNA was inserted in a gene GPI mannosyltransferase (VDAG_05742.1). Other genes included endoglucanase 1 (VDAG_07825.1), a transmembrane and coiled coil domain containing protein (VDAG_03258.1), a hydroxymethylglutaryl-CoA synthase (VDAG_10091.1), a C6 Zinc finger domain containing protein (VDAG_008079.1), and a Ras GTPase activator (VDAG_04078.1) (Table 3). The T-DNA was also inserted in genes encoding hypothetical proteins, some of which may have novel functions (Table 3). Importantly, analyses of sequences near the T-DNA integration sites suggested that T-DNA insertions were unbiased, since the integrations were scattered on seven of the eight chromosomes of V. dahliae (Table 3).
The right border of inserted T-DNA and its flanking genomic sequences were analyzed to determine how T-DNA was integrated in the mutants using Verticillium group database and the NCBI GenBank database. Out of 23 insertion sites analyzed, there was no T-DNA sequence truncation in 19 of them (~80%). The maximum truncation was two nucleotides in one site (Fig. 6).
Fig. 6.
Alignment of the right border (RB) sequences at the junction of T-DNA integration site of Verticillium dahliae transformants
RT-PCR on T-DNA Mutated Genes
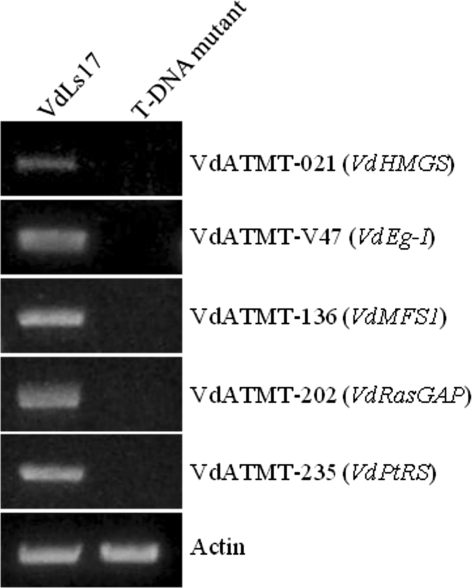
RT-PCR was carried out on five different genes affected by the T-DNA insertion. The results revealed that the transcripts of these five genes that encode endoglucanase 1 (VdEg-1), hydroxyl-methyl glutaryl-CoA synthase (VdHMGS), a major facilitator superfamily transporter 1 (VdMFS1), Ras GTPase activator (VdRasGAP), and a Prolyl-tRNA synthetase (VdPtRS) were not detected in the T-DNA mutants, and all these five genes transcripts were present in the wilt-type strain, VdLs17 (Fig. 7).
Fig. 7.
Reverse-transcriptase polymerase reaction analysis of transcription of a hydroxyl-methyl glutaryl-CoA synthase (VdHMGS), an endoglucanase 1 (VdEg-1), a major facilitator superfamily (VdMFS1) transporter 1, a Ras GTPase activator (VdRasGAP), and a Prolyl-tRNA synthetase (VdPtRS). Primers VdActF and VdActR were used for the Actin gene as a control
Discussion
The principal aim of this study was to generate random insertional mutants in V. dahliae using ATMT. In recent years, this approach has been applied to several fungi to generate mutants and subsequently indentify mutated genes [15, 16]. ATMT has several advantages compared with other transformation methods, mainly because of the versatility in selecting starting material, transformation efficiency, and high percentage of single copy T-DNA integration [15]. Using ATMT, genome-wide analysis of pathogenicity genes has been carried out in Magnaporthe oryzae [16].
ATMT has been exploited for random and targeted mutagenesis in many foliar fungal pathogens, but comparable studies with soilborne fungal pathogens lag behind. Therefore, development and implementation of an ATMT protocol for insertional mutagenesis of the soilborne pathogen V. dahliae represents an important step to characterize genes involved in pathogenicity and survival of soilborne fungi. We used a soilless Verticillium wilt assay to screen 181 mutants for changes in virulence. This assay is preferred because it is rapid compared with the conventional greenhouse assay in the soil medium (takes ~16 weeks) [29]. The soilless assay enabled the testing of 100 transformants in a single experiment. Many transformants (~60%) caused necrosis, wilting, and root discoloration similar to control plants inoculated with the wild-type strain. However, several mutants showed an altered pathogenicity phenotype on lettuce, including several mutant strains, which completely lost or had reduced virulence relative to the wild-type strain. T-DNA insertion in two of these loci eliminated or reduced the ability of these mutants to cause disease. In this study, we report a high frequency of isolating pathogenicity/virulence-defective mutants compared with other fungi [16, 35], because these mutants selected for pathogenicity assay showed interesting phenotypes on PDA medium, such as slow and fluffy growth, different colony color, and few or no microsclerotia production.
Among the genes identified through ATMT insertional mutagenesis, two had an insertion in genes that had previously been characterized and known to be involved in pathogenicity in other fungi and bacteria [36–40, 42, 43]. The flanking sequence from one of the mutants, VdATMT-V47, demonstrated that the T-DNA insertion was in predicted endoglucanase EG-1 gene in V. dahliae (VDAG_07825.1). Microscopic observations suggest that this mutant was unable to colonize the xylem vessels in inoculated plants. Plant pathogenic fungi, including V. dahliae, are known to produce a range of cell wall-degrading enzymes to facilitate infection and colonization [1]. Endoglucanase 1 (EG-1) is important for the depolymerization of plant cellulose [36, 37]. Deletion of major endoglucanase genes in phytopathogenic bacteria resulted in reduction or loss of virulence [38, 39]. In V. dahliae, higher endo-β-1,4-glucanase activity was correlated with aggressiveness [40]. Thus, the predicted EG-1 in V. dahliae (VdEg-1) plays an important role in plant penetration or in early stages of colonization. A detailed characterization of endoglucanase 1 in V. dahliae is currently underway.
The flanking sequence of VdATMT-021 mutant revealed that T-DNA inserted in the hydroxymethylglutaryl-CoA synthase gene in V. dahliae (VDAG_10091.1). Additional confirmatory pathogenicity screening and confocal microcopy suggested an important role of a hydroxymethylglutaryl-CoA synthase gene in the pathogenicity of V. dahliae. The hydroxymethylglutaryl-CoA synthase gene is essential for the sterol biosynthesis pathway in various organisms [41]. In Fusarium spp., this gene plays a role in trichothecene mycotoxin production [42, 43].
Other candidate pathogenicity-related genes were identified in this study, but these require additional screening for confirmation. For example, in mutant VdATMT-136, T-DNA was inserted in a major facilitator superfamily transporter (MFS) gene (VDAG_09647.1). In fungi, MFS transporters play a major role in toxic compound resistance, including fungicidal resistance [44, 45]. The MFS gene disruption mutant is potentially valuable for investigating the role of the VDAG_09647.1 gene in drug resistance, or resistance to plant-produced antimicrobial compounds. Other genes of interest encode mannosyltransferase (VDAG_05742.1), Ras GTPase activator (VDAG_04078.1), C6 Zinc finger domain-containing protein (VDAG_08079.1), and a transmembrane, coiled-coil domain-containing protein (VDAG_03258.1). These genes are required for proper fungal growth, cell wall construction, virulence, and possibly for asexual morphogenesis in various fungi [46–49].
An additional aim of this study was to investigate parameters for optimal ATMT insertional mutagenesis in V. dahliae. Transformation efficiency in V. dahliae displayed dependency on co-cultivation times, similar to the situation described for other fungal systems [15, 21]. In general, more transformants can be obtained by a longer co-cultivation period in most fungi [50, 51]. In this study, the transformation efficiency was improved by increasing the co-cultivation time up to 48 h. With 48 h of co-cultivation, an average of 450 transformants per 5 × 106 conidia/ml was generated in V. dahliae. ATMT generates a high percentage of transformants with a single copy T-DNA compared with other conventional methods, which makes it easier to isolate the tagged gene [15, 16]. In the current study, Southern blot analysis from randomly selected transformants showed that almost 69% of the transformants had a single copy of T-DNA integration at 36 h of co-cultivation. The percentage of single copy T-DNA insertion is similar to what was observed in other fungi including V. albo-atrum [21, 50–52]. The current study suggests that co-cultivations for 36 h as the optimum time for V. dahliae transformation to obtain an adequate number of single copy transformants.
In addition, the results suggest that iPCR is an efficient tool to identify the sequences adjacent to the inserted T-DNA in V. dahliae. Inverse-PCR is more efficient and may be a more sensitive method than TAIL-PCR in identifying the T-DNA flanking sequences in several fungi [32, 33]. Further analysis of iPCR sequences from the right border suggests that most of the sequences recovered from the transformants were intact without any truncation, which is similar to previous reports [51, 53]. Truncation of T-DNA right border sequences was less frequent probably because VirD2 protein of Agrobacterium protects this region from degradation [15, 53].
In conclusion, V. dahliae was successfully transformed using A. tumefaciens, and the transformation efficiency of 450 mutants per 5 × 106 conidia is comparable to that of various fungi, including V. albo-atrum [21]. This protocol is useful for generating a large number of mutants carrying a single randomly inserted copy of T-DNA thus facilitating the identification of V. dahliae genes directly or indirectly involved in pathogenesis and survival characteristics.
Acknowledgments
This research was supported in part by funding from the California Leafy Greens Research Program and the California Tomato Research Institute. The authors thank Rosa Marchebout, Francisco Hernandez, Amy Anchieta, and Dianna Hernandez for their technical assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Pegg GF, Brady BL. Verticillium wilts. New York: CABI Publishing; 2002. [Google Scholar]
- 2.Klosterman SJ, Atallah ZK, Vallad GE, Subbarao KV. Diversity, pathogenicity, and management of Verticillium species. Annual Review of Phytopathology. 2009;47:39–62. doi: 10.1146/annurev-phyto-080508-081748. [DOI] [PubMed] [Google Scholar]
- 3.Hayes R, Vallad GE, Subbarao KV. The inheritance of resistance to race 1 isolates of Verticillium dahliae in lettuce (Abst.) Hortscience. 2007;42:896. [Google Scholar]
- 4.Fradin EF, Zhang Z, Ayala JCJ, Castroverde CDM, Nazar RN, Robb J, Liu C-M, Thomma BPHJ. Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiology. 2009;150:320–332. doi: 10.1104/pp.109.136762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruthachalam K, Atallah ZK, Vallad GE, Klosterman SJ, Davis RM, Subbarao KV. Molecular variation among isolates of Verticillium dahliae and PCR-based differentiation of races. Phytopathology. 2010;100:1222–1230. doi: 10.1094/PHYTO-04-10-0122. [DOI] [PubMed] [Google Scholar]
- 6.Schaible L, Cannon OS, Waddoups V. Inheritance of resistance to Verticillium wilt in a tomato cross. Phytopathology. 1951;41:986–990. [Google Scholar]
- 7.Koike ST, Subbarao KV, Davis RM, Gordon TR, Hubbard JC. Verticillium wilt of cauliflower in California. Plant Disease. 1994;78:1116–1121. doi: 10.1094/PD-78-1116. [DOI] [Google Scholar]
- 8.Bhat RG, Smith RF, Koike ST, Wu BM, Subbarao KV. Characterization of Verticillium dahliae isolates and wilt epidemics of pepper. Plant Disease. 2003;87:789–797. doi: 10.1094/PDIS.2003.87.7.789. [DOI] [PubMed] [Google Scholar]
- 9.Rauyaree P, Ospina-Giraldo MD, Kang S, Bhat RG, Subbarao KV, Grant SJ, Dobinson KF. Mutation in VMK1, a mitogen-activated protein kinase gene, affect microsclerotia formation and pathogenicity in Verticillium dahliae. Current Genetics. 2005;48:109–116. doi: 10.1007/s00294-005-0586-0. [DOI] [PubMed] [Google Scholar]
- 10.Dobinson KF, Grant SJ, Kang S. Cloning and targeted disruption, via Agrobacterium tumefaciens-mediated transformation, of a trypsin protease gene from the vascular wilt fungus Verticillium dahliae. Current Genetics. 2004;45:104–110. doi: 10.1007/s00294-003-0464-6. [DOI] [PubMed] [Google Scholar]
- 11.Wang JY, Chai Y, Gou YY, Mao YB, Xu YH, Jiang WH, Chen XY. VdNEP, an elicitor from Verticillium dahliae, induces cotton plant wilting. Applied and Environmental Microbiology. 2004;70:4989–4995. doi: 10.1128/AEM.70.8.4989-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzima A, Paplomatas EJ, Rauyaree P, Kang S. Roles of the catalytic subunit of cAMP dependent protein kinase A in virulence and development of the soilborne plant pathogen Verticillium dahliae. Fungal Genetics and Biology. 2010;47:407–415. doi: 10.1016/j.fgb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Tzima, A., Paplomatas, E. J., Rauyaree, P., Ospina-Giraldo, M. D., & Kang, S. (2010b). VdSNF1, the sucrose non-fermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell wall degradation. Molecular Plant-Microbe Interactions,24, 129–142. [DOI] [PubMed]
- 14.Klimes A, Dobinson KF. A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae. Fungal Genetics and Biology. 2006;43:283–294. doi: 10.1016/j.fgb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Michielse CB, Hooykaas PJJ, van den Hondel CAMJJ, Ram AFJ. Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Current Genetics. 2005;48:1–17. doi: 10.1007/s00294-005-0578-0. [DOI] [PubMed] [Google Scholar]
- 16.Jeon J, Park SY, Chi MH, Choi J, Park J, Rho HS, Kim S, Goh J, Yoo S, Choi J, Park JY, Yi M, Yang S, Kwon MJ, Han SS, Kim BR, Khang CH, Park B, Lim SE, Jung K, Kong S, Maruthachalam K, Oh HS, Kim H, Kim S, Park J, Kang S, Choi WB, Kang S, Lee YH. Genome-wide functional analysis of pathogenicity genes in the rice blast fungus. Nature Genetics. 2007;39:561–565. doi: 10.1038/ng2002. [DOI] [PubMed] [Google Scholar]
- 17.Meyer V, Mueller D, Strowig T, Stahl U. Comparison of different transformation methods for Aspergillus giganteus. Current Genetics. 2003;43:371–377. doi: 10.1007/s00294-003-0406-3. [DOI] [PubMed] [Google Scholar]
- 18.Thon MR, Nuckles EM, Vaillancourt LJ. Restriction enzyme-mediated integration used to produce pathogenicity mutants of Colletotrichum graminicola. Molecular Plant-Microbe Interaction. 2000;13:1356–1365. doi: 10.1094/MPMI.2000.13.12.1356. [DOI] [PubMed] [Google Scholar]
- 19.de Groot MJA, Bundock P, Hooykaas PJJ, Beijersbergen AGM. Agrobacterium tumefaciens mediated transformation of filamentous fungi. Nature Biotechnology. 1998;16:839–842. doi: 10.1038/nbt0998-839. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Stone M, Schlagnhaufer C, Romaine CP. A fruiting body tissue method for efficient Agrobacterium transformation of Agaricus bisporus. Applied and Environmental Microbiology. 2000;66:4510–4513. doi: 10.1128/AEM.66.10.4510-4513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight CJ, Bailey AM, Foster GD. Agrobacterium-mediated transformation of the plant pathogenic fungus, Verticillium albo-atrum. Journal of Plant Pathology. 2009;91:745–750. [Google Scholar]
- 22.Hooykaas PJJ, Roobol C, Schilperoort RA. Regulation of the transfer of Ti-plasmids of Agrobacterium tumefaciens. Journal of General Microbiology. 1979;110:99–109. [Google Scholar]
- 23.Bundock P, Den Dulk-Ras A, Beijersbergen A, Hooykaas PJJ. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisae. EMBO Journal. 1995;14:3206–3214. doi: 10.1002/j.1460-2075.1995.tb07323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallad GE, Subbarao KV. Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology. 2008;98:871–885. doi: 10.1094/PHYTO-98-8-0871. [DOI] [PubMed] [Google Scholar]
- 25.Al-Samarrai TH, Schmid J. A simple method for extraction of fungal genomic DNA. Letters in Applied Microbiology. 2000;30:53–56. doi: 10.1046/j.1472-765x.2000.00664.x. [DOI] [PubMed] [Google Scholar]
- 26.Selden RF. Southern blotting and hybridization (basic protocol) In: Ausubel FM, Brent R, Kingston RE, Moor DD, Seidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. New York: Wiley; 1990. pp. 2.9.1–2.9.10. [Google Scholar]
- 27.Carroll A, Sweigard JA, Valent B. Improved vectors for selecting resistance to hygromycin. Fungal Genetics Newsletter. 1994;41:22. [Google Scholar]
- 28.Ochman H, Gerber AS, Hartl DL. Genetic application of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klosterman SJ, Hayes RJ. A soilless Verticillium wilt assay using an early flowering lettuce line. Plant Disease. 2009;93:691–698. doi: 10.1094/PDIS-93-7-0691. [DOI] [PubMed] [Google Scholar]
- 30.Brunner E, Domhof S, Langer F. Nonparametric analysis of longitudinal data in factorial experiments. New York: Wiley; 2002. [Google Scholar]
- 31.Shah DA, Madden LV. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology. 2004;94:33–43. doi: 10.1094/PHYTO.2004.94.1.33. [DOI] [PubMed] [Google Scholar]
- 32.White D, Chen W. Genetic transformation of Ascochyta rabiei using Agrobacterium-mediated transformation. Current Genetics. 2006;49:272–280. doi: 10.1007/s00294-005-0048-8. [DOI] [PubMed] [Google Scholar]
- 33.Meng Y, Patel G, Heist M, Betts MF, Tucker SL, Galadima N, Donofrio NM, Brown D, Mitchell TK, Li L, Xu JR, Orbach M, Thon M, Dean RA, Farman ML. A systematic analysis of T-DNA insertion events in Magnaporthe oryzae. Fungal Genetics and Biology. 2007;44:1050–1064. doi: 10.1016/j.fgb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 35.Talhinhas P, Muthumeenakshi S, Martins JN, Oliveira H, Sreenivasaprasad S. Agrobacterium-mediated transformation and insertional mutagenesis in Colletotrichum acutatum for investigating varied pathogenicity lifestyles. Molecular Biotechnology. 2008;39:57–67. doi: 10.1007/s12033-007-9028-1. [DOI] [PubMed] [Google Scholar]
- 36.De Lorenzo G, Castoria R, Cervone F. Fungal invasion enzymes and their inhibition. In: Esser K, Lemke PA, editors. Plant relationship, part-A. The mycota. Berlin: Springer-Verlag; 1997. pp. 61–83. [Google Scholar]
- 37.Valaskova V, Baldrian P. Degradation of cellulose and hemicelluloses by the brown rot fungus Piptoporus betulinus production of extracellular enzymes and characterization of the major cellulases. Microbiology. 2006;152:3613–3622. doi: 10.1099/mic.0.29149-0. [DOI] [PubMed] [Google Scholar]
- 38.Gough CL, Dow JM, Barber CE, Daniels MJ. Cloning of two endoglucanase genes of Xanthomonas campestris pv campestris: Analysis of the role of the major endoglucanase in pathogenesis. Molecular Plant-Microbe Interaction. 1988;1:275–281. doi: 10.1094/MPMI-1-275. [DOI] [Google Scholar]
- 39.Roberts DP, Denny TP, Schell MA. Cloning of the egl gene of Pseudomonas solanacearum and analysis of its role in phytopathogenicity. Journal of Bacteriology. 1988;170:1445–1451. doi: 10.1128/jb.170.4.1445-1451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novo M, Pomar F, Gayoso C, Merino F. Cellulase activity in isolates of Verticillium dahliae deferring in aggressiveness. Plant Disease. 2006;90:155–160. doi: 10.1094/PD-90-0155. [DOI] [PubMed] [Google Scholar]
- 41.de Souza W, Rodrigues JCF. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdisciplinary Perspectives on Infectious Diseases. 2009;2009:1–19. doi: 10.1155/2009/642502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goswami RS, Kistler HC. Heading for disaster: Fusarium graminearum on cereal crops. Molecular Plant Pathology. 2004;5:515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 43.Peplow AW, Tag AG, Garifullina GF, Beremand MN. Identification of new genes positively regulated by Tri19 and regulatory network for trichothecene mycotoxin production. Applied and Environmental Microbiology. 2003;69:2731–2736. doi: 10.1128/AEM.69.5.2731-2736.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapoor K, Rehan M, Kaushiki A, Pasrija R, Lynn AM, Prasad R. Rational mutational analysis of a multidrug MFS transporter CaMdr1p of Candida albicans by employing a membrane environment based computational approach. PLOS Computational Biology. 2009;5:1–11. doi: 10.1371/journal.pcbi.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad R, Kapoor K. Multidrug resistance in yeast Candida. International Review of Cytology. 2005;242:215–248. doi: 10.1016/S0074-7696(04)42005-1. [DOI] [PubMed] [Google Scholar]
- 46.Grimme SJ, Colussi PA, Taron CH, Orlean P. Deficiencies in the essential Smp3 mannosyltransferase block glycosylphosphatidylinositol assembly and lead to defect in growth and cell wall biogenesis in Candida albicans. Microbiology. 2004;150:3115–3128. doi: 10.1099/mic.0.27254-0. [DOI] [PubMed] [Google Scholar]
- 47.Harispe L, Portela C, Scazzocchio C, Penalva MA, Gorfinkiel L. Ras GTPase-activating protein regulation of actin cytoskeleton and hyphal polarity in Aspergillus nidulans. Eukaryotic Cell. 2008;7:141–153. doi: 10.1128/EC.00346-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parra IF, Vranes M, Kamper J, Martin JP. Biz1, a zinc finger protein required for plant invasion by Ustilago maydis, regulates the levels of a mitotic cyclin. Plant Cell. 2006;18:2369–2387. doi: 10.1105/tpc.106.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamura Y, Shim WB. The coiled-coil protein-binding motif in Fusarium verticillioides Fsr1 is essential for maize stalk rot virulence. Microbiology. 2008;154:1637–1645. doi: 10.1099/mic.0.2008/016782-0. [DOI] [PubMed] [Google Scholar]
- 50.Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, Kang S. Agrobacterium-mediated transformation of Fusarium oxysporum: An efficient tool for insertional mutagenesis and gene transfer. Phytopathology. 2001;91:172–180. doi: 10.1094/PHYTO.2001.91.2.173. [DOI] [PubMed] [Google Scholar]
- 51.Maruthachalam K, Nair V, Rho HS, Choi J, Kim S, Lee YH. Agrobacterium tumefaciens-mediated transformation in Colletotrichum falcatum and C. acutatum. Journal of Microbiology and Biotechnology. 2008;18:234–241. [PubMed] [Google Scholar]
- 52.Rho HS, Kang S, Lee YH. Agrobacterium tumefaciens-mediated transformation of the plant pathogenic fungus, Magnaporthe grisea. Molecules and Cells. 2001;12:407–411. [PubMed] [Google Scholar]
- 53.Choi J, Park J, Jeon J, Chi MH, Goh J, Yoo SY, Park J, Jung K, Kim H, Park SY, Rho HS, Kim S, Kim BR, Han SS, Kang S, Lee YH. Genome-wide analysis of T-DNA integration into the chromosomes of Magnaporthe oryzae. Molecular Microbiology. 2007;66:371–382. doi: 10.1111/j.1365-2958.2007.05918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]