Abstract
Background
Obesity is associated with high rates of disability in the general population. The nature of the relationship between obesity and disability in lupus, a condition with a high background rate of disability, is unknown.
Methods
Data were from two interviews, 4 years apart, of a longitudinal cohort of individuals with SLE (n=716 women). Body mass index (BMI) was calculated from self-reported height and weight; obesity was classified by usual (BMI≥30) and revised (BMI≥26.8) definitions. Three measures of functioning were examined: SF-36 Physical Function (PF) subscale, Valued Life Activities (VLA) Disability questionnaire, and employment. Multivariate analyses controlled for demographics, SLE duration and disease activity, glucocorticoid use, depression, and comorbidities. Prospective analyses also controlled for baseline function.
Results
At BMI≥30, 27.8% were obese; at BMI≥26.8, 40.6% were obese. Regardless of obesity definition, obese women exhibited poorer baseline function, with decrements ranging from 20%-33%, depending on the functional measure and obesity definition. With BMI≥26.8, adjusted SF-36 PF scores were 4.3 points lower for obese women (p<.0001), VLA difficulty was 0.09 higher (p=.01), and odds of employment were 80% of non-obese women (OR=0.8 [95% CI 0.5, 1.1]). At 4-year follow-up, women who were obese at baseline had poorer function and experienced greater functional declines.
Conclusion
Obesity was associated with clinically significant negative effects on function, both concurrently and prospectively. This negative impact occurred at a lower BMI than is often considered problematic clinically. Because of the high rate of SLE-related disability, addressing preventable risk factors such as obesity may improve long-term SLE outcomes.
Body composition has been implicated as an important factor in the development of functional limitations and disability. The primary pathways by which body composition affects functioning are through an excess of fat mass relative to lean, or through disproportionately low lean mass (muscle-wasting, or sarcopenia) (1-5). Obesity, in particular, is associated with increased functional limitations, both self-reported and performance-based, and greater disability(2, 6-17).
Both disability and obesity appear to be common in systemic lupus erythematosus (SLE)(18-21). A recent study of the same cohort used in the current study found that among individuals with SLE, 91% experienced disability in at least one valued life activity, and almost half were unable to perform one or more valued life activities(21). Studies have also reported an increased risk of work disability(22-24), and significant impact on the ability to perform activities at home or work(18, 25). Few studies have addressed body composition or obesity in SLE. One report cited a 39% prevalence of obesity among a group of women with lupus(20). A more recent estimate reported the prevalence of obesity from 29% to 50%, depending of the method of ascertainment, with the highest rate based on the most accurate method (dual energy x-ray absorptiometry [DXA])(21). In spite of the high rates of disability and obesity in lupus, no studies have addressed the contribution of obesity to disability in SLE.
The goals of these analyses were to examine functioning among women with SLE classified as obese and to identify the prospective impact of obesity on functioning and functional decline.
Methods
Subjects
The sample for the present study was drawn from female participants in the UCSF Lupus Outcomes Study (LOS). Participants in the LOS had formerly participated in a study of genetic risk factors for SLE outcomes (26, 27) and were recruited from both clinical and community-based sources, including UCSF-affiliated clinics (22%), non-UCSF rheumatology offices (11%), lupus support groups and conferences (26%), and newsletters, websites and other forms of publicity (41%). SLE diagnoses have been verified by medical record review. Participants are interviewed annually by telephone; annual retention averages 93%. Baseline data for these analyses are from the wave 2 interview (n = 832; 753 women), and follow-up data are from wave 6 (n =779; 715 women). All study procedures were approved by the UCSF Committee on Human Research.
At baseline, 26 women met the criterion for underweight (BMI<18.5). Because underweight may also be associated with poor functioning, but for potentially different reasons than obesity, these women were excluded from these analyses. There were an additional 11 participants with missing data for key variables, leaving a final baseline sample of 716, of whom 568 were followed to Wave 6.
Variables
Body mass index (BMI)
Height was self-reported at baseline. Weight was self-reported at each interview. BMI was calculated as weight (kg) / height (m)2(28). Obesity was defined from BMI using two criteria. The first criterion was the one commonly used, BMI≥30 kg/m2 (29). The second was BMI≥26.8 kg/m2, a revised obesity criterion recently proposed for women with SLE based on data regarding body composition from DXA analyses of a subset of these women(21). This lower criterion was found to correspond better with the percentage of body fat associated with threshold of obesity than a BMI of ≥30 kg/m2.
Functioning
Three measures, which assess different aspects of functioning, were examined:
(1) SF-36 Physical Function subscale(30). The Physical Function subscale includes 10 items assessing actions such as lifting and carrying, bending or kneeling, walking, and climbing stairs. Scores are standardized to range from 0 – 100, with a mean of 50, standard deviation of 10, and higher scores reflecting better function.
(2) Valued life activity (VLA) disability(18). The VLA disability scale consists of 21 items, for which respondents rate difficulty in performance on a 4-point scale (0 = no difficulty, 3 = unable to perform). Activities that individuals deem unimportant to them or that they do not perform for reasons unrelated to lupus are not rated and are not included in scoring. The mean difficulty score is calculated based on items rated, and ranges from 0 – 3, with higher scores reflecting greater disability.
(3) Employment. Participants who reported that they were working part- or full-time were classified as currently employed. Employment was examined only for women younger than age 65.
Other variables
Socio-demographic characteristics (age, race/ethnicity, education, income, and marital status) and smoking status were obtained from the baseline LOS telephone interview. Disease activity was assessed during annual telephone interviews using the Systemic Lupus Activity Questionnaire (SLAQ) a validated, self-report measure of disease activity in SLE (31, 32). One item of the SLAQ queries unintentional weight loss, so sensitivity analyses were conducting using a modified version of the SLAQ excluding this item. No differences were noted in results, so all results presented use the entire SLAQ. Disease duration at baseline was calculated based on date of diagnosis obtained from medical record reviews or, if not available in medical records, from participant self-report. Current glucocorticoid use was assessed by interview (at baseline, simple yes/no for current use; at follow-up, yes/no for current use, plus report of highest dose in the previous year). Depressive symptoms were measured with the Center for Epidemiologic Studies Depression (CESD) scale (33), using a score of ≥ 24 as a cut-point for possible depression (34). The following comorbid conditions were assessed by self-report: hypertension, heart disease, diabetes, cancer, respiratory disease, fibromyalgia, and renal disease.
Analysis
Differences in background characteristics and functioning between obese and non-obese women were tested first with t-tests and chi-square analyses. To examine the independent contribution of obesity to function at baseline, multivariate linear or logistic regression models were constructed for each measure of functioning, adjusting for age, race/ethnicity, education, household income, marital status, disease duration, smoking, current glucocorticoid use, SLAQ score, depression, and comorbid conditions. Changes in obesity classifications (e.g., not obese at baseline→obese at follow-up) were tabulated; mean weight changes were calculated for each group. To examine the prospective contribution of baseline obesity to functioning at follow-up, two multivariate regression models were constructed for each measure of functioning. First, to estimate change in functioning alone, the model included only the baseline value of the function measure. The second model added age, race/ethnicity, education, household income, marital status, disease duration, smoking, glucocorticoid use at baseline, highest glucocorticoid use in the follow-up year, baseline SLAQ score, baseline depression, and baseline comorbid conditions. Sensitivity analyses were conducted by eliminating individuals who no longer met the obesity criteria at follow-up. Results of these analyses were not substantively different, and are not shown. Each of the above analyses was performed for each of the two obesity criteria.
Results
Subject characteristics
Mean age of the 716 women at baseline was 48.1 (± 12.6) years (Table 1). The majority (71.9%) were white non-Latino, 7.3% were African American, 8.2% were Asian, and 7.1% were Latino. Thirteen percent had incomes below the poverty level. 37.4% had a history of smoking, and 9.5% were current smokers. The average duration of SLE was 13.6 (±8.5) years, and the average SLAQ score was 12.7 (±7.9). Roughly three quarters of the sample (72.0%) were taking oral glucocorticoids at the time of assessment.
Table 1.
Baseline characteristics of interview cohort (n = 716)
| Obese (BMI ≥ 30) |
Obese (BMI ≥26.8) |
||||||
|---|---|---|---|---|---|---|---|
| Total** (n = 716) |
No (n = 517) (72.2%) |
Yes (n = 199) (27.8%) |
p | No (N = 425) (59.4%) |
Y es (n = 291) (40.6%) |
p | |
| Demographic | |||||||
| Age, years * | 48.1 (12.6) | 47.5 (13.1) | 49.6 (11.3) | .03 | 46.9 (13.1) | 49.8 (11.6) | .002 |
| Race/ethnicity † | |||||||
| White | 71.9 (515) | 71.3 (367) | 28.7 (148) | .40 | 59.6 (307) | 40.4 (208) | .87 |
| Hispanic | 7.1 (51) | 64.7(33) | 35.3 (18) | .26 | 43.1 (22) | 56.9 (29) | .02 |
| African-American | 7.3 (52) | 69.2 (36) | 30.8 (16) | .63 | 50.0 (26) | 50.0 (26) | .19 |
| Asian | 8.2 (59) | 91.5 (54) | 8.5 (5) | .0002 | 81.4 (48) | 18.6 (11) | .0003 |
| Education < college degree † | 14.4 (103) | 13.5 (70) | 16.6 (33) | .34 | 12.9 (55) | 16.5 (48) | .19 |
| Income below poverty † | 12.8 (91) | 12.2 (63) | 14.1 (28) | .53 | 11.6 (49) | 14.5 (42) | .26 |
| Married † | 58.5 (419) | 59.6 (308) | 55.8 (111) | .40 | 60.2 (256) | 56.0 (163) | .28 |
| Smoking | |||||||
| Ever smoked † | 37.4 (268) | 39.5 (185) | 46.1 (83) | .13 | 37.8 (147) | 46.7 (121) | .03 |
| Current smoker † | 9.5 (68) | 9.5 (49) | 9.6 (19) | .99 | 8.5 (36) | 11.0 (32) | .30 |
| Health-related | |||||||
| SLAQ score * | 12.7 (7.9) | 11.7 (7.8) | 15.5 (7.4) | <.0001 | 11.4 (7.9) | 14.6 (7.5) | <.0001 |
| Disease duration, years * | 13.6 (8.5) | 13.9 (8.8) | 12.7 (7.9) | .08 | 13.9 (8.7) | 13.2 (9.2) | .31 |
| Childhood onset of SLE † | 10.9 (78) | 12.0 (62) | 8.0 (16) | .14 | 12.0 (51) | 9.3 (27) | .27 |
| Current use of oral glucocorticoids † |
72.0 (304) | 74.4 (218) | 66.7 (86) | .13 | 77.2 (183) | 65.4 (121) | .009 |
| Depressed (CESD≥24) † | 25.0 (178) | 21.1 (108) | 35.2 (70) | .0002 | 20.4 (86) | 31.7 (92) | .0008 |
| Comorbid conditions †, § | <.0001 | <.0001 | |||||
| 0 | 28.8 (206) | 33.9 (175) | 15.6 (31) | 36.9 (157) | 16.8 (49) | ||
| 1 | 33.8 (242) | 34.4 (178) | 32.2 (64) | 32.2 (137) | 36.1 (105) | ||
| 2 | 20.0 (143) | 19.5 (101) | 21.1 (42) | 18.6 (79) | 22.0 (64) | ||
| 3+ | 17.5 (125) | 12.2 (63) | 31.2 (62) | 12.2 (52) | 25.1 (73) | ||
| Loss to follow-up at Wave 6 | 20.7 (148) | 22.1 (114) | 17.1 (34) | .15 | 21.9 (93) | 18.9 (55) | .35 |
mean (SD)
% (n)
From the following: hypertension, heart disease, diabetes, cancer, respiratory disease, fibromyalgia, and renal disease
26 women who were classified as underweight (BMI < 18.5) were excluded from these analyses. 11 were dropped for missing data on key variables.
With obesity defined as BMI ≥ 30 kg/m2, 27.8% of the cohort was obese at baseline; with the revised obesity cut-point of BMI ≥ 26.8 kg/m2, 40.6% of the women were obese. Using the BMI≥30 definition of obesity, approximately one-third of each ethnic group was obese, except for the Asian group, where only 8.5% met the obesity criterion. Using the lower BMI cutpoint, obesity was significantly more common among Latino women (56.9%) and significantly less common among Asian women (18.6%). High levels of depressive symptoms were significantly more common in obese women, as were a greater number of comorbid conditions, regardless of the obesity definition. SLAQ scores were also higher for obese women. Loss to follow-up was not significantly different between women who were and were not obese at baseline.
Baseline functioning
Unadjusted analyses revealed significant differences in all three function measures between obese and non-obese women, regardless of how obesity was defined (Table 2). In each case, obese women exhibited worse function. With BMI≥30, functional decrements ranged from 22% (on SF-36 PF; mean score for obese=32.5, mean score for non-obese=46.7) to 33% (VLA disability) for obese women. With BMI≥26.8, differences were only slightly smaller, ranging from 20% (SF-36 PF) to 31% (VLA). Significant differences persisted after adjustment for age, race/ethnicity, education, household income, marital status, smoking, disease duration, glucocorticoid use, SLAQ scores, depression, and comorbid conditions. With BMI≥30, SF-36 PF scores were 4.2 points lower (p<.0001), VLA mean difficulty was 0.08 points higher (p=.03), and odds of employment were 80% of non-obese women (OR=0.8 [95% CI 0.5, 1.3]), although the latter difference was not statistically significant. With BMI≥26.8, differences in SF-36 and VLA difficulty were roughly equivalent (−4.3, p<.0001, and 0.09, p=.01), respectively, but still significantly significant, and the odds of employment for obese women were 80% of non-obese women (OR=0.7 [0.5, 1.1]).
Table 2.
Baseline differences in functioning according to obesity (n = 716)
| Obese (BMI ≥ 30) |
Obese (BMI ≥ 26.8) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted¶ |
Unadjusted |
Adjusted¶ |
|||||||
| No (N = 517) |
Yes (n = 199) |
Difference | p§ | β (p) | No (N = 425) |
Yes (n = 291) |
Difference | p§ | β (p) | |
| SF-36 PF * |
41.7
(12.1) |
32.5
(11.0) |
−9.2 | <.0001 |
−4.2
(<.0001) |
42.6
(12.0) |
34.1
(11.4) |
−8.5 | <.0001 |
−4.3
(<.0001) |
| VLA mean difficulty* |
0.74
(0.65) |
1.10
(0.60) |
+0.36 | <.0001 |
0.08
(.03) |
0.71
(0.64) |
1.03
(0.64) |
+0.32 | <.0001 |
0.09
(.001) |
| OR (95% CI) |
OR (95% CI) |
|||||||||
| Currently employed† |
53.4
(250) † |
38.2
(68) |
+15.2 | .0006 | 0.8 (0.5, 1.3) |
55.3
(214) |
40.2
(104) |
+15.1 | .0002 |
0.8
(0.5, 1.1) |
mean (SD)
% (n). Includes only women younger than age 65 at baseline (n = 646).
p-value from t-test or chi-square analysis comparing obese yes/no.
Adjusted for age, race/ethnicity, education, household income, marital status, disease duration, smoking, glucocorticoid use, SLAQ score, depression, and comorbid conditions in multivariate linear or logistic regression analyses.
Note: Bolded values represent statistically significant differences between obese/non-obese
Functioning at follow-up
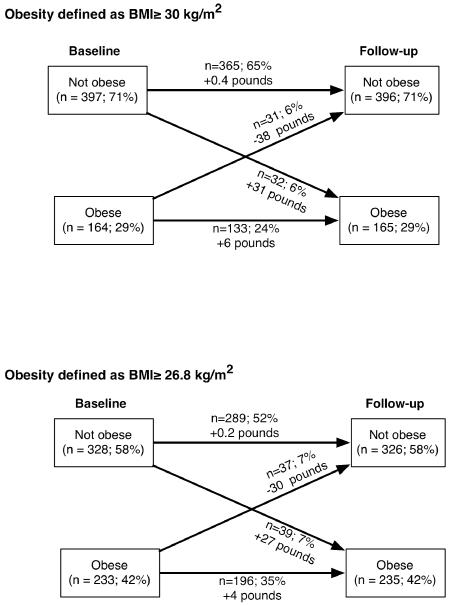
The vast majority of women remained in the same obesity classification category from baseline to follow-up – 89% did not change categories when obesity was defined as BMI≥30 (65% remained not obese and 24% remained obese) and 87% did not change when obesity was defined as BMI≥26.8 (52% remained not obese and 35% remained obese) (Figure 1). Approximately equal numbers of women shifted from non-obese to obese and from obese to non-obese. Mean absolute changes in weight were small among women whose classification did not change (+0.4 pounds or +0.2 pounds for women who were not obese, and +6.3 pounds or +4.1 pounds for obese women). Weight gains and losses were more substantial for women whose classification changed (+30.7 pounds or +26.6 pounds for women who shifted from non-obese to obese; −38.2 pounds or −29.6 pounds for women who shifted from obese to non-obese).
Figure 1.
Transitions in obesity from baseline to follow-up and mean weight changes
At 4-year follow-up, obese women also had significantly worse function according to the unadjusted analyses (Table 3). The decrements in SF-36 and VLA difficulty were equivalent to those noted at baseline. However, the decrement in employment increased from 15.2% at baseline to 20.0% at follow-up for women with BMI≥30, and from 15.1% at baseline to 22.3% at follow-up for women with BMI≥26.8. When follow-up scores were adjusted for baseline values, providing an estimate of the difference in functional changes between obese and non-obese women, the results suggested that obese women experienced greater functional declines than non-obese women. After adjusting for baseline function, sociodemographic and disease characteristics, including SLAQ score, depression, and comorbidities, women with BMI≥30 reported changes in SF-36 PF scores 6% greater (adjusted difference −2.5 / non-obese score of 41.7) and changes in VLA difficulty 13% greater (adjusted difference 0.09 / non-obese score of 0.67) than non-obese women, and were 40% less likely to be employed (OR for employment at follow-up, controlling for baseline employment = 0.60). Taking into account the same covariates, women with BMI≥26.8 reported changes in SF-36 PF 4% greater than non-obese women and were 50% less likely to be employed. Women with BMI≥26.8 also reported changes in VLA difficulty 5% greater than non-obese women, but this difference was not statistically significant.
Table 3.
Functioning at follow-up based on obesity status at baseline (n = 568)
| Obese (BMI ≥ 30) | Obese (BMI ≥ 26.8) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted1 |
Adjusted2 |
Unadjusted |
Adjusted1 |
Adjusted2 |
|||||||
| No (n=403) |
Yes (n=165) |
Difference | p§ | β (p) | β (p) | No (n=332) |
Yes (n=236) |
Difference | p§ | β (p) | β (p) | |
| SF-36 PF |
41.7
(12.3) * |
32.7
(11.3) |
−9.0 | <.0001 |
−2.7
(.001) |
−2.4
(.003) |
42.6
(12.1) |
34.2
(11.9) |
−8.4 | <.0001 |
−2.0
(.01) |
−1.7
(.03) |
| VLA mean difficulty |
0.67
(0.62) |
1.03
(0.59) |
+0.36 | <.0001 |
0.12
(.002) |
0.10
(.01) |
0.65
(0.61) |
0.95
(0.63) |
+0.30 | <.0001 | .06 (.13) |
.04 (.32) |
| OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
OR (95% CI) |
|||||||||
| Currently employed† |
55.0%
(191) |
35.0%
(49) |
−20% | <.0001 |
0.5
(0.3, 0.8) |
0.6
(0.3, 0.9) |
58.2%
(170) |
35.9%
(70) |
−22.3% | <.0001 |
0.4
(0.3, 0.7) |
0.5
(0.3, 0.8) |
Adjusted for baseline value.
Adjusted for age, race/ethnicity, education, income, marital status, disease duration, smoking, glucocorticoid use, SLAQ score, depression, comorbid conditions, and baseline functioning in multivariate linear or logistic regression analyses
mean (SD)
% (n). Includes only women younger than age 65 at follow-up (n = 487).
p-value from t-test or chi-square analysis comparing obese yes/no.
Note: Bolded values represent statistically significant differences between obese/non-obese
Is there a critical value in the BMI-function relationship?
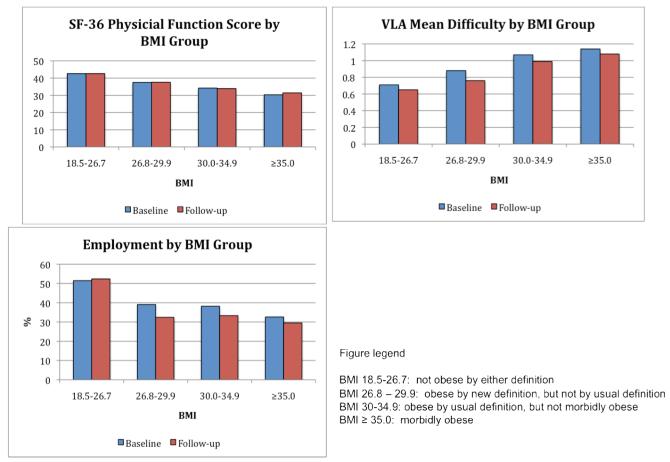
Figure 2 shows mean unadjusted values for each of the functional measures at baseline and follow-up with the cohort divided into four BMI groups – Group 1: BMI 18.5-26.7 (those who would not be considered obese by either definition); Group 2: BMI 26.8-29.9 (obese by the revised definition only); Group 3: BMI 30.0-34.9 (obese by the traditional definition, but not morbidly obese); and Group 4: BMI ≥35.0 (morbidly obese)(29). Each measure shows a substantial functional decrement when moving from the lowest BMI group to the next group (12% for SF-36 PF and 24% for VLA difficulty and employment), but further patterns of functional decrements differed among measures. For the SF-36 PF, each step in the BMI gradient was associated with a relatively comparable functional decrement, ranging from 9% to 12% at baseline and 7% to 12% at follow-up. At baseline, VLA difficulty exhibited similar decrements for the first two gradients (Group 1 to Group 2, 24%; and Group 2 to Group 3, 22%), with a further decrement of only 7% at the last BMI gradient (Group 3 to Group 4). At follow-up, a similar pattern was noted (17%, 30%, 9%). For employment, the largest decrements were noted between Group 1 (BMI 18-5-26.7) and Group 2 (BMI 26.8-29.9), with differences between the two groups of 24% at baseline and 38% at follow-up. There were only slight differences at the next BMI gradient, moving from Group 3 to Group 4, and another considerable decrement in employment noted at the final BMI increase from Group 3 (15% at baseline and 11% at follow-up).
Figure 2.
Function by BMI groups at baseline and follow-up.
Discussion
Function was significantly worse among women with lupus who were obese, compared to those who were not obese, regardless of the definition of obesity or the measure of functioning. Moreover, these differences were not static: obese women experienced a greater decline in functioning over the four-year follow-up period than non-obese women, even taking initial functioning into account. These differences existed even after controlling for factors that might be associated with either obesity or functional difficulties, such as glucocorticoid use and SLE disease activity.
We used two BMI criteria to classify obesity, the well-accepted cut-point of BMI ≥ 30 kg/m2, as well as a newly suggested cut-point of BMI ≥ 26.8 kg/m2(21). The lower cut-point was identified in a previous analysis, using data from a subset of the current cohort, as more accurately reflecting the proportion of body fat associated with the threshold of obesity. In that previous analysis, the lower BMI cut-point was shown to identify cardiovascular risk as well as the accepted BMI cut-point of 30. In the current study, both criteria identified significant differences in baseline functioning for all three measures, indicating that women who might not be considered obese using the traditional BMI cut-point are still at elevated risk for functional problems. Both BMI criteria also identified differences in functional decline between obese and non-obese women. These findings provide additional evidence that, for women with lupus, a lower BMI cut-point might be useful in identifying those at risk for potential negative health outcomes.
While these analyses focused on the impact of obesity on function, it appears that there is a steady increase in functional problems as BMI increases above the normal range. Importantly, this impact does not begin at the usual BMI cut-point for obesity. Evidence of this phenomenon is provided first by the results we obtained using the lower BMI cut-point of 26.8 kg/m2, which is quite close to the standard cut-point for overweight (25.0 kg/m2). Plots of functional measures also showed increasing gradients of impairment and disability as BMI increased, although the patterns of functional decrements varied among measures of functioning.
We examined functioning using three measures that tap different aspects of functioning. The most consistent impact of obesity on functioning was noted for the SF-36 Physical Function subscale, which is a measure of functional limitations. The effect of obesity was also strong for the two disability measures, but less consistent, perhaps reflecting some degree of adaptation to functional limitations. Perhaps most striking was the association between obesity and current work status. Women who were obese at baseline were 30% less likely to be working than women who were not obese, and were about half as like to be working by follow-up. Whether this situation was due solely to the functional or health effects of obesity, there were subtle workplace discriminations due to body size, or changes in disease status that led to both becoming obese and job loss, we cannot determine.
This study has several strengths. Data are drawn from a large, community-based cohort of women with verified SLE diagnoses. We analyzed multiple measures that assessed different aspects of function. The longitudinal nature of our cohort permitted analysis of the prospective impact of obesity on functioning. Examination of both the traditional and revised BMI cut-points demonstrated the impact of body fatness on functioning at a lower level than might be expected.
The study also has limitations that should be considered. First, all data are self-reported. The self-report of height and weight, from which BMI is calculated, might be particularly suspect. However, in a previous analysis of a sample of this cohort, we found that BMI calculated from self-report and in-person measurements were highly correlated (r = .98). Other studies have also reported adequate correlations between BMI calculated from direct measurement and self-report(35-37). We do not know whether obesity developed before or after SLE diagnoses, but it is possible that the relative timing of obesity and SLE diagnosis may affect the impact of obesity on functioning. The LOS may not be representative of all individuals with SLE. However, participants were recruited from community sources as well as from medical centers, and so may be more representative of the universe of women with SLE than samples drawn only from tertiary medical centers. The relationship between obesity and functioning may differ among racial/ethnic groups. In our sample, we found that Asian women were less likely to be obese by either definition and that Hispanic women were more likely to be obese at least by the revised definition. We did not have large enough samples of either ethnic group to conduct separate analyses, but we did control for race/ethnicity in our multivariate analyses. We did not examine the impact of underweight. In population studies, individuals whose BMI falls below 18.5 kg/m2 have functional decrements that are, in some cases, as large as those associated with obesity. However, we did not have a sufficient number of women whose BMI fell into the underweight category to conduct multivariate analysis of that group. An additional limitation to these analysis is incomplete information regarding current and, perhaps more importantly, cumulative dosages of glucocorticoids. We did account for use/non-use of glucocorticoids at baseline and the highest dose received during the follow-up year, but we were not able to distinguish individuals with histories of prolonged exposure to high levels of glucocorticoids, which might be expected to affect body composition. Finally, men with SLE were not included in these analyses. The proportion of men (n=79 at baseline; 9%) in our cohort is equivalent to the prevalence of SLE in men, yet is still small for stratified multivariate analysis. In addition, our previous analysis comparing BMI with DXA did not include men because the number of men was too small (n=12) for meaningful analysis. Thus, we did not have a DXA-equivalent BMI for men for the current analyses. Future studies are needed to determine both the body composition characteristics of men with SLE and the impact of those characteristics on functioning.
In summary, obesity is associated with a significant negative impact on functioning among women with lupus, both cross-sectionally and prospectively. This negative impact was seen in terms of functional limitations, disability, and work status. Perhaps most notably, this negative impact occurred at a BMI lower than is usually considered problematic clinically or in the general population. Results from these analyses confirm our earlier suggestion that use of this lower cut-point to define obesity may prove useful in identifying women at risk of adverse health outcomes, such as disability. Because of the high rates of disability in SLE, identifying preventable risk factors, such as overweight and obesity, has the potential to decrease the negative impact of the disease on functioning.
Significance and Innovation.
Obesity is associated with high rates of disability in the general population, but the relationship has not been studied in SLE, a condition with high background rates of disability.
Previous studies have shown a high prevalence of obesity in women with SLE.
Obesity was associated with a clinically negative impact on function concurrently.
At 4-year follow-up, women who were obese at baseline experienced significantly greater functional declines.
Acknowledgments
This research was supported by NIH/NIAMS grant P60 AR053308.
References
- 1.Okoro C, Hootman J, Strine T, Balluz L, Mokad A. Disability, arthritis, and body weight amongadults 45 years and older. Obes Res. 2004;12:854–61. doi: 10.1038/oby.2004.103. [DOI] [PubMed] [Google Scholar]
- 2.Ferraro K, Su Y, Gretebeck R, Black D, Badylak S. Body mass index and disability in adulthood: a 20-year panel study. Am J Public Health. 2002;92:834–40. doi: 10.2105/ajph.92.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan L, Daviglus M, Liu K, Pirzada A, Garside D, Schiffer L, et al. BMI and health-related quality of life in adults 65 years and older. Obes Res. 2004;12:69–76. doi: 10.1038/oby.2004.10. [DOI] [PubMed] [Google Scholar]
- 4.Heo M, Allison D, Faith M, Zhu S, Fontaine K. Obesity and quality of life: mediating effects of pain and comorbidities. Obes Res. 2003;11:209–16. doi: 10.1038/oby.2003.33. [DOI] [PubMed] [Google Scholar]
- 5.Ford E, Moriarty D, Zack M, Mokdad A, Chapman D. Self-reported body mass index and health-related quality of life: findings from the Behavioral Risk Factor Surveillance System. Obes Res. 2001;9:21–31. doi: 10.1038/oby.2001.4. [DOI] [PubMed] [Google Scholar]
- 6.Visser M, Langlois J, Guralnick J, Cauley J, Kronmal R, Robbins J, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health study. Am J Clin Nutr. 1998;68:584–90. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 7.Sturm R, Ringel J, Andreyeva T. Increasing obesity rates and disability trends. Health Aff. 2004;23:199–205. doi: 10.1377/hlthaff.23.2.199. [DOI] [PubMed] [Google Scholar]
- 8.Sturm R. Increases in clinically severe obesity in the United States, 1986-2000. Arch Intern Med. 2003;163:2146–8. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 9.Visser M, Harris T, Langlois J, Hannan M, Roubenoff R, Felson D, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol: Med Sci. 1998;53:M214–M21. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 10.Zamboni M, Turcato E, Santana H, Maggi S, harris T, Bietrobelli A, et al. The relationship between body composition and physical performance in older women. J Am Geriatr Soc. 1999;47:1403–9. doi: 10.1111/j.1532-5415.1999.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 11.Sternfeld B, Ngo L, Satariano W, Tager I. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156:110–21. doi: 10.1093/aje/kwf023. [DOI] [PubMed] [Google Scholar]
- 12.Broadwin J, Goodman-Gruen D, Slymen D. Ability of fat and fat-free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49:1641–5. doi: 10.1046/j.1532-5415.2001.t01-1-49273.x. [DOI] [PubMed] [Google Scholar]
- 13.LaCroix A, Guralnik J, Berkman L, Wallace R, Satterfield S. Maintaining mobility in late life. II. Smoking, alcohol consumption, physical activity, and body mass index. Am J Epidemiol. 1993;137:858–69. doi: 10.1093/oxfordjournals.aje.a116747. [DOI] [PubMed] [Google Scholar]
- 14.Launer L, Harris T, Rumpel C, Madans J. Body mass index, weight changes, and risk of mobility disability in middle-aged and older women. The epidemiologic follow-up study of NHANES I. JAMA. 1994;271:1093–8. [PubMed] [Google Scholar]
- 15.Friedmann J, Elasy T, Jensen G. The relationship between body mass index and self-reported functional limitation among older adults: a gender difference. J Am Geriatr Soc. 2001;49:398–403. doi: 10.1046/j.1532-5415.2001.49082.x. [DOI] [PubMed] [Google Scholar]
- 16.Weil E, Wachterman M, McCarthy E, Davis R, O’Day B, Lezzoni L, et al. Obesity among adults with disabling conditions. JAMA. 2002;288:1265–8. doi: 10.1001/jama.288.10.1265. [DOI] [PubMed] [Google Scholar]
- 17.Zoico E, Di Francesco V, Guralnick J, Mazzali G, Bortolani A, Guariento S, et al. Physical disability and muscular strength in relation to obesity and different body composition indexes in a sample of healthy elderly women. Int J Obes. 2004;28:234–41. doi: 10.1038/sj.ijo.0802552. [DOI] [PubMed] [Google Scholar]
- 18.Katz P, Morris A, Trupin L, Yazdany J, Julian L, Yelin E. Disability in valued life activities among individuals with systemic lupus erythematosus. Arthritis Rheum (Arthritis Care Res) 2008;59:465–73. doi: 10.1002/art.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kipen Y, Strauss B, Morand E. Body composition in systemic lupus erythematosus. Br J Rheumatol. 1998;37:514–9. doi: 10.1093/rheumatology/37.5.514. [DOI] [PubMed] [Google Scholar]
- 20.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus. 2000;9:170–5. doi: 10.1191/096120300678828226. [DOI] [PubMed] [Google Scholar]
- 21.Katz P, Gregorich S, Yazdany J, Trupin L, Yelin E, Criswell L. Obesity and its measurement in a community-based sample of women with systemic lupus erythematosus. Arthritis Care Res. 2010;63:261–8. doi: 10.1002/acr.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yelin E, Trupin L, Katz P, Criswell L, Yazdany J, Gillis J, et al. Work dynamics among persons with systemic lupus erythematosus. Arthritis Rheum (Arthritis Care Res) 2007;57:56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker K, Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology. 2009;48:281–4. doi: 10.1093/rheumatology/ken477. [DOI] [PubMed] [Google Scholar]
- 24.Mok C, Cheung M, Ho L, Yu K, To C. Risk and predictors of work disability in Chinese patients with systemic lupus erythematosus. Lupus. 2008;17:1103–7. doi: 10.1177/0961203308094280. [DOI] [PubMed] [Google Scholar]
- 25.Boomsma M, Bijl M, Stegeman C, Kallenberg C, Hoffman G, Tervaert J. Patients’ perceptions of the effects of systemic lupus erythematosus on health, function, income, and interpersonal relationships: a comparison with Wegener’s granulomatosis. Arthritis Rheum (Arthritis Care Res) 2002;47:196–201. doi: 10.1002/art.10341. [DOI] [PubMed] [Google Scholar]
- 26.Freemer M, King TJ, Criswell L. Association of smoking with dsDNA autoantibody production in systemic lupus erythematosus. Ann Rheum Dis. 2006;65:581–4. doi: 10.1136/ard.2005.039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorburn C, Prokunina-Olsson L, Sterba K, Lum R, Seldin M, Alarcon-Riquelme M, et al. Association of PCDC1 genetic variation with risk and clinical manifestations of systemic lupus erythematosus in a multiethnic cohort. Genes Immun. 2007 Mar 8; doi: 10.1038/sj.gene.6364383. doi:10.1038/sj.gene.6364383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Word Health Organization. Status WECoP . The use and interpretation of anthropometry physical status: the use and interpretation of anthropmetry: report of a WHO expert committee. Geneva, Switzerland: 1995. 1995. [Google Scholar]
- 29.World Health Organization . Obesity: preventing and managing the global epidemic. World Health Organization; Geneva: 2000. [PubMed] [Google Scholar]
- 30.Ware JJ, Snow K, Kosinski M, Gandek B. SF-36 Health Survey: manual and interpretation guide. The Health Institute, New England Medical Center; Boston, Massachusetts: 1993. [Google Scholar]
- 31.Karlson E, Daltroy L, Rivest C, Ramsey-Goldman R, Wright E, Patrtridge A, et al. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus. 2003;12:280–6. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 32.Yazdany J, Yelin E, Panopalis P, Trupin L, Julian L, Katz P. Validation of the systemic lupus erythematosus activity questionnaire in a large obsevational cohort. Arthritis Rheum (Arthritis Care Res) 2008;59:136–43. doi: 10.1002/art.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 34.Julian L, Gregorich S, Tonner C, Yazdany J, Trupin L, Criswell L, et al. Using the CES-D to screen for depression in SLE. Arthrits Care Res. 2011 doi: 10.1002/acr.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dekkers J, van Wier M, Hendriksen I, Twisk J, van Mechelen W. Accuracy of self-reported body weight, height and waist circumference in a Dutch overweight working population. BMC Med Res Methodol. 2008;8:69. doi: 10.1186/1471-2288-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craig B, Adams A. Accuracy of body mass index categories based on self-reported height and weight among women in the United States. Matern Child Health J. 2009;13:489–96. doi: 10.1007/s10995-008-0384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burton N, Brown W, Dobson A. Accuracy of body mass index estimated from self-reported height and weight in mid-aged Australian women. Aust N Z J Public Health. 2010;34:620–3. doi: 10.1111/j.1753-6405.2010.00618.x. [DOI] [PubMed] [Google Scholar]