Abstract
The individual roles of estradiol (E) and progesterone (P) in the control of food intake and body weight in ovariectomized (OVX) rats were investigated. Six groups of OVX Sprague-Dawley rats (n=9/group) were assigned to one of three 4-day cyclic hormone treatments: two groups were treated with E benzoate; two groups were treated with P; two groups were treated with both (EP). All rats had continuous access to chow and water throughout this 4-week study. One group of rats within each hormone treatment condition was fed chow ad libitum, and the second was subjected to a binge schedule: chow ad libitum plus 1-h access to an optional fat source on Monday, Wednesday, and Friday. A seventh OVX group (n = 8) received the oil vehicle and chow. This group was included to monitor body weight and to verify hormone efficacy. The main findings were: (1) relative to rats receiving only P, E alone or EP attenuated 24-h chow intake tonically and cyclically, i.e. intake on Day 4, which models estrus, was lower in E and EP than in P, and also was lower than intake on Day 2, which models diestrus. In contrast, (2) neither E nor EP detectably affected optional fat intake during the 1-h fat access period relative to rats receiving only P when data were collapsed across the entire study. However, (3) E and EP had large effects on fat intake relative to P during the 1-h fat access period at the start of the study, but not at the end, when bingeing was fully established. (4) E and EP led to lower and apparently normal levels of body weight compared to rats receiving only the oil vehicle or only P. These results indicate that (1) administration of E alone has similar effects as co-administration of E and P on feeding and body weight in rats bingeing on fat, (2) with or without P, the inhibitory effects of E on meal size are compromised when bingeing on fat, and (3) the effects of E on binge size change dynamically as bingeing develops.
Keywords: binge eating, bulimia, ovarian hormones, female, fat intake
Introduction
Ovarian cycling affects food intake in both humans and animals, mainly via estrogens. [1–3]. Estrogens have both tonic and cyclic inhibitory effects on eating [1,4,5]. Tonic inhibition is evidenced by the increases in food intake and adiposity that occur with ovariectomy (OVX) in animals and by the increase in adiposity after menopause in women [1–3,6–8]. Cyclic inhibition is evidenced by changes in food intake across the estrous cycle in rats and across the menstrual cycle in women. Female rats and mice eat less during the estrus phase, which occurs just after estrogens peak, and eat more during early diestrus, when estrogen levels are lower [1,2]. In women, food intake decreases in the peri-ovulatory phase, when estrogen levels are highest, and increases in the luteal phase, when both progestin and estrogen levels are high [9–11]. Treatment with cyclic regimens of estradiol (E) normalizes food intake and body weight in OVX rats [1–3,5]. Physiological levels of progesterone (P), on the other hand, do not affect eating in OVX rats; however, pharmacological P treatment can reverse the inhibitory effect of E on eating [3,9].
Ovarian hormones also modulate binge eating. In humans, the frequency of binge eating varies with the menstrual cycle, with higher binge frequency occurring during the luteal phase and menses [12–14]. A negative association between E level and binge frequency, and a positive association between P level and binge frequency, were reported in women with bulimia nervosa (BN) [15]. In addition, in a community sample, higher emotional eating scores (consistent with binge eating) on an eating behavior questionnaire were obtained when E was low and P was high [16]. Associations between E and binge size have not been reported.
In current animal models of binge-type eating, opportunities to binge are experimentally controlled, so that binge size rather than binge frequency is the outcome measure. We previously reported that binge size was tonically, but not cyclically, reduced in OVX rats treated with both E and P [17]. In the same rats, total daily food intake was reduced both tonically and cyclically, suggesting that the modulatory effects of E on food intake differ depending upon the conditions under which food is consumed. However, the individual contributions of E and P to binge eating were not ascertained in that study. Although E appears sufficient to explain the contribution of ovarian function to normal eating, in women with BN, cyclic changes in P levels have been independently associated with increased binge frequency [15] and emotional-eating scores [16]. Therefore, it is possible that P contributed to the loss of cyclic inhibitory effects of E on binge size in our previous report [17].
Here we sought to determine the individual and combined effects E and P on food intake in bingeing rats. We hypothesized that: (1) binge size would be reduced tonically in E and EP rats relative to P rats, (2) binge size would not be cyclically reduced in any of the groups, (3) daily energy intake also would be reduced tonically in E and EP rats relative to P and control oil vehicle rats, and (4) that daily energy intake, in contrast to binge intake, would be cyclically reduced in E and EP, but not in P rats [17,18].
Materials and Methods
1. Subjects
Female Sprague-Dawley rats (Harlan, Indianapolis, IN; 60 days of age) were individually housed in stainless-steel cages with ad libitum access to water and pelleted chow (Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN; macronutrient content (kcal/kg diet, percent of calories): protein (936, 28%), fat (405, 12%), carbohydrate (1960, 60%); 3.3 kcal/g). The vivarium was maintained at 22±2°C with a 12/12h light-dark cycle (lights off at 1900 h). All procedures were approved by the Pennsylvania State University Institutional Animal Care and Use Committee.
2. Ovariectomy (OVX)
After one-week adaptation to the vivarium, rats were given a single period of overnight access to fat (Crisco® shortening [hydrogenated vegetable oil], J.M. Smucker Co., Orrville, OH; 9.17 kcal/g) in a bowl clipped to the front of the home cage. This was done to prevent neophobia during the rest of the study. In addition, the overnight fat intake data were used to group the rats after the surgery. Chow and water were available during the overnight fat access. Three days later, the rats were anaesthetized (1 ml/kg body weight, IP) with a mixture of 70 mg/kg ketamine (Phoenix Science Inc., St. Joseph, MO) and 2 mg/kg xylazine (Phoenix Science), with 0.2 ml/kg supplements given as needed, and bilaterally OVX using a dorsal approach. Specifically, the surgical area was shaved and then cleaned by alternate iodine and 70% ethanol solutions for a total of 3 cycles. A midline dorsal incision (2.0–3.0 cm) was made through the skin about halfway between the shoulder blades and tail base. Parallel incisions (0.5–1.0 cm) were then made through the underlying abdominal musculature on both sides of the rat, about 2.0 cm lateral to the midline. The ovaries were pulled out through the incisions, the uterine horn and blood vessels were clamped, and a 4-knot ligature was placed around the blood vessels and uterine horn just distal to the clamp. The ovaries were the excised and the clamp released. After checking for bleeding, the uterine horn was replaced into the abdominal cavity. Absorbable suture material (4-0 vicryl or a generic equivalent) was used to close the muscle layer. Surgical staples were used to close the skin; these were removed after 5–7 d.
3. Experimental design
Sixty-two OVX rats were used. After 5 days of postoperative recovery from OVX, rats were matched for body weight and fat intake during the single pre-surgical overnight exposure (above) and divided into seven groups. Two groups (n=9/group) were maintained on the same 4-day hormone treatment cycle as EP groups in our previous study; that is, subcutaneous injection with 17-β-estradiol-benzoate (E, Sigma, 2 µg/100 µl sesame oil) on day 2 followed by progesterone (P, Sigma, 500 µg/100 µl sesame oil) on day 3 [17]; two groups (n=9/group) had only E injections and another two groups (n=9/group) had only P injections. These doses of E and P produce near-physiological levels of E [18] and P [19], and the cyclic regimen models the typical 4-d estrous cycle of intact rats; that is Day 1: Diestrus 1, Day 2: Diestrus 2, Day 3: Proestrus, Day 4: Estrus [17]. Cyclic EP also maintains sexual receptivity (lordosis) in OVX rats [18], and normal body weight and food intake in OVX rats fed chow or allowed to binge on fat [17,18]. Hormone treatments were continued throughout the experiment. The seventh group (n=8) had oil vehicle injections on days 2 and 3. This group was maintained on chow throughout the study and was included to monitor body weight gain as an indicator of hormone treatment efficacy.
After four hormone treatment cycles, rats within each hormone treatment group were assigned to one of two feeding protocols: Chow only (chow available ad libitum with no fat access) and our standard high-restriction “binge” protocol (chow available ad libitum with fat provided 1 h/day on Mon, Weds and Fri, 2 h prior to lights off). The experimental groups are summarized in Table 1. Chow intake was measured every 24 h, as well as during the 1-h fat access period on Mon, Weds, and Fri in all groups. Fat intake was measured during the 1-h fat access period on Mon, Weds and Fri in the H groups. Body weight was measured before OVX, daily during the 6 days post OVX and once a week thereafter.
Table 1.
Experimental Groups
| Group | Hormone Treatment | Fat Access | |||
|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | ||
| EPC | Estradiol | Progesterone | None | ||
| EPH | Estradiol | Progesterone | High-restriction | ||
| EC | Estradiol | Oil | None | ||
| EH | Estradiol | Oil | High-restriction | ||
| PC | Oil | Progesterone | None | ||
| PH | Oil | Progesterone | High-restriction | ||
4. Data analysis
Data were analyzed using SAS 9.1 for Windows (SAS institute, Cary, NC). The outcomes analyzed were 1-h fat intake (kcal), 1-h chow intake (kcal), 1-h total energy intake (kcal), 24-h chow intake (kcal), 24-h total energy intake (kcal), and body weight (g). All data are presented as means ± SEM. Fat intake data were analyzed via 2-way ANOVA, since only the fat access groups consumed the fat (hormone treatment × cycle day); chow data and total energy intake data were analyzed via 3-way ANOVA, since these analyses included both the chow and the fat-access groups (diet group × hormone treatment × cycle day). Cycle day 2 data were calculated by averaging all of the day 2 data for each 4-day hormone treatment cycle across the 4-week study. Similarly, intake data on day 4 were averaged across all 4 weeks. Cycle day 2 data and day 4 data were compared to examine the cyclic effects of E, EP and P because day 2 models the diestrus phase, in which hormone effects are minimal, and day 4 models the estrus phase, in which hormone effects are maximal. Significant differences among groups on each day were assessed using preplanned LS means comparison, corrected for the number of comparisons being made. For three comparisons, alpha was set at 0.05/(3−1) = 0.025; for six comparisons, alpha was set at 0.05/(6−1) = 0.01. T-tests were used to determine differences between days 2 and 4 within each group. Since six t-tests were needed for some of the intake comparisons (for instance 24-h chow intake, which included all 6 groups), alpha was set at 0.05/(6−1) = 0.01 for all Day 2 vs. Day 4 comparisons.
To determine if the hormones affected binge intake differently at the start of the study (before bingeing was established), and end of the study (after bingeing was established), day 4 intakes on weeks 1 and 4 of treatment in groups with access to fat were analyzed by 2-way ANOVA (hormone treatment × day of study). Day 4 was used because the effects of E are maximal on day 4. Tukey’s post hoc tests following the 1-way ANOVA were used to determine significant differences among groups on each day. T-tests, with alpha set at 0.05/(3−1) = 0.025 were used to determine differences between the first Day 4 and the last Day 4 within each group.
Body weights before OVX and 5 and 45 d after OVX were analyzed by 2-way ANOVA (group × time). Cumulative energy intake across the entire study was analyzed via 1-way ANOVA, followed by Tukey’s HSD for determination of significant differences among the groups.
Effect sizes were determined using Cohen’s d, based upon formulas described by Thalheimer & Cook (2002) [20].
Results
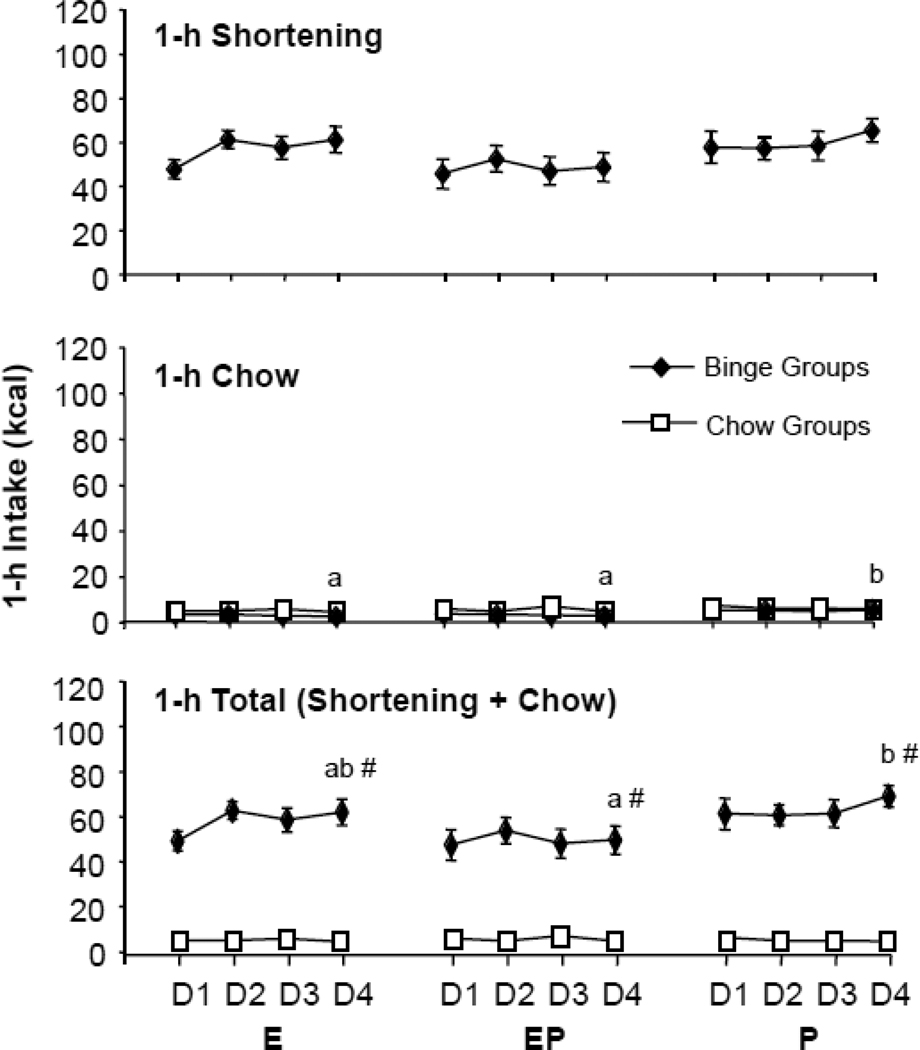
1. 1-h energy intake averaged across the entire study (Fig. 1)
Figure 1.
Effect of cyclic hormone treatment and fat access schedule on 1-h fat (shortening) (top), chow (middle) and total (bottom) energy intake. D=Day, E=Estradiol, P=Progesterone, EP=Estradiol/Progesterone. Small letters indicate tonic effects of estradiol in the binge groups; different letters indicate significant differences among the groups. There was no effect of cycle day on intake. # indicates significant differences between binge and chow groups within any given hormone treatment. For clarity only Day 4 comparisons are shown.
1-h fat intake (Fig. 1, top panel)
One-h fat intake under binge conditions was unaffected by hormone treatment or day (main effect of hormone treatment, F (2,24) = 1.33, p>0.05; main effect of day, F (1,24) = 0.63, p>0.05]. However, there was an interaction effect [F (2,24) = 3.43, p<0.05], apparently due to slight, but not significant, reductions in fat intake on Day 4 in the EPH group (Day 2: 52.6 ± 5.9 kcal, Day 4: 48.9 ± 6.5 kcal) and increases on Day 4 in the PH group (Day 2: 57.4 ± 5.3 kcal; Day 4: 65.5 ± 5.4 kcal) (Fig. 1, top panel). Intake of the EH group was unaffected by cycle day (Day 2: 61.3 ± 3.9 kcal; Day 4: 61.4 ± 5.9 kcal). Thus, relative to treatment with P alone, treatment with E resulted in neither tonic nor cyclic effects on 1-h fat intake in bingeing rats.
1-h chow (Fig. 1, middle panel)
One-h chow intake during the fat-access period was low, as expected, never exceeding an average of 4 g for any group (Day 4 intakes: EC 2.4 ± 0.4, EPC 2.8 ± 0.5, PC 3.9 ± 0.8, EH 0.7 ± 0.3, EPH 0.9 ± 0.6, PH 3.6 ± 1.0 g). Even so, the two E-treated binge groups (E, EP) consumed significantly less chow during the 1-h period relative to the group that received only P [main effect of hormone treatment F(2,48) = 5.20, p<0.01; LS means comparisons indicated significant differences only in the binge groups, p<0.01]. That is, in contrast to its lack of effect on fat intake, E tonically reduced chow intake relative to P alone even during this brief 1-h period. There were no main effects of or interactions with day for the 1-h chow intake. Finally, as expected, 1-h chow intake was significantly reduced in the groups with fat access relative to the groups with no fat access [3-way ANOVA: main effect of fat access F(1,48) = 5.97, p<0.05].
1-h total (Fig. 1, bottom panel)
One-h total intake (chow plus fat), was unaffected by hormone treatment [main effect of hormone treatment F (2,48) = 2.18, p>0.05], but was significantly greater in the groups with fat access [main effect of fat access F(1,48) = 382.54, p < 0.0001], due to the large amount of fat consumed by those groups. There was a significant 3-way interaction among fat access schedule, hormone treatment, and cycle day [F(2,48) = 3.82, p <0.05]. Each of the binge groups consumed significantly more energy during the fat access period relative to the respective chow group receiving the same hormone treatment on both Day 2 and Day 4 of the cycle (LS means comparisons: p < 0.0001 for each). In addition, the EPH group consumed significantly less than the PH group, but only on Day 4 (LS means comparisons: p <0.005]. In spite of these differences, t-tests revealed no statistically significant differences in total 1-h intake between Day 2 and Day 4 for any group, i.e. total intake during the 1-h access period was not reduced in the day modeling estrus (day 4) compared to the day modeling diestrus (day 2) in either binge or control rats.
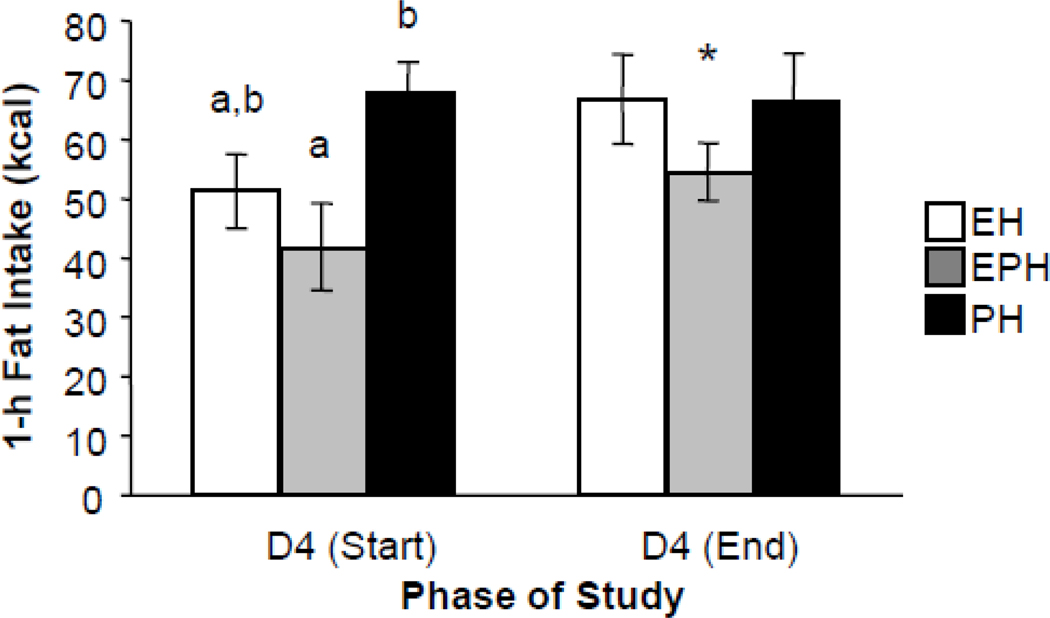
2. 1-h fat intake at the beginning and end of the study (Fig. 2)
Figure 2.
Effect of hormone treatment on 1-h fat intake on the first and last Day 4 of the study. D=Day, E=Estradiol, P=Progesterone, EP=Estradiol/Progesterone. One-h intake differed among the groups at the start of the study, but not at the end of the study. Different small letters indicate significant differences among the groups. Asterisk indicates a significant difference between intakes on Day 4 (start) and Day 4 (end) in the EPH group.
The failure to see differences in fat intake among the hormone treated groups (above) may have been due to averaging the data across the study, since the binge behavior escalates across time [21]. Thus, an additional analysis of Day 4 data at the start and end of the study was done (see Methods).
On the first Day 4, the EPH group consumed significantly less than PH, with EH intakes intermediate [main effect of group F(2,24) = 4.53; p < 0.05] (Fig 2). Effect size analysis showed that the effects of E on the first Day 4, relative to P, were large in both the EH and EPH groups (Cohen’s d: −0.73 and −1.05, respectively) (Table 2). In contrast, there were no significant differences among the groups on the last Day 4 of the study, and the effects of E relative to P alone were small (Cohen’s d: 0.02 in EH rats) to moderate (Cohen’s d: −0.45 in EPH rats).
Table 2.
First D4, Last D4 fat intake analyses.
| 1-hr Fat: First D4 | |||||
|---|---|---|---|---|---|
| Group | Intake (kcal±SE) | ANOVA F, p | LS Means | Cohen's d* | Effect Size |
| EH | 51.3 (6.3) | F(2,24) = 4.53 | AB | 0.73 | large |
| EPH | 41.7 (7.3) | p 0.0214 | B | 1.05 | large |
| PH | 68.0 (4.9) | A | |||
| 1-hr Fat: Last D4 | |||||
|---|---|---|---|---|---|
| Group | Intake (kcal±SE) | ANOVA F, p | Tukey's HSD |
Cohen's d* | Effect Size |
| EH | 66.8 (7.6) | F(2,24) = 1.05 | NS | −0.02 | small |
| EPH | 54.3 (4.9) | p 0.3640 | NS | 0.45 | medium |
| PH | 66.4 (8.0) | NS | |||
relative to PH
There was a significant effect of day [F(1,24) = 4.32, p<0.05], due to increased intake on the last Day 4 of the study relative to the first Day 4 in the groups treated with E. Specifically, intake in the EH group increased by 15.6 ± 8.0 kcal across the study, although this was not significant (first Day 4 vs. last Day 4 t = −1.95, p>0.05) and in the EPH group by 12.6 ± 4.4 kcal (first Day 4 vs. last Day 4 t = −2.83, p <0.05). In contrast, the PH groups changed little (−1.6 ± 8.9 kcal, first Day 4 vs. last Day 4, t = 0.18, p>0.05). Effect size analysis showed that the change in intake across the study in both groups treated with estradiol, relative to the group treated with progesterone alone, was moderate (Cohen’s d: 0.50 for both EH and EPH).
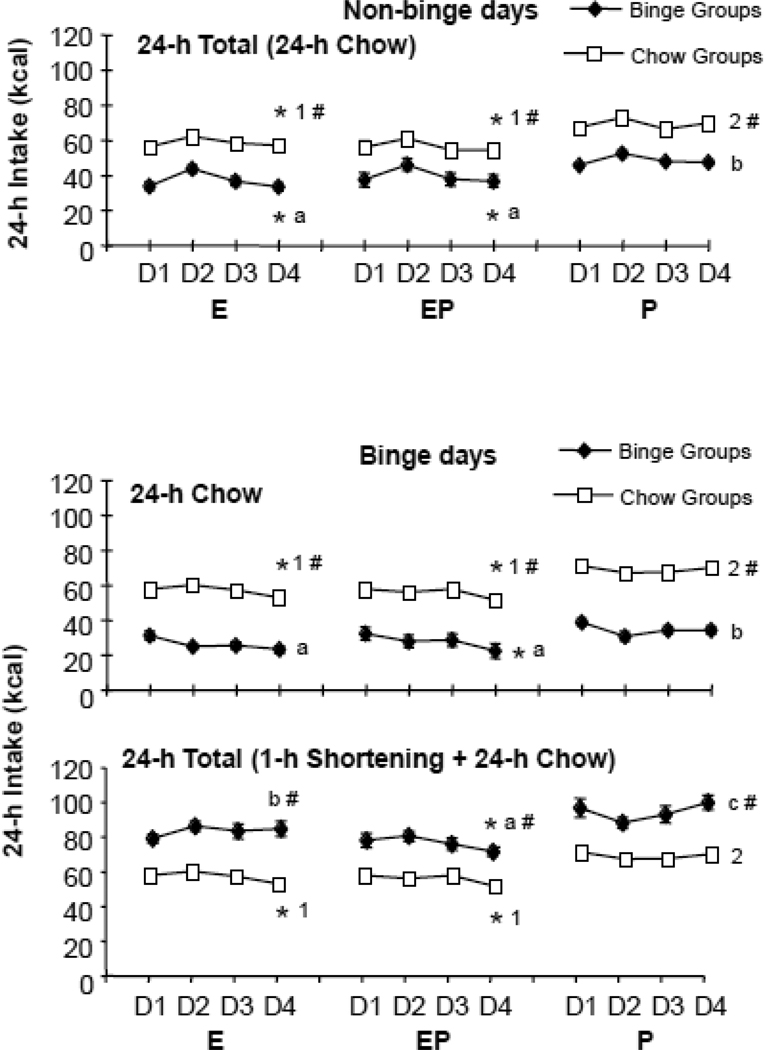
3. 24-h energy intake averaged across the entire study (Fig. 3)
Figure 3.
Effect of cyclic hormone treatment on daily energy intake. Top: 24-h total intake, i.e. 24-h total energy intake on days that binge rats did not have access to the optional fat. Middle: 24-h chow intake on days that binge rats did have access to the optional fat. Bottom: 24-h total energy intake (1-h fat plus chow) on days that binge rats had access to the optional fat. Note that 1-h fat intake is shown in Fig 1. D=Day, E=Estradiol, P=Progesterone, EP=Estradiol/Progesterone. Small letters indicate tonic effects of estradiol in the binge groups; different letters indicate significant differences among the groups. Numbers indicate tonic effects of estradiol in the chow groups; different numbers indicate significant differences among the groups. * indicates cyclic effect of estradiol in each group, i.e. intake on Day 4 significantly less than intake on Day 2. # indicates significant difference between binge and chow group within each hormone treatment. For clarity only Day 4 comparisons are shown.
24-h chow on non-binge days (Fig 3, top panel)
On the days that the H rats did not have access to fat, 24-h chow intake, i.e. 24-h total intake, was significantly affected by hormone treatment [main effect of hormone treatment F(2, 48) = 21.56, p < 0.0001]. This was the result of lower intakes in the groups treated with E, relative to those treated with P alone. LS Means comparisons among individual groups on Day 4 of the cycle showed that the EC, EPC, EH, and EPH groups each consumed significantly less than the relevant P comparison group (PC and PH, respectively) (p < 0.01 for each). In addition, intakes in the EC and EPC group were not statistically different, nor were intakes in the EH and EPH groups. To summarize, E exerted a tonic inhibitory effect on 24-h chow intake on non-binge days relative to effects of P alone that was not significantly reduced by the addition of P.
Intake was also significantly affected by cycle day [main effect of cycle day F(1,48) = 181.15, p < 0.0001], due to reduced intake on Day 4 relative to Day 2 in the rats treated with E (t-tests comparing Day 2 to Day 4: t = 3.69, 7.96, 7.76, and 13.3 for EC, EPC, EH, and EPH, respectively; p < 0.01 for all), but not in the rats treated with P alone (t-tests comparing Day 2 to Day 4, ns for PC, PH). As a result, cycle day interacted significantly with hormone treatment [F(2,48) = 6.87, p<0.005], and with fat access [F(1,48) = 11.21, p<0.005], but there was no significant 3-way interaction among the factors. Overall, these results indicate that, in addition to tonic effects, E exerted cyclic effects on 24-h chow intake on non-binge days.
Chow intake was significantly affected by diet, with the fat access groups consuming less than the groups without access to the optional source of fat [3-way ANOVA: main effect of fat access F(1,48) = 143.17, p < 0.0001; LS Means comparison p < 0.0001 for each C group (EC, EPC, PC) compared to the binge group receiving the same hormone treatment (EH, EPH, PH, respectively)].
24-h chow on binge days (Fig 3, middle panel)
Results for 24-h chow intake on binge days were similar to those reported above for non-binge days. Intake was significantly affected by hormone treatment [main effect of hormone treatment F(2, 48) = 21.56, p < 0.0001], due to reduced intake in the groups treated with E relative to those treated with P alone. Specifically, LS Means comparisons on Day 4 showed that groups treated with estradiol (EC, EPC and EH, EPH) consumed significantly less chow than their respective P comparison group (PC and PH, respectively) (p < 0.01 for each). In addition, EC and EPC were not statistically different from each other, nor were EH and EPH. Thus, E exerted a tonic effect on 24-h chow intake on binge days, in a manner similar to that on non-binge days, relative to P alone. Furthermore, groups that received E and P consumed amounts comparable to groups that received only E, i.e. progesterone did not reverse estradiol’s inhibitory effect.
Binge day chow intake was also significantly affected by cycle day [main effect of cycle day F(1,48) = 13.46, p<0.001], due to reduced intake on Day 4 relative to Day 2 in 3 of the 4 groups treated with E (t-tests comparing Day 2 to Day 4: t = 3.77, 4.10, and 4.60 for EC, EPC, and EPH, respectively; p<0.01 for all). Intake did not change significantly in the EH group or in the rats treated with P alone. As a result of these differences, cycle day interacted significantly with hormone treatment [F(2,48) = 22.00, p < 0.0001]. These results indicate that, in addition to its tonic effects, E exerted cyclic effects on 24-h chow intake on binge days in most groups.
As expected, groups with access to fat ate less chow than groups without [main effect of fat access F(1,48) = 321.41, p < 0.0001; LS Means comparison p < 0.0001 for each C group (EC, EPC, PC) compared to the binge group receiving the same hormone treatment (EH, EPH, PH, respectively)].
24-h total intake (1-h fat + 24-h chow) on binge days (Fig 3, bottom panel)
Total energy intake on binge days was affected by hormone treatment [main effect of hormone treatment F(2,48) = 24.09, p < 0.0001], due to reduced intakes in the E and EP groups relative to the P group. Specifically, Day 4 LS means comparisons showed that each group receiving estradiol (EC, EPC and EH, EPH) consumed less than its P comparison group (PC and PH, respectively) (p < 0.001 for all). As described above, 24-h intakes in EC and EPC (i.e., chow intakes) were not statistically different from each other. EH rats consumed an amount that was less than PH, but greater than EPH, i.e. EPH < EH < PH (p < 0.01, LS Means), due primarily to the relatively high fat intake in the EH group (see 1-h fat data, above). In summary, effects of E on total intake paralleled the effects reported above for 24-h chow, i.e. tonic reductions relative to treatment with P alone.
Fat access, cycle day and hormone treatment interacted [cycle × hormone, F(2,48) = 18.18, p < 0.0001; fat access × cycle × hormone, F(2,48 = 3.99, p <0.05]. 24-h total intakes were lower on Day 4 than on Day 2 in 3 of the 4 groups treated with E (Day 2-Day 4 t = 3.77, 4.10, 4.65 for EC, EPC, and EPH, respectively; t = p<0.01 for all). Intakes on Day 2 and Day 4 were not statistically different in EH and in groups treated with P alone.
Finally, 24-h intake was significantly increased by fat access [main effect of fat access F(1,48) = 171.11, p < 0.0001, and LS Means comparisons: p < 0.0001 for each group with fat access compared to chow control with same hormone treatment].
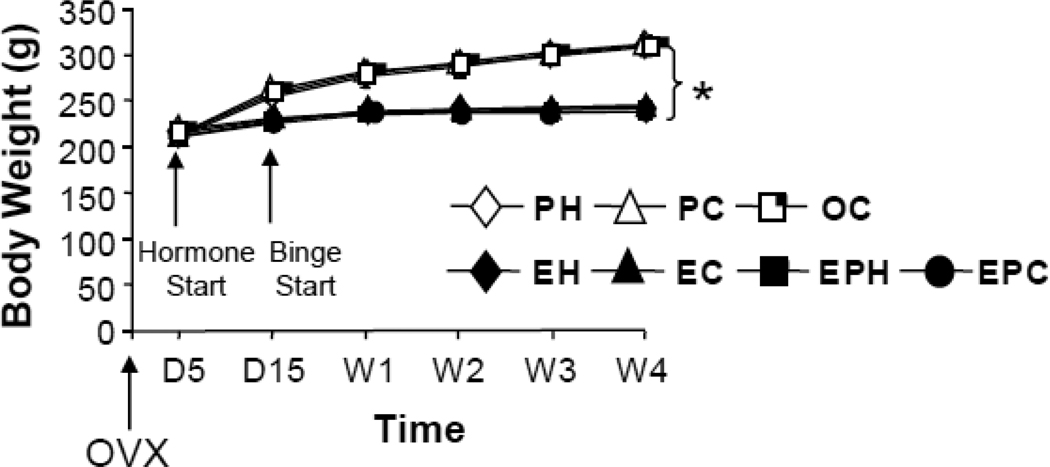
4. Body Weight (Figure 4)
Figure 4.
Body weight at day 5 postovariectomy and weekly across this 4-week study. D=Day, W=Week. * indicates significant difference at the end of the study (W4) between the groups treated with estradiol and the groups treated with P or oil vehicle, i.e. PH=PC=OC > EH=EC=EPH=EPC.
Body weight varied as a function of group [main effect F(6,55) = 10.71, p < 0.0001] and of time [main effect F(2,110) = 629.41, p < 0.0001], with the two factors interacting significantly [interaction F(12,110) = 35.14, p < 0.0001]. Significant differences among the groups emerged between 5 and 45 days post-OVX, demonstrating the efficacy of the hormone treatments. Specifically, P and oil vehicle rats weighed significantly more (LS means, p < 0.0001) than E and EP rats (PH: 307±4 g; PC: 311±4 g; OC: 309±11 g; EH: 244±3 g; EC: 243±5 g; EPH: 242±3 g; EPC: 238±3 g) (Fig 4). There were no significant differences among the 4 groups treated with E or EP, or among the 3 groups treated with P or oil.
5. Cumulative Energy Intake
Cumulative energy intake by the end of the study varied as a function of group in a manner similar to that of body weight. The 1-way ANOVA revealed an effect of group (F(6, 55) = 25.60, p < 0.0001). Post-hoc analysis using Tukey’s HSD showed that P and oil vehicle rats consumed significantly more cumulative total energy across the study than rats that had been treated with E and EP (PH: 1895 ± 33 kcal; PC: 1920 ± 39 kcal; OC: 1907 ± 51 kcal; EH: 1579 ± 36 kcal; EC: 1607 ± 24 kcal; EPH: 1538 ± 93 kcal; EPC: 1560 ± 30 kcal). In addition, there were no significant differences among the 4 groups treated with E or EP, or among the 3 groups treated with P or oil.
Discussion
This study confirmed a previous report from this lab that the effects of ovarian steroid hormones on food intake differ depending upon the conditions under which eating occurs [17]. The present study further demonstrated that administration of E alone had effects that were similar to co-administration of E and P, and both are distinct from P alone. Specifically, E exerted normal tonic and cyclic inhibitory effects on chow ingestion, but these were attenuated when rats were bingeing on fat. In addition, the present results indicate that the effects of E change dynamically as bingeing develops.
The present study replicated our previous report that 1-h intake was not cyclically reduced in EP-treated binge rats [17]. We have extended those previous findings by showing that actions of P do not account for the loss of E’s cyclic effects on binge intake, i.e. 1-h intake was not cyclically reduced in rats treated with either E alone or EP. The loss of cyclicity was not due to a generalized reduction in the efficacy of E: 24-h chow intake still showed cyclic effects in the bingeing rats, and body weight was significantly lower in rats treated with E or EP relative to rats treated with P or oil. Thus, the loss of the eating inhibitory effect of estradiol was specific to the binge episodes. The reduced efficacy of estradiol was not due to the short duration of the 1-h measurement period because estradiol tonically reduced chow intake during the 1-h fat-access period, even though 1-h chow intakes were quite low in these non-food-deprived rats. Finally, the reduced efficacy of estradiol on binge intake was not due to an inability of estradiol to influence fat consumption, per se. At the start of the study, estradiol had a large intake reducing effect on fat consumption in both the E and EP groups (Cohen’s d 0.73 and 1.95, respectively), relative to the group treated with P alone. However, by the end of the study, these effects were attenuated (Cohen’s d −0.02 and 0.45, respectively; see Table 2). In addition, combined treatment of OVX rats with EP previously has been shown to have cyclic effects on fat intake in non-bingeing rats that had chow and fat everyday [17]. In summary, the influence of progesterone, generalized loss of efficacy, the short measurement period, or the choice of a pure fat as the binge food, cannot explain the loss of estradiol cyclicity in bingeing rats.
The lack of cyclic changes in binge size in E-treated rats here was surprising because under non-binge conditions, the cyclic inhibitory effect of estradiol is manifested as reduced meal size, often with opposite effects (i.e., increases) in meal frequency [18,22,23]. In contrast, here binge size remained unchanged across E-treatment cycles. Thus, the normal cyclic inhibitory effects of E on meal size are abrogated during binge-type consumption of large fatty meals. The mechanisms that account for this are not yet known (but see Yu et al., 2008 for discussion of possibilities). E’s effects on meal frequency may also be different under binge-type conditions. This speculation is based upon reports that E levels and binge frequency in women were negatively associated across the menstrual cycle [15]. Binge frequency was experimentally fixed in our study. It is possible, however, that under conditions in which rats can vary binge frequency E might reduce binge frequency.
In addition to an attenuation of E’s cyclic effects, bingeing also attenuated E’s tonic effects (relative to progesterone alone) in the present study. This result was unexpected. Since physiological levels of P are not thought to affect eating [1–3], we hypothesized that E and EP binge rats would have smaller 1-h intakes than P binge rats, similar to the reduced intake of EP rats compared to vehicle-treated OVX rats in our previous report [17]. There are two possible reasons that this did not occur. First, it may be that the generally large binge sizes in this study masked hormone effects. In our previous study [17], binge size in EP rats was significantly smaller (38.9±4.2 kcal) than that of vehicle-treated rats (63.8±4.5 kcal), but was comparable to binge size in intact female rats (35~40 kcal) [24]. Furthermore, binge size in the vehicle-control rats in that study were comparable to those in much larger male rats under similar limited access conditions (~60 kcal) [21,25]. In the present study, however, binge size was larger in E and EP rats than previously reported: 52.7±6.0 kcal in EP rats, 61.9±5.2 kcal in E binge rats, with binge size in the P rats being even larger, 64.2±5.8 kcal. Perhaps such large binges approach the physiological capacity of the stomach for rats of this size [26] and, therefore, may have produced a ceiling effect. A second and potentially more interesting reason for our negative result is that, similar to E, P (or its metabolites) may inhibit binge size relative to vehicle-treated controls, in a manner similar to that which has been reported for cocaine [27].
One-hour energy intakes on day 4 were compared at the start and at the end of the study in order to determine if the effects of hormone treatment changed with experience under binge-type conditions. At the start of the study, EP rats consumed significantly less than P rats; rats treated with E alone tended to do so as well, but this did not achieve statistical significance. Nevertheless, as mentioned above, the effects of the hormone treatments relative to P were large (as assessed by Cohen’s d) in both groups (see Table 2). By the end of the study, 1-h binge intakes had increased in the EP and E rats and no longer were different from those in P rats. A re-analysis of previous data [17] revealed a similar dynamic effect. That is, at the start of the previous study, binge rats treated with EP consumed significantly less energy during the 1-h fat-access period than did binge rats treated with vehicle, whereas at the end (6 weeks later), there no longer was a difference between the groups (unpublished analysis). Taken together, these data indicate that E exerts an inhibitory effect on the consumption of fatty food offered in a limited-access schedule that elicits bingeing when rats have had little experience with the schedule, but not when they have had more experience with it. Thus, experience with binge conditions progressively weakens the estrogenic inhibition of eating in rats, i.e. bingeing weakens normal inhibitory control, which then serves to exacerbate the bingeing. Whether or not a similar phenomenon occurs in humans who binge eat has not been reported. Indeed, this would be difficult to determine because most people who binge present clinically only after the behavior has already escalated.
In contrast to the lack of effects on binge-type consumption of fat, E alone or co-administrated with P tonically and cyclically reduced 24-h chow intake relative to rats treated with P alone. This was expected because, although larger doses of P sometimes reduce the eating-inhibitory effect of E [28], more physiological doses do not [1–3]. It is interesting to note, however, that the present results indicate that E can selectively control the consumption of non-binge foods (e.g. chow) under binge-type conditions in which it does not control consumption of the binge food.
Body weight was assessed throughout this study to verify efficacy of the hormone treatments. Administration of either E or EP, but not P, prevented OVX-induced body weight gain in both binge and non-binge rats, confirming several previous reports [17,18,23,29]. P or vehicle-treated rats gained about 60 g across this 4-wk study, whereas the E and EP rats gained only about 30 g. The body weight differences were reflected in the total cumulative energy intake across the study, i.e. intakes were significantly greater in the P or vehicle-treated rats than in the rats treated with estradiol (E or EP). Furthermore, the weight gain in the E and EP groups was comparable to weight gain in intact female binge rats and EP-treated OVX binge rats in previous studies [17,24]. Thus, normal levels of E with or without P can maintain normal body weight and cumulative energy intake in OVX rats even under binge-type conditions.
One potential limitation of this study is the use of pure shortening as the binge food. Although people do not typically binge on pure shortening, it works well as a binge food in rats. Rats find pure sources of fat to be palatable, although binge-like ingestion and intake simply due to palatability can be distinguished [21,24,25]. It is important to consider the type of food consumed because bingeing on fat and bingeing on sugar or on sugar-fat combinations can result in different pharmacological profiles [30–32]. Thus, different mechanisms likely operate during binges that include different types of food [33], which might alter the hormonal or other macronutrient-specific controls of eating [34]. Therefore, generalizations based on the present results, in which fat comprised the binge, to other circumstances in which other palatable foods comprise the binge, should be made with caution.
In summary, cyclic treatment of OVX rats with E alone or co-administrated with P resulted in maintenance of body weight, as well as both tonic and cyclic inhibition of chow intake, but neither tonic nor cyclic inhibition of binge-type consumption of fat, once bingeing was fully established. In contrast, P alone had no effect on either food intake or body weight in binge OVX rats. This indicates that E is the primary ovarian hormone responsible for food intake and body weight regulation under binge-type conditions in rats. Furthermore, the present results indicate that the eating inhibitory effects of E are compromised, independent of P modulation, by experience with bingeing on a high-fat food. Thus, although human studies indicate that binge frequency is inversely related to E [15], the present results suggest that, in experienced bingers, binge size is unaffected by E.
Research Highlights.
Estradiol alone or when combined with progesterone has similar effects on feeding and body weight in bingeing rats.
The eating inhibitory effects of estradiol are compromised when bingeing on fat.
The effects of estradiol on binge size change dynamically as bingeing develops.
Acknowledgement
The authors thank Allison Brown for assistance with ovariectomies and thank Kim Feeney, Lauren Chuday, Niloufar Mishani, and Neh Deidre Molyneaux for assistance with data collection. This work was supported by R01-MH6794301 (RLC), as well as the Women in Science and Engineering Research Program (WISER) funded by the Pennsylvania Space Grant Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geary N. The estrogenic inhibition of eating. In: Sticker E, Woods SC, editors. Neurobiology of food and fluid intake. vol 14. New York: Kluwer Academic Publishing; 2004. pp. 305–343. [Google Scholar]
- 2.Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- 4.Drewett RF. The meal patterns of the oestrous cycle and their motivational significance. Q J Exp Psychol. 1974;26:489–494. doi: 10.1080/14640747408400438. [DOI] [PubMed] [Google Scholar]
- 5.Drewett RF. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim Behav. 1973;21:772–780. doi: 10.1016/s0003-3472(73)80103-4. [DOI] [PubMed] [Google Scholar]
- 6.Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes Relat Metab Disord. 2000;24:226–231. doi: 10.1038/sj.ijo.0801118. [DOI] [PubMed] [Google Scholar]
- 7.Ley CJ, Lees B, Stevenson JC. Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr. 1992;55:950–954. doi: 10.1093/ajcn/55.5.950. [DOI] [PubMed] [Google Scholar]
- 8.Tremollieres FA, Pouilles JM, Ribot CA. Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am J Obstet Gynecol. 1996;175:1594–1600. doi: 10.1016/s0002-9378(96)70111-4. [DOI] [PubMed] [Google Scholar]
- 9.Geary N, Lovejoy J. Sex differences in energy metabolism, obesity, and eating behavior. In: Becker JBBK, Geary N, Hampson E, Herman JP, Young EA, editors. Sex differences in the brain: from genes to behavior. Oxford, UK: Oxford University Press; 2008. pp. 253–274. [Google Scholar]
- 10.Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol Behav. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- 11.Dye L, Blundell JE. Menstrual cycle and appetite control: implications for weight regulation. Hum Reprod. 1997;12:1142–1151. doi: 10.1093/humrep/12.6.1142. [DOI] [PubMed] [Google Scholar]
- 12.Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: relation to menstrual-cycle phase and cortisol levels. Psychol Med. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- 13.Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. Am J Psychiatry. 1987;144:1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- 14.Price WA, Torem MS, DiMarzio LR. Premenstrual exacerbation of bulimia. Psychosomatics. 1987;28:378–379. doi: 10.1016/s0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- 15.Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychol Med. 2006:1–11. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- 16.Klump KL, Keel PK, Culbert KM, Edler C. Ovarian hormones and binge eating: exploring associations in community samples. Psychol Med. 2008:1–9. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Z, Geary N, Corwin RL. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiol Behav. 2008;95:501–507. doi: 10.1016/j.physbeh.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 19.al-Dahan MI, Thalmann RH. Progesterone regulates gamma-aminobutyric acid B (GABAB) receptors in the neocortex of female rats. Brain Res. 1996;727:40–48. [PubMed] [Google Scholar]
- 20.Thalheimer W, Cook S. How to calculate effect sizes from published research articles: A simplified methodology. 2002 www.work-learning.com. [Google Scholar]
- 21.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 22.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 23.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 24.Dimitriou SG, Rice HB, Corwin RL. Effects of limited access to a fat option on food intake and body composition in female rats. Int J Eat Disord. 2000;28:436–445. doi: 10.1002/1098-108x(200012)28:4<436::aid-eat12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Corwin RL. Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite. 2004;42:139–142. doi: 10.1016/j.appet.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Bull LS, Pitts GC. Gastric capacity and energy absorption in the force-fed rat. J Nutr. 1971;101:593–596. doi: 10.1093/jn/101.5.593. [DOI] [PubMed] [Google Scholar]
- 27.Anker JJ, Zlebnik NE, Carroll ME. Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacology (Berl) doi: 10.1007/s00213-010-1968-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade GN. Some effects of ovarian hormones on food intake and body weight in female rats. J Comp Physiol Psychol. 1975;88:183–193. doi: 10.1037/h0076186. [DOI] [PubMed] [Google Scholar]
- 29.Blaustein JD, Gentry RT, Roy EJ, Wade GN. Effects of ovariectomy and estradiol on body weight and food intake in gold thioglucose-treated mice. Physiol Behav. 1976;17:1027–1030. doi: 10.1016/0031-9384(76)90028-7. [DOI] [PubMed] [Google Scholar]
- 30.Berner LA, Bocarsly ME, Hoebel BG, Avena NM. Baclofen suppresses binge eating of pure fat but not a sugar-rich or sweet-fat diet. Behav Pharmacol. 2009 doi: 10.1097/FBP.0b013e328331ba47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corwin RL, Wojnicki FH. Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol. 2009;20:537–548. doi: 10.1097/FBP.0b013e3283313168. [DOI] [PubMed] [Google Scholar]
- 32.Wong KJ, Wojnicki FH, Corwin RL. Baclofen, raclopride, and naltrexone differentially affect intake of fat/sucrose mixtures under limited access conditions. Pharmacol Biochem Behav. 2009;92:528–536. doi: 10.1016/j.pbb.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009;139:623–628. doi: 10.3945/jn.108.097584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geary N. Is the control of fat ingestion sexually differentiated? Physiol Behav. 2004;83:659–671. doi: 10.1016/j.physbeh.2004.08.041. [DOI] [PubMed] [Google Scholar]