Abstract
Our previous studies show that insulin-like growth factor-1 (IGF-1) can either protect against or increase lipopolysaccharide (LPS)-induced damage in the developing brain, depending on the dose, when it is co-administered with LPS through intracerebral injection. To further explore effects of IGF-1 on central inflammation associated brain injury, IGF-1 was administered through intranasal infusion in the current study. Postnatal day 5 (P5) rats were exposed to LPS at a dose of 1 μg/g body weight or sterile saline through intracerebral injection. Recombinant human insulin-like growth factor-1 (rhIGF-1) at a dose of 50 μg/pup or vehicle was administered intranasally 1 or 2 hr after the LPS injection. Neonatal LPS exposure resulted in oligodendrocyte (OL) and white matter injury in the P6 or P21 rat brain. The damages include dilatation of lateral ventricles, pyknotic cell death, loss of OL progenitor cells and mature OLs in the cingulum area, and impairment of myelination at the corpus callosum area. Neurological dysfunctions were observed in juvenile rats with neonatal LPS exposure. Intranasal IGF-1 treatment at either 1 or 2 hr after LPS exposure significantly attenuated LPS-induced brain injury and improved some behavioral deficits. Intranasal IGF-1 treatment also reduced infiltration of polymorphonuclear leukocytes and activation of microglia in the rat brain 24 hr after LPS exposure, but it did not prevent the elevation in concentrations of interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNFα) in the LPS-exposed rat brain during the first 24 hr. This is an indication that direct anti-inflammation might not be the primary mechanism for the protection of IGF-1, and other mechanisms, such as anti-apoptotic effects, are likely involved in its protective effects.
Keywords: insulin-like growth factor-1, intranasal administration, LPS, oligodendrocyte, apoptotic cell death, inflammatory cytokine
Cerebral white matter damage or periventricular leukomalacia (PVL) is one of the most devastating conditions in preterm infants. It is estimated that approximately 60,000 infants (1.5% of the 4,000,000 yearly live births) are born with a birth weight less than 1500g, and based on MRI data at least 50% of them exhibit some degree of cerebral white matter damage (Volpe 2003). The pathogenesis of PVL is not completely understood, but investigators believe that hypoxia-ischemia and infection/inflammation are two primary causes (Leviton and Dammann 2004; Khwaja and Volpe 2008). Therefore, several animal models have been developed based on these two factors. We previously reported that intracerebral delivery of lipopolysaccharide (LPS) preferentially induces white matter damage, loss of immunoreactivity of immature oligodendrocyte (OL) markers, increased size of lateral ventricles, delayed myelination and neurological dysfunctions (Cai et al., 2003; Fan et al., 2005b; Pang et al., 2003).
Pre-oligodendrocytes (Pre-OLs) or late OL progenitor cells are the major cell type selectively damaged in PVL (McQuillen et al., 2004; Back et al., 2002). Therefore, protection of pre-OLs could be the primary strategy for PVL treatment. Insulin-like growth factor-1 (IGF-1) appears to be a plausible candidate for such a purpose due to its potent survival effect. IGF-1 has been reported to protect OLs from various insults, including TNFα cytotoxicity (Pang et al., 2007), growth factor deprivation (Cui et al., 2005) and excitotoxicity (Ness et al., 2004). Exogenous IGF-1 has been shown to protect against ischemic brain damage in both the adult (Dempsey et al., 2003; Schäbitz et al., 2001) and newborn animals (Brywe et al., 2005; Cao et al., 2003; Guan et al, 2000; Lin et al., 2005) when injected directly into the brain. However, our in vivo study showed that when IGF-1 was co-administered with LPS, it can either protect against or increase LPS-induced damage in the developing brain, depending on the dose (Pang et al., 2010). Since IGF-1 was co-administered with LPS through intracerebral injection in our study, the local concentration of IGF-1 could be high at the injection site and consequently affect the action of IGF-1. We and other investigators have shown that IGF-1 can be delivered to the rat or mouse brain along olfactory and trigeminal pathways with intranasal administration (Thorne et al., 2004; Lin et al., 2009) and that intranasally delivered IGF-1 protects against cerebral hypoxic-ischemic injury (Liu et al., 2001a, b; Lin et al., 2009, 2011) or other neurodegenerative damages (Vig et al., 2006). Therefore, the objective of the current study was to test whether a single dose of IGF-1 delivered through intranasal infusion in the neonatal rat can provide protection against LPS-induced oligodendrocyte and white matter injury and improve neurological functions in juvenile animals.
1. Experimental procedures
1.1 Chemicals
Unless otherwise stated, all chemicals used in this study were purchased from Sigma (St. Louis, MO). Recombinant human IGF-1 (rhIGF-1) was purchased from Cell Sciences (Canton, MA). Monoclonal mouse antibodies against O4 oligodendrocyte or myelin basic protein (MBP, a marker of myelination), as well as the terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) staining kit were acquired from Millipore (Temecula, CA). Monoclonal mouse antibodies against rat OX42 (a marker of microglia) or CD43 (a marker of polymorphonuclear cells, PMN), and against adenomatous polysis coli (clone CC1) (APC-CC1, a marker of mature oligodendrocytes) were obtained from Serotec (Raleigh, NC) and Calbiochem (San Diego, CA), respectively. ELISA kits for immunoassay of IL-1β, TNFα or rhIGF-1 in the rat brain and the Caspase-3 colorimetric assay kit were purchased from R&D Systems (Minneapolis, MN).
1.2 Animals and drug treatment
Timed pregnant Sprague-Dawley rats arrived in the laboratory on day 19 of gestation. Animals were maintained in a room with a 12-h light/dark cycle and at constant temperature (22 ± 2°C). The day of birth was defined as postnatal day 0 (P0). After birth, the litter size was adjusted to ten pups per litter to minimize the effect of litter size on body weight and brain size. Intracerebral injection of LPS to 5-day old rat pups was performed as described previously (Cai et al., 2003; Fan et al., 2005b; Pang et al., 2003). Under light anesthesia with isoflurane (1.5%), LPS (1 μg/g, from Escherichia coli, serotype 055: B5) in sterile saline (total volume of 2 μl) was administered to the rat brain at the location of 1.0 mm posterior and 1.0 mm left to the bregma, and 2.0 mm deep to the scalp at the left hemisphere in a stereotaxic apparatus with a neonatal rat adapter. The injection site was aimed at the area just above the left cingulum. The injection was completed in 5 min and the needle was kept in this position for an additional 2 min and then retrieved slowly out of the brain. The wound was sutured and the pups were placed on a thermal blanket (34°C–35°C) for recovery before being returned to their dams. The dose of LPS has been shown to produce reproducible white matter brain injury (Cai et al., 2003; Fan et al., 2005b; Pang et al., 2003). The control rats were injected with the same volume of sterile saline. All animals survived the intracerebral injection. Each dam had the same litter size (10 pups) and equal numbers of LPS-treated and saline-treated rat pups were included in a litter. All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. Every effort was made to minimize the number of animals used and their suffering.
Intranasal administration of rhIGF-1 in the rat pup was performed as previously described (Lin et al., 2009). Briefly, P5 SD rat pups were placed on their backs under light anesthesia with isoflurane (5% for induction and 1.5% for maintenance). After pups were sedated, 50 μg of rhIGF-1 dissolved in 5 μl PBS containing 0.1% BSA was given into the left naris using a fine tip. This dose of IGF-1 has been shown to protect the neonatal rat brain from hypoxic-ischemic injury as demonstrated in our previous studies (Lin et al., 2009; 2011). The pups were then maintained sedated with isoflurane for 5 min to ensure that they stayed on their backs. All pups woke up within 1–2 min upon withdrawal of isoflurane and were returned to their dams. For pups in the control group, 0.1% BSA was given by intranasal administration. In an initial study to determine whether rhIGF-1 administered through intranasal infusion can penetrate into the rat brain, pups were decapitated at 30 min following intranasal infusion of IGF-1 at 0, 1 or 2 h after intracerebral injection of LPS or saline and the brain was collected. Selection of the 30-min duration is based on previous reports that concentration of IGF-1 delivered through intranasal infusion reaches its peak at that time in the rat brain (Thorne et al., 2004). The brain (without the cerebellum) was separated into three parts: olfactory bulbs (OB), frontal brain (FB), and posterior brain (PB). The FB and PB were separated coronally at the bregma level. Brain tissue was stored at −80°C for determination of brain concentrations of rhIGF-1 using an ELISA kit (R&D systems, Minneapolis, MN) at a later time. The initial study showed that concentrations of rhIGF-1in the rat brain were not affected by the time elapsed following LPS exposure (see results and Fig. 1 for details). Because post-treatment is of more therapeutic significance, rhIGF-1 or vehicle was administered at 1 or 2 hr after the intracerebral injection of LPS or saline in all our studies.
Figure 1.
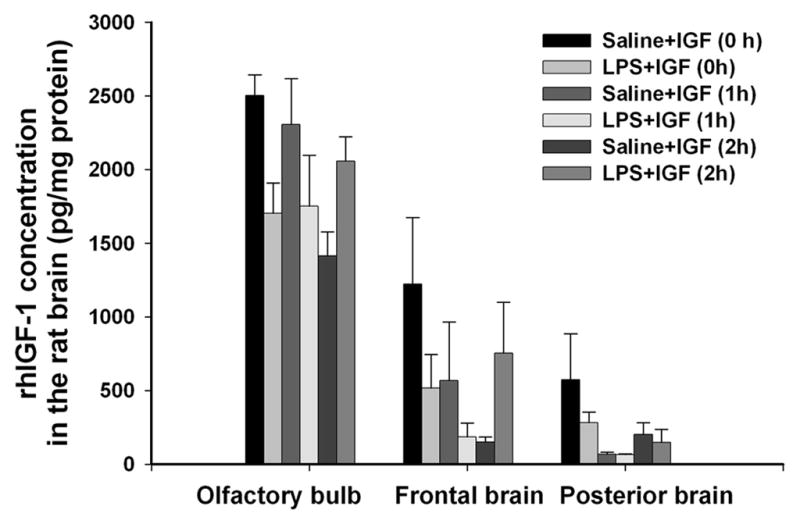
Concentrations of rhIGF-1 in different parts of the rat brain 30 min after intranasal administration of rhIGF-1 in the initial study. Intranasal infusion of rhIGF-1 was performed at 0, 1 or 2 hr after the intraceberal injection of LPS or sterile saline in P5 rats. Each group contained 5 animals. rhIGF-1 was detected in the brain of rhIGF-1-infused animals, but not in that of the vehicle (0.1% BSA)-infused animals, from either the saline- or the LPS-injected group. rhIGF-1 concentrations in the FB and the PB of saline- or LPS-injected rats brain were lower than that in the OB. No significant differences in rhIGF-1 concentrations were observed among the treatment groups within respective brain region.
1.3 Brain section preparation and immunohistochemistry
Rat pups were sacrificed by transcardiac perfusion with normal saline followed by PBS-buffered 4% paraformaldehyde (PFA) for brain section preparation or by decapitation for fresh brain tissue (without the cerebellum) collection 24 hr (P6) after the LPS exposure or 16 days (P21) later following the completion of behavioral tests. After fixation in PFA over night at 4°C, the brain was equilibrated in cryopretection solution containing 10~30% sucrose at 4°C. Frozen coronal brain sections at 10 μm of thickness were prepared in a cryostat. These sections were used for immunohistochemistry except for O4 staining. Free-floating coronal brain sections at 40 μm of thickness were prepared in a freezing sliding microtone and used for Nissl staining and pre-OL surface antigen O4 immunostaining.
For immunohistochemical staining, primary antibodies were used at the following dilution: O4, 1μg/ml; MBP, 1:500; CD43, OX42, 1:200; or APC-CC1, 1: 20. Sections were incubated with primary antibodies at 4°C overnight, and then incubated with appropriate secondary antibodies conjugated with various fluorescent dyes. For visualization of MBP, sections were incubated with biotinylated secondary antibody and then incubated in ABC complex (Vector, Burlingame, CA) with diaminobenzidine tetrahydrochloride as a chromogen. The results were examined under a light or fluorescent microscope at appropriate wavelengths. Sections incubated in the absence of primary antibodies served as negative controls.
1.4 Detection of cell death
Cell death was detected using Nissl staining and the TUNEL kit. Double labeling of O4 cells was performed to test if the neonatal LPS exposure resulted in apoptotic death of OL progenitors. O4 immunostaining was first performed as described above, with rhodamine as the fluorophore. The free-floating sections were then incubated with digoxigenin-linked terminal deoxynucleotidyl transferase at 37°C for 1 hr followed by incubation with FITC-labeled anti-digoxigenin for 30 min at room temperature. Results were examined with a fluorescence microscope. The TUNEL positive cells show a green color and the O4 positive cells show a red color.
To further confirm that the TUNEL and O4 double-labeled cells were indicative of apoptotic cell death, caspase-3 activity was determined in the rat forebrain 2, 6 and 24 hr after LPS injection in animals with or without intranasal IGF-1 treatment by a commercial assay kit (R&D systems), following the manufacturer’s instruction. Briefly, forebrain tissues were homogenized in 4 volumes of lysis buffer and the homogenate was kept on ice for 10 min. After centrifugation, 50 μl supernatant was mixed with the same volume of reaction buffer and 5 μl of substrate in wells of a 96-well plate. Following incubation at 37°C for 1 hr, activity of cleaved caspase-3 was determined colorimetrically with a microplate reader at 405 nm. Caspase-3 activity in the naïve rat brain was determined as the baseline level and that from other groups is expressed as a percentage of the baseline level.
1.5 ELISA
Concentrations of IL-1β and TNFα in the rat brain at 2, 6 and 24 hr after the LPS exposure were determined as markers of LPS-induced inflammatory responses by ELISA. Concentrations of rhIGF-1 in the rat brain 30 min after intranasal infusion of rhIGF-1 were also determined by ELISA. ELISA was performed following the manufacturer’s instruction. Briefly, brain tissues were homogenized in 5 volumes of ice-cold PBS (pH 7.2) and centrifuged at 12,000 g for 20 min, at 4°C. The supernatant was then collected and total protein was determined by the Bradford method. Data were acquired using a 96-well plate reader. The cytokine contents are expressed as pg cytokine/mg protein.
1.6 Analysis of immunostaining data
Our previous studies indicate that in the current neonatal rat model, LPS injection produces preferential white matter injury primarily in the corpus callosum, the periventricular area, and the white matter tract of the forebrain (Cai et al., 2003; Fan et al., 2005b; Pang et al., 2003). In the present study, therefore, brain sections at the bregma level and the middle dorsal hippocampus level from rats sacrificed 1 (P6) or16 days (P21) after the LPS injection were used for determination of all pathological changes.
To compare the size of lateral ventricles, Nissl stained sections at the bregma level were scanned by a densitometer (Bio-Rad, Hercules, CA) and areas of the left and right ventricles as well as that of the whole brain section were measured (Fan et al., 2005c). The ratio between the area of the left and the right ventricles and that of the whole brain section was calculated as the ventricle size index.
For quantification of the density of pyknotic cells, O4+, O4+ and TUNEL double-labeled, APC-CC1+ OLs, OX42+ microglia and CD43+ PMN cells in the rat forebrain, brain sections at the bregma and the middle dorsal hippocampal levels were used for cell counting. Three digital microscopic images at a high-power view (100×, 0.0768 mm2) were randomly captured at the cingulum and the corpus callosum area, where OLs have abundant distributions (Fan et al., 2008b) and is close to the LPS injection site. The number of positively stained cells in the three images was counted and averaged. Three consecutive sections at each of the two levels (the bregma and the middle dorsal hippocampus) were examined by an observer blind to the treatment and the mean value of cell counting was used to represent one single brain. For convenience of comparison among the treatment groups, results were converted to cells per mm2.
For semi-quantification of MBP immunostaining, images of MBP staining at the corpus callosum from three consecutive sections at the bregma level were acquired under a low-power microscopic view (4×). Thickness of the MBP staining at three locations (the middle line, the inner corner of the left and right ventricles, see Fig 4D for details) was measured using an NIH image analysis software, and averaged to represent one single brain.
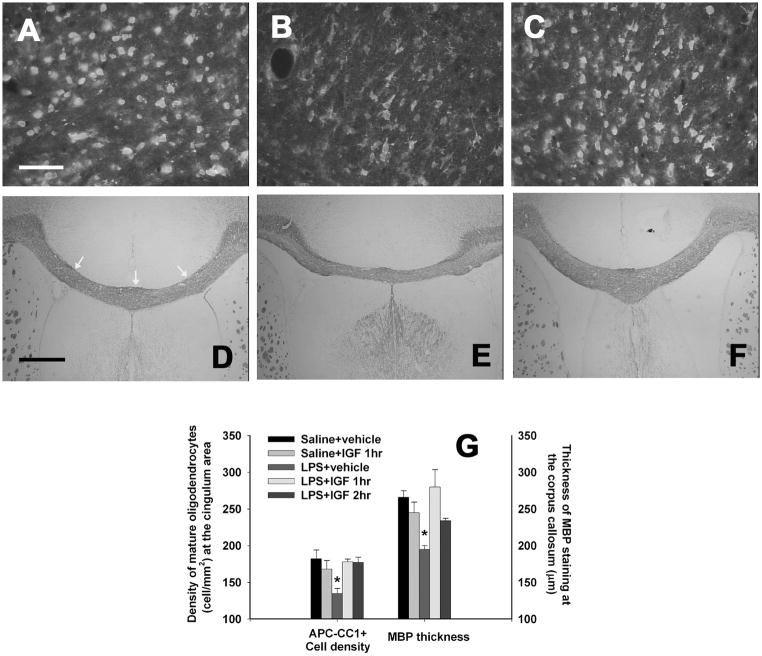
Figure 4.
Density of mature oligodendrocytes and myelination in the P21 rat brain following perinatal LPS exposure. LPS exposure on P5 significantly decreased the density of mature oligodendrocytes (APC-CC1+) at the cingulum area in the P21 rat brain (B), as compared to that in the control group (A). IGF treatment at either 1 or 2 h after the LPS exposure significantly reversed effects of LPS (C). Neonatal LPS exposure also reduced the thickness of MBP staining at the corpus callosum (E), suggesting myelination was impaired in these rat brains, as compared to the control group (D). IGF treatment at either 1 or 2 h after the LPS exposure prevented the reduction in the thickness of MBP staining (F). The thickness of MBP staining was determined at three locations of each brain sections: the middle of the corpus callosum, the inner corner of the bilateral ventricles (as indicated by the three white arrows in D). The three measurements were averaged to represent a single section. The quantitative data of the density of mature oligodendrocytes and the thickness of MBP staining are presented in G (n=6 for each group). *p<0.05 vs other groups. Scale bar: A–C, 50 μm; D–F, 500 μm.
1.7 Neurobehavioral testing
To determine if intranasal IGF-1 treatment improves neurological functions, neurobehavioral performance was tested on P20 and P21. Behavioral tests included the movement initiation test, vibrissa-elicited forelimb-placing test, passive avoidance test, pole test, and beam walking test. Our previous studies have shown that performance of the juvenile rat in these tests was impaired following neonatal LPS exposure (Fan et al., 2008a, c). Details of these tests have been described in our previously study (Fan et al., 2011). All tests were performed by an investigator who was unaware of the treatment of animals.
1.7.1 Movement initiation test
Movement initiation for each forelimb was assessed to test the forelimb function (Fleming et al., 2004). The animal was held by its torso with its hindlimbs and one forelimb lifted above the surface of the table so that the weight of the animal’s body was supported by the other forelimb alone. The animal was allowed to initiate stepping movements in a 60 sec period for one forelimb and then the other in a balanced order. The time to initiate one step was recorded for each forelimb and initiation times for both forelimbs were averaged to create one response latency score.
1.7.2 Vibrissa-elicited forelimb-placing test
This test was used to measure forelimb placing deficit upon stimulation of the rat’s vibrissae to trigger a placing response (Schallert and Woodlee, 2005). Rats use their vibrissae to gain bilateral information about the proximal environment and this information is integrated between the hemispheres. In the cross-midline test of forelimb placing, the animal was gently held by its torso, but was turned sideways so that the vibrissae were perpendicular to the surface of the table. The downwardly oriented limb was gently restrained by the experimenter as the downwardly oriented vibrissae were brushed against a table edge once per trial for 10 trials. The percentage of trials in which the rat successfully places its forepaw onto the tabletop was recorded for each side. Intact animals place the forelimbs of both sides quickly onto the counter top with 100% success in all variants of this test. If an animal struggled during testing, the data were not included in the overall analysis.
1.7.3 Passive avoidance
It gives information about learning and memory capabilities as well as maturation of the inhibitory process (Hermans et al., 1992). On P20, rats were trained in a step-down type of passive avoidance apparatus. The experimental chamber was made of plexiglass. The floor of the chamber was made of parallel 2-mm-caliber stainless steel rods spaced 1 cm apart from each other and connected with an electric shock generator. The safe part was a piece of wood board placed at a corner of the chamber above the metal rods. Each animal was placed initially on the safe platform. When the rat stepped down onto the floor, it was given a foot shock (1 s, 0.5 mA). Although the rats repeatedly stepped up and down, they eventually remained on the board. The number of shocks required to retain an individual animal on the board for 2 min was recorded as a measure of acquisition of passive avoidance. The next day rats were placed on the wood board, the time elapsed (retention latency) before the rat stepped down to the floor was recorded as a measure of memory of the acquired passive avoidance. Following the avoidance test, locomotor activity was determined in an open field test as previously described (Fan et al., 2005a).
1.7.4 Pole test
This test was used to assess the maturation of ascending and descending skills (Altman & Sudarshan, 1975). The rat was confronted with a situation in which it had to turn around and climb down a pole (diameter: 1.7 cm, length: 40 cm) (Fan et al., 2008c). The rat was placed under a cork ball installed at the top of the pole with its head held upwards. Each animal was given three trials and the time to turn round and to reach the platform at the bottom was measured as response latency (cut-off time: 60 sec). The mature pattern of descending consists of turning around on the rod, releasing grip with the forepaws, rotating of the trunk and supporting of the body during this maneuver by the hindpaws, then descend with the head in the leading position. During the three trials, maturation of skill was assessed by a score defined as following: 0, falling down; 1, descending down with the head up; 2, descending down with the release of grip with the forepaws; 3, descending down with head turning around.
1.7.5 Beam Walking Test
Motor coordination and balance were assessed by measuring the ability of the animals to traverse a graded series of narrow beam (different size) to reach an enclosed safety platform (Altman and Sudarshan, 1975). The test consisted of elevated platforms connected by a 60 cm long square wood beam with a width of 3.0 or 1.2 cm (Fan et al., 2008a). The graded difficulty of motor coordination and balance can be measured by different beam widths. The beam was placed horizontally, 50 cm above the bench surface, with one end mounted on a narrow support and the other end attached to a goal box with a litter of pups. A sawdust-filled box at the base served as protection for the falling pups. A light (60 Watt) was positioned above and to one side of the start of the beam. A litter of pups was placed on the goal box and one pup at a time was removed and placed on the start platform. On the day of testing, each pup was given three trials. The time spent on each beam was recorded for pups that were able to traverse the beam and join their littermates. The cut-off time was 60 sec.
2.8 Statistics
Data were presented as the mean ± standard error of the mean (SEM) and analyzed by one-way or two-way ANOVA (LPS or saline vs IGF-1 or vehicle) followed by the Student-Newman-Keuls test. Since caspase-3 activity in the saline-injected group was at the baseline level and concentrations of IL-1β and TNFα in the saline-injected rat forebrain were undetectable, the caspase-3 activity data and the cytokine data were only analyzed within the LPS-injected group (IGF-1 or vehicle treatment vs time). The behavioral data were analyzed by one-way ANOVA (for movement initiation, forelimb-placing, passive avoidance, pole, and beam walking tests) or by two-way repeated measures ANOVA for body weight data determined continuously at different postnatal days, followed by Student-Newman-Keuls test. The significance level was set at p<0.05.
2. Results
2.1 rhIGF-1 administered through intranasal infusion could reach the neonatal brain
rhIGF-1 was not detected in any part of the brain in any vehicle-treated animals from either saline- or LPS-injected group, but was detected in all rat brains intranasally administered with rhIGF-1. As shown in Fig 1, high concentration (1500 ~ 2500 pg/mg protein) of rhIGF-1 was detected in the OB of saline-or LPS-injected rat brain 30 min after intranasal administration of rhIGF-1, regardless whether rhIGF-1 was applied immediately, 1 hr or 2 hr after LPS injection. rhIGF-1 concentrations in the FB and the PB of saline- or LPS-injected rats brain was lower than that in the OB. rhIGF-1 concentration ranged from 200 to 1200 pg/mg protein in the FB and from 70 to 500 pg/mg protein in the PB. When rhIGF-1 was administered 2 hr after intracerebral injection of LPS, rhIGF-1 concentration in the OB or FB of LPS-injection rat brain had an increasing trend as compared to that in the saline-injected group, but the difference was not statistically significant. No significant differences in rhIGF-1 concentrations were observed among the treatment groups within the respective brain region.
2.2 Intranasal infusion of rhIGF-1 attenuated the LPS-induced injury in the developing rat brain
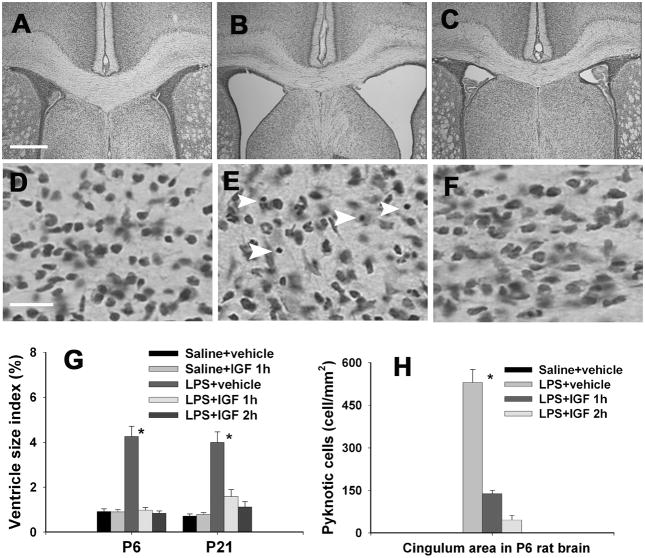
Intracerebral administration of LPS in the neonatal rat brain resulted in enlargement of both lateral ventricles in the rat brain at both 24 hr (P6) and 16-day (P21) after the injection as compared to the control group. The representative images of the dilated bilateral ventricles in the LPS-injected P6 rat brain and the saline-injected rat brain are shown in Figs. 2B and 2A, respectively. Intranasally administered IGF-1 reduced dilatation of ventricles (Fig. 2C). Ventricle size index was determined to quantify ventricle dilatation. While IGF-1 did not affect the ventricle size index in the saline-injected group, intranasal IGF-1 infusion at either 1 or 2 hr after LPS injection reduced the elevation of ventricle size index in the LPS-injected P6 or P21 rat brain to the control level (Fig. 2G). Perinatal LPS exposure also significantly increased the number of pyknotic cells in the forebrain white matter area (Fig. 2E, white arrow heads indicated) and the cortical area (data not shown) 24 h after the exposure. No pyknotic cells were detected in the saline-injected rat brain (Figs. 2D & 2H). Intranasal IGF-1 administration at 1 or 2 h after LPS injection reduced the number of pyknotic cells (Figs. 2F & 2H).
Figure 2.
Ventricle enlargement and the number of pyknotic cells in the cingulum area of the P6 or P21 rat brain. Intracerebral injection of LPS or sterile saline was performed in P5 rat pups and intranasal infusion of rhIGF-1or vehicle was performed 1 or 2 hr after the LPS injection. Nissl stained brain sections showed that perinatal LPS exposure resulted in a significant increase in ventricle size in the P6 rat brain (B) as compared to the control rat brain (A). Intranasal IGF-1 administration at 1 or 2 hr after LPS injection reduced dilatation of ventricles (C). Ventricle size index (area of the ventricles/area of whole brain sections at the bregma level) in the P6 and P21 rat brain is presented in G. Perinatal LPS exposure also significantly increased the number of pyknotic cells (indicated by white arrow heads) in the white matter area (E) and the cortical area 24 hr after the exposure. No pyknotic cells were detected in the saline-treated rat brain (D). Intranasal IGF-1 administration at 1 or 2 h after LPS injection reduced the number of pyknotic cells (F), as determined 24 hr after the LPS exposure. Quantitative data of pyknotic cells in the P6 rat brain are presented in H. * p<0.05 vs the other groups. Each group contained 6 animals. Scale bar: A–C, 500 μm; D–F, 20 μm.
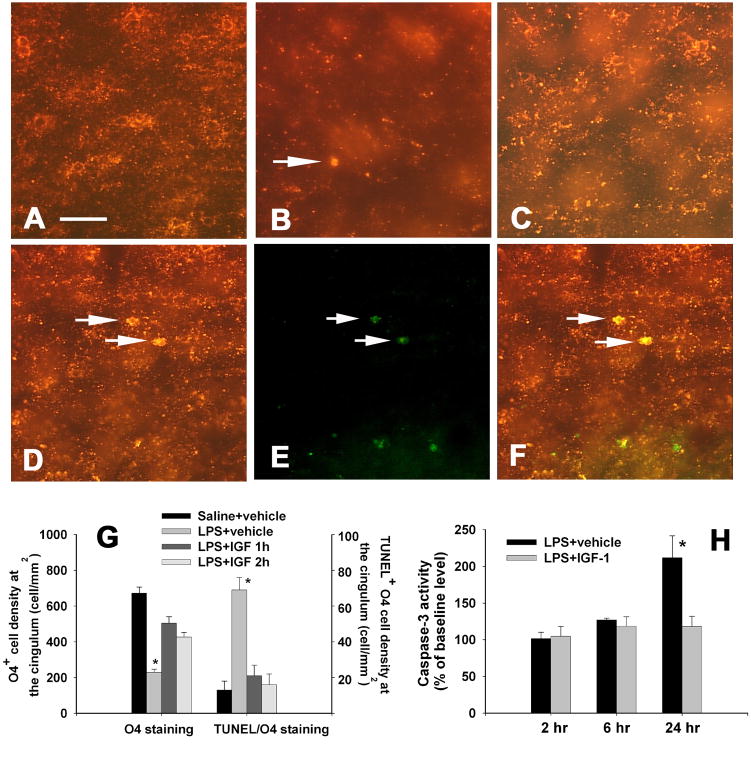
Late OL progenitor cells (O4+/O1−), which have been proposed to be the major target in cerebral white matter injury in human infants (Back et al., 2002), represent the predominant OL lineage stage in the rat cerebral hemispheres between P2 to P7 (Back et al., 2002). Abundant O4 positive cells, which has positive staining primarily localized at the cell membrane and processes, were observed in the P6 control rat brain, mostly at the corpus callosum (Fig. 3A) and subcortical white matter tract (data not shown). LPS exposure significantly reduced O4 positive staining in the P6 rat brain. Perinatal LPS exposure reduced O4+ cell density at the cingulum area and resulted in dying O4 cells, which displayed acute degenerative features including apparent condensation of the cell body and appearance of immunoreactivity to O4 antibody at both the plasma membrane and the cytoplasm (Lin et al., 2004; Figs. 3B & 3G) 24 h after the injection. Intranasal IGF-1 treatment attenuated the loss of O4+ cells (Figs. 3C & 3G). TUNEL and O4 double-labeling (indicated by white arrows in Figs. 3D-3F) showed that many dying O4 cells were under the process of apoptotic cell death. Quantification of the density of TUNEL and O4 double-labeled cells in the cingulum area indicated that LPS exposure increased the number double-labeled cells by more than five-fold as compared to the control (69.5±7.0 vs 13.0±5.1 cells per mm2, Fig. 3G). Intranasal infusion of rhIGF-1 at either 1 or 2 hr after the LPS exposure significantly reduced the number of TUNEL and O4 double-labeled cells in this area (21.2±6.0 and 16.2±6.1 cell per mm2, Fig. 3G). These double-labeling data were supported by the measurement of cleaved caspase-3 activity in the forebrain by ELISA. At 2 or 6 hr after the LPS exposure caspase-3 activity in the LPS-injected rat brain was similar to that in the control rat brain, but it increased about 2-fold in the rat brain 24 hr after LPS injection (Fig. 3H). Intranasal IGF-1 infusion at 1 hr after the LPS injection did not alter caspase-3 activity at 2 or 6 hr after the LPS injection, but decreased caspase-3 activity in the rat forebrain to the control level at 24 hr after the LPS injection, suggesting that the reduced loss of O4+ cells observed at 24 hr was associated with inhibition of caspase-3 activation.
Figure 3.
Oligodendrocyte cell death and caspase-3 activity in the P6 rat brain. Perinatal LPS exposure reduced O4+ cell density at the cingulum area and resulted in dying O4 cells (white arrow indicated, featured with apparent condensation of the cell body and appearance of immunoreactivity to O4 antibody at both the plasma membrane and the cytoplasm) 24 h after the injection (B), as compared to that in the control rat brain (A). Intranasal IGF-1 treatment at 2 h after the LPS exposure attenuated the loss of O4+ cells (C). TUNEL (E) and O4 (D) double-labeling showed that many dying O4 cells were under the process of apoptotic cell death (indicated by white arrows in merged image F). The quantitative data of O4+ cell density and TUNEL positive O4 cell density are presented in G (n=6 for each group). *p<0.05 vs the other groups. Neonatal LPS increased caspase-3 activity in the rat forebrain 24 h, but not 2 or 6 h, after the LPS exposure, whereas intranasal IGF-1 treatment at 1 hr after the LPS injection prevented the increase in caspase-3 activity (H). *p<0.05 vs the LPS+IGF-1 group in H. Scale bar: A-F, 50 μm.
Neonatal LPS exposure caused damage to not only late OL progenitor cells, but also mature OLs and myelination in the corpus callosum area. As shown in Fig.4, LPS exposure reduced the density of mature OLs in the P21 rat brain (Figs. 4B & 4G), determined by the APC-CC1 positive staining, as compared to that in the control rat brain (Fig. 4A). LPS exposure also decreased the thickness of MBP staining at the corpus callosum area of the P21 rat brain (Figs. 4E & 4G). Intranasal infusion of rhIGF-1 at either 1 or 2 hr after the LPS injection reversed adverse effects of neonatal LPS exposure on the density of mature OLs (Figs. 4C & 4G) and myelination at the corpus callosum (Figs. 4F & 4G).
2.3 Intranasal IGF-1 infusion ameliorated microglial activation and infiltration of PMN cells after neonatal LPS exposure
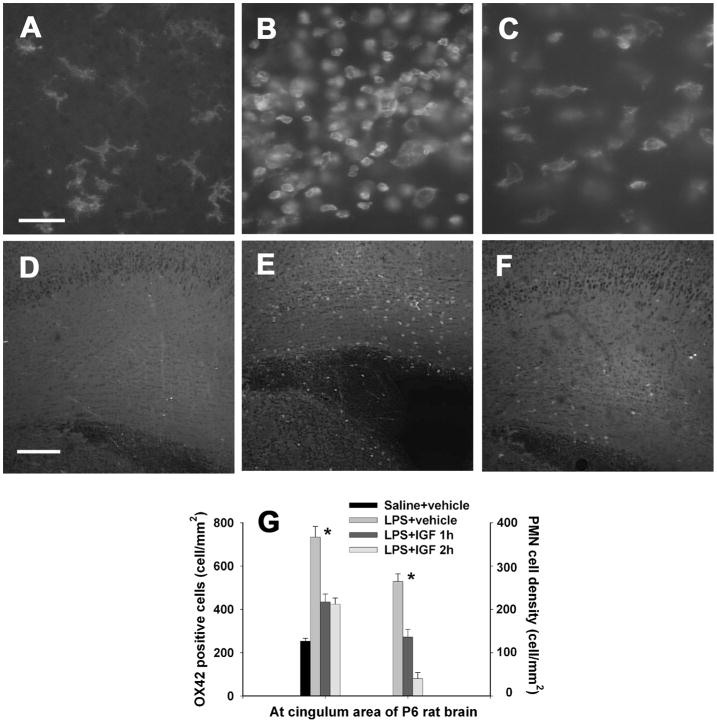
As we reported previously (Fan et al., 2005b; 2008b), neonatal LPS exposure induced massive activation of microglia and infiltration of PMN cells in the forebrain white matter 24 hr following the LPS injection. In the control rat brain, most OX42 positive microglia were at the resting status with a small rod shaped cell body with fine and ramified processes (Fig. 5A) and no CD43 positive PMN cells were detectable (Fig 5D). Microglial activation, as indicated by the increased number of OX42 positive cells and the morphological alterations, was observed in the cingulum area 24 hr after the LPS exposure (Figs. 5B & 5G). The activated microglia had an enlarged soma with few blunt or no processes. Infiltration of PMN cells, as evidenced by the increased number of CD43 positive cells, was also found in the cingulum area (Figs. 5E & 5G). The increased PMN cell infiltration was observable even at P21 (data not shown). Intranasal infusion of rhIGF-1 at either 1 or 2 hr after the LPS injection significantly reduced the number of activated microglia (Figs. 5C & 5G) and the number of infiltrated PMN cells (Figs. 5F & 5G) in the rat brain.
Figure 5.
Density of OX42 positive microglia (A–C) and infiltrated CD43 positive PMN cells (D–F) in the P6 rat brain. Perinatal LPS exposure resulted in a significant increase in the number of microglia, which have a round shape (B) as compared to the control rat brain (A) 24 h after the LPS exposure. IGF-1 administration at 2 hr after LPS injection reduced microglial activation (C). Most microglia in the control rat brain and some microglia in the LPS+IGF rat brain have a ramified shape. No CD43+ PMN cells were detectable in the control rat brain (D). Perinatal LPS exposure significantly increased the number of PMN cells at the cingulum area of the rat brain 24 h after LPS injection (E). IGF-1 treatment at 2 hr after LPS injection reduced PMN infiltration (F). The quantitative data of OX42+ microglia density and CD43+ PMN cell density in the rat brain are presented in G (n=6 for each grtoup). *p<0.05 vs the other groups. Scale bar: A–C, 50 μm; D–F, 200 μm.
2.4 Intranasal administration of rhIGF-1 improved some neurological functions in the juvenile rat
Body weight change
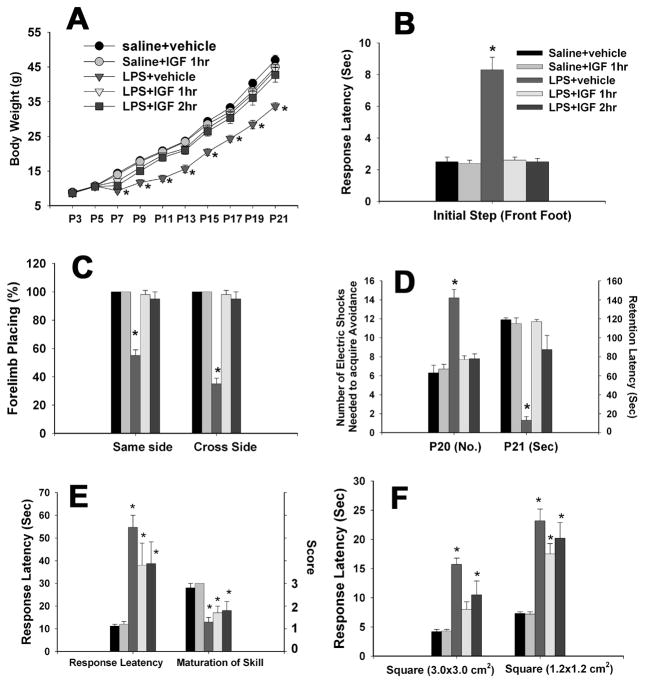
Intracerebral injection of LPS in P5 rats resulted in a significant body weight loss from P7 to P21 (Fig. 6A). Administration of IGF-1 through intranasal infusion at either 1 or 2 hr after the LPS injection prevented LPS-induced weight loss.
Figure 6.
Neurobehavioral tests following neonatal LPS exposure and IGF-1 treatment. Each group contained 6 animals. LPS exposure in P5 rats resulted in a significant body weight loss from P7 toP21 and administration of IGF-1 through intranasal infusion at either 1 or 2 hr after the LPS injection prevented LPS-induced weight loss (A). Neonatal LPS exposure also impaired the performance of P20 or P21 rats in the movement initiation test (B), the vibrissa-elicited forelimb-placing test (C) and the passive avoidance test (D). Intranasal infusion with rhIGF-1 at either 1 or 2 h after the LPS exposure reversed the LPS-induced impairment. *p<0.05 vs the other groups. Neonatal LPS exposure also impaired performance of rats in the pole test (E) and the bean walking test (F), but treatment with IGF-1did not improve performance in these two tests. *p<0.05 vs the saline+vehicle group in E and F.
Movement initiation test
In saline-exposed groups including those infused with IGF-1 or vehicle 1 hr after the saline injection, all P21 rats initiated stepping movements in 1~3 sec for one forelimb in a balanced order (Fig. 6B). Neonatal exposure to LPS resulted in a delayed response in stepping initiation (about 8 sec) at P21. Intranasal infusion of IGF-1 at either 1 or 2 hr after the LPS injection prevented LPS-induced delay in stepping initiation.
Vibrissa-elicited forelimb-placing test
In saline-exposed groups including those infused with IGF-1 or vehicle 1 hr after the saline injection, all rats succeeded in vibrissa-elicited forelimb-placing test (~100%) for both the same side and the cross side stimuli on P21 (Fig. 6C). The success rate of the vibrissa-elicited forelimb-placing test in the LPS-injected group was significantly lower than that in the saline-exposed groups. Intranasal infusion of IGF-1 at either 1 or 2 hr after the LPS injection prevented LPS-induced failure in vibrissa-elicited forelimb-placing.
Passive avoidance
As shown in Fig. 6D, neonatal LPS exposure increased the number of electric foot shocks needed to retain the rat on the safe board at P20, as compared to those exposed to saline followed by infusion with either vehicle or IGF-1 on P5. Neonatal LPS exposure also impaired memory of the acquired avoidance, as indicated by the significantly shortened response latency at P21 (<20 sec vs 120 sec). Post-treatment with rhIGF-1 at either 1 or 2 hr after the LPS injection attenuated the LPS-induced learning deficits and memory impairment. No differences in locomotor activity among groups were observed on P20 and P21 (data not shown), indicating that the observed differences in learning and memory was unlikely due to possible alterations in locomotor activity.
Pole test
Neonatal LPS exposure increased the response latency while decreased the maturation of skill in P21 rats as compared to the saline-exposed groups including those infused with IGF-1 or vehicle 1 hr after the saline injection (Fig 6E). Intranasal infusion of IGF-1 at either 1 or 2 hr after the LPS injection failed to improve the response latency and maturation of skill.
Beam walking tests
All P21 rats in the saline-exposed groups including those infused with IGF-1 or vehicle 1 hr after the saline injection succeeded the beam walking tests in less than 5 or10 sec for the 3.0×3.0 or the 1.2×1.2 cm2 square beam respectively. Neonatal LPS exposure increased the time elapse required for rats to travel through either beam (Fig. 6F). Intranasal infusion of IGF-1 at1 or 2 hr did not improve the rat performance in the beam walking test.
2.5 rhIGF-1 administered through intranasal infusion did not have acute anti-inflammatory effects
As we reported above, intranasal infusion of IGF-1 attenuated inflammatory responses as indicated by the decrease in microglial activation and infiltration of PMN cells in the LPS-exposed rat brain (Fig. 5). This observation raises a question as to whether IGF-1 might have direct anti-inflammatory effects. To investigate such a possibility, we determined production of inflammatory cytokines, IL-1β and TNFα in the forebrain of LPS-exposed rats with or without the IGF-1 treatment (Fig. 7). No IL-1β or TNFα was detectable in the rat brain following saline injection or saline plus IGF-1 intranasal infusion (data not shown). Concentrations of IL-1β and TNFα in the rat brain increased at 2 hr after LPS injection and reached the peak level (~1200 and ~90 pg/mg protein, respectively) at 6 hr. By 24 hr after the LPS injection, the IL-1β concentration in the rat brain decreased to ~600 pg/mg protein while the TNFα level returned to an undetectable level. No differences in IL-1β or TNFα concentrations in the rat brain were found between LPS-exposed rats infused with vehicle and those infused with IGF-1, suggesting that IGF-1 did not directly have an acute anti-inflammatory effect.
Figure 7.
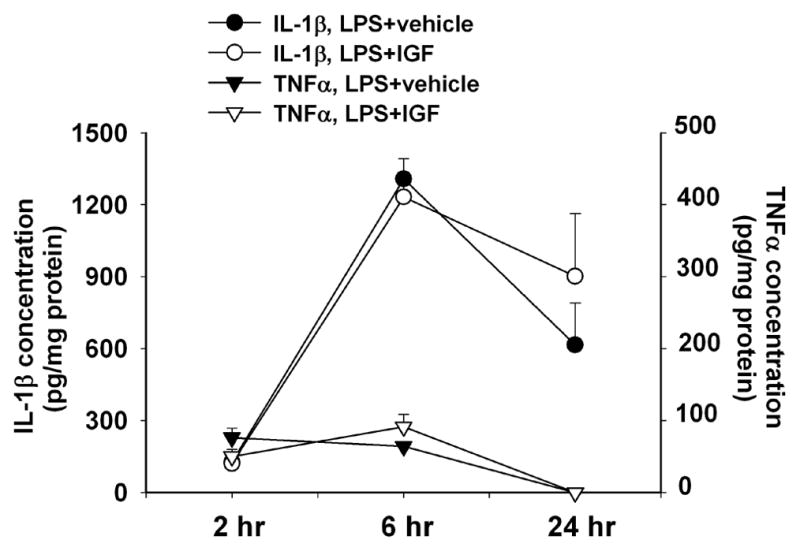
rhIGF-1 did not alter neonatal LPS exposure-induced elevation of inflammatory cytokine levels in the rat brain. Each group included 5 rat brains. Concentration of IL-1β or TNFα was not detectable by ELISA in the rat brain injected with saline or saline plus IGF-1 intranasal infusion (data not shown). Concentrations of IL-1β and TNFα in the rat brain increased at 2 hr after LPS injection and reached the peak level at 6 hr. By 24 hr after the LPS injection, the IL-1β concentration in the rat brain decreased to ~600 pg/mg protein while the TNFα level returned to an undetectable level. IGF-1 treatment did not alter the expression profile of IL-1β or TNFα stimulated by LPS.
3. Discussion
The major finding of the current study is that exogenous IGF-1 administered through intranasal infusion even at 2 hr after the LPS exposure can provide protection against LPS-induced injury in the developing brain, as evidenced by decreases in dilatation of lateral ventricles, pyknotic cell death (Fig 2), loss of OL progenitor cells (Fig. 3) and mature OLs, and impairment of myelination (Fig 4), and by improved neurobehavioral performance (Fig. 6). Our previous study showed that whether the treatment of exogenous IGF-1 has protective or detrimental effects on LPS-induced injury in the neonatal rat brain largely depends on its dose, i.e. IGF-1 is beneficial at low doses, but detrimental at high doses (Pang et al., 2010). In the current study, the protection of IGF-1 against LPS-induced injury was observed at an IGF-1 concentration range of ng/ml. Thirty minutes after intranasal infusion of IGF-1 at a dose of 50 μg per rat, the concentrations of IGF-1 in the P5 rat brain were 200–1200 and 70–500 pg/mg protein in FB and PB, respectively. Based on the determined protein concentration (~ 4 mg/ml) and the dilution factor (5 volume of PBS added), the above mentioned concentrations of IGF-1 are equivalent to 4–24 ng/ml in FB and 1.4–10 ng/ml in PB. Interestingly, this range of IGF-1 concentration (1–100 ng/ml) is the dose range at which many in vitro studies have shown that IGF-1 protects OLs from various insults (Pang et al., 2007; Cui et al., 2005; Ness et al., 2004; Wood et al., 2007). The dose effects of IGF-1 in neuroprotection have also been reported in several in vivo studies. Brywe et al. (2005) reported that administration of IGF-1 through intracerebral injection protects against hypoxic-ischemic brain damage only at a high dose (50 μg per P7 rat). IGF-1 delivered through intranasal infusion at a total dose of 600 μg over a 48 hr period improves behavior and Purkinje cell pathology in SCA1 mice, an animal model of spinocerebellar ataxia (Vig et al. 2006). Intranasal infusion of IGF-1 at a dose of 150, but not at 37.5 μg per animal, attenuated hypoxic-ischemic brain injury in adult rats (Liu et al., 2001a). Contrarily, Cao et al. (2003) showed that IGF-1 at a lower dose (3 μg per animal), but not a high dose (30 μg per animal), could prevent loss of OLs and myelin in fetal sheep subjected to hypoxia-ischemia. More recently, Escartin et al. (2007) reported that IGF-1 at a high dose (0.25 μg/h intracerebroventricular infusion for 5 days) exacerbated neuronal damage induced by 3-nitropropionic acid in a rat model of Huntington’s disease. However, in all these studies the dose of IGF-1 was referred to the administered dose, not the dose of IGF-1 actually reaching the brain. When the dose of IGF-1 actually reaching the brain was considered, the protective dose of IGF-1 in a neonatal rat model of hypoxia-ischemia (Lin et al., 2009) or the effective dose of IGF-1 in the adult rat brain (Thorne et al., 2004) also falls into a range of ng/ml. These studies together with our current data, suggest that the actual dose of IGF-1 that reaches the brain may depend on the amount of IGF-1 administered, specific animal models, route of administration and pathological conditions. To investigate the dose effect of IGF-1 in neuroprotection, it is more appropriate to be based on the actual dose of IGF-1 that reaches the brain.
We found in a previous study that IGF-1 at a low dose (0.5 μg per pup) protects the rat brain from LPS-induced OL and white matter injury, but also has some adverse effects (Pang et al., 2010). In that study, IGF-1 was co-administered with LPS through intracerebral injection, and even at the low dose used, the local concentration at the injection site could still be quite high. Such a locally elevated IGF-1 concentration could also have adverse effects. For example, increased hemorrhage and increased infiltration of PMN cells due to the breaking down of the blood brain barrier (BBB) were observed following co-injection of IGF-1 with LPS, while this dose of IGF-1 attenuated LPS-induced damage to OLs and the white matter (Pang et al., 2010). In the present study, no hemorrhage or increased PMN cell infiltration was found in any rat brain following intranasal infusion of IGF-1 and instead, intranasal IGF-1 infusion reduced infiltration of PMN cells. IGF-1, a promising treatment for various brain injuries (Guan, 2008), has been shown to provide neuroprotection to newborn rats (Brywe et al 2005; Lin et al., 2005) and fetal sheep (Cao et al., 2003; Guan et al., 2001; Johnston et al., 1996) when administered centrally. However, IGF-1 does not efficiently cross the BBB and delivery of IGF-1 to the brain, especially the neonatal brain, remains a substantial obstacle for its potential therapeutic uses. Intracerebral injection of IGF-1 apparently is not a practically useful approach for clinic use. Several studies have shown that IGF-1 can be successfully delivered into the brain through intranasal infusion and protect the brain from various injuries (Liu et al., 2001a, b; Lin et al., 2009, 2011; Vig et al., 2006). Mechanisms involved in delivery of IGF-1 through intranasal administration remain to be understood. Putatively, unique anatomic and physiologic attributes in the olfactory region of nasal passages may provide both extracellular and intracellular pathways into the CNS bypassing the blood brain barrier. The dendritic processes of the olfactory sensory neurons are directly exposed to the external environment in the upper nasal passage while their axons project through perforations in the cribriform plate of the ethmoid bone to synaptic glomeruli in the olfactory bulb (OB). A direct extracellular pathway between the nasal passages and the brain was first conclusively demonstrated for horseradish peroxidase (Balin et al., 1986). Several potential pathways have been proposed: (1) adsorptive or receptor-mediated endocytosis into olfactory sensory neurons followed by intracellular transport to the OB, (2) non-specific fluid phase endocytosis into olfactory sensory neurons followed by intracellular transport to OB and (3) extracellular diffusion into the olfactory submucosa along open intracellular clefts in the olfactory epithelium with subsequent diffusion into OB (Thorne et al., 1995). LPS is known to further enhance permeability of the BBB in the neonatal brain. In the current study, in spite of different treatments (LPS or saline exposure) and different rhIGF-1 infusion time (0, 1, or 2 hr after LPS injection), rhIGF-1 concentrations in the respective part of the rat brain were not significantly different (Fig. 1), suggesting that penetration of intranasally infused rhIGF-1 into the brain might not need to cross the BBB. This observation is consistent with above mentioned potential pathways and is an indication that intranasal delivery of protein may bypass the BBB (Liu et al., 2001a). Regardless of the mechanisms involved, results from the current study have further demonstrated that intranasal infusion of IGF-1 is a non-invasive, but effective way to deliver proteins like IGF-1 into the brain and provides protection against LPS-induced injury in the neonatal brain. This non-invasive intranasal administration of IGF-1 provides a feasible approach to give post-treatment for brain injury associated with central inflammation and it is potentially of great significance in clinical practice.
One of the mechanisms involved in the neuroprotection of IGF-1 is promotion of cell survival and prevention of apoptotic cell death (Crowder and Freeman, 1998; Fukunaga and Kawano 2003; Feldman et al, 1997; Russel et al., 1998). Survival-promoting effects are proposed to be elicited by activation of intracellular signaling cascades such as phophatidylinositol-3 kinase (PI3K) pathways (Feldman et al, 1997; Russel et al., 1998). Activation of PI3K leads to phosphorylation and activation of Akt (pAkt), which can inhibit the pro-apoptotic agents such as Bad, caspase 9, caspase 3, Forkhead transcription factors and NF-kB (Fukunaga and Kawano, 2003). Data from our previous studies (Lin et al., 2005; 2009) and from other investigators (Brywe et al., 2005; Cao et al., 2003; Cui et al., 2005; Wood et al., 2007) support that protection of IGF-1 against hypoxia-ischemia induced injury to OLs is through activation of the PI3K/Akt signaling pathway, inhibition of caspase-3 activation and reduction of apoptotic cell death. Our previous studies also show that anti-apoptotic cell death is involved in protection of IGF-1 against LPS-induced damage to OL progenitor cell in vivo (Pang et al., 2010) or TNFα-induced death of OL progenitor cells in vitro (Pang et al., 2007). Results from the present study show that intranasal administered rhIGF-1 reduced the number of pyknotic cells and the number of TUNEL positive O4 cells in white matter and prevented the increase in caspase-3 activity following LPS exposure (Figs. 2&3). These data once again indicate that prevention of apoptotic death of OL progenitor cells may play a major role in the IGF-1 protection observed in the present study. Increased IGF-1 production through liposomal IGF-1 gene transfer has been shown to modulate pro- and anti-inflammatory cytokine expression in the burn wound and improve the healing (Spies et al., 2001), suggesting that IGF-1 may decrease local inflammation. In the current study, reduced activation of microglia and infiltration of PMN cells following LPS exposure, as markers of inflammation, were observed in the IGF-1 treated rat brain (Fig. 5), suggesting that IGF-1 might have anti-inflammatory effects. Therefore, we examined the inflammatory cytokine levels following LPS exposure in the rat brain with or without IGF-1 treatment. Our results showed that IGF-1 treatment did not alter the expression profile of IL-1β and TNFα induced by LPS exposure (Fig. 7), indicating that IGF-1 does not directly have an acute anti-inflammatory effect in this experimental model and the attenuated activation of microglia and PMN cell infiltration observed 24 h after IGF-1 administration might be a consequence secondary to the reduced cell death in the white matter, which could have induced further inflammation. The different animal models, methods of delivery of IGF-1 and timing of cytokine determination may explain the differences in effects of IGF-1 on inflammatory cytokine expression stimulated by the insult between the current study and that reported by Spies et al. (2001).
In summary, rhIGF-1 was successfully delivered into the P5 neonatal rat brain through intranasal infusion. Intranasally delivered rhIGF-1 attenuated LPS-induced injury to OLs and white matters, and improved neurological dysfunction in juvenile rats. Attenuation of LPS-induced OL cell death might contribute to the neuroprotective effect of rhIGF-1, but it does not directly have acute anti-inflammatory effects.
Highlights.
Neonatal LPS exposure results in oligodendrocyte and white matter injury.
rhIGF-1 administered through intranasal infusion can penetrate into the neonatal rat brain.
Intranasal infusion of IGF-1 up to 2 hr after LPS exposure attenuates LPS-induced brain injury.
Intranasal infusion of IGF-1 improved neurobehavioral deficits induced by LPS in juvenile rats.
rhIGF-1 attenuates LPS-induced apoptotic cell death, but not LPS-induced acute immune responses.
Acknowledgments
This work was supported by grants from NIH HD 35496 and NS 54278.
Abbreviations
- BBB
blood brain barrier
- FB
front brain
- IL-1β
interleukin-1 beta
- LPS
lipopolysacharride
- MBP
myelin basic protein
- OB
olfactory bulb
- OL
oligodendrocyte
- P5
postnatal day 5
- PFA
paraformaldehyde
- PMN
polymorphonuclear
- PVL
periventricular leukomalacia
- PB
posterior brain
- rhIGF-1
recombinant human insulin-like growth factor-1
- TNFα
tumor necrosis factor alpha
- TUNEL
terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balin BJ, Broadwell RD, Salcman M, el-Kalliny M. Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J Comp Neurol. 1986;251:260–280. doi: 10.1002/cne.902510209. [DOI] [PubMed] [Google Scholar]
- Brywe KG, Mallard C, Gustavsson M, Hedtjärn M, Leverin AL, Wang X, Blomgren K, Isgaard J, Hagberg H. IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur J Neurosci. 2005;21:1489–1502. doi: 10.1111/j.1460-9568.2005.03982.x. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Cao Y, Gunn AJ, Bennet L, Wu D, George S, Gluckman PD, Shao XM, Guan J. Insulin-like growth factor (IGF)-1 suppresses oligodendrocyte caspase-3 activation and increases glial proliferation after ischemia in near-term fetal sheep. J Cereb Blood Flow Metab. 2003;23:739–747. doi: 10.1097/01.WCB.0000067720.12805.6F. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui QL, Zheng WH, Quirion R, Almazan G. Inhibition of Src-like kinases reveals Akt-dependent and -independent pathways in insulin-like growth factor I-mediated oligodendrocyte progenitor survival. J Biol Chem. 2005;280:8918–28. doi: 10.1074/jbc.M414267200. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Sailor KA, Bowen KK, Türeyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87:586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- Escartin C, Boyer F, Bemelmans AP, Hantraye P, Brouillet E. IGF-1 exacerbates the neurotoxicity of the mitochondrial inhibitor 3NP in rats. Neurosci Lett. 2007;425:167–172. doi: 10.1016/j.neulet.2007.08.031. [DOI] [PubMed] [Google Scholar]
- Fan L-W, Chen R-F, Mitchell HJ, Lin RCS, Simpson KL, Rhodes PG, Cai Z. α-phenyl-n-tert-butyl-nitrone attenuates lipopolysaccharide-induced brain injury and improves neurological reflexes and early sonsorimotor behavioral performance in juvenile rat. J Neurosci Res. 2008a;86:3536–3547. doi: 10.1002/jnr.21812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L-W, Lin S, Pang Y, Lei M, Zhang F, Rhodes PG, Cai Z. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res. 2005a;165:80–90. doi: 10.1016/j.bbr.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Fan L-W, Mitchell HJ, Tien L-T, Zheng B, Pang Y, Rhodes PG, Cai Z. α-phenyl-n-tert-butyl-nitrone reduces lipopolysaccharide-induced white matter injury in the neonatal rat brain. Dev Neuro. 2008b;68:365–378. doi: 10.1002/dneu.20591. [DOI] [PubMed] [Google Scholar]
- Fan L-W, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neurosci. 2005b;133:1359–68. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Fan L-W, Pang Y, Lin S, Tien L-T, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J Neurosci Res. 2005c;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- Fan L-W, Tien L-T, Mitchell HJ, Rhodes PG, Cai Z. α-phenyl-n-tert-butyl-nitrone ameliorates hippocampal injury and improves learning and memory in juvenile rats following neonatal exposure to lipopolysaccharide. Eur J Neurosci. 2008c;27:1475–1484. doi: 10.1111/j.1460-9568.2008.06121.x. [DOI] [PubMed] [Google Scholar]
- Fan L-W, Tien L-T, Zheng B, Pang Y, Lin R, Simpson K, Ma T, Rhodes PG, Cai Z. Dopaminergic neuronal injury in the adult rat brain following neonatal exposure to lipopolysaccharide and the silent neurotoxicity. Brain Behav Immunity. 2011;25:286–297. doi: 10.1016/j.bbi.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman EL, Sullivan KA, Kim B, Russell JW. Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiol Dis. 1997;4:201–214. doi: 10.1006/nbdi.1997.0156. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Zhu C, Fernagut PO, Mehta A, Dicarlo CD, Seaman RL, Xhesselet MF. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol. 2004;187:418–429. doi: 10.1016/j.expneurol.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Kawano T. Akt is a molecular target for signal transduction therapy in brain ischemic insult. J Pharmacol Sci. 2003;92:317–327. doi: 10.1254/jphs.92.317. [DOI] [PubMed] [Google Scholar]
- Guan J. Insulin-like growth factor-1 and its derivatives: potential pharmaceutical application for ischemic brain injury. Recent Patents CNS Drug Discov. 2008;3:112–127. doi: 10.2174/157488908784534630. [DOI] [PubMed] [Google Scholar]
- Guan J, Bennet L, George S, Wu D, Waldvogel HJ, Gluckman PD, Faull RL, Crosier PS, Gunn AJ. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21:493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- Guan J, Gunn AJ, Sirimanne ES, Tuffin J, Gunning MI, Clark R, Gluckman PD. The window of opportunity for neuronal rescue with insulin-like growth factor-1 after hypoxia-ischemia in rats is critically modulated by cerebral temperature during recovery. J Cereb Blood Flow Metab. 2000;20:513–519. doi: 10.1097/00004647-200003000-00010. [DOI] [PubMed] [Google Scholar]
- Hermans RH, Hunter DE, McGivern RF, Cain CD, Longo LD. Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol Teratol. 1992;14:119–129. doi: 10.1016/0892-0362(92)90060-n. [DOI] [PubMed] [Google Scholar]
- Johnston BM, Mallard EC, Williams CE, Gluckman PD. Insulin-like growth factor-1 is a potent neuronal rescue agent after hypoxic-ischemic injury in fetal lambs. J Clin Invest. 1996;97:300–308. doi: 10.1172/JCI118416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal ED. 2008;93:F153–161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Dammann O. Coagulation, inflammation, and the risk of neonatal white matter damage. Pediatr Res. 2004;55:541–545. doi: 10.1203/01.PDR.0000121197.24154.82. [DOI] [PubMed] [Google Scholar]
- Lin S, Fan L-W, Pang Y, Rhodes PG, Mitchell HJ, Cai Z. IGF-1 protects oligodendrocyte progenitor cells and improves neurological functions following cerebral hypoxia-ischemia in the neonatal rat. Brain Res. 2005;1063:15–26. doi: 10.1016/j.brainres.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Lin S, Fan LW, Rhodes PG, Cai Z. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Exp Neurol. 2009;217:361–70. doi: 10.1016/j.expneurol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Rhodes PG, Cai Z. Whole body hypothermia broadens the therapeutic window of intranasally administered IGF-1 in a neonatal rat model of cerebral hypoxia-ischemia. Brain Res. 2011;1385:246–256. doi: 10.1016/j.brainres.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Rhodes PG, Lei M, Zhang F, Cai Z. α-phenyl-N-tert-butyl-nitrone attenuates hypoxic-ischemic white matter injury in the neonatal rat brain. Brain Res. 2004;1007:132–141. doi: 10.1016/j.brainres.2004.01.074. [DOI] [PubMed] [Google Scholar]
- Liu X, Fawcett JR, Thorne RG, DeFor TA, Frey WH., 2nd Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001a;187:91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- Liu XF, Fawcett JR, Thorne RG, Frey WH., 2nd Non-invasive intranasal insulin-like growth factor-I reduces infarct volume and improves neurologic function in rats following middle cerebral artery occlusion. Neurosci Lett. 2001b;308:91–94. doi: 10.1016/s0304-3940(01)01982-6. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, Ferriero DM. Selective vulnerability in the developing central nervous system. Pediatr Neurol. 2004;30:227–235. doi: 10.1016/j.pediatrneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Ness JK, Scaduto RC, Jr, Wood TL. IGF-I prevents glutamate-mediated bax translocation and cytochrome C release in O4+ oligodendrocyte progenitors. Glia. 2004;46:183–94. doi: 10.1002/glia.10360. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–14. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Pang Y, Zheng B, Campbell L, Fan L-W, Cai Z, Rhodes PG. IGF-1 can either protect or deteriorate LPS-induced damage to the developing rat brain. Pediatr Res. 2010;67:579–584. doi: 10.1203/PDR.0b013e3181dc240f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Zheng B, Fan L-W, Rhodes PG, Cai Z. IGF-1 protects oligodendrocyte progenitors against TNFalpha-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55:1099–1107. doi: 10.1002/glia.20530. [DOI] [PubMed] [Google Scholar]
- Russell JW, Windebank AJ, Schenone A, Feldman EL. Insulin-like growth factor-I prevents apoptosis in neurons after nerve growth factor withdrawal. J Neurobiol. 1998;36:455–467. doi: 10.1002/(sici)1097-4695(19980915)36:4<455::aid-neu1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Schäbitz WR, Hoffmann TT, Heiland S, Kollmar R, Bardutzky J, Sommer C, Schwab S. Delayed neuroprotective effect of insulin-like growth factor-I after experimental transient focal cerebral ischemia monitored with MRI. Stroke. 2001;32:1226–1233. doi: 10.1161/01.str.32.5.1226. [DOI] [PubMed] [Google Scholar]
- Schallert T, Woodlee MT. Motor systems: Orienting and Placing. In: Whishaw IQ, Kolb B, editors. The Behaviour of the Laboratory Rat: A Handbook with Tests. Oxford University Press; New York: 2005. pp. 129–140. [Google Scholar]
- Spies M, Nesic O, Barrow RE, Perez-Polo JR, Herndon DN. Liposomal IGF-1 gene transfer modulates pro- and anti-inflammatory cytokine mRNA expression in the burn wound. Gene Therapy. 2001;8:1409–1415. doi: 10.1038/sj.gt.3301543. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Emory CR, Ala TA, Frey WH., 2nd Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692:278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neurosci. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Vig PJ, Subramony SH, D’Souza DR, Wei J, Lopez ME. Intranasal administration of IGF-I improves behavior and Purkinje cell pathology in SCA1 mice. Brain Res Bull. 2006;69:573–579. doi: 10.1016/j.brainresbull.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatri. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- Wood TL, Loladze V, Altieri S, Gangoli N, Levison SW, Brywe KG, Mallard C, Hagberg H. Delayed IGF-1 administration rescues oligodendrocyte progenitors from glutamate-induced cell death and hypoxic-ischemic brain damage. Dev Neurosci. 2007;29:302–310. doi: 10.1159/000105471. [DOI] [PubMed] [Google Scholar]