Abstract
The primary purpose of this study was to examine pathways from prenatal cigarette exposure to physiological regulation at 2 months of age. Specifically, we explored the possibility that any association between prenatal cigarette exposure and infant physiological regulation was moderated by fetal growth, prenatal or postnatal environmental tobacco smoke (ETS) exposure or maternal depressive symptomatology during pregnancy. We evaluated whether exposed infants who were also exposed to ETS after birth, were small for gestational age (SGA) or had mothers with higher depressive symptoms during pregnancy had the highest levels of physiological dysregulation. Respiratory sinus arrhythmia (RSA) was obtained from 234 (166 exposed and 68 nonexposed) infants during sleep. As expected, cigarette-exposed infants had significantly lower RSA than nonexposed infants. This association was not moderated by prenatal or postnatal ETS exposure, or maternal depressive symptomatology during pregnancy. However, small for gestational age status did moderate this association such that nonexposed infants who were not small for gestational age had a significantly higher RSA than nonexposed small for gestational age infants and exposed infants. These findings provide additional evidence that prenatal cigarette exposure is directly associated with dysregulation during infancy.
Keywords: Physiological Regulation, Prenatal Cigarette Exposure
Introduction
Recent estimates suggest that 15% of women smoke during pregnancy (SAMHSA, 2010). Prenatal cigarette exposure is associated with a range of adverse perinatal outcomes (Cornelius & Day, 2000; Ernst, Moolchan, & Robinson, 2001) including disruptions in regulatory processes. Cigarettes deliver chemical toxins to the fetus via the maternal bloodstream and increase norepinephrine and dopamine in the central catecholaminergic systems of the developing brain (Lichtensteiger, Ribary, Schlumpf, Odermatt, & Widmer, 1988). These regions are involved in neurobehavioral functions such as regulatory activities and reactivity to stress (Kinney, O'Donnal, Kriger & White, 1993; Robbins, 1997) including autonomic functioning in early infancy (Fried & Makin, 1987; Jacobson, Fein, Jacobson, Schwartz, & Dowler, 1984; Mansi et al., 2007; Schuetze & Zeskind, 2001; Schuetze & Eiden, 2006; Yolton et al., 2009). The neural regulation of autonomic functioning is sensitive to a range of perinatal factors including substance use during pregnancy and infant risk variables. Heart rate is one variable that is believed to reflect autonomic function and neurological integrity in young infants. The central nervous system modulates heart rate by the sympathetic and parasympathetic nervous systems. The parasympathetic branch of the autonomic nervous system maintains homeostasis by regulating sympathetic excitation. Variability in heart rate (HR), in particular, is considered to reflect autonomic function and neural status in young infants. Respiratory Sinus Arrhythmia (RSA) is a measure of heart rate variability (HRV) that occurs at the frequency of respiration and is believed to reflect parasympathetic influence on HRV via the vagus nerve (e.g., Porges, 1996). Thus, RSA at rest (baseline RSA) is a measure of the ability of an individual to maintain physiological homeostasis during periods of minimal external stimulation and the capacity of the nervous system to react.
RSA, as a measure of physiological regulation, is considered to be an index of the capacity to self-regulate (Porges, 1991) and is predictive of behavioral measures of regulation later in infancy and childhood. Infants with higher baseline RSA have more organized stress responses and, therefore, the ability to more optimally react to environmental demands (Porges, 1991). For example, higher baseline RSA during infancy is associated with increased positive and negative emotional reactivity and better emotional regulation during environmental stimulation (Stifter & Fox, 1990; Stifter, Spinrad, & Braungart-Rieker, 1999) and with increased self-soothing (Fox, 1989; Huffman et al., 1998). Thus, RSA may be a particularly useful index of the impact prenatal exposure to cigarettes has on regulation during early infancy. Obtaining this measure of physiological regulation during periods of minimal stimulation from the external environment is particularly relevant during early infancy. According to Porges (1996), competence in maintaining homeostasis by regulating autonomic processes such as temperature, respiration, blood pressure, and sleep is critical for numerous aspects of development including emotional and social development. Furthermore, measures of HR and HRV are state dependent. For example, RSA is most pronounced during quiet sleep (Katona & Jih, 1975; Schechtman, Harper, & Kluge, 1989).
A few existing studies have found an association between heart rate measures and maternal cigarette smoking during pregnancy. One study noted disturbances in autonomic nervous system functioning as a result of cigarette exposure using spectral analysis of heart rate during sleep among 6–16 week old infants (e.g., Franco, Chabanski, Szliwowski, Dramaix, & Kahn, 2000). Maternal smoking during pregnancy is also predictive of higher HR overall and during quiet and active sleep, lower long-term HRV (Schuetze & Zeskind, 2001) and lower RSA during sleep in neonates (Schuetze & Eiden, 2006). It is not clear, however, if the effects of prenatal cigarette exposure on measures of HR persist beyond the neonatal period. A number of developmental theorists have highlighted that a major biobehavioral shift occurs at 2 months of age (e.g., Emde & Gaensbauer, 1981). Basic physiologic responses become more organized around this time. Thus, one goal of this study was to examine the association between prenatal exposure to cigarettes and RSA at 2 months of age.
Increasingly, studies highlight the importance of examining both potential moderators of the association between prenatal cigarette exposure and regulatory processes among cigarette-exposed infants. Evidence is accumulating that suggest that fetal growth may moderate the association between prenatal cigarette exposure and infant dysregulation. There are consistent findings that maternal cigarette smoking during pregnancy is associated with a decrease in birthweight, even after controlling for confounding variables such as maternal age, weight and SES (Thompson et al., 2001). In fact, maternal smoking during pregnancy was identified as the single largest preventable risk factor for intrauterine growth retardation among infants born in developed countries (Kramer, 1998). Nicotine interacts with receptors in placental vasculature resulting in decreased placental blood flow and fetal vasoconstriction which, in turn, disrupts the delivery of oxygen and nutrients to the fetus. This reduced blood flow leads to fetal malnutrition and has been implicated as a causal mechanism for the effects of prenatal cigarette exposure on poor fetal growth (Weitzman, Gortmaker & Sobol, 1992). Reduced fetal growth has been linked to regulatory difficulties during infancy that persist into early childhood. For example, children born small for gestational age (SGA) have poorer developmental outcomes including neurobehavioral and regulatory deficits during the neonatal period (Figueras et al., 2009), and perceptual and behavioral problems, and language and attentional deficits during the school-aged years (Larroque, Bertais, Czernichow, & Leger, 2001; O'Keefe, O'Callaghan, Williams, Najman, & Bor, 2003; Pryor, Silva & Brooke, 1994. Thus, fetal growth may moderate any association between prenatal cigarette exposure and physiological regulation. In other words, fetal growth may interact with exposure status such that cigarette-exposed infants who are small for gestational age may be at highest risk for poor autonomic regulation.
A growing body of evidence suggests that problematic maternal characteristics may exacerbate the association between prenatal cigarette exposure and child outcomes (Wakschlag & Hans, 2002). Depression during pregnancy may be particularly relevant. Cigarette smoking has consistently been associated with higher levels of depressive symptomatology among the general population (Fergusson, Goodwin & Horwood, 2003) as well as among pregnant women (Rodriquez, Bohlin & Lindmark, 2000; Schuetze & Eiden, 2006; Zhu & Valbo, 2002). The importance of considering the potential impact of symptoms of maternal depression during pregnancy on infant developmental outcomes is underscored by the rapidly increasing body of literature that shows a range of nonoptimal developmental outcomes among infants of women with increased symptoms of depression. Although the majority of work focused on the influence of postpartum depression on infant development, there is growing recognition that prenatal exposure to maternal depression has the potential to negatively impact infant developmental outcomes. According to the prenatal programming hypothesis, characteristics of the prenatal environment such as maternal negative affect-based physiological changes impact the fetal brain leading to nonoptimal behavioral and physiological regulation (Zeanah, Boris & Larrieu, 1997). One way in which maternal depression may impact fetal biology is through the release of catecholamines that constrict maternal blood vessels resulting in a diminished blood flow to the fetus and restriction of oxygen and nutrients (Van den Bergh, Mulder, Mennes & Glover, 2005). This placental vasoconstriction can disrupt development of the central nervous system leading to problems with physiological and behavioral regulation. For example, infants of mothers who were depressed during pregnancy have higher cortisol levels (Field et al., 2004) and lower vagal tone (Field, Fox, Nawrocki, & Gonzalez, 1995) during the neonatal period. Thus, maternal prenatal depression may moderate the association between prenatal cigarette exposure and infant regulation.
Exposure to environmental tobacco smoke (ETS) may also impact physiological regulation in young infants. There are two types of ETS exposure that are relevant to the development of young infants: maternal ETS exposure during pregnancy and postnatal ETS exposure of the child. Pregnant women who live with or spend time with smokers are themselves exposed to ETS and, consequently, expose their fetuses to ETS. After birth, infants are exposed to cigarette side-stream smoke that contains higher concentrations of toxins than the mainstream smoke and readily enters the infant's bloodstream (Gillies, Kristmundsdottir, Wilcox, & Pearson, 1986). Consequently, exposure to ETS may have significant physiological and neurological influences on the infant postnatally. Studies have consistently found a link between maternal ETS exposure during pregnancy and adverse perinatal outcomes such as reduced fetal growth (Cornelius & Day, 2000; Dejin-Karlsson, Hanson, Ostergren, Sjobrg & Marsal, 1999; NCI, 1999). Slotkin et al. (2002) reported that postnatal ETS exposure was associated with up-regulation in the number of nicotine receptors, which are associated with neurobehavioral functioning in the brain using a primate model of prenatal and ETS exposure. Evidence for this hypothesis is provided by findings that a combination of prenatal exposure to maternal cigarette smoking and postnatal ETS exposure is associated with the highest rates of conduct disorder (Maughan, Taylor, Taylor, Butler & Bynner, 2001). Thus, there may be an increased risk for altered neurobehavioral processes in human infants exposed to ETS. Thus, ETS exposure may moderate the association between prenatal cigarette exposure and physiological regulation such that prenatally exposed infants with higher postnatal ETS exposure will have less optimal physiological regulation.
The purpose of the present study was to examine the association between prenatal cigarette exposure and infant physiological regulation at 2 months of age. We tested the possibility that the association between prenatal cigarette exposure and infant physiological regulation would be moderated by small for gestational age (SGA) status, maternal depression during pregnancy or prenatal or postnatal ETS exposure. Specifically, we hypothesized that the association between cigarette exposure and infant regulation would be stronger under conditions of SGA status, high prenatal or postnatal ETS exposure or high maternal depressive symptomatology.
1. Method
1.1 Sample Selection
Women who presented for care at the prenatal clinic of a large urban hospital were asked to complete a screening form during their first prenatal appointment. Women who were eligible were invited to participate in an ongoing longitudinal study of maternal health and child development. Initial eligibility criteria included: less than 20 weeks gestation, maternal age of 18 or older, and no multiple fetuses. Additional eligibility criteria were: no illicit drug use (other than cannabis), no heavy alcohol use (more than 1drink/day on average or 4 drinks on one occasion) after pregnancy recognition, and no heavy marijuana use (more than 1 joint/day on average) after pregnancy recognition. Women who agreed to participate were scheduled for four appointments: one at the end of each trimester of pregnancy and one at 2 months postpartum. At the end of each month of recruitment, the closest matching non-smoker (based upon age and education) was invited to participate. Smokers were over-sampled so that one non-smoker was recruited for every two smokers (taking the average of age and education of both). Participants included a total of 234 mothers and their infants. Of these dyads, 166 included infants prenatally exposed to cigarettes through maternal smoking, and 68 included infants not exposed to cigarettes.
1.2 Procedure
As described above, all mothers were screened prenatally for initial eligibility and matching criteria. Informed written consent was obtained from interested, eligible mothers. Assessments were conducted at 2 months of infant age. Data from the prenatal interviews and from the maternal interview and physiological assessment conducted at the 2-month visit were included in these analyses. The study protocol was approved by the appropriate institutional review board. Participants were informed that data confidentiality was protected by a Federal Certificate of Confidentiality issued by the National Institute on Drug Abuse. Participants received a $30.00 check, a $10.00 infant toy, and $10.00 gift certificate at the 2 month visit for their participation.
1.3 Infant Growth and Risk Status
Three measures of growth were taken by obstetrical nurses in the delivery room: birth weight (gm), birth length (cm), and head circumference (cm). Medical chart review at the time of recruitment was used to complete the Obstetrical Complications Scale (OCS; Littman & Parmelee, 1978), a scale designed to assess perinatal risk factors. Higher numbers indicate a more optimal score. SGA was defined as having a birthweight less than the 10th percentile for gestational age using a standard singleton curve (Alexander, Kogan, Martin, & Papiernik, 1998).
1.4 Maternal Substance Use
Maternal pregnancy smoking status was determined through a combination of self-report, meconium and maternal saliva results. To obtain self-report data, participants were interviewed in a private setting by trained interviewers. At each prenatal interview and at the postnatal interviews, the Timeline Follow-Back Interview (TLFB Sobell & Sobell, 1995) was used to assess maternal substance use. Participants were provided a calendar and asked to identify events of personal interest (i.e., holidays, birthdays, vacations, etc.) as anchor points to aid recall. This method has been established as a reliable and valid method of obtaining longitudinal data on substance-use patterns, has good test-retest reliability, and is highly correlated with other intensive self-report measures (Brown et al., 1998). At each prenatal appointment, the TLFB was used to gather daily tobacco, alcohol, and cannabis use for the previous three months. Women who smoked blunts were asked how many joints they could have rolled from the amount of marijuana in the blunt. Thus, self-reported data spanned 3 months prior to conception through delivery.
Maternal saliva was collected at each prenatal interview to provide objective evidence of recent exposure. The saliva specimens were analyzed by a commercial laboratory for cotinine, the primary nicotine biomarker, with enzyme-linked immunosorbent with a 10ng/mL cutoff (ELISA at the first prenatal interview only for the first 32 women recruited into the study) or liquid chromatography-tandem mass spectrometry (LC-MSMS). Maternal saliva was used to determine maternal smoking status, and was not used for identification of ETS exposure.
After birth, meconium specimens were collected from soiled diapers twice daily until the appearance of milk stool, transferred to storage containers, and frozen until transport to the National Institute on Drug Abuse for analysis. Meconium specimens were assayed with a validated LS-MSMS method for nicotine, cotinine, or trans-3-hydroxycotinine (OHCOT; Gray, Shakleya, & Huestis, 2009). Quantification limits were 2.5 ng/g nicotine, 1 ng/g cotinine, and 5 ng/g OHCOT. The same assay also permitted quantification of opiate, cocaine, and amphetamine biomarkers, including morphine, codeine, 6-acetylmorphine, hydrocodone, hydromorphone, oxycodone, methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenyl-pyrrolidine, buprenorphine, norbuprenorphine, cocaine, benzoylecgonine, cocaethylene, m-hydroxybenzoylecgonine, methamphetamine, amphetamine, and p-hydroxyamphetamine (Gray, Shakleya, & Huestis, 2009).
Mothers were included in the smoking group if self-reports were positive, even if saliva results (38% of women in the smoking group) or meconium results (28%) were negative. Similarly, mothers who reported that they did not smoke but had positive saliva samples (1% of women in the smoking group) were included in the smoking group. There were no women who reported no smoking but had infants with positive meconium samples. Of the women in the smoking group, 76% had at least one positive saliva sample (67% in the first trimester, 67% in the second trimester and 66% in the third trimester), 72% had an infant with a positive meconium sample, and 99% reported smoking.
1.5 Environmental Tobacco Exposure
Frequency of ETS exposure in the past 7 days obtained during the maternal interview conducted at the end of the first trimester of pregnancy (between 12–20 weeks gestation) was the primary measure of maternal environmental tobacco smoke exposure (M-ETS). Frequency of exposure is an important indicator of amount of exposure in pregnancy that may by most critical for the fetus. Women were asked about the number of days in the past week that they were in the same room, in the same car, or outside with someone who was smoking. Responses to these three variables were averaged to create a composite measure of average frequency of ETS exposure (range 0–7). This composite measure had high internal consistency, Cronbach's alpha = .90.
Postnatal ETS was assessed for infants (I-ETS) during their postnatal visit using infant saliva samples and maternal report of postnatal smoking. Infant saliva samples, which were obtained on 214 infants, assessed cotinine levels. Cotinine, a metabolite of nicotine, is a biochemical marker which effectively quantifies exposure to cigarettes (Jarvis, 1989). It can be detected at low concentrations and is specific to tobacco (Feyerabend & Russell, 1990). Salivary cotinine concentrations are equivalent to those in the blood (Jarvis et al., 1984) and, thus, are an accurate, yet noninvasive, way of measuring ETS exposure. Saliva samples were collected by placing eye spears (BD Opthalmology “Visispears” (product #581089), marketed by Salimetrics as “Sorbettes” (product #5029)) in the mouth of infants. These samples were placed in a storage vial and immediately placed in −80 degrees C freezer. The advantage of this measure is that it quantifies exposure to cigarette smoke from all possible sources including other household smokers. However, since saliva samples were not available from all infants, maternal report of the mean number of cigarettes smoked after birth until the 2-month laboratory visit, obtained from the TFLB, also provided a measure of infant postnatal ETS exposure.
1.6 Symptoms of Maternal Depression
Symptoms of maternal depression were assessed during the prenatal interviews at the end of the second and third trimesters. Maternal depressive symptomatology was assessed using the Beck Depression Inventory-II (BDI-II; Beck, Steer & Brown, 1996). The BDI-II is a widely accepted self-report measure to assess the severity of depressive symptoms. It has high reliability in a range of populations and high internal consistency and well-established construct validity (Sharp & Lipsky, 2002). Scores on the BDI obtained during the two prenatal interviews were highly correlated (r = .68, p < .001) and were, thus, averaged to provide one score indicating average depression during pregnancy.
1.7 Infant Physiological Assessment
At 2 months of age, infants were tested in a quiet examining room by examiners blind to group status. Because behavioral state can impact heart rate, all testing was conducted while infants were in a sleep state, assessed with a 6-point scale (Brazelton, 1984). Only infants in States 1 (Quiet Sleep) and 2 (Active Sleep) were tested. In order to ensure that infants remained in a sleep state throughout the testing session, behavioral state was time-sampled and recorded every 30 seconds.
A five-channel Bioamp (James Long Company, Caroga Lake, NY) recorded respiration and electrocardiograph (ECG) data. Disposable electrodes were triangulated on the infant's chest. A respiration bellows was placed at the bottom of the sternum (zyphoid process) to measure inspiration and expiration. The infant was then placed in a bassinet. Following a five- minute period of undisturbed acclimation to the equipment and bassinet, 10 minutes of undisturbed physiological data were recorded on-line directly into a data acquisition computer.
IBI Analysis software (James Long Company, Caroga Lake, NY) processed the HR data and calculated RSA. HR samples, which were collected every 10 ms, were used to calculate mean HR per one-second period. A level detector was triggered at the peak of each R-wave. The interval between sequential R-waves was calculated to the nearest millisecond. Data files of R-wave intervals were later manually edited to remove incorrect detection of the R-wave or movement artifacts which occurred in less than 1% of cases since infants were asleep during data collection. The software computes RSA using respiration and interbeat interval (IBI) data as suggested by Grossman (1983). The difference between maximum IBI during expiration and the minimum IBI during inspiration was calculated. The difference, measured in seconds, is considered to be a measure of RSA, and is measured twice for each respiration cycle (once for each inspiration and once for each expiration). The time for inspirations and expirations is assigned as the midpoint for each. The time for each arrhythmia sample is assigned as the midpoint between an inspiration time and an expiration time. The software synchronizes with respiration and is, thus, relatively insensitive to arrhythmia due to tonic shifts in heart rate, thermoregulation, and baroreceptor. Average RSA and HR variables were calculated for the 10-minute period of sleep.
1.8 Analytic Strategy
First, associations between group status and demographics; infant growth outcomes; maternal ETS exposure during pregnancy and maternal use of alcohol, cigarettes, and marijuana; and infant physiological regulation (baseline RSA) were examined using analyses of variance (ANOVAs) or multivariate analyses of variance (MANOVAs). MANOVAs were used when multiple theoretically associated constructs were the dependent measures in order to control for high Type I error rate. These were followed by analyses of potential confounds using correlations. Confounds with significant bivariate associations with RSA or those of theoretical value (e.g., other prenatal substance use) were used as covariates in all remaining analyses (if they were associated at p < .10). Separate linear regression analyses were then conducted to examine possible dose-response effects of prenatal cigarette exposure during each trimester on RSA. Finally, hierarchical multiple regressions were used to examine if maternal depressive symptomatology, fetal growth (SGA) or prenatal or postnatal exposure to ETS moderated the association between prenatal cigarette and other substance exposure and infant RSA.
2. Results
2.1 Sample Characteristics
Mothers ranged in age from 18 to 39 (M = 24.5, SD = 5.0). Maternal race was 52% African-American, 30% Caucasian, 18% Hispanic, and 8% other or mixed race with several mothers reporting more than one race. Fifty-eight percent of the women were married or living with their partner, while the remainder of the sample reported being in a relationship, but not living with their partner. 29% of the women had less than a high-school education, 30% completed high-school, 30% completed some college courses, 8% had a vocational degree or technical training degree and 4% had a bachelor's degree.
Descriptive statistics for the demographic and substance use variables for mothers of the two groups are presented in Table 1. Mothers who smoked cigarettes during pregnancy did not differ in age, educational level, race or marital status from non-smoking mothers. However, mothers who smoked during pregnancy consumed significantly more alcohol during the first two trimesters of pregnancy and marginally more alcohol during the third trimester. Mothers who smoked cigarettes during pregnancy also smoked significantly more marijuana during the first trimester and showed a trend towards smoking more marijuana during the second trimester of pregnancy.
Table 1.
Group Differences for Maternal Characteristics and Substance Use during Pregnancy
| Non-Smokers | Smokers | |||||
|---|---|---|---|---|---|---|
| n = 68 | n = 166 | |||||
| Variables | M | SD | M | SD | F | p |
| Demographic Characteristics | ||||||
| Age (years) | 23.79 | 5.02 | 24.82 | 4.97 | 2.06 | .15 |
| Education (years completed) | 12.13 | 1.23 | 12.08 | 1.62 | .05 | .83 |
| Cigarette Use During Pregnancy (cigarettes/day) | ||||||
| First trimester | 0 | 0 | 7.66 | 6.28 | 100.97 | .001 |
| Second trimester | 0 | 0 | 4.04 | 4.7 | 50.18 | .001 |
| Third trimester | 0 | 0 | 3.87 | 5.34 | 35.55 | .001 |
| Salivary Cotinine Levels (ng/g) | ||||||
| First Trimester | 0 | 0 | 62.64 | 83.26 | 42.35 | .001 |
| Second Trimester | 0 | 0 | 62.68 | 91.29 | 32.45 | .001 |
| Third Trimester | 0 | 0 | 56.38 | 66.93 | 49.54 | .001 |
| Alcohol Use During Pregnancy (standard drinks/day) | ||||||
| First trimester | .04 | .10 | .22 | .59 | 6.91 | .01 |
| Second trimester | .002 | .01 | .005 | .02 | 2.27 | .13 |
| Third trimester | .003 | .02 | .004 | .016 | .35 | .55 |
| Average Marijuana Use During Pregnancy (joints smoked/day) | ||||||
| First trimester | .19 | .95 | .75 | 1.68 | 6.57 | .01 |
| Second trimester | .01 | .04 | .15 | .49 | 5.53 | .02 |
| Third trimester | 0 | 0 | .08 | .34 | 3.24 | .07 |
| Maternal ETS Exposure during Pregnancy (# of reported days of ETS exposure during past 7 days) | 1.42 | .55 | 2.86 | 1.29 | 73.79 | .001 |
| Maternal Depressive Symptoms during Pregnancy – Average BDI | 14.43 | 7.8 | 16.16 | 7.84 | 2.35 | .13 |
Group differences for infant characteristics are presented in Table 2. Infants who were prenatally exposed to cigarettes had a significantly lower score on the OCS than nonexposed infants indicating a higher number of perinatal complications. Exposed infants also had a significantly lower birthweight and smaller head circumference at birth and had significantly higher salivary cotinine levels than nonexposed infants. There were no group differences in gestational age, birthlength or Apgar scores at 1 and 5 minutes after birth.
Table 2.
Group Differences for Infant Characteristics
| Non-Exposed | Exposed | |||||
|---|---|---|---|---|---|---|
| n = 68 | n = 166 | |||||
| Variables | M | SD | M | SD | F | p |
| Infant Characteristics | ||||||
| Gestational age (weeks) | 39.13 | 1.26 | 38.86 | 1.68 | 1.34 | .25 |
| Birth Weight (grams) | 3389.12 | 554.41 | 3204.65 | 542.65 | 5.44 | .02 |
| Percent preterm (<37 weeks gestational age) | 4% n = 3 |
7% n = 2 |
X2 = .43 | .32 | ||
| Percent small for gestational age (SGA) | 13% n = 9 |
15% n = 24 |
X2 = .19 | .49 | ||
| Birth Length (cm) | 49.98 | 5.47 | 50.05 | 3.07 | .02 | .90 |
| Birth Head Circumference (cm) | 35.04 | 3.26 | 33.84 | 2.38 | 8.81 | .003 |
| Apgar – 1 minute | 8.5 | 1.08 | 8.47 | 1.02 | .03 | .85 |
| Apgar – 5 minutes | 8.89 | .45 | 8.95 | .28 | 1.07 | .30 |
| Obstetrical Complications Scale | 93.1 | 15.6 | 84.17 | 15.93 | 15.08 | .001 |
| Gender (% male) | 42% | 50% | ||||
| Respiratory Sinus Arrhythmia | .02 | .03 | .014 | .012 | 6.73 | .01 |
| Meconium – trans- 3'- Hydroxycotinine (ng/g) | 0 | 0 | 78.69 | 66.85 | ||
| Meconium – Cotinine (ng/g) | 0 | 0 | 64.73 | 69.34 | ||
| Meconium – Nicotine (ng/g) | 0 | 0 | 62.60 | 72.57 | ||
| Postnatal salivary cotinine levels (ng/Ml) | .81 | 1.01 | 4.80 | 8.13 | 15.57 | .001 |
| ETS exposure – mean number of maternal cigarettes smoked per day postnatally (maternal report) | .17 | .80 | 5.39 | 5.2 | 67.45 | .001 |
2.2 Examination of Potential Covariates
We examined the relation of maternal demographic variables (age, education, parity) and maternal substance use during pregnancy (alcohol and marijuana) to the average number of cigarettes smoked during each trimester of pregnancy and to infant RSA at 2 months of age. Cigarette smoking (average number of cigarettes per day) during the first trimester was significantly associated with alcohol (average number of standard drinks per day), r = .19, p = .004, and marijuana (average number of joints per day), r = .17, p = .011, during the first trimester and to cigarette smoking (average number of cigarettes per day) during the second, r = .76, p < .001, and third trimesters, r = .68, p < .001. Cigarette smoking during the first trimester was also associated with the number of years of education completed, r = −.17, p = .014, and with maternal age, r = .16, p = .018. Consequently, alcohol and marijuana use during the first trimester and maternal age and education were included as covariates in all subsequent analyses of RSA.
2.3 Association between Cigarette Smoking and RSA
Results of a one-way Analysis of Variance (ANOVA) indicated that infants who were prenatally exposed to cigarettes had a significantly lower RSA during rest than nonexposed infants (see Table 2). Linear regression analyses were then conducted to examine a possible dose-response effect on RSA (see Table 3). Because of the high association between average number of cigarettes smoked during each of the three trimesters of pregnancy (rs ranged from .72 to .76), these three variables were not included as predictors in the same regression analysis. Thus, we conducted three separate linear regression analyses (one for each trimester of pregnancy) on RSA. Maternal demographic variables (age and education) were entered in the first step followed by the prenatal substance exposure variables (alcohol and marijuana). After controlling for these covariates, only the average number of cigarettes smoked during the first trimester significantly predicted RSA, standardized beta = −.27, p = .01, such that higher cigarette use during the first trimester was associated with a reduced RSA at 2 months of age. Consequently, only the variable indicating first trimester cigarette smoking was used for subsequent moderational analyses.
Table 3.
Dose-response Analyses by Trimester of Exposure
| Predictor Variables | Standardized Beta | Change in R2 | F |
|---|---|---|---|
| Regression 1: First trimester exposure and RSA | |||
| 1. Maternal Age | .053 | ||
| Maternal Education | .005 | ||
| 2. Prenatal Alcohol | −.22 | ||
| Prenatal Marijuana | .136 | .053 | .95 |
| 3. Smokers vs. Nonsmokers* | −.266 | .064 | 2.11 |
|
| |||
| Regression 2: Second trimester exposure and RSA | |||
| 1. Maternal Age | .056 | ||
| Maternal Education | .018 | ||
| 2. Prenatal Alcohol | −.061 | ||
| Prenatal Marijuana | .107 | .014 | .27 |
| 3. Smokers vs. Nonsmokers | −.143 | .019 | 1.08 |
|
| |||
| Regression 3: Third trimester exposure and RSA | |||
| 1. Maternal Age | .056 | ||
| Maternal Education | .018 | ||
| 2. Prenatal Alcohol | −.093 | ||
| Prenatal Marijuana | −.047 | .014 | .24 |
| 3. Smokers vs. Nonsmokers | −.186 | .031 | 1.41 |
p < 0.05
2.4 Moderational Analyses
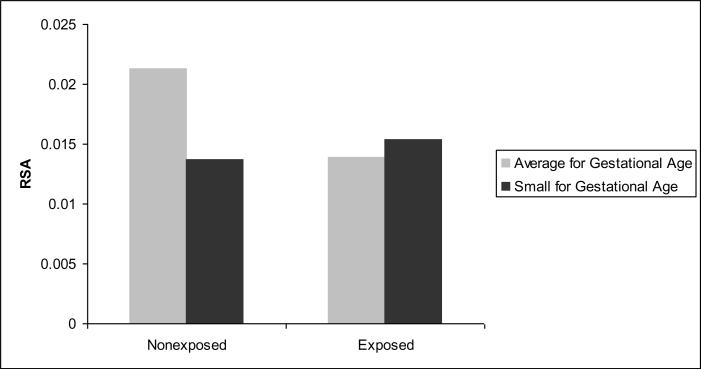
Next, we conducted analyses to examine if the association between prenatal exposure to cigarettes and physiological regulation would be moderated by maternal depressive symptomatology, fetal growth (SGA), prenatal exposure to ETS or postnatal exposure to ETS (infant cotinine and maternal report of infant ETS exposure were analyzed in separate analyses) using five separate regression analyses (see Table 4). The covariates described above were entered in the first step, followed by cigarette exposure and the moderator in the second step, and the interaction term in the third step. These analyses indicated no significant moderation by maternal depressive symptomatology, prenatal ETS exposure, or by postnatal ETS exposure, using either the maternal report or infant cotinine variable. However, the analysis on SGA indicated that SGA was a significant moderator (see Figure 1).
Table 4.
Moderational Analyses for RSA
| Predictor Variables | Standardized Beta | Change in R2 | F |
|---|---|---|---|
| Regression 1: Moderator - BDI | |||
| Step 1 | |||
| Prenatal Alcohol Exposure+ | −.215 | ||
| Prenatal Marijuana Exposure | .136 | ||
| Maternal Age | .053 | ||
| Maternal Education | .005 | .95 | |
| Step 2 | |||
| Smokers vs. Nonsmokers+ | −.23 | ||
| BDI | .144 | .058 | 1.31 |
| Step 3 | |||
| Interaction Term | −.53 | .034 | 1.54 |
| Regression 2: Moderator - SGA | |||
| Step 1 | |||
| Prenatal Alcohol Exposure+ | −.215 | ||
| Prenatal Marijuana Exposure | .136 | ||
| Maternal Age | .053 | ||
| Maternal Education | .005 | .95 | |
| Step 2 | |||
| Smokers vs. Nonsmokers+ | −.21 | ||
| SGA | .029 | .04 | 1.09 |
| Step 3 | |||
| Interaction Term* | .484 | .074 | 2.29 |
| Regression 3: Moderator – I-ETS (maternal self-report) | |||
| Step 1 | |||
| Prenatal Alcohol Exposure+ | −.215 | ||
| Prenatal Marijuana Exposure | .136 | ||
| Maternal Age | .053 | ||
| Maternal Education | .005 | .95 | |
| Step 2 | |||
| Smokers vs. Nonsmokers | −.199 | ||
| I-ETS | −.028 | .04 | 1.08 |
| Step 3 | |||
| Interaction Term | .284 | .001 | .33 |
| Regression 4: Moderator – I-ETS (salivary cotinine) | |||
| Step 1 | |||
| Prenatal Alcohol Exposure+ | −.215 | ||
| Prenatal Marijuana Exposure | .136 | ||
| Maternal Age | .053 | ||
| Maternal Education | .005 | 1.01 | |
| Step 2 | |||
| Smokers vs. Nonsmokers* | −.284 | ||
| I-ETS | .103 | .06 | 1.34 |
| Step 3 | |||
| Interaction Term | −.349 | .004 | .54 |
| Regression 4: Moderator – M-ETS | |||
| Step 1 | |||
| Prenatal Alcohol Exposure+ | −.215 | ||
| Prenatal Marijuana Exposure | .136 | ||
| Maternal Age | .053 | ||
| Maternal Education | .005 | 1.01 | |
| Step 2 | |||
| Smokers vs. Nonsmokers | −.106 | ||
| M-ETS | −.146 | .20 | 1.41 |
| Step 3 | |||
| Interaction Term | −.187 | .001 | 1.19 |
p < .10
p < 0.05
Figure 1.
Interaction effect for Small for Gestational Age (SGA) status on RSA
3. Discussion
The primary focus of the present study was to explore the association between early cigarette exposure and infant physiological measures at 2-months of age. Although previous studies found reduced long-term heart rate variability (Schuetze & Zeskind, 2001) and reduced RSA among cigarette-exposed neonates, the findings of the present study suggest that altered physiological regulation in cigarette-exposed infants extends beyond the neonatal period into early infancy. More specifically, the present findings indicated that prenatal cigarette exposure during early pregnancy was directly associated with infant RSA at 2 months of age. Higher first trimester cigarette exposure was associated with lower RSA during sleep which is indicative of decreased parasympathetic influence (e.g., Fox & Porges, 1985). When assessed during rest, baseline RSA is believed to index the ability of an infant to react both physiologically and behaviorally to external stimuli (Porges, Doussard-Roosevelt, Portales & Suess, 1994; Stifter, Fox, & Porges, 1989). Thus, a lower RSA may make it difficult for infants to regulate themselves in the face of environmental challenge. This is further supported by the fact that baseline measures of RSA are related differentially to temperament reactivity in toddler and preschool-aged children (Calkins, 1997). Thus, the increased level of physiological arousal seen in exposed infants may have implications for their interactions with external stimuli as they get older.
The few existing studies on older children suggest that altered regulatory processes are not limited to infancy and that both prenatal exposure and ETS exposure have a significant impact on the regulatory system in later childhood as evidenced by higher rates of child behavior problems (Day et al., 2000; Wakschlag & Hans, 2002; Weitzman, Gortmaker, & Sobol, 1992) including conduct disorder (CD; Wakschlag, Leventhal, Cook & Pickett, 2000) and delinquency (Wakschlag, Pickett, Cook, Benowitz, & Leventhal, 2001) in children. These findings persist even after controlling for a wide number of potential confounds and further strengthen the hypothesis that altered regulatory processes seen in infants exposed to cigarettes are persistent rather than transitory developmental effects. Future studies should, therefore, explore whether these difficulties with physiological regulation during periods of minimal external stimulation during early infancy are one pathway to externalizing behavior problems in older exposed children. It is important to note that these findings do not support the idea of an indirect effect of prenatal exposure to cigarettes through maternal depressive symptomatology or ETS exposure on physiological regulation during infancy. However, SGA status did moderate the association between prenatal exposure to cigarettes and RSA such that nonexposed infants who were not small for gestational age had a significantly higher RSA than exposed infants, regardless of SGA status, and than nonexposed small for gestational age infants. Thus, poor fetal growth negatively impacted RSA for nonexposed infants but not for exposed infants. These findings suggest that there is no additional effect of fetal growth on RSA beyond that of the effect of prenatal exposure to cigarettes. This suggests that, at least initially, the neurodevelopmental effects of prenatal exposure to cigarettes on the developing brain are the primary influence on physiological regulation during infancy. As infants develop, postnatal experiences such as the quality of the caregiving environment and parenting characteristics may interact with the effects of prenatal cigarette exposure to influence regulatory processes. Future studies are needed to examine these potential developmental pathways among cigarette exposed children beyond early infancy.
It is important to note some of the limitations of this study. One limitation concerns the measurement of substance exposure. Although care was taken in the present study to identify exposure to cigarettes in this sample, the accurate assessment of substance use is always difficult. Pregnant women are often hesitant to divulge information regarding the use of substances during pregnancy. Although this is widely acknowledged as a factor with illicit substances, the discrepancy between infant cotinine levels and maternal self-report of cigarette exposure suggests that this is also the case for legal, more socially accepted substances such as cigarettes. This may reflect increased knowledge by pregnant women that cigarettes, including ETS exposure, can be harmful for their infants such that they are hesitant to admit to these behaviors. To address this issue, multiple indices of cigarette exposure were used including self-report using the reliable Timeline Followback Interview, as well as analysis of infant cotinine and meconium levels. Although each of these measures has its own limitations, when used in combination, these measures provide the most accurate method for identifying substance exposure. A second limitation is that the measures of symptoms of maternal depression and anxiety were based on maternal self-report. Consequently, women may have misrepresented their levels of symptomatology. However, it is noteworthy that there are significant group differences in several of these measures.
Despite these limitations, the present findings are important because they provide additional support for the influence of prenatal exposure to cigarettes on neurobehavioral functioning beyond the first month of life. The lower RSA in infants prenatally exposed to cigarettes is suggestive of a compromised nervous system. These findings may have implications for later development as deficits in physiological regulation during rest have been associated with subsequent emotional and behavioral regulatory problems in older infants and children.
Highlights
This study examined pathways from prenatal cigarette exposure to physiological regulation at 2 months of age.
Respiratory sinus arrhythmia (RSA) was obtained from 234 (166 exposed and 68 nonexposed) infants during sleep.
Cigarette-exposed infants had significantly lower RSA than nonexposed infants.
This association was not moderated by prenatal or postnatal environmental tobacco smoke exposure, or maternal depressive symptomatology during pregnancy.
Small for gestational age status did moderate this association such that nonexposed infants who were not small for gestational age had a significantly higher RSA than nonexposed small for gestational age infants and exposed infants.
Acknowledgements
The authors thank the parents and children who participated in this study and research staff who were responsible for conducting numerous assessments with these families. Special thanks to Drs. Amol Lele for collaboration on data collection at Women and Children's Hospital of Buffalo. This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (Intramural Research Program and grant number 1 R01 DA019632-01).
The study was funded by a grant from the National Institute on Drug Abuse.
Abbreviations:
- ETS
Environmental Tobacco Exposure
- RSA
Respiratory Sinus Arrhythmia
- SGA
Small for Gestational Age
- HRV
Heart Rate Variability
- HR
Heart Rate
- TLFB
Timeline Followback Interview
- ELISA
Enzyme-linked Immunosorbent Assay
- LC-MSMS
Liquid Chromatography-tandem Mass Spectrometry
- OHCOT
trans-3-hydroxycotinine
- OCS
Obstetrical Complications Scale
- BDI
Beck Depression Inventory
- IBI
Interbeat Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement There is no conflict of interest.
References
- Alexander GR, Kogan M, Martin J, Papiernik E. What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin Obstet Gynec. 1998;41:114–25. doi: 10.1097/00003081-199803000-00017. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory—II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Brazelton TB. Neonatal Behavioral Assessment Scale. 2nd ed. Lippincott; Philadelphia: 1984. Clinics in Developmental Medicine, No. 50. [Google Scholar]
- Brown RA, Burges ES, Sales SD, Whiteley JA, Evans DM, Miller I. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12:101–12. [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Dev Psychobiol. 1997;31:125–35. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Day NL. The effects of tobacco use during and after pregnancy on exposed children and the relevance of these findings for alcohol research. Alc Health Res World. 2000;24:242–49. [PMC free article] [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, Cornelius MD. Effects of prenatal tobacco exposure on preschoolers' behavior. J Dev Behav Ped. 2000;21:180–88. [PubMed] [Google Scholar]
- Dejin-Karlsson E, Hanson BS, Ostergren P, Sjoberg N, Marsal K. Does passive smoking in early pregnancy increase the risk of small-for-gestational-age infants? Am J Pub Health. 1998;88:1523–7. doi: 10.2105/ajph.88.10.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon K. Vagal regulation of heart rate in the prediction of outcome for very low birth weight preterm infants. Child Dev. 1997;68:173–86. [PubMed] [Google Scholar]
- Emde RN, Gaensbauer TJ. Some emerging models of emotion in early infancy. In: Immelman K, Barlow G, Petrinovich L, Main M, editors. Behavioral Development. Cambridge University Press; Cambridge, England: 1981. [Google Scholar]
- Ernst M, Moolchan ER, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adol Psychiat. 2001;40:630–41. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Trupin L. Passive and active maternal smoking during pregnancy as measured by serum cotinine and postnatal smoke exposure: II. Effect on neurodevelopment at age 5 years. Am J Epidem. 1995;142:S19–29. doi: 10.1093/aje/142.supplement_9.s19. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Goodwin RD, Horwood LJ. Major depression and cigarette smoking: Results of a 21-year longitudinal study. Psychol Med. 2003;33:1357–67. doi: 10.1017/s0033291703008596. [DOI] [PubMed] [Google Scholar]
- Feyerabend C, Russell MAH. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. Pharm. 1990;42:450–452. doi: 10.1111/j.2042-7158.1990.tb06592.x. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, et al. Prenatal depression effects on the fetus and the newborn. Inf Beh Dev. 2004;2:216–29. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Field R, Fox NA, Pickens J, Nawrocki T, Gonzalez Vagal tone in infants of depressed mothers. Dev Psychopath. 1995;7:227–31. [Google Scholar]
- Figueras F, Oros D, Cruz-Martinez R, Padilla N, Haernandez-Andrade E, Botet F, Costas-Moragas C, Gratacos E. Neurobehavior in term, small-for-gestational age infants with normal placental function. Peds. 2009;124:934–41. doi: 10.1542/peds.2008-3346. [DOI] [PubMed] [Google Scholar]
- Franco P, Chabanski S, Szliwowski H, Dramaix M, Kahn A. Influence of maternal smoking on autonomic nervous system in healthy infants. Ped Res. 2000;47:215–20. doi: 10.1203/00006450-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Fried PA, Makin JE. Neonatal behavioral correlates of prenatal exposure to marihuana, cigarettes, and alcohol in a low risk population. Neurobeh Toxicol Teratol. 1987;9:1–7. doi: 10.1016/0892-0362(87)90062-6. [DOI] [PubMed] [Google Scholar]
- Fox NA. The development of emotion regulation: Biological & behavioral considerations. Monographs of the Society for Research in Child Development. 1994;59(Nos. 2–3) Serial No. 240. [PubMed] [Google Scholar]
- Fox NA, Porges SW. The relationship between developmental outcome and neonatal heart period patterns. Child Dev. 1985;56:28–37. [PubMed] [Google Scholar]
- Gillies PA, Kristmundsdottir F, Wilcox B, Pearson JCG. The quantification of passive exposure to smoking amongst children. Hyg. 1986;5:14–6. [PubMed] [Google Scholar]
- Gray TR, Shakleya DM, Huestis MA. A liquid chromatography tandem mass spectrometry method for the simultaneous quantification of 20 drugs of abuse and metabolites in human meconium. Anal Bioanal Chem. 2009;393:1977–90. doi: 10.1007/s00216-009-2680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman D. Respiration, stress and cardiovascular function. Psychophys. 1983;20:284–99. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Haug K, Irgens LM, Skjaerven R, Markestad T, Baste V, Schreuder P. Maternal smoking and birthweight: Effect modification of period, maternal age and smoking. Acta Obstet Gynec Scand. 2000;79:485–89. [PubMed] [Google Scholar]
- Huffman LC, Bryan Y, del Carmen R, Pederson F, Doussard-Roosevelt J, Porges SW. Infant temperament and cardiac vagal tone: Assessments at 12 weeks of age. Child Dev. 1998;69:624–35. [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. Neonatal correlates of prenatal exposure to smoking, caffeine, and alcohol. Inf Behav Dev. 1984;7:253–65. [Google Scholar]
- James Long Company; Caroga Lake, NY: 1999. [Google Scholar]
- Jarvis MJ. Application of biochemical intake markers to passive smoking measurement and risk estimation. Mut Res. 1989;222:101–110. doi: 10.1016/0165-1218(89)90023-2. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Biochemical markers of smoke absorption and self reported exposure to passive smoking. J Epidem Comm Health. 1984;38:335–339. doi: 10.1136/jech.38.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona PG, Jih F. Respiratory sinus arrhythmia: Noninvasive measure of parasympathetic control. J App Physiol. 1975;39:801–05. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- Kinney HC, O'Donnal TJ, Kriger P, White WS. Early developmental changes in (3H) nicotine binding in human brainstem. Neurosci. 1993;55:1127–30. doi: 10.1016/0306-4522(93)90326-b. [DOI] [PubMed] [Google Scholar]
- Kramer MS. Intrauterine growth and gestational duration determinants. Ped. 1987;80:502–11. [PubMed] [Google Scholar]
- Larroque B, Bertrais S, Czernichow P, Leger J. School difficulties in 20-year-olds who were born small for gestational age at term in a regional cohort study. Peds. 2001;108:111–5. doi: 10.1542/peds.108.1.111. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger W, Ribary U, Schlumpf M, Odermatt B, Widmer HR. Prenatal adverse effects of nicotine on the developing brain. In: Boer GJ, Feenstra MGP, Mirmiran M, Swaab DF, Van Haaren F, editors. Progress in Brain Research. Vol. 73. Elsevier Science Publishers; 1988. [DOI] [PubMed] [Google Scholar]
- Littman A, Parmelee B. Medical correlation of infant development. Ped. 1978;61:474. doi: 10.1542/peds.61.3.470. [DOI] [PubMed] [Google Scholar]
- Mansi G, Raimondi F, Pichini S, Capasso L, Sarno M, Zuccaro P, Pacifici R, Garcia-Algar O, Romano A, Paludetto R. Neonatal urinary cotinine correlates with behavioral alterations in newborns prenatally exposed to tobacco smoke. Ped Res. 2007;61:257. doi: 10.1203/pdr.0b013e31802d89eb. [DOI] [PubMed] [Google Scholar]
- Maughan B, Taylor C, Taylor A, Butler N, Bynner J. Pregnancy smoking and childhood conduct problems: A causal association? J Child Psychol Psychiat. 2001;42:1021–28. doi: 10.1111/1469-7610.00800. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (NCI) Smoking and Tobacco Monograph No. 10. U S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: 1999. Health effects of exposure to environmental tobacco smoke: The report of the California Environmental Protection Agency. NIH Pub. No. 9904645. [Google Scholar]
- O'Keeffe MJ, O'Callaghan M, Williams GM, Najman JM, Bor W. Learning, cognitive, and attentional problems in adolescents born small for gestational age. Peds. 2003;112:301–307. doi: 10.1542/peds.112.2.301. [DOI] [PubMed] [Google Scholar]
- Porges SW. Vagal tone: An automatic mediator of affect. In: Garber J, Dodge KA, editors. The development of emotion regulation and dysregulation. Cambridge University Press; Cambridge, England: 1991. pp. 111–28. [Google Scholar]
- Porges SW. Physiological regulation in high-risk infants: A model for assessment and potential intervention. Dev Psychopath. 1996;8:43–58. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Portales AL, Suess PE. Cardiac vagal tone: Stability and relation to difficultness in infants and three-year-olds. Dev Psychobiol. 1994;27:289–300. doi: 10.1002/dev.420270504. [DOI] [PubMed] [Google Scholar]
- Pryor J, Silva PA, Brooke M. Gorwth, development and behaviour in adolescents born small-for-gestational-age. J Paediatr Child Health. 1995;31 doi: 10.1111/j.1440-1754.1995.tb00847.x. [DOI] [PubMed] [Google Scholar]
- Ramsey DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants' adrenocorticol reactivity to stress. J Ped Psychol. 1996;21:833–40. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. Arousal systems and attentional processes. Bio Psychol. 1997;45:57–71. doi: 10.1016/s0301-0511(96)05222-2. [DOI] [PubMed] [Google Scholar]
- Rodriquez A, Bohlin G, Lindmark G. Psychosocial predictors of smoking and exercise during pregnancy. J Rep Inf Psychol. 2000;18:203–23. [Google Scholar]
- Schechtman VL, Harper RM, Kluge KA. Development of heart rate variation over the first 6 months of life in normal infants. Ped Res. 1989;26:343–46. doi: 10.1203/00006450-198910000-00011. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden RD. The association between maternal smoking and secondhand exposure and autonomic functioning at 2–4 weeks of age. Inf Beh Dev. 2006;29:32–43. doi: 10.1016/j.infbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Zeskind PS. Relation between prenatal exposure to cigarettes and behavioral and physiological measures of autonomic regulation in neonates. Infancy. 2001;2:371–83. doi: 10.1207/S15327078IN0203_5. [DOI] [PubMed] [Google Scholar]
- Sharp LK, Lipsky MS. Screening for depression across the lifespan: A review of measures for use in primary care settings. Am Fam Phys. 2002;66:1001–8. [PubMed] [Google Scholar]
- Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke up-regulates nicotinic cholinergic receptors in monkey brain. Dev Brain Res. 2002;133:175–79. doi: 10.1016/s0165-3806(02)00281-x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol Timeline Followback Users' Manual. Addiction Research Foundation; Toronto, Canada: 1995. [Google Scholar]
- Stifter CA, Fox NA. Infant reactivity: Physiological correlates of newborn and 5-month temperament. Dev Psychol. 1990;26:582–8. [Google Scholar]
- Stifter CA, Fox NA, Porges SW. Facial expressivity and vagal tone in five-and ten-month-old infants. Inf Beh Dev. 1989;12:127–37. [Google Scholar]
- Stifter CA, Spinrad T, Braungart-Rieker J. Toward a developmental model of child compliance: The role of emotion regulation in infancy. Child Dev. 1999;70:21–32. doi: 10.1111/1467-8624.00003. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, MD: 2010. Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4856Findings. [Google Scholar]
- Thompson JMD, Clark PM, Robinson E, Becroft DMO, Pattison NS, Glavish N, Pryor JE, Wild CJ, Rees K, Mitchell EA. Risk factors for small-for-gestational-age babies: The Auckland Birthweight Collaborative Study. J Paediatr Child Health. 2001;37:369–75. doi: 10.1046/j.1440-1754.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, Mulder EJH, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioral development of the fetus and child: Links and possible mechanisms. A review. Neurosci Biobeh Rev. 2005;29:237–58. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Wakschlag L, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: A developmental framework. Dev Psychopath. 2002;14:351–69. doi: 10.1017/s0954579402002092. [DOI] [PubMed] [Google Scholar]
- Wakschlag L, Leventhal B, Cook E, Jr, Pickett K. Intergenerational health consequences of maternal smoking. Econ Neurosci (TEN) 2000;2:47–54. [Google Scholar]
- Wakschlag L, Pickett K, Cook E, Benowitz N, Leventhal B. Maternal smoking behavior during pregnancy and severe antisocial behavior in offspring: A review. Am J Pub Health. 2002;92:966–74. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman M, Gortmaker S, Sobol A. Maternal smoking and behavior problems of children. Ped. 1992;90:343–49. [PubMed] [Google Scholar]
- Yolton K, Khoury J, Xu Y, Succop P, Lanphear B, Bernert JT, Lester B. Low-level prenatal exposure to nicotine and infant neurobehavior. Neurotox Teratol. 2009;31:356–63. doi: 10.1016/j.ntt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Boris NW, Larrieu JA. Infant development and developmental risk: A review of the past 10 years. J Am Acad Child Adol Psychiat. 1997;36:165–78. doi: 10.1097/00004583-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Zhu S, Valbo A. Depression and smoking during pregnancy. Add Behav. 2002;27:649–58. doi: 10.1016/s0306-4603(01)00199-x. [DOI] [PubMed] [Google Scholar]