Abstract
Neurological disorders are common, costly, and can cause enduring disability. Although mostly unknown, a few environmental toxicants are recognized causes of neurological disorders and subclinical brain dysfunction. One of the best known neurotoxins is methylmercury (MeHg), a ubiquitous environmental toxicant that leads to long-lasting neurological and developmental deficits in animals and humans. In the aquatic environment, MeHg is accumulated in fish, which represent a major source of human exposure. Although several episodes of MeHg poisoning have contributed to the understanding of the clinical symptoms and histological changes elicited by this neurotoxicant in humans, experimental studies have been pivotal in elucidating the molecular mechanisms that mediate MeHg-induced neurotoxicity. The objective of this mini-review is to summarize data from experimental studies on molecular mechanisms of MeHg-induced neurotoxicity. While the full picture has yet to be unmasked, in vitro approaches based on cultured cells, isolated mitochondria and tissue slices, as well as in vivo studies based mainly on the use of rodents, point to impairment in intracellular calcium homeostasis, alteration of glutamate homeostasis and oxidative stress as important events in MeHg-induced neurotoxicity. The potential relationship among these events is discussed, with particular emphasis on the neurotoxic cycle triggered by MeHg-induced excitotoxicity and oxidative stress. The particular sensitivity of the developing brain to MeHg toxicity, the critical role of selenoproteins and the potential protective role of selenocompounds are also discussed. These concepts provide the biochemical bases to the understanding of MeHg neurotoxicity, contributing to the discovery of endogenous and exogenous molecules that counteract such toxicity and provide efficacious means for ablating this vicious cycle.
Keywords: methylmercury, neurotoxicity, oxidative stress, glutamate, calcium, selenium, selenoproteins, glutathione peroxidase
Introduction
Neurological disorders represent an important health concern faced by society, accounting for great numbers of hospitalizations and disabilities (Schmidt, 2007). Although still debatable, evidence has pointed to a potential link between exposures to chemical contaminants and neurological disorders/mental illness, such as autism (Landrigan, 2010), Parkinson’s disease (Barlow et al., 2007), Alzheimer’s disease (Coppede and Migliore, 2010), amyotrophic lateral sclerosis (Johnson and Atchison, 2009), just to name a few. Important chemical contaminants reported to cause neurotoxic effects in humans at environmentally relevant levels of exposure include polychlorinated biphenyls (Schantz et al., 2003), arsenic (Wasserman et al., 2004), lead (Lanphear et al., 2005), manganese (Takser et al., 2003), pesticides (Rauh et al., 2006), polycyclic aromatic hydrocarbons (Perera et al., 2006) and mercury compounds (Grandjean et al., 1997; Debes et al., 2006).
Mercury (Hg) is present in the environment due to either natural events or anthropogenic sources. It exists within the environment in three different chemical forms: elemental mercury vapor, inorganic mercury salts, and organic mercury (Clarkson, 1997). The distribution, toxicity and metabolism of mercury are greatly dependent on its chemical form. Organic mercury compounds, such as methylmercury (MeHg), have been extensively studied because they are able to reach high levels in the central nervous system (CNS), leading to neurotoxic effects (Aschner et al., 2007; Clarkson and Magos, 2006).
MeHg is an environmental contaminant that has been shown to cause neurological deficits in both animals and humans (Clarkson et al., 2003; Farina et al., 2010). As a result of the biomethylation of mercury compounds released mainly from anthropogenic sources in the aquatic environment, MeHg-containing fish represent a major source of human exposure. Therefore, fishing communities, which commonly depend greatly on fish for food, can be exposed to toxic levels of MeHg (Clarkson et al., 2003).
Human poisoning to MeHg was first reported around 150 years ago in laboratory accidents (Edwards, 1865). Several years later, catastrophic epidemics from environmental MeHg contamination in Japan (Harada, 1978) and Sweden (Westöö, 1966) were reported. Subsequently, exposure was documented in Iraq where locals consumed bread prepared from seeds treated with a fungicide containing MeHg (Bakir et al., 1973), causing a large outbreak of human poisoning. Similar incidents occurred in Guatemala, Pakistan, and Ghana (for a review, see Clarkson, 2002). Observed among the initial symptoms of MeHg poisoning were paresthesias, constriction of visual fields, impairment of hearing and speech, cerebellar ataxia and psychiatric symptomatology (Ekino et al., 2007; Murata et al., 2007).
Although the aforementioned episodes contributed to the understanding of the main clinical symptoms and histological changes elicited by MeHg poisoning in humans, the molecular mechanisms involved have been elucidated mainly based on studies with laboratory animals. Moreover, important aspects related to MeHg’s absorption, distribution, storage, biotransformation and elimination were also clarified due to the development of experimental studies. This mini-review discusses the importance of experimental studies to the understanding of molecular mechanisms related to MeHg-induced neurotoxicity. The correlation between biochemical and behavioral effects in MeHg-exposed animals, the relationship between oxidative stress and glutamate dyshomeostasis, and the potential use of antioxidant selenocompounds to counteract MeHg-induced neurotoxicity in experimental models are the focus of this mini-review.
Behavioral hallmarks
Behavioral parameters detected in MeHg-exposed animals have been essential to the understanding of the bases of several clinical manifestations observed in humans. In the early 1950s, an epidemiological investigation at the Minamata district showed that several cats fed a diet that primarily consisted of fish presented a severe condition named “dancing disease”, which was characterized by movement disorders, convulsions and death (Kitamura et al., 1957). Another epidemiological report showed that fish-feeding cats that lived on Indian Reserves in Northwestern Ontario, Canada, developed acute neurological symptoms characterized by ataxic gait, abnormal movements, uncontrolled howling, and seizures (Takeuchi et al., 1977). The foregoing evidences (Kitamura et al., 1957; Takeuchi et al., 1977) pointed to motor impairment as an important behavioral sign observed in MeHg-poisoned animals, corroborating data in humans (Ekino et al., 2007; Murata et al., 2007). Such symptoms were also observed in experimental studies using cats (Charbonneau et al., 1976), dogs (Mattsson et al., 1981), mice (Inouye et al., 1985; Dietrich et al., 2004); rats (Rocha et al., 1993; Farina et al., 2005), monkeys (Rice, 1996) and zebrafish (Danio rerio) (Samson et al., 2001). Although experimental studies on MeHg-exposed animals have shown that behaviors related to visual (visual contrast sensitivity task; Burbacher et al., 2005), cognitive (passive avoidance task; Ferraro et al., 2009) and emotional (depression-like behavior evaluated in the forced swimming test; Onishchenko et al., 2007) functions are also affected by MeHg exposures, movement disorders, consisting mainly of ataxia and loss of balance, have been extensively reported in experimental studies using MeHg-exposed animals (Dietrich et al., 2004, Farina et al., 2005; Lucena et al., 2007) and have been used as an important behavioral parameter to correlate with histological/cellular damage and/or biochemical changes (Franco et al., 2006; Carvalho et al., 2007, Carvalho et al., 2008), as well as to study potential protective/antidotal strategies to counteract MeHg-induced toxicity (Farina et al., 2005; Martins et al., 2009).
As mentioned previously, fishing communities, which commonly present a fish-based diet, can be exposed to toxic levels of MeHg (Clarkson et al., 2003). Accordingly, oral exposure represents a major form by which human population is exposed to MeHg. It is noteworthy that MeHg absorption in the gastrointestinal (GI) tract is around 90–95%, which is significantly higher when compared to the GI absorption of inorganic salts of mercury from food (Nielsen, 1992). Taking these kinetic properties into consideration, and aiming to better understand the movement alterations elicited by MeHg exposure, our group standardized an exposure protocol based on the intoxication of Swiss mice through the ingestion of MeHg (Dietrich et al., 2004). In this protocol, methylmercury (II) chloride (CH3HgCl) is diluted in drinking water and animals are allowed to drink the MeHg solution, ad libitum. We have observed that the liquid ingestion of MeHg-exposed mice is similar to that of control animals (allowed to drink tap water) when MeHg concentrations do not exceed 40 mg/L (please, consider that the use of different strains or species should give different effects). Using higher concentrations (e.g. 60 mg/L), mouse liquid ingestion is significantly decreased probably due to the metallic taste of MeHg which causes food aversion in the mice. Based on the daily liquid ingestion of an adult Swiss mouse (around 6.5 ml/day) and on its body weight (around 45 g), a daily MeHg dose of 7 mg/kg body weight can be calculated for animals exposed to a 40 mg/L MeHg solution, ad libitum. This dose is similar to those in toxicological studies based on either intraperitoneal or subcutaneous injections (Kobayashi et al., 1981; Verschaeve and Léonard, 1984; Stringari et al., 2006). The advantage of using an oral exposure protocol is its similarity with the most common human exposure condition, as well as the absence of daily injections and the associated discomfort. The protocol is also practical for relatively long-term exposure periods (several months if using low doses). From an environmental point of view, this is important because long-term exposures to MeHg in experimental studies better represent the chronic exposure in humans, who are normally exposed to the neurotoxicant for months or years.
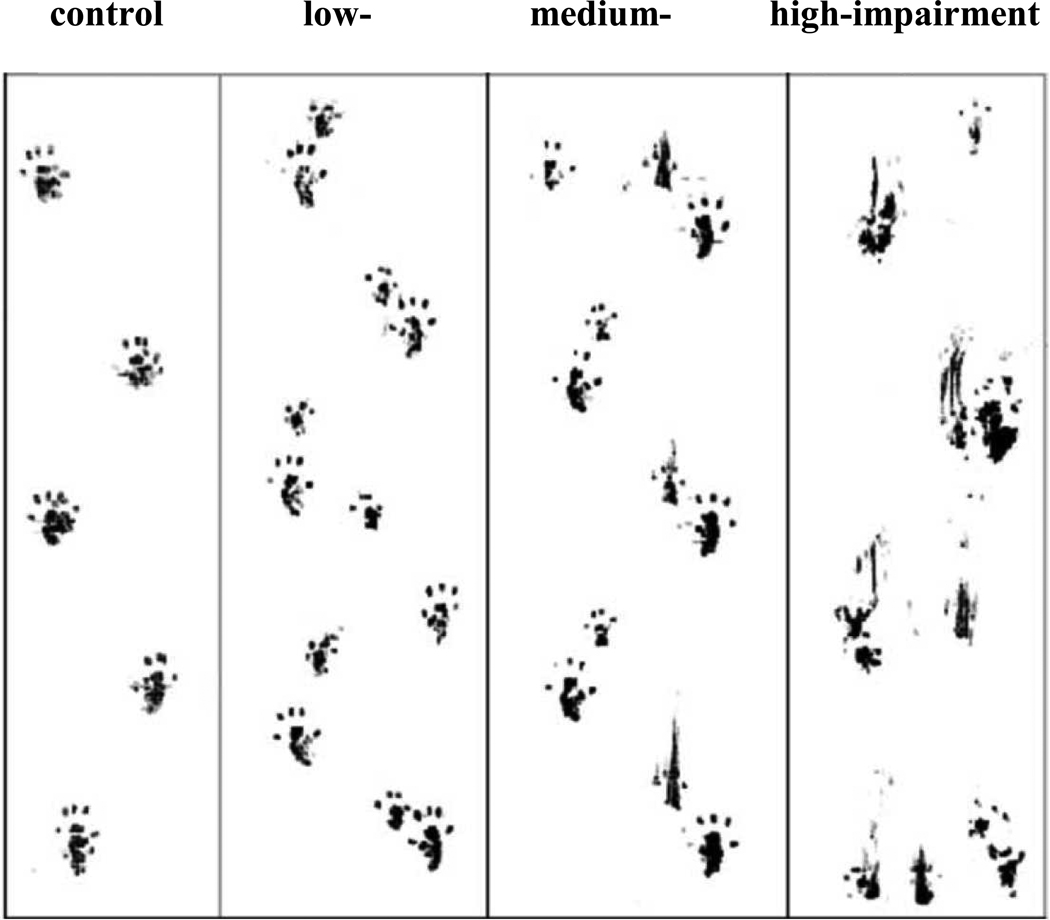
As already mentioned, motor alterations represent an important behavioral outcome of MeHg poisoning in both animals (Charbonneau et al., 1976; Mattsson et al., 1981; Inouye et al., 1985; Dietrich et al., 2004; Rocha et al., 1993; Farina et al., 2005; Rice, 1996) and humans (Ekino et al., 2007; Murata et al., 2007). The aforementioned experimental protocol (Dietrich et al., 2004) is able to induce significant deficits on motor performance in Swiss mice, which can be observed with different low-cost tests, such as the footprint (Carter et al., 1999), beam walking (Perry et al., 1995) and rotarod (Jones and Roberts, 1968) tests. Figure 1 depicts the progressive loss of the motor coordination (normal gait) in animals exposed to MeHg in drinking water.
Figure 1. Progressive motor impairment in MeHg exposed mice.
Animals were exposed to methylmercury (CH3Hg+; MeHg) (40 mg/L, diluted in drinking water) (Dietrich et al., 2004). The figure represents a footprint test evaluation in a randomly selected animal. Low-, medium- and high-impairment represent footprint tests performed at 7, 14 and 21 days after the beginning of the treatment, respectively.
Gender is an important variable to take into consideration when studying MeHg-exposed animals and evaluating motor impairments. An interesting finding obtained from mice exposed to MeHg via drinking water is that female mice are more resistant to MeHg-induced motor impairment compared to males (Malagutti et al., 2009). Consistent with this observation, when studied in the rotarod apparatus, two-week exposure to MeHg in drinking water (40 mg/L, ad libitum) caused a significant motor impairment in male but not female adult Swiss mice (Malagutti et al., 2009). Data from other experimental studies (Gimenez-Llort et al., 2001) have also pointed to more profound neurotoxic effects (spontaneous and dopamine-stimulated locomotor activity) of MeHg in males, corroborating epidemiological studies in humans exposed to MeHg (McKeown-Eyssen et al., 1983; Grandjean et al., 1998). Since the co-administration of 17 β-estradiol protected male mice against the neurotoxic effects induced by oral MeHg exposure (Malagutti et al., 2009), it is reasonable to suggest that sex steroids afford neuroprotective effects.
Another important characteristic of the proposed protocol (exposure of Swiss mice to MeHg through the ingestion of contaminated water) is that the mercury levels in central structures of these mice closely mimic those in brains obtained from autopsied humans (Myers et al., 1995; Franco et al., 2006), reflecting upon the environmental significance of the exposure protocol.
Molecular mechanisms of neurotoxicity in experimental animal models
As mentioned above, experimental studies in either in vitro or in vivo models have been crucial in elucidating the molecular and cellular mechanisms of neurotoxicity elicited by MeHg. Pioneering in vivo studies with 203Hg showed that the brain uptake of MeHg in rats is enhanced by continuous L-cysteine infusion (Aschner and Clarkson, 1987). This study brought about the hypothesis that MeHg may be transported from the blood to the CNS across the blood-brain barrier (BBB) by the L-type neutral amino acid carrier transport (LAT) system. A few years later, Kerper and collaborators (1992) observed that MeHg entered the rat brain as a cysteine complex via the LAT system as a result of the close mimicry between the MeHg/L-cysteine (MeHg-Cys) complex and L-methionine, a substrate for the LAT amino acid transport system. More recently, an in vitro experimental study using cultured CHO-k1 (Chinese hamster ovary) cells showed that the overexpression of the L-type large neutral amino acid transporter, LAT1, was associated with increased uptake of MeHg in the presence of L-cysteine, as well as reduced cellular viability (Yin et al., 2008). These observations culminated in the conclusion that MeHg-L-cysteine conjugate (MeHg-Cys) is a substrate for the LAT1 system, which actively transports MeHg across membranes and is responsible, at least in part, for the high Hg levels found in the brain after exposures. Indeed, although other transporters have been reported to contribute to MeHg transport (Whu, 1996; Bridges and Zalups, 2010), LAT1 seems to be the main, if not the only, transporter responsible for MeHg transport from peripheral tissues to the CNS (Kerper et al., 1992). Interestingly, a recent in vitro study in tissue slices showed that methionine pre-treatment displayed a protective effect against the toxic effects induced by MeHg and/or MeHg-Cys on mitochondrial function and cell viability, suggesting that methionine can be considered a potential strategy to the treatment of acute MeHg exposure (Roos et al., 2011).
Not only the transport and metabolism of MeHg, but also its major molecular targets and biochemical effects have been elucidated in experimental studies. Of particular importance, in vivo studies with rats showed that MeHg combines covalently with sulfhydryl (thiol) groups from plasma cholinesterase, leading to the enzyme inhibition (Hastings et al., 1975). After this important observation, several in vitro and in vivo experimental studies showed that sulfhydryl-containing enzymes are inhibited by MeHg (Kanda et al., 1976; Magour et al., 1986; Kageyama et al., 1986; Rocha et al., 1993; Kung et al., 1987). These observations led to the notion that the direct chemical interaction among MeHg and thiol groups from proteins and non-protein molecules, such as glutathione (GSH; γ -glutamyl-cysteinyl-glycine), plays a crucial role in MeHg-induced neurotoxicity (for a review, see Aschner and Syversen, 2005).
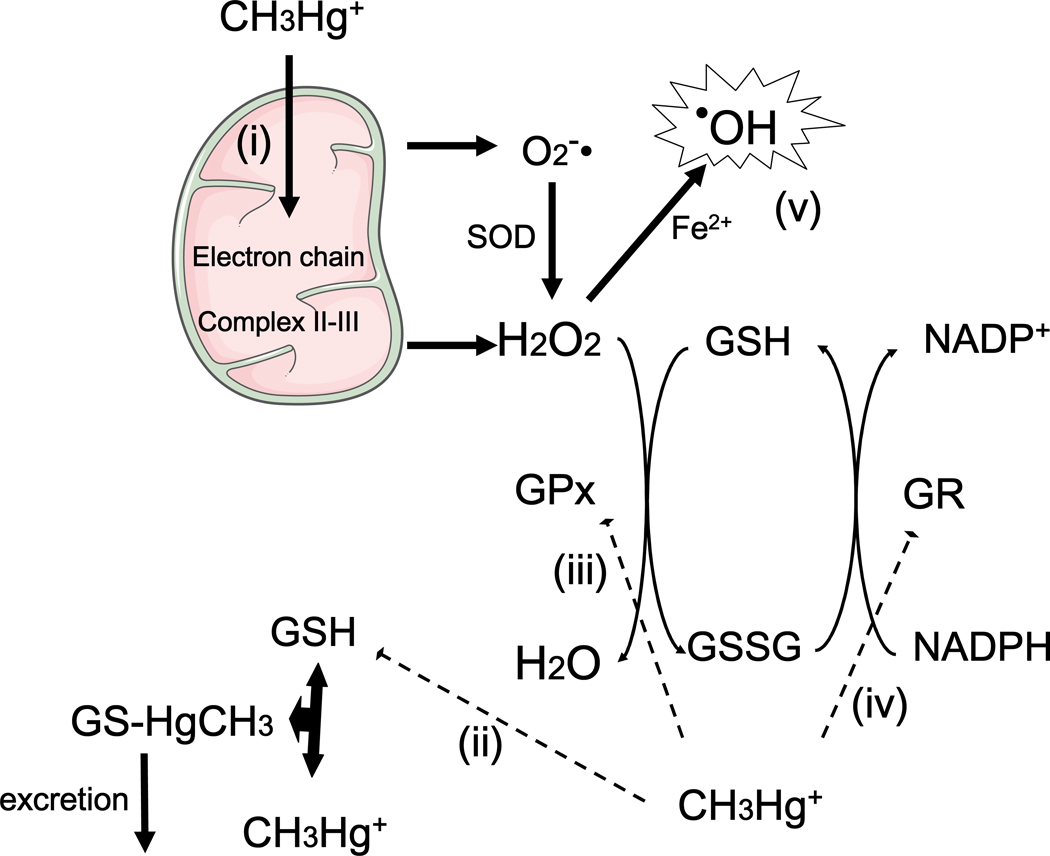
The antioxidant GSH system is an important target in mediating MeHg neurotoxicity (Kaur et al., 2006; Stringari et al., 2008). GSH is the most abundant intracellular low molecular weight thiol compound in all tissues, including the CNS (Dringen, 2000). GSH is present at the low millimolar range (1–10 mM) in some mammalian cells (Cooper and Kristal, 1997). Its reducing capacity is determined by the nucleophilic properties of its thiol group and its antioxidant role is sustained by the presence of several enzymes that catalyze its interaction with endogenous and xenobiotic electrophilic molecules (Zhu et al., 2006). Of particular importance, glutathione peroxidase (GPx) and glutathione reductase (GR) are central enzymes involved with detoxification of peroxides and reduction of glutathione disulphide (oxidized glutathione; GSSG), respectively (Dringen, 2000). The precise activities of these enzymes, as well as the maintenance of a normal thiol status, represented mainly by the GSH/GSSG ratio, are crucial for protecting cells against oxidative damage. With particular emphasis on the toxicity induced by MeHg, it is known that its mercury atom directly interacts with the thiol group of GSH, leading to the formation of an excretable GS-HgCH3 complex (Ballatori and Clarkson, 1982). This interaction has been proposed to decrease the levels of GSH, which could contribute to the occurrence of oxidative stress (Franco et al., 2007). Consistent with this hypothesis, experimental studies based on in vitro approaches have reported decreased GSH levels after MeHg exposure. This effect was detected in non-neuronal cell lines (Amonpatumrat, 2008), neuronal and glial primary cultures (Kaur et al., 2006) and isolated mitochondria from the mouse brain (Franco et al., 2007). From a molecular point of view, decreased GSH levels, which can occur as a consequence of GS-HgCH3 complex formation, will cause increased reactive species (H2O2, nitric oxide, etc.) generation and oxidative damage in a plethora of biomolecules (nucleic acids, lipids and proteins). However, it is important to note that MeHg can induce oxidative stress due to its direct interaction with nucleophilic protein groups (Farina et al., 2010), even in the absence of significant changes in GSH levels or GSH/GSSG ratio.
Experimental in vivo studies have also reported decreased GSH levels in the CNS (cerebellum) after MeHg exposure in mice, although this effect was dependent upon ontogeny: weanling animals were more susceptible than adults (Franco et al., 2006). Another interesting in vivo study showed that prenatal MeHg exposure hampered the normal maturation of the antioxidant GSH system during the early postnatal period (Stringari et al., 2008). In fact, in utero exposure to MeHg, which did not alter cerebral GSH levels and GR activity at birth, inhibited the developmental profile of the cerebral GSH antioxidant system during the early postnatal period. Decreased GSH levels and reduced GR activity were observed in the brain of MeHg-exposed animals at weaning (postnatal day 21). Additional studies are required to affirm if this event is permanent or if levels of GSH and GR activity are normalized as the animals reach adulthood. Nevertheless, this effect is consistent with additional molecular mechanism by which MeHg affects the GSH antioxidant system in the developing CNS, thereby rendering the brain more susceptible to oxidants. MeHg’s effects on GSH homeostasis are depicted in Figure 2.
Figure 2. Effects of MeHg on the GSH antioxidant system.
(i) Methylmercury (CH3Hg+; MeHg) disrupts mitochondrial electron transport chain, leading to increased formation of reactive oxygen species, such as hydrogen peroxide (H2O2) and superoxide anion (O2•−) (Franco et al., 2007; Mori et al., 2007). (ii) MeHg also reacts with reduced glutathione (GSH), leading to GSH depletion due to the formation of a MeHg–GSH (GS–HgCH3) complex, which is excreted from the body. MeHg hampers the physiological increase in glutathione reductase (GR) and glutathione peroxidase (GPx) activities in the rodent CNS during the early postnatal period (iii and iv) (Stringari et al., 2008), but also decreases GPx activity in adult animals (Farina et al., 2003a, Franco et al., 2009). All these events (i–iv) culminate in increased ROS generation and oxidative stress (v).
Glutamate dyshomeostasis in the CNS represents another critical target in MeHg-induced neurotoxicity (for a review, see Aschner et al., 2007). Glutamate is the major excitatory neurotransmitter in the mammalian CNS, where it plays key roles in development, learning, memory and response to injury (Featherstone, 2010). However, glutamate at high concentrations at the synaptic cleft acts as a toxin, inducing neuronal injury and death (Meldrum, 2000; Ozawa et al., 1998). Glutamate-mediated neurotoxicity has been dubbed as “excitotoxicity”, referring to the consequence of the overactivation of the N-methyl D-aspartate (NMDA)–type glutamate receptors, leading to increased Na+ and Ca2+ influx into neurons (Choi, 1992; Pivovarova & Andrews, 2010). Increased intracellular Ca2+ levels are associated with the generation of oxidative stress and neurotoxicity (Lafon-Cazal, 1993; Ceccatelli et al., 2010). Accordingly, the control of extracellular levels of glutamate dictates its physiological/pathological actions and this equilibrium is maintained primarily by the action of glutamate transporters (such as GLAST, GLT1, and EAAC1) located on astrocytic cell membranes, which remove the excitatory neurotransmitter from the synaptic cleft, keeping its extracellular concentrations below toxic levels (Anderson and Swanson, 2000; Maragakis and Rothstein, 2001; Szydlowska & Tymianski, 2010).
Advances in understanding the role of glutamate dyshomeostasis in MeHg-induced neurotoxicity was derived from in vitro studies with cultured cells (neurons and astrocytes), as well as tissue slices, isolated synaptosomes and synaptic vesicles. Experimental in vitro approaches have shown that MeHg readily inhibits glutamate uptake into cultured astrocytes (Brookes and Kristt, 1989; Aschner et al., 2000). MeHg also inhibits the uptake of glutamate into rat synaptic vesicles (Porciúncula et al., 2003) and cerebral cortical slices (Moretto et al., 2005a), suggesting that increased glutamate levels in the extracellular milieu could represent a biochemical consequence of MeHg exposure. In agreement, experimental ex vivo studies showed decreased glutamate uptake into cerebral cortex slices in both adult (Farina et al., 2003a) and weanling (Manfroi et al., 2004) mice exposed to MeHg. MeHg also increases the spontaneous release of glutamate from mouse cerebellar slices (Reynolds and Racz, 1987) and cultured neuronal cells (Vendrell et al., 2007), suggesting that increased glutamate release also contributes to elevated extracellular glutamate levels after MeHg exposure. The molecular mechanisms mediating increased glutamate release and decreased glutamate uptake in MeHg toxicity are not yet completely understood. However, evidence shows that hydrogen peroxide, whose levels are increased during MeHg exposure, can be a crucial molecule involved in the oxidative and inhibitory effects on astrocyte glutamate transporters (Allen et al., 2001). On the other hand, increased glutamate release seems to depend, at least in part, on the decreased vesicular uptake of glutamate, probably due to the direct inhibition of H+-ATPase activity (Porciúncula et al., 2003). Interestingly, the foregoing experimental in vitro evidences were confirmed by in vivo studies with microdialysis probes implanted in the frontal cortex of adult Wistar rats (Juarez et al., 2002), showing increased levels of extracellular glutamate after MeHg exposure.
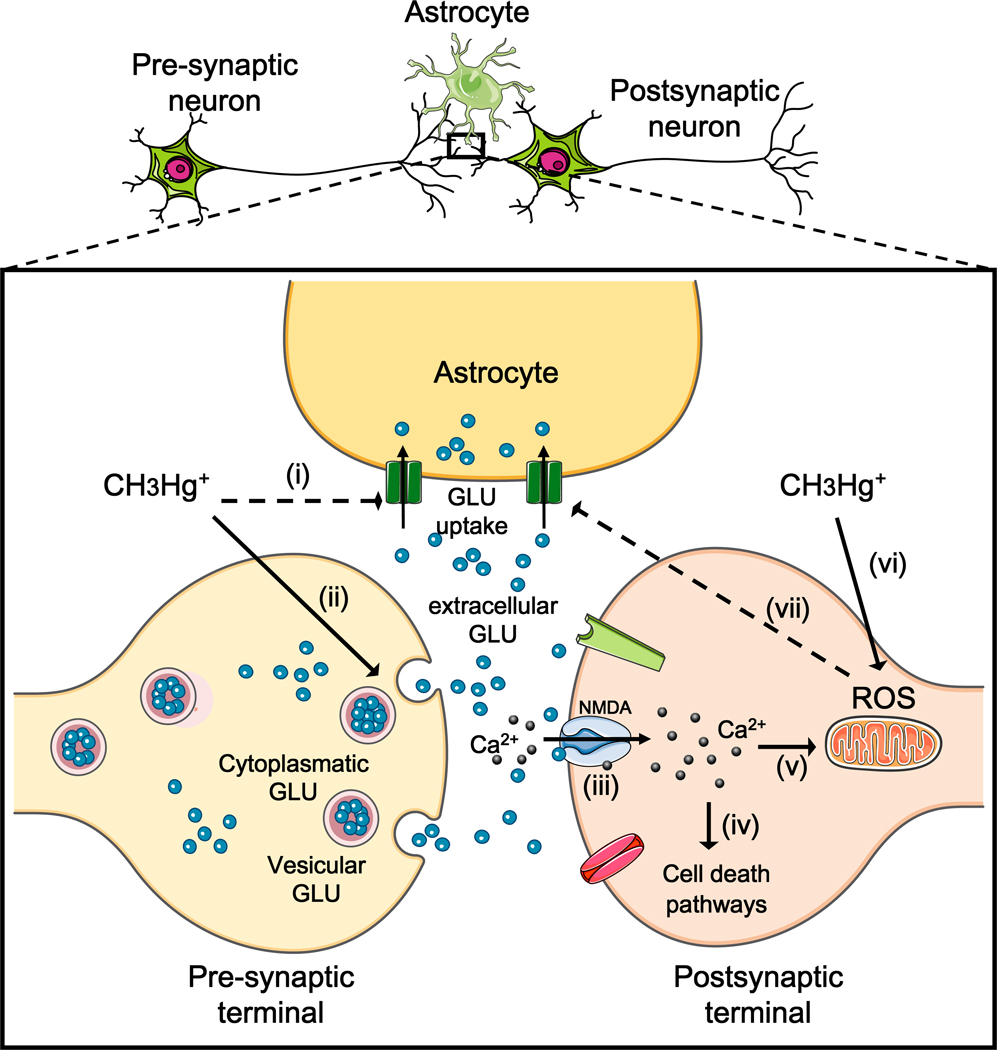
All the aforementioned observations are consistent with the ability of MeHg to increase extracellular glutamate levels. The overactivation of NMDA-type glutamate receptors increases Ca2+ influx into neurons, therefore leading to the activation of important pathways involved with cell death. Alternatively, Ca2+ can be taken up by mitochondria, where it may stimulate the generation of reactive oxygen species (ROS) (Reynolds and Hastings, 1995). Consistent with this hypothesis, several studies corroborates MeHg’s ability to induce mitochondrial dysfunction and consequent generation of hydrogen peroxide (Mori et al., 2007), which by itself is a potent inhibitor of astrocytic glutamate uptake (Allen et al., 2001). Thus, three important events related to MeHg-induced neurotoxicity [(i) Ca2+ dyshomeostasis, (ii) glutamate dyshomeostasis, and (iii) increased ROS generation/oxidative stress] represent interrelated events that can feed forward upon each other. This connection between the aforementioned events is depicted in Figure 3.
Figure 3. Interrelated association among MeHg-induced oxidative stress, Ca2+ and glutamate dyshomeostasis.
The figure shows a tripartite synapses, where methylmercury (CH3Hg+; MeHg) inhibits astrocytic glutamate uptake (i) and increases glutamate release (ii), leading to elevated extracellular glutamate levels. High levels of extracellular glutamate overactivate N-methyl D-aspartate (NMDA)-type glutamate receptor (iii). Overactivation of NMDA glutamate receptors leads to increased influx of Ca2+ into postsynaptic neurons, causing activation of cell death pathways (Hidalgo and Donaso, 2008). Alternatively, Ca2+ taken up by mitochondria may cause mitochondrial dysfunction and increased reactive oxygen species (ROS) generation (v). This last event is also directly stimulated by MeHg (vi), which seems to be related to misbalance in the electron transport chain (Mori et al., 2007). Increased levels of ROS (mainly H2O2) can directly decrease astrocytic glutamate uptake (vii) (Allen et al., 2001), contributing to excitotoxicity. GLU = glutamate.
Neurodevelopmental toxicity in experimental models
Experimental and epidemiological evidence indicates that compared to the adult CNS the developing CNS is more susceptible to several toxicants (Costa et al., 2004; Grandjean and Landrigan, 2006). With particular emphasis on the developmental neurotoxicity caused by MeHg, significant attention has been directed to this topic due to the long-term consequences of prenatal exposure to this organometal on child development in communities with chronic low level dietary exposure (Castoldi et al., 2008; Debes et al., 2006). In order to better understand the neurotoxicity induced by MeHg-exposure during the developmental period, different experimental protocols have been developed. At the beginning of the 1970s, Mansour and collaborators (1973) showed that MeHg is transferred across the placenta from pregnant rats to their fetuses. Few years later, a study on the pharmacokinetics of MeHg in the maternal-fetal unit in mice showed that the fetal accumulation of mercury increased with fetal age and peak fetal mercury was reached 3 days post MeHg administration (Oslon and Massaro, 1977), suggesting that the transfer of MeHg from pregnant mice to the fetus can occur within a relative short period (few days). Another interesting experimental study with pregnant mice exposed to MeHg showed significant changes in biochemical parameters in the fetuses’ brain and absent changes in the mothers’ brain (Watanabe et al., 1999). The results from Watanabe’s work clearly showed that the transfer of MeHg from the pregnant animals to their fetuses via the placenta may lead to neurotoxicity in the offspring with no evident neurotoxic signs in their respective dams. Interestingly, epidemiological studies corroborated this observation in humans as well (Myers and Davidson, 2000), reinforcing the idea that the developing CNS is most susceptible to the deleterious effects of MeHg.
Epidemiological evidence indicates that prenatal MeHg exposure in humans may cause permanent neurological deficits (Debes et al., 2006). Experimental studies on the prenatal effects of MeHg showed a significant disruption in the postnatal development of the GSH antioxidant system (Stringari et al., 2008). Moreover, these authors observed significant delayed and long-lasting neurochemical changes during the suckling period following gestational MeHg exposure, even in the presence of extremely low levels of mercury in the brain (near to control values). These observations are consistent with the ability of exposure to MeHg during critical periods of development to trigger enduring and long-lasting (into adulthood) biochemical changes associated with oxidative stress (Debes et al., 2006).
Although astrocytes are the preferential site of MeHg accumulation (Charleston et al., 1996; Aschner, 1996), neurons seem to be more susceptible to MeHg-induced toxicity. With a particular emphasis on the developing brain, neuronal cells from different brain structures, such as hippocampus, cerebellum and cerebral cortex, have been reported as potential targets for the toxic effects elicited by MeHg (Burke et al., 2006; Gao et al., 2006; Falluel-Morel et al., 2007; Dasari & Yuan, 2009). With respect to the histological changes observed after MeHg exposure, significant reductions of the number of neurons have been particularly described (Carvalho et al., 2008; Falluel-Morel et al., 2007).
Although brain development is more dramatic during fetal life, it continues for years postnatally and additional exposure can occur when a mother breast-feeds or the child consumes fish (Myers et al., 2009). Thus, experimental studies on the developmental neurotoxicity elicited by postnatal MeHg-exposures appear to be also of toxicological significance. In this context, an experimental study on the lactational exposure and neonatal kinetics of MeHg in mice showed that postnatal exposure to MeHg via breast-feeding raises important toxicological concerns because of the high absorption of MeHg in the gastrointestinal tract, as well as its low excretion rate in pups (Sundberg et al., 1999). Taking into account that no neurotoxic signs were evaluated in Sundberg’s work (1999), we conducted two experimental studies aimed at understanding the exclusive contribution of the lactational exposure to MeHg to neurotoxicity in the offspring (Manfroi et al., 2004; Franco et al., 2006). Female mice (genitors) were orally exposed to MeHg from the first day after parturition until postnatal day 21 (weaning period). Subsequently, weanling pups were exposed to MeHg exclusively by breast-feeding. The eventual pups’ consumption of MeHg-containing water was excluded because the dispensing ends of the water tubes were not accessible to pups even at postnatal day 21. The major results from these two studies were that lactational exposure to MeHg induced several behavioral changes (mainly related to the motor function), as well as decreased glutamate uptake into cerebellar slices and increased cerebellar oxidative stress (Manfroi et al., 2004; Franco et al., 2006). These studies showed that the exclusive lactational exposure to MeHg caused neurotoxicity in the offspring and reinforced the link between glutamate dyshomeostasis, oxidative stress and movement deficits, which was previously observed in adult animals (Farina et al., 2003a; Farina et al., 2005). Although the foregoing studies on the lactational exposure to MeHg suggest that the presence of this toxicant in the breast milk can lead to neurotoxicity in experimental protocols with rodents, further epidemiological investigations are necessary to examine the occurrence of such events in humans.
MeHg interacts with selenol groups
As mentioned above, MeHg interacts with thiol groups from proteins and non-protein molecules, and such interaction plays a crucial role in MeHg-induced oxidative stress and neurotoxicity (Aschner and Syversen, 2005). Nevertheless, it is difficult to understand how the equimolar interaction between MeHg and GSH (the major low molecular weight thiol antioxidant) could lead to oxidative stress. In fact, GSH is present in some mammalian cells at concentrations near to 10 millimolar (Cooper and Kristal, 1997) and MeHg has been reported to cause oxidative damage in cultured neuronal cells when added to the culture medium at concentrations as low as 300 nanomolar (Farina et al., 2009). Moreover, in vivo experimental studies have shown reduced cerebellar (Franco et al., 2006) and cerebral (Stringari et al., 2008) GSH levels in MeHg-exposed animals whose cerebellar/cerebral mercury levels were in the low micromolar range. Taking into account these observations, it is reasonable to assume that nucleophilic groups other than GSH’s thiol could be more reactive toward MeHg, allowing for the formation of high affinity complexes, thus leading to dysfunction of important cellular molecules with nucleophilic properties. In agreement with this hypothesis, thiol groups from specific proteins [tyrosine phosphatase (Sumi, 2008) and creatine kinase (Wang et al., 2001)] are more nucleophilic and, consequently, more reactive than GSH (Farina et al., 2010). Toxicological consequences of this phenomenon have already been reported (Glasser et al., 2010). Nonetheless, mammalian cells posses another group of proteins, named selenoproteins, whose nucleophilicity is in general higher than sulfhydryl-containing proteins. Selenoproteins are usually involved in redox reactions, where selenocysteine (a strong nucleophilic amino acid) is an active-site residue essential for catalytic activity (Lu and Holmgren, 2009). Selenocysteine possesses a selenol group (-SeH) that is generally more reactive than thiols (-SH), including towards mercury (Sugiura et al., 1976). Experimental studies have been pursued in order to understand the chemical interaction between MeHg and selenol groups in the biological environment in an attempt to elucidate selenol’s role in MeHg-induced neurotoxicity. In 1977, an in vivo experimental study with rats showed that the cerebral activity of glutathione peroxidase, a selenoprotein responsible for peroxide detoxification, was significantly decreased after MeHg exposure (Prohaska and Ganther, 1977). Interestingly, this effect was prevented by the equimolar administration of selenite. After this pioneering work, several studies showed that the selenium-mercury interaction presents important toxicological significance (for a review, see Khan and Wang, 2009). Recently, an experimental in vitro study with primary cultures of cerebellar neurons showed that glutathione peroxidase (isoform 1; GPx1) is an initial molecular target of low-dose MeHg (Farina et al., 2009). In fact, GPx1 activity was decreased in MeHg-treated neurons with no changes in several biochemical parameters that are normally affected by high-dose MeHg exposures. In addition, the decreased enzyme activity was a consequence of direct inhibitory effects, which were probably related to mercury-selenol interactions. Interestingly, these events were responsible for enhanced susceptibility to peroxides, increased lipid peroxidation and neuronal death (Farina et al., 2009). In agreement, another recent study established that glutathione peroxidase also serves as a crucial molecule involved with MeHg-induced neurotoxicity (Franco et al., 2009). In addition to the direct interaction between MeHg and the selenol group of GPx, another molecular mechanism has been proposed to explain the reduced enzyme’s activity after MeHg exposure. Consistent with this hypothesis, cultured cells showed that MeHg induces a “selenium-deficient-like” condition, which affects GPx1 synthesis through a posttranscriptional effect (Usuki et al., 2010). Collectively, these effects point to the selenoenzyme GPx as an important molecular target in MeHg-induced neurotoxicity, which seems to be affected by MeHg earlier than other thiol-containing proteins. This effect, which is probably related to the higher nucleophilicity and softness of selenols when compared to thiols, reinforces the idea that the selenium-mercury interaction possesses an important role in the neurotoxicity induced by MeHg. In agreement with the foregoing data on MeHg and GPx, thioredoxin reductase (TrxR), another important antioxidant selenoprotein, has also been reported to be a molecular target involved in MeHg toxicity (Carvalho et al. 2008; 2011; Wagner et al., 2010). Based on both in vivo and in vitro experiments, the authors propose that MeHg can bind to selenocysteine residues present in the catalytic site of TrxR, thus causing enzyme inhibition that can compromise the redox state of cells.
Taking advantage of the Se-Hg interaction in biological systems, experimental studies have been directed at the potential protective effects of organic selenocompounds against MeHg–induced toxicity (Farina et al., 2003a, Farina et al., 2003b; Moretto et al., 2005a; Moretto et al., 2005b; de Freitas et al., 2009). These studies were performed based on the observation that organic selenocompounds, such as ebselen and diphenyl diselenide, are metabolized in vivo to selenol-containing intermediates, representing potential candidates to interact with MeHg in a high affinity way, thus minimizing its interaction with thiol-containing biomolecules. The development of studies on the search for potential therapeutic/antidotal strategies against MeHg toxicity is motivated by the fact that there are no effective treatments available which completely abolish the toxic effects of MeHg. Studies based on in vitro protocols showed that ebselen, an organic selenium compound, prevented MeHg-induced inhibition of glutamate uptake into rat cortico-cerebral slices (Moretto et al., 2005a). Ebselen also prevented MeHg-induced inhibition of glutamate uptake under in vivo conditions (Farina et al., 2003a), corroborating the in vitro data (Moretto et al., 2005a). More recently, an experimental in vitro approach showed that ebselen protected against MeHg-induced inhibition of glutamine uptake and mitochondrial collapse in primary cultures of rat astrocytes (Yin et al., 2011). It is noteworthy that ebselen has been reported to offer borderline but significant efficacy against excitotoxicity in Phase III studies, when the neurological outcomes of patients were determined in a short period after the neuropathological insult (Yamaguchi et al., 1998; Lee et al., 1999).The neuroprotective activity of ebselen has been attributed to its GPx-like activity (Suzuki, 2009). However, clinical studies on ebselen are practically absent within the last decade. This most likely reflects the potential toxicity of this compound in relatively high levels, observed under in vitro and in vivo conditions (Yang et al., 2000; Farina et al., 2004). Alternatively, it is possible that the neuroprotective effects of ebselen observed close to the ischemic episodes could not be confirmed after a more prolonged longitudinal follow-up of the patients. Nevertheless, it is reasonable to suggest that when used in low dosages it may serve as an efficient antidotal therapy in MeHg poisoning due to the high affinity of MeHg for its selenol groups. In addition to ebselen, another interesting and promising organic selenocompound is diphenyl diselenide. This compound presents GPx-like activity and has been reported to present significant beneficial effects in several experimental pathological conditions (for a review, see Nogueira et al., 2004; Nogueira and Rocha, 2010). With particular emphasis on the toxicity elicited by MeHg, an in vivo experimental study showed that diphenyl diselenide administration was able to decrease the mercury levels in the liver, kidneys and brain of MeHg-treated mice (de Freitas et al., 2009), suggesting that it possesses direct chelating effects. Such effects are responsible, at least in part, for the increased excretion of MeHg from the body.
In summary, organic selenocompounds represent an interesting class of molecules for further investigation as potential therapeutic agents to treat MeHg poisoning. This is most likely a consequence of their ability to interact with endogenous and exogenous thiols to form “selenol intermediates”, which possess GPx-like activity (Nogueira et al., 2004) thus forming stable complexes with MeHg, rendering them more excretable. Additionally, ebselen and diphenyl diselenide can be reduced by TrxR, which generates the selenol of ebselen and selenophenol, respectively (Zhao et. 2002; Freitas et al. 2010), the nucleophylic intermediates that can bind to MeHg. Although these selenocompounds have been reported to display beneficial effects against MeHg-induced neurotoxicity in experimental studies, further research is necessary to evaluate the efficacy and safety of such compounds in humans.
Concluding remarks
Since the occurrence of the first outbreaks of MeHg poisoning in humans, the development of experimental studies has been instrumental in advancing the understanding of its metabolism and toxicokinetics, as well as the molecular mechanisms involved with its neurotoxic effects. Studies on the behavioral profile of MeHg-exposed animals have corroborated the clinical symptoms observed in humans poisoned by this toxicant. Moreover, the behavioral changes caused by MeHg exposure in animals (mainly represented by motor impairments) have been closely correlated to the biochemical alterations observed in these animals. In vitro and in vivo experimental approaches using cultured cells or living animals were also instrumental in demonstrating that MeHg entered the CNS complexed with the amino acid cysteine. Since this complex is structurally similar to methionine, MeHg is able to enter in neural cells mainly through the L-type neutral amino acid carrier transport (LAT) system by a mimicry mechanism. In addition, experimental studies were important in showing that MeHg is transported across the placenta from pregnant animals to their fetuses. Based initially on in vitro studies, the molecular mechanisms related to MeHg-induced neurotoxicity were partially elucidated. In this regard, glutamate and calcium dyshomeostasis, as well as ROS generation, are three important and interrelated phenomena that mediate a toxic cycle that culminates in neuronal death. These events were also proven to occur in in vivo conditions. Moreover, experimental studies were important in showing that MeHg reacted with selenol groups with high affinity, and that MeHg’s interaction with selenoproteins, such as the antioxidant enzymes glutathione peroxidase and thioredoxin reductase contribute to the increased oxidative stress. Finally, experimental studies have shown that organic selenocompounds, such as ebselen and diphenyl diselenide, represent promising molecules that counteract the toxic effects elicited by MeHg, given their GPx-like activities and their potential chelating effects after biotransformation to selenol-containing molecules. Although experimental studies have been highly instrumental in shedding novel information on aspects involved in MeHg-induced neurotoxicity, further research on the role of microglial cells and neuroinflammation, as well as potential therapeutic/antidotal strategies is warranted.
Acknowledgements
The authors would like to thank the colleagues/co-authors who contributed to several studies referenced in this mini-review. These studies were funded in part by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and from the National Institute of Environmental Health Sciences (USA). The FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)” # 01.06.0842-00 and INCT for Excitotoxicity and Neuroprotection-MCT/CNPq are especially appreciated. MA was supported by NIH grant R01 ES07331.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H-D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001;902(1):92–100. doi: 10.1016/s0006-8993(01)02375-7. [DOI] [PubMed] [Google Scholar]
- Amonpatumrat S, Sakurai H, Wiriyasermkul P, Khunweeraphong N, Nagamori S, Tanaka H, Piyachaturawat P, Kanai Y. L-glutamate enhances methylmercury toxicity by synergistically increasing oxidative stress. J Pharmacol Sci. 2008;108(3):280–289. doi: 10.1254/jphs.08118fp. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32(1):1–14. [PubMed] [Google Scholar]
- Aschner M, Clarkson TW. Mercury 203 distribution in pregnant and nonpregnant rats following systemic infusions with thiol-containing amino acids. Teratology. 1987;36(3):321–328. doi: 10.1002/tera.1420360308. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res. 2007;40(3):285–291. doi: 10.1590/s0100-879x2007000300001. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T. Methylmercury: recent advances in the understanding of its neurotoxicity. Ther Drug Monit. 2005;27(3):278–283. doi: 10.1097/01.ftd.0000160275.85450.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Yao CP, Allen JW, Tan KH. Methylmercury alters glutamate transport in astrocytes. Neurochem Int. 2000;37(2–3):199–206. doi: 10.1016/s0197-0186(00)00023-1. [DOI] [PubMed] [Google Scholar]
- Aschner M. Methylmercury in astrocytes - what possible significance? Neurotoxicology. 1996;17(1):93–106. [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181(96):230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Developmental changes in the biliary excretion of methylmercury and glutathione. Science. 1982;216(4541):61–63. doi: 10.1126/science.7063871. [DOI] [PubMed] [Google Scholar]
- Barlow BK, Cory-Slechta DA, Richfield EK, Thiruchelvam M. The gestational environment and Parkinson' s disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol. 2007;23(3):457–470. doi: 10.1016/j.reprotox.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health B Crit Rev. 2010;13(5):385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes N, Kristt DA. Inhibition of amino acid transport and protein synthesis by HgCl2 and methylmercury in astrocytes: selectivity and reversibility. J Neurochem. 1989;53(4):1228–1237. doi: 10.1111/j.1471-4159.1989.tb07419.x. [DOI] [PubMed] [Google Scholar]
- Burbacher TM, Grant KS, Mayfield DB, Gilbert SG, Rice DC. Prenatal methylmercury exposure affects spatial vision in adult monkeys. Toxicol Appl Pharmacol. 2005;208(1):21–28. doi: 10.1016/j.taap.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Burke K, Cheng Y, Li B, Petrov A, Joshi P, Berman RF, Reuhl KR, DiCicco-Bloom E. Methylmercury elicits rapid inhibition of cell proliferation in the developing brain and decreases cell cycle regulator, cyclin E. Neurotoxicology. 2006;27(6):970–981. doi: 10.1016/j.neuro.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington's disease mutation. J Neurosci. 1999;19(8):3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CM, Chew EH, Hashemy SI, Lu J, Holmgren A. Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity. J Biol Chem. 2008;283(18):11913–11923. doi: 10.1074/jbc.M710133200. [DOI] [PubMed] [Google Scholar]
- Carvalho CM, Lu J, Zhang X, Arner ES, Holmgren A. Effects of selenite and chelating agents on mammalian thioredoxin reductase inhibited by mercury: implications for treatment of mercury poisoning. FASEB J. 2011;25(1):370–381. doi: 10.1096/fj.10-157594. [DOI] [PubMed] [Google Scholar]
- Carvalho MC, Franco JL, Ghizoni H, Kobus K, Nazari EM, Rocha JB, Nogueira CW, Dafre AL, Muller YM, Farina M. Effects of 2,3-dimercapto-1-propanesulfonic acid (DMPS) on methylmercury-induced locomotor deficits and cerebellar toxicity in mice. Toxicology. 2007;239(3):195–203. doi: 10.1016/j.tox.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Carvalho MC, Nazari EM, Farina M, Muller YM. Behavioral, morphological, and biochemical changes after in ovo exposure to methylmercury in chicks. Toxicol Sci. 2008;106(1):180–185. doi: 10.1093/toxsci/kfn158. [DOI] [PubMed] [Google Scholar]
- Castoldi AF, Johansson C, Onishchenko N, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo L. Human developmental neurotoxicity of methylmercury: impact of variables and risk modifiers. Regul Toxicol Pharmacol. 2008;51(2):201–214. doi: 10.1016/j.yrtph.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Charbonneau SM, Munro IC, Nera EA, Armstrong FA, Willes RF, Bryce F, Nelson RF. Chronic toxicity of methylmercury in the adult cat. Interim report. Toxicology. 1976;5(3):337–349. doi: 10.1016/0300-483x(76)90052-4. [DOI] [PubMed] [Google Scholar]
- Charleston JS, Body RL, Bolender RP, Mottet NK, Vahter ME, Burbacher TM. Changes in the number of astrocytes and microglia in the thalamus of the monkey Macaca fascicularis following longterm subclinical methylmercury exposure. Neurotoxicology. 1996;17(1):127–138. [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110 Suppl 1:11–23. doi: 10.1289/ehp.02110s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L, Myers GJ. The toxicology of mercury--current exposures and clinical manifestations. N Engl J Med. 2003;349(18):1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Kristal BS. Multiple roles of glutathione in the central nervous system. Biol Chem. 1997;378(8):793–802. [PubMed] [Google Scholar]
- Coppede F, Migliore L. Evidence linking genetics, environment, and epigenetics to impaired DNA repair in Alzheimer' s disease. J Alzheimers Dis. 20(4):953–966. doi: 10.3233/JAD-2010-1415. [DOI] [PubMed] [Google Scholar]
- Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP. Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol. 2004;44:87–110. doi: 10.1146/annurev.pharmtox.44.101802.121424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Freitas AS, Funck VR, Rotta Mdos S, Bohrer D, Morschbacher V, Puntel RL, Nogueira CW, Farina M, Aschner M, Rocha JB. Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Res Bull. 2009;79(1):77–84. doi: 10.1016/j.brainresbull.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28(5):536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Dietrich MO, Mantese CE, Anjos Gd, Souza DO, Farina M. Motor impairment induced by oral exposure to methylmercury in adult mice. Environmental Toxicology and Pharmacology. 2005;19(1):169–175. doi: 10.1016/j.etap.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62(6):649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Edwards GN. Two cases of poisoning by mercuric methide. Saint Bartholomew’s Hosp Rep. 1865;1:10. [Google Scholar]
- Ekino S, Ninomiya T, Imamura K, Susa M. Methylmercury causes diffuse damage to the somatosensory cortex: how to diagnose Minamata disease. Seishin Shinkeigaku Zasshi. 2007;109(5):420–437. [PubMed] [Google Scholar]
- Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, Dicicco-Bloom E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem. 2007;103(5):1968–1981. doi: 10.1111/j.1471-4159.2007.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Rocha JBT, Aschner M. Oxidative stress and methylmercury-induced neurotoxicity. Indianapolis: John Wiley & Sons; 2010. pp. 357–385. [Google Scholar]
- Farina M, Campos F, Vendrell I, Berenguer J, Barzi M, Pons S, Sunol C. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol Sci. 2009;112(2):416–426. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- Farina M, Dahm KC, Schwalm FD, Brusque AM, Frizzo ME, Zeni G, Souza DO, Rocha JB. Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen. Toxicol Sci. 2003b;73(1):135–140. doi: 10.1093/toxsci/kfg058. [DOI] [PubMed] [Google Scholar]
- Farina M, Franco JL, Ribas CM, Meotti FC, Missau FC, Pizzolatti MG, Dafre AL, Santos AR. Protective effects of Polygala paniculata extract against methylmercury-induced neurotoxicity in mice. J Pharm Pharmacol. 2005;57(11):1503–1508. doi: 10.1211/jpp.57.11.0017. [DOI] [PubMed] [Google Scholar]
- Farina M, Frizzo ME, Soares FA, Schwalm FD, Dietrich MO, Zeni G, Rocha JB, Souza DO. Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicol Lett. 2003a;144(3):351–357. doi: 10.1016/s0378-4274(03)00242-x. [DOI] [PubMed] [Google Scholar]
- Farina M, Soares FA, Zeni G, Souza DO, Rocha JB. Additive pro-oxidative effects of methylmercury and ebselen in liver from suckling rat pups. Toxicol Lett. 2004;146(3):227–235. doi: 10.1016/j.toxlet.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Featherstone DE. Intercellular Glutamate Signaling in the Nervous System and Beyond. ACS Chemical Neuroscience. 2009;1(1):4–12. doi: 10.1021/cn900006n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Tomasini MC, Tanganelli S, Mazza R, Coluccia A, Carratu MR, Gaetani S, Cuomo V, Antonelli T. Developmental exposure to methylmercury elicits early cell death in the cerebral cortex and long-term memory deficits in the rat. Int J Dev Neurosci. 2009;27(2):165–174. doi: 10.1016/j.ijdevneu.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Franco JL, Braga HC, Stringari J, Missau FC, Posser T, Mendes BG, Leal RB, Santos AR, Dafre AL, Pizzolatti MG, Farina M. Mercurial-induced hydrogen peroxide generation in mouse brain mitochondria: protective effects of quercetin. Chem Res Toxicol. 2007;20(12):1919–1926. doi: 10.1021/tx7002323. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy AC, Marques MR, Dafre AL, Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med. 2009;47(4):449–457. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Franco JL, Posser T, Dunkley PR, Dickson PW, Mattos JJ, Martins R, Bainy AC, Marques MR, Dafre AL, Farina M. Methylmercury neurotoxicity is associated with inhibition of the antioxidant enzyme glutathione peroxidase. Free Radic Biol Med. 2009;47(4):449–457. doi: 10.1016/j.freeradbiomed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Franco JL, Teixeira A, Meotti FC, Ribas CM, Stringari J, Garcia Pomblum SC, Moro AM, Bohrer D, Bairros AV, Dafre AL, Santos AR, Farina M. Cerebellar thiol status and motor deficit after lactational exposure to methylmercury. Environ Res. 2006;102(1):22–28. doi: 10.1016/j.envres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Gao Y, Yan CH, Yu XD, Wu SH. Effects of perinatal exposure to methylmercury on the structure of hippocampus and cerebellum in young rats. Wei Sheng Yan Jiu. 2006;35(4):402–405. [PubMed] [Google Scholar]
- Gimenez-Llort L, Ahlbom E, Dare E, Vahter M, Ogren S, Ceccatelli S. Prenatal exposure to methylmercury changes dopamine-modulated motor activity during early ontogeny: age and gender-dependent effects. Environ Toxicol Pharmacol. 2001;9(3):61–70. doi: 10.1016/s1382-6689(00)00060-0. [DOI] [PubMed] [Google Scholar]
- Glaser V, Leipnitz G, Straliotto MR, Oliveira J, dos Santos VV, Wannmacher CM, de Bem AF, Rocha JB, Farina M, Latini A. Oxidative stress-mediated inhibition of brain creatine kinase activity by methylmercury. Neurotoxicology. 31(5):454–460. doi: 10.1016/j.neuro.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F. Cognitive performance of children prenatally exposed to "safe" levels of methylmercury. Environ Res. 1998;77(2):165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Harada M. Congenital Minamata disease: intrauterine methylmercury poisoning. Teratology. 1978;18(2):285–288. doi: 10.1002/tera.1420180216. [DOI] [PubMed] [Google Scholar]
- Hastings FL, Lucier GW, Klein R. Methylmercury-cholinesterase interactions in rats. Environ Health Perspect. 1975;12:127–130. doi: 10.1289/ehp.7512127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10(7):1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- Inouye M, Murao K, Kajiwara Y. Behavioral and neuropathological effects of prenatal methylmercury exposure in mice. Neurobehav Toxicol Teratol. 1985;7(3):227–232. [PubMed] [Google Scholar]
- Johnson FO, Atchison WD. The role of environmental mercury, lead and pesticide exposure in development of amyotrophic lateral sclerosis. Neurotoxicology. 2009;30(5):761–765. doi: 10.1016/j.neuro.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BJ, Roberts DJ. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J Pharm Pharmacol. 1968;20(4):302–304. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Juarez BI, Martinez ML, Montante M, Dufour L, Garcia E, Jimenez-Capdeville ME. Methylmercury increases glutamate extracellular levels in frontal cortex of awake rats. Neurotoxicol Teratol. 2002;24(6):767–771. doi: 10.1016/s0892-0362(02)00270-2. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Onoyama Y, Kano E. Effects of methyl mercuric chloride and sulfhydryl inhibitors on phospholipid synthetic activity of lymphocytes. J Appl Toxicol. 1986;6(1):49–53. doi: 10.1002/jat.2550060111. [DOI] [PubMed] [Google Scholar]
- Kanda F, Kamikashi T, Ishibashi S. Competitive inhibition of hexokinase isoenzymes by mercurials. J Biochem. 1976;79(3):543–548. doi: 10.1093/oxfordjournals.jbchem.a131098. [DOI] [PubMed] [Google Scholar]
- Kaur P, Aschner M, Syversen T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology. 2006;27(4):492–500. doi: 10.1016/j.neuro.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Kerper LE, Ballatori N, Clarkson TW. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am J Physiol. 1992;262(5 Pt 2):R761–R765. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- Khan MAK, Wang F. Mercury-selenium compounds and their toxicological significance: Toward a molecular understanding of the mercury-selenium antagonism. Environmental Toxicology and Chemistry. 2009;28(8):1567–1577. doi: 10.1897/08-375.1. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Miyata C, Tomita M, Date S, Ueda K, Misumi H, Kojima T, Minamoto H, Kurimoto S, Noguchi Y, Nakagawa R. Epidemiological investigation of the unknown central nervous disorder in the Minamata district. Kumamoto Med. J. 1957;31(1):1–9. [Google Scholar]
- Kobayashi H, Yuyama A, Matsusaka N, Takeno K, Yanagiya I. Neuropharmacological effect of methylmercury in mice with special reference to the central cholinergic system. Jpn J Pharmacol. 1981;31(5):711–718. doi: 10.1254/jjp.31.711. [DOI] [PubMed] [Google Scholar]
- Kung MP, Kostyniak P, Olson J, Malone M, Roth JA. Studies of the in vitro effect of methylmercury chloride on rat brain neurotransmitter enzymes. J Appl Toxicol. 1987;7(2):119–121. doi: 10.1002/jat.2550070208. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364(6437):535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 22(2):219–225. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738 Suppl):A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284(2):723–727. doi: 10.1074/jbc.R800045200. [DOI] [PubMed] [Google Scholar]
- Lucena GM, Franco JL, Ribas CM, Azevedo MS, Meotti FC, Gadotti VM, Dafre AL, Santos AR, Farina M. Cipura paludosa extract prevents methyl mercury-induced neurotoxicity in mice. Basic Clin Pharmacol Toxicol. 2007;101(2):127–131. doi: 10.1111/j.1742-7843.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Magour S. Studies on the inhibition of brain synaptosomal Na+/K+-ATPase by mercury chloride and methyl mercury chloride. Arch Toxicol Suppl. 1986;9:393–396. doi: 10.1007/978-3-642-71248-7_77. [DOI] [PubMed] [Google Scholar]
- Malagutti KS, da Silva AP, Braga HC, Mitozo PA, Soares dos Santos AR, Dafre AL, de Bem AF, Farina M. 17[beta]-estradiol decreases methylmercury-induced neurotoxicity in male mice. Environmental Toxicology and Pharmacology. 2009;27(2):293–297. doi: 10.1016/j.etap.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Manfroi CB, Schwalm FD, Cereser V, Abreu F, Oliveira A, Bizarro L, Rocha JB, Frizzo ME, Souza DO, Farina M. Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol Sci. 2004;81(1):172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- Mansour MM, Dyer NC, Hoffman LH, Schulert AR, Brill AB. Maternal-fetal transfer of organic and inorganic mercury via placenta and milk. Environ Res. 1973;6(4):479–484. doi: 10.1016/0013-9351(73)90061-3. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Arch Neurol. 2001;58(3):365–370. doi: 10.1001/archneur.58.3.365. [DOI] [PubMed] [Google Scholar]
- Martins Rde P, Braga Hde C, da Silva AP, Dalmarco JB, de Bem AF, dos Santos AR, Dafre AL, Pizzolatti MG, Latini A, Aschner M, Farina M. Synergistic neurotoxicity induced by methylmercury and quercetin in mice. Food Chem Toxicol. 2009;47(3):645–649. doi: 10.1016/j.fct.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson JL, Miller E, Alligood JP, Koering JE, Levin SG. Early effects of methylmercury on the visual evoked response of the dog. Neurotoxicology. 1981;2(3):499–514. [PubMed] [Google Scholar]
- McKeown-Eyssen GE, Ruedy J, Neims A. Methyl mercury exposure in northern Quebec. II. Neurologic findings in children. Am J Epidemiol. 1983;118(4):470–479. doi: 10.1093/oxfordjournals.aje.a113652. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130(4S Suppl):1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Moretto MB, Funchal C, Santos AQ, Gottfried C, Boff B, Zeni G, Pureur RP, Souza DO, Wofchuk S, Rocha JB. Ebselen protects glutamate uptake inhibition caused by methyl mercury but does not by Hg2+ Toxicology. 2005a;214(1–2):57–66. doi: 10.1016/j.tox.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Moretto MB, Funchal C, Zeni G, Rocha JB, Pessoa-Pureur R. Organoselenium compounds prevent hyperphosphorylation of cytoskeletal proteins induced by the neurotoxic agent diphenyl ditelluride in cerebral cortex of young rats. Toxicology. 2005b;210(2–3):213–222. doi: 10.1016/j.tox.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Mori N, Yasutake A, Hirayama K. Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity. Arch Toxicol. 2007;81(11):769–776. doi: 10.1007/s00204-007-0209-2. [DOI] [PubMed] [Google Scholar]
- Murata K, Dakeishi M, Shimada M, Satoh H. Assessment of intrauterine methylmercury exposure affecting child development: messages from the newborn. Tohoku J Exp Med. 2007;213(3):187–202. doi: 10.1620/tjem.213.187. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Tanner MA, Marsh DO, Cernichiari E, Lapham LW, Berlin M, Clarkson TW. Summary of the Seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicology. 1995;16(4):711–716. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW. Does methylmercury have a role in causing developmental disabilities in children? Environ Health Perspect. 2000;108 Suppl 3:413–420. doi: 10.1289/ehp.00108s3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GJ, Thurston SW, Pearson AT, Davidson PW, Cox C, Shamlaye CF, Cernichiari E, Clarkson TW. Postnatal exposure to methyl mercury from fish consumption: a review and new data from the Seychelles Child Development Study. Neurotoxicology. 2009;30(3):338–349. doi: 10.1016/j.neuro.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers GJDP, Cox C, Shamlaye CF, Tanner MA, Marsh DO, Cernichiari E, Lapham LW, Berlin M, Clarkson TW. Summary of the Seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicology. 1995;16(4):6. [PubMed] [Google Scholar]
- Nielsen JB, Andersen O. The toxicokinetics of mercury in mice offspring after maternal exposure to methylmercury--effect of selenomethionine. Toxicology. 1992;74(2–3):233–241. doi: 10.1016/0300-483x(92)90142-2. [DOI] [PubMed] [Google Scholar]
- Nogueira CW, Rocha JoBT. Diphenyl diselenide a janus-faced molecule. Journal of the Brazilian Chemical Society. 21:2055–2071. [Google Scholar]
- Nogueira CW, Zeni G, Rocha JB. Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev. 2004;104(12):6255–6285. doi: 10.1021/cr0406559. [DOI] [PubMed] [Google Scholar]
- Olson FC, Massaro EJ. Pharmacodynamics of methyl mercury in the murine maternal/embryo:fetal unit. Toxicol Appl Pharmacol. 1977;39(2):263–273. doi: 10.1016/0041-008x(77)90159-4. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Tamm C, Vahter M, Hokfelt T, Johnson JA, Johnson DA, Ceccatelli S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci. 2007;97(2):428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54(5):581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect. 2006;114(8):1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry TA, Torres EM, Czech C, Beyreuther K, Richards S, Dunnett SB. Cognitive and motor function in transgenic mice carrying excess copies of the 695 and 751 amino acid isoforms of the amyloid precursor protein gene. Alzheimer's Res. 1995;1:5–14. [Google Scholar]
- Porciuncula LO, Rocha JB, Tavares RG, Ghisleni G, Reis M, Souza DO. Methylmercury inhibits glutamate uptake by synaptic vesicles from rat brain. Neuroreport. 2003;14(4):577–580. doi: 10.1097/00001756-200303240-00010. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Ganther HE. Interactions between selenium and methylmercury in rat brain. Chem Biol Interact. 1977;16(2):155–167. doi: 10.1016/0009-2797(77)90125-9. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15(5 Pt 1):3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Racz WJ. Effects of methylmercury on the spontaneous and potassium-evoked release of endogenous amino acids from mouse cerebellar slices. Can J Physiol Pharmacol. 1987;65(5):791–798. doi: 10.1139/y87-127. [DOI] [PubMed] [Google Scholar]
- Rice DC. Sensory and cognitive effects of developmental methylmercury exposure in monkeys, and a comparison to effects in rodents. Neurotoxicology. 1996;17(1):139–154. [PubMed] [Google Scholar]
- Rocha JB, Freitas AJ, Marques MB, Pereira ME, Emanuelli T, Souza DO. Effects of methylmercury exposure during the second stage of rapid postnatal brain growth on negative geotaxis and on delta-aminolevulinate dehydratase of suckling rats. Braz J Med Biol Res. 1993;26(10):1077–1083. [PubMed] [Google Scholar]
- Samson JC, Goodridge R, Olobatuyi F, Weis JS. Delayed effects of embryonic exposure of zebrafish (Danio rerio) to methylmercury (MeHg) Aquat Toxicol. 2001;51(4):369–376. doi: 10.1016/s0166-445x(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Sausen de Freitas A, de Souza Prestes A, Wagner C, Haigert Sudati Js, Alves D, Oliveira Porciúncula L, Kade IJ, Teixeira Rocha JB. Reduction of Diphenyl Diselenide and Analogs by Mammalian Thioredoxin Reductase Is Independent of Their Gluthathione Peroxidase-Like Activity: A Possible Novel Pathway for Their Antioxidant Activity. Molecules. 15(11):7699–7714. doi: 10.3390/molecules15117699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausen de Freitas A, de Souza Prestes A, Wagner C, Haigert Sudati Js, Alves D, Oliveira Porciúncula L, Kade IJ, Teixeira Rocha JoB. Reduction of Diphenyl Diselenide and Analogs by Mammalian Thioredoxin Reductase Is Independent of Their Gluthathione Peroxidase-Like Activity: A Possible Novel Pathway for Their Antioxidant Activity. Molecules. 15(11):7699–7714. doi: 10.3390/molecules15117699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111(3):357–576. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CW. Environmental connections: a deeper look into mental illness. Environ Health Perspect. 2007;115(8):A404, A6–A10. doi: 10.1289/ehp.115-a404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringari J, Meotti FC, Souza DO, Santos AR, Farina M. Postnatal methylmercury exposure induces hyperlocomotor activity and cerebellar oxidative stress in mice: dependence on the neurodevelopmental period. Neurochem Res. 2006;31(4):563–569. doi: 10.1007/s11064-006-9051-9. [DOI] [PubMed] [Google Scholar]
- Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M, Farina M. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol. 2008;227(1):147–154. doi: 10.1016/j.taap.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Hojo Y, Tamai Y, Tanaka H. Letter: Selenium protection against mercury toxicity. Binding of methylmercury by the selenohydryl-containing ligand. J Am Chem Soc. 1976;98(8):2339–2341. doi: 10.1021/ja00424a059. [DOI] [PubMed] [Google Scholar]
- Sumi D. Biological Effects of and Responses to Exposure to Electrophilic Environmental Chemicals. J.Health Sci. 2008;54(3):267–272. [Google Scholar]
- Sundberg J, Jonsson S, Karlsson MO, Oskarsson A. Lactational exposure and neonatal kinetics of methylmercury and inorganic mercury in mice. Toxicol Appl Pharmacol. 1999;154(2):160–169. doi: 10.1006/taap.1998.8566. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, D'Itri FM, Fischer PV, Annett CS, Okabe M. The outbreak of Minamata disease (methyl mercury poisoning) in cats on Northwestern Ontario reserves. Environ Res. 1977;13(2):215–228. doi: 10.1016/0013-9351(77)90098-6. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24(4–5):667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- Usuki F, Yamashita A, Fujimura M. Posttranscriptional defects of antioxidant selenoenzymes cause oxidative stress under methylmercury exposure. J Biol Chem. doi: 10.1074/jbc.M110.168872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrell I, Carrascal M, Vilaro MT, Abian J, Rodriguez-Farre E, Sunol C. Cell viability and proteomic analysis in cultured neurons exposed to methylmercury. Hum Exp Toxicol. 2007;26(4):263–272. doi: 10.1177/0960327106070455. [DOI] [PubMed] [Google Scholar]
- Verschaeve L, Leonard A. Dominant lethal test in female mice treated with methyl mercury chloride. Mutat Res. 1984;136(2):131–136. doi: 10.1016/0165-1218(84)90155-1. [DOI] [PubMed] [Google Scholar]
- Wang PF, McLeish MJ, Kneen MM, Lee G, Kenyon GL. An unusually low pK(a) for Cys282 in the active site of human muscle creatine kinase. Biochemistry. 2001;40(39):11698–11705. doi: 10.1021/bi011208f. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH. Water arsenic exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2004;112(13):1329–1333. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol Teratol. 1999;21(1):83–88. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- Westoo G. Determination of methylmercury compounds in foodstuffs. I. Methylmercury compounds in fish, identification and determination. Acta Chem Scand. 1966;20(8):2131–2137. doi: 10.3891/acta.chem.scand.20-2131. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29(1):12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- Yang CF, Shen HM, Ong CN. Ebselen induces apoptosis in HepG(2) cells through rapid depletion of intracellular thiols. Arch Biochem Biophys. 2000;374(2):142–152. doi: 10.1006/abbi.1999.1574. [DOI] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, Aschner M. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem. 2008;107(4):1083–1090. doi: 10.1111/j.1471-4159.2008.05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Lee E, Ni M, Jiang H, Milatovic D, Rongzhu L, Farina M, Rocha JB, Aschner M. Methylmercury-induced alterations in astrocyte functions are attenuated by ebselen. Neurotoxicology. 2011;32(3):291–299. doi: 10.1016/j.neuro.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Holmgren A. A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J Biol Chem. 2002;277(42):39456–39462. doi: 10.1074/jbc.M206452200. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhang L, Xi X, Zweier JL, Li Y. 4-Hydroxy-2-nonenal upregulates endogenous antioxidants and phase 2 enzymes in rat H9c2 myocardiac cells: protection against overt oxidative and electrophilic injury. Free Radic Res. 2006;40(8):875–884. doi: 10.1080/10715760600778694. [DOI] [PubMed] [Google Scholar]