Abstract
Background
Diabetic macular edema (DME) is a leading cause of blindness in the developed world. Sirolimus has been shown to inhibit the production, signaling, and activity of many growth factors relevant to the development of diabetic retinopathy. This phase I/II study assesses the safety of multiple subconjunctival sirolimus injections for the treatment of DME, with some limited efficacy data.
Methods
In this phase I/II prospective, open-label pilot study, five adult participants with diabetic macular edema involving the center of the fovea and best-corrected ETDRS visual acuity score of ≤74 letters (20/32 or worse) received 20 μl (440 μg) of subconjunctival sirolimus at baseline, month 2 and every 2 months thereafter, unless there was resolution of either retinal thickening on OCT or leakage on fluorescein angiography. Main outcome measures included best-corrected visual acuity and central retinal thickness on OCT at 6 months and 1 year, as well as safety outcomes.
Results
Repeated subconjunctival sirolimus injections were well-tolerated, with no significant drug-related adverse events. There was no consistent treatment effect related to sirolimus; one participant experienced a 2-line improvement in visual acuity and 2 log unit decrease in retinal thickness at 6 months and 1 year, two remained essentially stable, one had stable visual acuity but improvement of central retinal thickness of 1 and 3 log units at 6 months and 1 year respectively, and one had a 2-line worsening of visual acuity and a 1 log unit increase in retinal thickness at 6 months and 1 year. Results in the fellow eyes with diabetic macular edema, not treated with sirolimus, were similar.
Conclusions
Subconjunctival sirolimus appears safe to use in patients with DME. Assessment of possible treatment benefit will require a randomized trial.
Keywords: Sirolimus, Diabetic retinopathy, Macular edema, mTOR
Introduction
Diabetic macular edema (DME), which develops in approximately 20% of diabetic patients within 15 years of diagnosis [1], can result in progressive vision loss if left untreated, with up to 33% of patients losing 3 lines of vision over 3 years [2]. Focal and grid photocoagulation has been regarded as the standard of care since its efficacy was first described in the Early Treatment of Diabetic Retinopathy Study (ETDRS) in 1985. However, macular laser photocoagulation becomes difficult to perform safely when leaking microaneurysms are located peri-foveally, and in approximately 20% of eyes there is continued worsening of visual acuity despite treatment [3]. Given these limitations, further investigation into newer modalities of treatment is warranted.
While a large recent clinical trial of serial ranibizumab injections focused on the role of vascular endothelial growth factor (VEGF) inhibition in the treatment of DME, and challenged the notion of laser photocoagulation as first-line treatment [4], there are currently no FDA-approved ophthalmic pharmaceuticals in use for DME. Although the pathophysiology of DME is not fully understood, inflammatory mediators may play a significant role in the development of diabetic retinopathy and DME. Diabetic retinopathy has been suggested to be a low-grade inflammatory disease, with leukocyte adhesion to the retinal vasculature possibly resulting in retinal vascular dysfunction [5, 6]. Therefore, a potential pharmaceutical approach to the treatment of DME involves exploiting this biochemical pathway of leukocyte and cytokine inhibition. Leukocyte adhesion can lead to endothelial cell damage, vascular leakage, and histopathological changes. Retinal inflammation also probably involves prostaglandin production mediated by cyclo-oxygenase-2 [4–6]. These factors, in addition to retinal ischemia, hemodynamic changes, pericyte loss, and other cellular and protein signaling elements, contribute to fluid extravasation from damaged retinal microvasculature [7].
Sirolimus, also known as rapamycin, is a macrolide derived from a bacterium found in a soil sample from Easter Island, or Rapa Nui. While originally used as an antifungal agent, sirolimus eventually became known for its immunosuppressive properties. Sirolimus impacts multiple steps in the angiogenic pathway by blocking T-lymphocyte activation, smooth muscle proliferation, and endothelial cell proliferation, which occur in response to angiogenic and cytokine stimulation [8, 9]. Specifically, the drug binds to immunophilin FK binding protein 12 (FKBP-12), and the resulting complex inhibits mTOR (mammalian target of rapamycin), a multifunctional serine-threonine kinase that plays a critical role in regulating basic cellular functions such as cell proliferation, survival, mobility, and angiogenesis [10]. Inhibition of mTOR also results in blockade of interleukin-2 mediated signal transduction pathways, preventing cell cycle progression, as well as an upstream blockade of VEGF production. Sirolimus can modulate the contribution of progenitor stem cells to angiogenesis, reduce permeability, blunt complement-mediated effects, and down-regulate genes involved in the inflammatory process.
Sirolimus is currently widely used in medicine as an oral formulation (Rapammune© (2–5 mg/day) for immunosuppression, and in coronary artery disease as sirolimus-eluting coronary stent (CYPHER©), which is coated with small doses of 71 to 314 μg of sirolimus [11, 12]. In a similar manner to the slow release of sirolimus that occurs with the CYPHER® stent, the formulation of sirolimus used in this study (22 μg/μl) forms a depot enabling the release of small amounts of sirolimus over an extended period of time.
Several pre-clinical studies have been performed using various routes of sirolimus administration. Macusight, the manufacturer of the formulation used in this study, has completed several ocular toxicity studies using both subconjunctival and intravitreal routes of administration of sirolimus in the rabbit model. Based on these animal studies, the manufacturer conducted a phase 1 clinical study evaluating safety and tolerability of a single sirolimus injection (22 μg/μl) given subconjunctivally or intravitreally in human participants with diabetic macular edema. For subconjunctival injections, doses varied from 10 to 80 μl. An overall improvement was found in mean best-corrected visual acuity at 90 days compared to baseline. Analysis of the post-injection conjunctival edema also revealed a direct relationship between the volume of drug given and the amount of conjunctival edema. In particular, doses greater than 40 μl (880 μg) were associated with an increase in conjunctival ballooning (unpublished results).
In the current study, the subconjunctival route was selected for its ease of administration and improved safety profile, given the mechanical risks of an intravitreal injection. Based on these results of phase I testing of a single subconjunctival injection, a dose of 20 μl (440 μg) was selected for this study of serial injections due to its efficacy, as well as the reduced risk of conjunctival inflammation and edema.
The dual effect of sirolimus on both VEGF and mTOR may have synergistic qualities in treating the complications of diabetic retinopathy. This five-participant pilot study assesses the safety of subconjunctival sirolimus injections in the treatment of DME, with some limited efficacy information.
Materials and methods
This phase I/II open label prospective pilot study was conducted at the National Eye Institute, National Institutes of Health, in Bethesda, Maryland. The study protocol adhered to the tenets of the Declaration of Helsinki, and institutional review board approval was obtained.
Study population
All eligible participants were adults over the age of 18 years with macular edema secondary to type 1 or 2 diabetes with a hemoglobin A1c of 12% or less within 1 month of the baseline visit. Study eye inclusion criteria included: (1) best-corrected ETDRS visual acuity score of ≤74 letters (20/32 or worse), (2) retinal thickening involving the center of the fovea due to DME on clinical examination, and deemed not refractory to further therapy according to the investigator’s clinical judgment, (3) retinal thickness on baseline spectral domain OCT measurement greater than 260 μm in the central subfield, (4) media clarity, pupillary dilation, and participant cooperation sufficient for adequate fundus photos. Participants were also required to practice two forms of birth control during the course of the study and for 3 months thereafter.
Principal exclusion criteria included: (1) co-existing ocular disease that precludes improvement in visual acuity or could alter macular edema, (2) cataract causing 3 or more lines of visual acuity loss, (3) history of macular photocoagulation within 12 weeks prior to study entry, (4) history of panretinal photocoagulation (PRP) within 4 months prior to study entry, or anticipated need for PRP in the 4 months following entry, (5) history of vitrectomy, or history of major intraocular surgery within the previous 6 months or anticipated in the 6 months following study entry, (6) evidence of external ocular infection, and (7) treatment with anti-VEGF injection or subtenon or intravitreal steroids in either eye within 3 months prior to study entry. If both eyes met the above criteria, preference in selecting the study eye was given to the treatment-naïve eye, or the eye with better visual acuity if eligible. Participants were also excluded if they had a history of chronic renal failure, were pregnant or breast-feeding, had unstable medical status, a history of cancer within the past 5 years that could be worsened by immunosuppression, had blood pressure greater than 180/110, had been treated with systemic anti-VEGF agents or steroids in the past year, or were on any medication that could significantly impact the P450 drug metabolism system.
Study design
In this prospective, open-label pilot study, five participants with DME received 20 μl (440 μg) of subconjunctival sirolimus at baseline and month 2, and every 2 months thereafter if retreatment criteria were satisfied. After the initial two injections, participants were evaluated every 2 months for over 1 year, and underwent injection unless there was significant clinical improvement deemed to be a treatment success, defined as one or more of the following: (1) no intraretinal cysts or fluid on optical coherence tomography (OCT), (2) 100% reduction in excess central macular thickness over 260 μm on OCT, and (3) no leakage on fluorescein angiography. At the month 4 visit, participants meeting the criteria of treatment failure (defined as loss of 15 or more letters of vision compared with baseline, or 50% or greater increase in total retinal OCT thickness at two consecutive visits) were also given focal laser therapy. However, at the month 4 visit, participants who were neither treatment successes nor failures received only sirolimus injections. From the month 6 visit and onwards, any participant not meeting treatment success criteria was allowed to receive focal laser therapy in addition to sirolimus. Steroid or anti-VEGF therapy was not permitted over the course of the study. Fellow eyes were permitted to receive laser treatment if macular edema or neovascularization developed, but steroid or anti-VEGF therapy was not allowed.
At each visit, participants underwent a complete ophthalmological examination, assessment of vital signs, Cirrus OCT (Carl Zeiss Meditec, Dublin, CA, USA) testing, color fundus photography and intravenous fluorescein angiography (IVFA). Sirolimus serum trough levels were measured at the final close-out safety visit. A full medical assessment and physical examination was performed at the baseline visit, as well as measurement of hemoglobin A1c (HbA1c), chemistry 20, lipid profile, and complete blood count. Routine bloodwork was repeated at months 2, 6, and 12. In addition, a 1-month safety visit was required, and a final closeout safety visit was required 2 months after final treatment. Safety variables and adverse events were recorded at each visit. All patients were followed to a common termination date defined by when the last patient enrolled reached the 14-month visit.
Administration of sirolimus
The formulation of sirolimus used in this study was MS-R001 (MacuSight, Inc.). It was supplied frozen in single-use vials, and thawed immediately prior to use. Injection volume given was 20 μl, which corresponded to 440 μg of sirolimus.
Using sterile technique, 20 μl of sirolimus was drawn into a 0.3 ml Becton-Dickinson (BD Medical, Franklin Lakes, NJ, USA) insulin syringe. Following administration of topical antibiotics (ofloxacin 0.3% ophthalmic solution), topical anaesthetic (0.5% proparacaine hydrochloride ophthalmic solution) and speculum insertion, 20 μl of sirolimus was slowly injected into the subconjunctival space of the study eye. A cotton-tipped applicator was gently placed over the entry site for 1 minute following removal of the needle to prevent reflux, and the eyelid speculum was removed.
Statistical methods
The primary outcome measure was safety in this study, with a primary efficacy outcome defined to be a three-line improvement in ETDRS best-corrected visual acuity in the study eye at 6 months compared with baseline. Safety outcomes were assessed, including rate and severity of systemic and ocular toxicities, adverse events, infections, and need for withdrawal from the study.
Secondary outcome measures include the change in best-corrected visual acuity in the study eye at 1 year compared with baseline, change in the OCT retinal thickness of the study eye at 6 months and 1 year compared with baseline, and the change in macular fluid leakage on IVFA in the study eye at 6 months and 1 year compared with baseline.
Due to the small sample size of five participants, analyses are descriptive, and include tabulation of outcomes over the study period.
Results
The medical and baseline data of the five participants are listed in Table 1. All participants were older adults between the ages of 60 and 73 at enrollment, with a significant duration of type 2 DM ranging from 14 to 49 years. Three were female and two were male. The duration of DME was variable, ranging from 6 months to 8 years. Four of five participants had severe non-proliferative diabetic retinopathy, while one had moderate nonproliferative retinopathy. The severity of retinopathy remained unchanged over the study period. Blood glucose control also varied, with HbA1c values ranging between 6.6% and 10.1% at baseline. Two participants had an improvement in HbA1c of 1.8% over the course of study, while one participant’s HbA1c worsened slightly by 0.3% and the others remained stable. All participants had co-existing atherosclerotic risk factors; five had hypertension, and four had a history of elevated serum cholesterol levels. Four participants had undergone previous ocular treatment for DME in the study eye, including laser, subtenon steroid injections, and anti-VEGF agents.
Table 1.
Baseline characteristics of study participants Laser refers to focal macular laser treatment
| Participant | Study eye | Sex | Age (years) | Duration of DM (years) | HbA1c | Duration of DME (years) | Prior ocular treatment
|
|
|---|---|---|---|---|---|---|---|---|
| Study eye | Non-study eye | |||||||
| 1 | OS | Male | 73 | 49 | 6.8 | 8 | Focal laser, STT, bevacizumab | PPV, focal laser, STT, PRP, bevacizumab |
| 2 | OD | Female | 60 | 25 | 6.6 | 0.5 | None | None |
| 3 | OD | Female | 68 | 23 | 7.8 | 4 | Focal laser | Focal laser |
| 4 | OS | Male | 66 | 20 | 9.4 | 1 | Focal laser | Focal laser |
| 5 | OD | Female | 69 | 14 | 10.1 | 3 | Focal laser, STT | None |
STT=subtenon triamcinolone
PPV=pars plana vitrectomy
PRP=pan-retinal photocoagulation
All the participants were compliant with the study requirements. Due to the common termination date, follow-up ranged from 14 to 26 months. Four of the five participants received injections every 2 months throughout the study as none met the treatment success definition of a fluid free macula. Even though the remaining participant also did not become fluid free, she did not receive an injection at month 12 due to an acute hypoglycemic episode during that visit. No progression of lens opacities was observed, although one participant did elect to undergo cataract surgery after the primary outcome visit at 6 months for a pre-existing cataract that was causing glare symptoms. Tables 2 and 3 summarize the clinical outcomes for all five participants.
Table 2.
Study outcomes at baseline, month 6, month 12, and at the final visit in the study eye
| Participant | Study eye | Duration of follow-up (months) | Number of sirolimus injections | BCVA
|
OCT (μ)
|
Interventions after month 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 | Month 12 | Final visit | Baseline | Month 6 | Month 12 | Final visit | |||||
| 1 | OS | 22 | 11 | 20/100 | 20/63 | 20/40 | 20/40 | 525 | 420 | 355 | 261 | None |
| 2 | OD | 20 | 10 | 20/40 | 20/63 | 20/63 | 20/63 | 388 | 462 | 532 | 424 | CE/IOL, focal laser |
| 3 | OD | 16 | 7 | 20/50 | 20/40 | 20/40 | 20/40 | 366 | 358 | 342 | 356 | None |
| 4 | OS | 16 | 8 | 20/50 | 20/40 | 20/40 | 20/32 | 565 | 402 | 244 | 299 | Focal laser |
| 5 | OD | 14 | 7 | 20/50 | 20/40 | 20/63 | 20/50 | 440 | 403 | 385 | 459 | Focal laser |
CE/IOL=Cataract extraction with intra-ocular lens insertion
Table 3.
Study outcomes at baseline, month 6, month 12, and at the final visit in the fellow (non-study) eye
| Participant | Fellow eye | Duration of follow-up (months) | BCVA
|
OCT (μ)
|
Interventions during study period | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 6 | Month 12 | Final visit | Baseline | Month 6 | Month 12 | Final visit | ||||
| 1 | OD | 22 | 20/40 | 20/50 | 20/40 | 20/50 | 499 | 436 | 347 | 268 | Focal laser |
| 2 | OS | 20 | 20/25 | 20/25 | 20/25 | 20/25 | 287 | 287 | 236 | 260 | None |
| 3 | OS | 26 | 20/50 | 20/50 | 20/63 | 20/50 | 296 | 260 | 241 | 230 | Focal laser |
| 4 | OD | 26 | 20/20 | 20/25 | 20/20 | 20/20 | 277 | 271 | 270 | 271 | None |
| 5 | OS | 14 | 20/25 | 20/25 | 20/25 | 20/20 | 285 | 275 | 253 | 262 | None |
Adjunctive laser therapy
No participant in the study received laser at month 4 or was deemed a treatment failure at this visit. Participant 2 received four focal treatments beginning at month 6, participant 4 received two focal laser treatments at months 6 and 14, and participant 5 received one focal laser treatment at the 6-month visit.
Adverse events
In general, the study drug was well-tolerated by all participants. The primary drug-related adverse events included transient erythema and edema at the injection site. While the participants were not seen 1 week after injection, all of the participants upon active questioning reported some mild erythema and edema at the injection site, which resolved spontaneously within 5 to 7 days after injection. One participant reported eye pain and blurred vision after an injection. This was thought to be due to the mechanics of the injection as opposed to the sirolimus, as she only reported this after one injection. Other adverse events recorded were deemed not drug-related. These consisted primarily of abnormal laboratory values relating to the participants’ underlying medical co-morbidities. There was no consistent trend in the laboratory abnormalities. Examples include anemia in one participant, and hypoglycemic episodes and increased urea nitrogen in two participants thought to be related to their underlying diabetes. One of these participants went to the emergency room for his hypoglycemia, so this was reported as a severe adverse event. One participant had elevated triglycerides, and another had elevated serum cholesterol, both of which were deemed not related to the study drug.
Discussion
In this prospective five-participant phase I/II pilot study, repeated subconjunctival injections of 440 μg sirolimus were well-tolerated, with no significant drug-related adverse events or ocular complications. However, there was no consistent treatment effect; participant 1 experienced a 2-line improvement in visual acuity and 2 log unit decrease in retinal thickness at 6 months and 1 year, participants 3 and 5 remained essentially stable, participant 4 had stable visual acuity but improvement in central retinal thickness of 1 and 3 log units at 6 months and 1 year respectively, and participant 2 had a 2-line worsening of visual acuity and a 1 log unit increase in retinal thickness at 6 months and 1 year. No participant met the definition of treatment success at any study visit, and thus every participant received regular pro re nata injections over the course of study as per the study protocol, with the exception of one skipped injection for participant 3 due to acute hypoglycemia during her study visit. Due to the common termination date, some participants were followed for longer periods of time, and the total number of injections given ranged from seven to 11 injections, depending on the duration of follow-up.
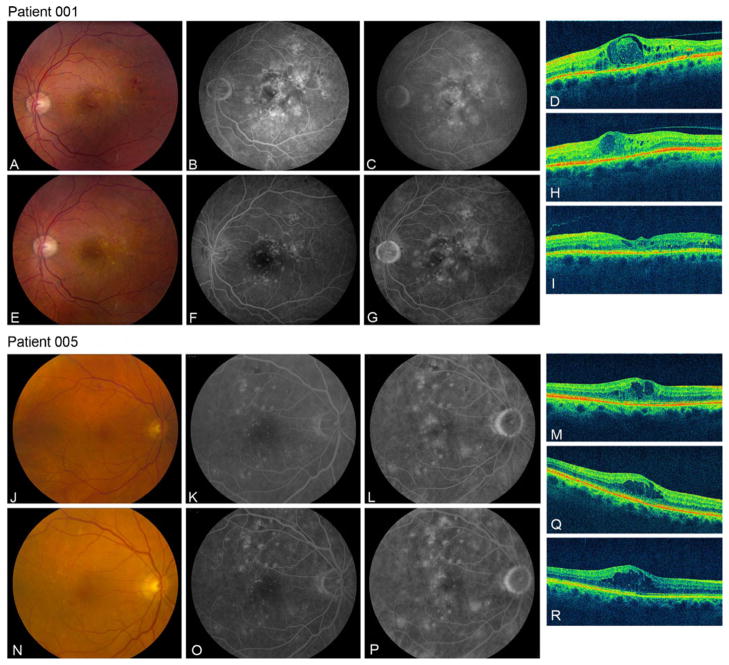
The only participant with definite improvement improved in both eyes, despite no change in glycemic control. A second participant had improvement in macular edema, despite poor glycemic control throughout the study. His fellow eye did not have diabetic macular edema. The apparent improvement in both cases may be related to previous laser treatment and the natural history of the diabetic macular edema. Systemic sirolimus levels were measured at the final safety close-out visit 2 months after the last sirolimus injection to assess systemic accumulation, and were undetectable in all five participants. However, systemic levels were not measured immediately after the injection. Diabetic macular edema is a polymorphous disease, with variability in both clinical presentation and angiographic features, as exemplified by our study population. DME can be categorized as diffuse microvascular leakage on IVFA from a leaky capillary bed, exudation from discrete microaneurysms, or somewhere along this spectrum. Review of the different angiographic features found in this study found that the two participants (1 and 4) who demonstrated anatomical improvement on optical coherence tomography at the 6-month and 1-year endpoints are similar in the more widespread nature of leakage on IVFA in their study eyes. This may suggest that a diffuse immunological process is involved, and, as a result, these eyes may be more responsive to an mTOR inhibitor such as sirolimus as compared with patients with discrete anatomical leaks in the form of focal microaneurysms, such as those observed in participants 2 and 5 (Fig. 1).
Fig. 1.
Baseline and month 6 fundus imaging and OCT for participants 1 and 5, Participant 1 has diffuse leakage on angiography at baseline (b,c), and large intraretinal cysts on OCT (d). At 6 months, angiographic leakage was reduced (f,g), and retinal thickness decreased by 105 microns on OCT (h). At the final visit of 22 months, the OCT showed a 264-micron decrease in central retinal thickness compared to baseline (i). Participant 5 had a perifoveal ring of microaneurysms on IVFA angiography at baseline (k,l) and at 6 months (o,p). Subconjunctival sirolimus did not result in a significant improvement in central retinal thickness in this patient at the 6-month visit (q) or at the final 14-month visit (r). a,j Color photograph at baseline; b,k early phase IVFA at baseline; c,l late-phase IVFA at baseline; d,m OCT at baseline; e,n color photograph at month 6; f,o early-phase IVFA at month 6; g,p late-phase IVFA at month 6; h,q OCT at month 6; i,r OCT at final visit (total follow-up varied due to common termination date design)
This study is one of the first to report the use of multiple subconjunctival sirolimus injections in participants with diabetic macular edema. Subconjunctival routes are advantageous in comparison to intravitreal injection, due to the ease of administration and reduced risk of the mechanical complications that can be associated with the intravitreal route. The small sample size of this study is a key limitation. But given the experimental nature of the treatment, a small sample size was selected to assess safety in an ethical and cost-effective manner in a small number of patients, prior to exposing a larger group to the potential risks of the investigational medication. Other limitations include the varying HbA1c (both between patients at baseline, and within an individual patient over the course of the study), and the variable duration of diabetes mellitus.. However, patients with both excellent (participant 1) and poor glycemic control (participant 4) had improvement in macular edema over the course of the study. Assessing the role of adjunctive laser therapy on response to sirolimus is also challenging, but focal laser was allowed for treatment failures for ethical reasons, and no participant received focal laser during the study until the primary outcome was met.
This pilot study suggests that subconjunctival sirolimus can be given safely in the treatment of patients with DME. Given the limited sample size, conclusions cannot be drawn regarding the efficacy of sirolimus in DME; these findings could also be attributed to the natural history of diabetic macular edema or laser treatment effects. A larger randomized clinical trial of sirolimus has been stopped by another center, apparently because of lack of efficacy (personal communication). The theoretical benefit of immunologic treatment remains, particularly in patients with diffuse angiographic leakage, but further study will be required to assess whether it can be an effective approach to treat diabetic macular edema.
Footnotes
Conflict of interest The authors have no financial conflict of interest. Although Macusight supplied the study drug, the authors have full control of all primary data, and agree to allow Graefe’s Archive for Clinical and Experimental Ophthalmology to review the data upon request.
Contributor Information
Nupura Krishnadev, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA.
Farzin Forooghian, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA.
Catherine Cukras, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA.
Wai Wong, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA.
Leorey Saligan, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA.
Emily Y. Chew, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA
Robert Nussenblatt, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA.
Frederick Ferris, III, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA.
Catherine Meyerle, Email: meyerlec@nei.nih.gov, National Eye Institute, National Institutes of Health, Bldg 10 Magnuson, Rm 10S-235, Bethesda, MD 20892, USA.
References
- 1.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464–1474. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 2.Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796–1806. [PubMed] [Google Scholar]
- 3.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–1449. 9, e1–10. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociuk N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18(12):1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 5.Adamis AP. Is diabetic retinopathy an inflammatory disease? Br J Ophthalmol. 2002;86(4):363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin MS, Nagineni CN, Hooper LC, Detrick B, Hooks JJ. Cyclooxygenase-2 gene expression and regulation in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2001;42 (10):2338–2346. [PubMed] [Google Scholar]
- 7.Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653–2664. doi: 10.2337/diacare.26.9.2653. [DOI] [PubMed] [Google Scholar]
- 8.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31(5):335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 9.Sehgal SN. Rapamune (Sirolimus, rapamycin): an overview and mechanism of action. Ther Drug Monit. 1995;17 (6):660–665. doi: 10.1097/00007691-199512000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Napoli KL, Taylor PJ. From beach to bedside: history of the development of sirolimus. Ther Drug Monit. 2001;23(5):559–586. doi: 10.1097/00007691-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23):1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 12.Holmes DR, Jr, Leon MB, Moses JW, Popma JJ, Cutlip D, Fitzgerald PJ, Brown C, Fischell T, Wong SC, Midei M, Snead D, Kuntz RE. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation. 2004;109(5):634–640. doi: 10.1161/01.CIR.0000112572.57794.22. [DOI] [PubMed] [Google Scholar]