Abstract
Rationale
Preweanling rats exhibit robust one-trial cocaine-induced behavioral sensitization; however, it is uncertain whether other psychostimulants can also induce sensitization in young rats using the one-trial procedure.
Objective
The purpose of this study was to determine whether methamphetamine, methylphenidate, and D-amphetamine are capable of inducing one-trial locomotor sensitization in preweanling rats.
Methods
In a series of four experiments, rats were pretreated with cocaine (30 mg/kg), methamphetamine (2–12 mg/kg), methylphenidate (5–20 mg/kg), or amphetamine (5 mg/kg) before being placed in a novel activity chamber or the home cage on PD 19. Rats were then challenged with the same psychostimulant (20 mg/kg cocaine, 1–8 mg/kg methamphetamine, 2.5–7.5 mg/kg methylphenidate, or 1–2 mg/kg amphetamine) on PD 21, with distance traveled being measured for 180 min. In a separate experiment, rats were pretreated with methamphetamine on PD 16–19 and challenged with methamphetamine on PD 21.
Results
Only cocaine, but not various dose combinations of other psychostimulants, was able to produce one-trial behavioral sensitization in preweanling rats. Context-dependent locomotor sensitization was also evident if rats were pretreated with methamphetamine on PD 16–19 and tested on PD 21.
Conclusions
It is uncertain why only cocaine was able to induce one-trial locomotor sensitization in preweanling rats, but it is possible that: (a) the neural circuitry mediating sensitization differs according to psychostimulant, (b) cocaine is more readily associated with environmental contexts than other psychostimulants, or (c) affinity and pharmacokinetic factors may underlie cocaine’s ability to induce one-trial behavioral sensitization in preweanling rats.
Keywords: Behavioral sensitization, Preweanling rats, Cocaine, Methamphetamine, Methylphenidate D-Amphetamine
Introduction
There is a growing realization that psychostimulant drugs differentially affect the developing and mature brains of humans and other animals (Andersen 2005). Although various developmental stages in the rat have translational relevance to humans, there is a renewed focus on the late preweanling period because it is approximately analogous to late childhood in humans (Smith and Morrell 2008). During this developmental stage, a surprising percentage of children in the United States (~7%) illicitly sample psychostimulants (e.g., cocaine, methamphetamine, methylphenidate, and amphetamine) and other drugs of abuse (Johnston et al. 2005; Smith and Morrell 2008; Wilens et al. 2008). Repeated exposure to psychostimulant drugs can cause a variety of neural and behavioral changes in young and adult animals (Laviola et al. 1999; Andersen 2005). One of the most frequently studied of these phenomena is behavioral sensitization, which is defined as a progressive enhancement in behavioral responsiveness that occurs after repeated treatment with a psychostimulant drug (Robinson and Becker 1986; Kalivas and Stewart 1991). Behavioral sensitization has been studied intensively, in part, because it is hypothesized to be an important component of the addiction process (Robinson and Berridge 1993, 2008; Wolf and Ferrario 2010).
In a typical behavioral sensitization procedure, adult rats or mice are given repeated administrations of a psychostimulant drug (e.g., cocaine, amphetamine, methylphenidate, methamphetamine, etc.) across multiple pretreatment days and sensitized responding to a challenge injection of the psychostimulant is assessed days, weeks, or months later (Kalivas et al. 1988; Kuribara and Uchihashi 1994; Browman et al. 1998; Yang et al. 2007). Using this procedure, adult rats are capable of showing a sensitized locomotor response many months after initial drug discontinuation (Leith and Kuczenski 1982; Paulson et al. 1991). Testing sessions often last 2 or more hours and sensitized responding is exhibited across the breadth of the session (i.e., tolerance-like effects are not evident, although sensitized stereotypy may be prominent) (Segal and Kuczenski 1987; Kalivas and Duffy 1993). Interestingly, environmental conditioning factors can modulate the strength of the sensitized response, since behavioral sensitization is often more robust when drug pretreatment and testing occur in the same previously novel environment (Post et al. 1981; Carey and Gui 1998). Drug–environment pairings are not necessary for behavioral sensitization, however, because sensitized responding can be observed in adult rats even when the psychostimulant is never associated with the testing environment (Vezina and Stewart 1990; Partridge and Schenk 1999).
Adult rats and mice will also exhibit locomotor sensitization if given a single pretreatment injection of cocaine or amphetamine (Weiss et al. 1989; McDougall et al. 2005). With this procedure, sensitized responding is typically assessed 1 or 2 days after drug pretreatment (Weiss et al. 1989; McDougall et al. 2007), although one-trial sensitization has been shown to persist for at least 3–4 weeks (Robinson et al. 1982). In adult rats, environmental conditioning factors appear to gain in importance when the drug pretreatment phase consists of a single psychostimulant administration (for discussion, see Pert et al. 1990; White et al. 1998). For example, behavioral sensitization was not evident if drug pretreatment and testing occurred in distinctly different environments or if drug pretreatment occurred in the home cage (Battisti et al. 1999a, 1999b; McDougall et al. 2009b). In contrast, adult rats and mice pretreated with cocaine or amphetamine in a novel environmental context showed a sensitized response when subsequently challenged with a psychostimulant in the same environment (Jackson and Nutt 1993; Battisti et al. 2000).
Preweanling rats given repeated administrations of cocaine, amphetamine, or methylphenidate also exhibit behavioral sensitization (McDougall et al. 1994, 1999; Snyder et al. 1998). As with adult animals, the sensitized responding of young rats is more robust when drug pretreatment and testing occur in the same previously novel environment (Wood et al. 1998; Zavala et al. 2000; see also Fujiwara et al. 1987; Kolta et al. 1990). Although often viewed as being qualitatively similar, the behavioral sensitization exhibited by preweanling and adult rats differs in meaningful ways (for a review, see Tirelli et al. 2003). For example, both the magnitude and persistence of the sensitized response appears to be reduced in preweanling rats relative to adults (McDougall et al. 1999; Zavala et al. 2000). More recently, Smith and Morrell (2008) have suggested that preweanling rats do not exhibit true adult-like behavioral sensitization. Instead, they reported that after three once-daily cocaine treatments 22-day-old rats exhibit an initial increase in locomotor activity that lasts for 30 min, followed by a “tolerance-like response” that was apparent for the remainder of the 3-h testing session (Smith and Morrell 2008). Adult rats, in comparison, showed a day-dependent increase in cocaine-induced locomotor activity that was evident across the entire 3-h testing period (Smith and Morrell 2008).
Preweanling rats, like adults, are capable of exhibiting one-trial cocaine-induced behavioral sensitization, however the sensitized responding of the two age groups differs in at least one important respect. Specifically, only preweanling rats show robust context-independent sensitization when tested using the one-trial procedure (McDougall et al. 2009a; Herbert et al. 2010). This age-dependent difference suggests that either (a) associative processes do not modulate the one-trial sensitization of young rats or (b) the nature of the cocaine–environment pairings differs according to age (McDougall et al. 2009b; Herbert et al. 2010). To further delineate the factors influencing one-trial behavioral sensitization, we attempted to determine if psychostimulant drugs other than cocaine (i.e., methamphetamine, methylphenidate, and amphetamine) are capable of inducing one-trial context-specific or context-independent sensitization during the preweanling period. In order to better describe the pattern of sensitized responding, locomotor activity was measured across a 3-h testing session. Lastly, using a standard multitrial sensitization procedure (i.e., incorporating 4 pretreatment days), we examined whether repeated treatment with methamphetamine would induce behavioral sensitization in preweanling rats (in previous developmental studies only cocaine-, methylphenidate-, and amphetamine-induced sensitization was assessed using a multitrial procedure). It was initially hypothesized that (a) all of the psychostimulants tested (i.e., cocaine, methamphetamine, methylphenidate, and amphetamine) would induce one-trial context-independent sensitization, and (b) context-dependent behavioral sensitization would be evident after a 4-day regimen of methamphetamine.
Materials and methods
Subjects
Subjects were 370 male and female rats of Sprague–Dawley descent (Charles River, Hollister, CA) that were born and bred at California State University, San Bernardino (CSUSB). Litters were culled to ten pups on PD 4. Except during testing, rat pups were kept with the dam and littermates in large polycarbonate maternity cages (56×34×22 cm) with wire lids and Tek-Fresh® bedding (Harlan, Indianapolis, IN). Food and water were freely available. The colony room was maintained at 22–24°C and kept under a 12 L:12 D cycle. Testing was done in a separate experimental room and was conducted during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research” (National Research Council 2003) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in commercially available (Coulbourn Instruments, Allentown, PA) activity monitoring chambers (25.5×25.5×41 cm), consisting of acrylic walls, a plastic floor, and an open top. Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (locomotor activity). Photobeam resolution was 0.76 cm, with the position of each rat being determined every 100 ms.
Drugs
(−)-Cocaine hydrochloride, (+)-methamphetamine hydrochloride, methylphenidate hydrochloride, and D-amphetamine sulfate were purchased from Sigma (St. Louis, MO). All drugs were dissolved in saline and injected intraperitoneally (IP) at a volume of 5 ml/kg.
Procedure
Experiment 1: One-trial cocaine sensitization
In Experiment 1 (N=24), PD 19 rats in the Cocaine-Activity group were taken to the testing room and injected with cocaine (30 mg/kg, IP) before being placed in the activity chambers. Distance traveled was measured for 30 min. These rats were then returned to the home cage and injected with saline 30 min later. Rats in the Cocaine-Home groups were injected with saline before being placed in the activity chambers and injected with cocaine (30 mg/kg, IP) 30 min after being returned to the home cage. The Acute Control group received saline in both the activity chamber and home cage. In all cases, “home” refers to the normal maternity cage that includes both the dam and littermates.
To determine the occurrence of behavioral sensitization, all rats were given a test day injection of 20 mg/kg cocaine 48 h after drug pretreatment (i.e., on PD 21). After cocaine challenge, rats were immediately placed in activity chambers where distance traveled was recorded. For all experiments, test day performance was measured for 180 min.
Experiment 2: One- and four-trial methamphetamine sensitization
The procedures were similar to those described for Experiment 1, except that various doses of methamphetamine were used. In Experiment 2a (N=72), PD 19 rats were injected with methamphetamine (2, 4, 8, or 12 mg/kg, IP) either before placement in the activity chambers (i.e., the Methamphetamine-Activity groups) or 30 min after being returned to the home cage (i.e., the Methamphetamine-Home groups). Saline was administered at the other time point, so that all rats received two injections on PD 19. Acute control rats were injected with saline in both the activity chamber and home cage. On PD 21, all rats received a challenge injection of 2 mg/kg methamphetamine and distance traveled was measured to determine the occurrence of behavioral sensitization.
In Experiment 2b (N=72), PD 19 rats were injected with methamphetamine (4 mg/kg, IP) either before being placed in the activity chambers (i.e., the Methamphetamine-Activity groups) or 30 min after being returned to the home cage (i.e., the Methamphetamine-Home groups). The 4 mg/kg dose of methamphetamine was based on the results of Experiment 2a. Acute control rats received saline in both the activity chamber and home cage. On PD 21, the pretreatment groups were further subdivided and rats received a challenge injection of 1, 4, or 8 mg/kg (IP) methamphetamine prior to behavioral testing. Although adult rats and mice are typically challenged with a low to moderate dose of methamphetamine (1–4 mg/kg) on the test day (Suzuki et al. 1997; Ago et al. 2007; Clifford et al. 2009), a broader dose range of methamphetamine was used in the present experiment to increase the likelihood of detecting a sensitized response.
Experiment 2c (N=32) was undertaken in order to determine whether preweanling rats would exhibit methamphetamine-induced behavioral sensitization if the pretreatment phase was extended to 4 days. On PD 16–19, rats were injected with methamphetamine (2 or 4 mg/kg, IP) before being placed in the activity chambers (i.e., the Methamphetamine-Activity groups) and were then injected with saline 30 min after being returned to the home cage. No groups were treated with methamphetamine in the home cage; however, Acute Control groups were included. On PD 21, the Methamphetamine-Activity and Acute Control groups were injected with either 1 or 2 mg/kg methamphetamine and locomotor activity was measured to determine the occurrence of behavioral sensitization.
Experiment 3: One-trial methylphenidate sensitization
The procedures were similar to those described for Experiment 2, except that various doses of methylphenidate were used. In Experiment 3a (N=56), PD 19 rats were injected with methylphenidate (5, 10, or 20 mg/kg, IP) either before placement in the activity chambers (i.e., the Methylphenidate-Activity groups) or 30 min after being returned to the home cage (i.e., the Methylphenidate-Home groups). Acute Control rats were injected with saline in both the activity chamber and home cage. On PD 21, all rats received a challenge injection of 2.5 mg/kg methylphenidate immediately prior to placement in the activity chambers.
In Experiment 3b (N=72), PD 19 rats were injected with methylphenidate (10 mg/kg, IP) either before being placed in the activity chambers (i.e., the Methylphenidate-Activity groups) or 30 min after being returned to the home cage (i.e., the Methylphenidate-Home groups). The 10 mg/kg dose of methylphenidate was based on the results of Experiment 3a. Acute Control rats received saline in both the activity chamber and home cage. On PD 21, the pretreatment groups were further subdivided and rats received a challenge injection of 2.5, 5, or 7.5 mg/kg (IP) methylphenidate prior to behavioral testing.
Experiment 4: One-trial amphetamine sensitization
In Experiment 4 (N=42), PD 19 rats in the Amphetamine-Activity group were taken to the test room and injected with amphetamine (5 mg/kg, IP) before being placed in the activity chambers. Because of amphetamine’s long half-life, distance traveled was measured for 60 min. These rats were then returned to the home cage and injected with saline 30 min later. Rats in the Amphetamine-Home group were injected with saline before being placed in the activity chambers and then injected with amphetamine (5 mg/kg, IP) 30 min after being returned to the home cage. The Acute Control group received saline in both the activity chamber and home cage. On PD 21, the pretreatment groups were further subdivided and rats received a challenge injection of 1 or 2 mg/kg (IP) amphetamine prior to behavioral testing.
Statistics
For all experiments, omnibus repeated-measures analyses of variance (ANOVAs) were used for the statistical analysis of distance traveled data. Because experiments typically included distinct subsets of treatment groups, smaller one-and two-way ANOVAs were used when appropriate. When the assumption of sphericity was violated, as determined by Mauchly’s test of sphericity, the Huynh–Feldt epsilon statistic was used to adjust the degrees of freedom (Huynh and Feldt 1976). Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a”. Post hoc analysis of distance traveled data was done using Tukey tests (P<0.05).
Litter effects were controlled through both experimental design and statistical procedures. In most circumstances, no more than one subject per litter was found in a particular group. In situations where this rule was violated (e.g., analyses of the pretreatment day), a single litter mean was calculated from multiple littermates assigned to the same group (Holson and Pearce 1992; Zorrilla 1997). In all cases, litter was used as the unit of analysis for statistical purposes (Zorrilla 1997). With this statistical model each litter, rather than each rat, is treated as an independent observation (i.e., a within analysis using one value/condition/litter). With only one exception, all experiments were provided with eight litters of rat pups (n=8 subjects per group). The exception was Experiment 4, in which seven litters were assigned (n=7 subjects per group). For each experiment, a nearly equal number of male and female preweanling rats were assigned to each group. Preliminary between-subjects analyses indicated that distance traveled data did not differ according to sex, so this variable was not included in subsequent analyses.
Results
Experiment 1: One-trial cocaine sensitization
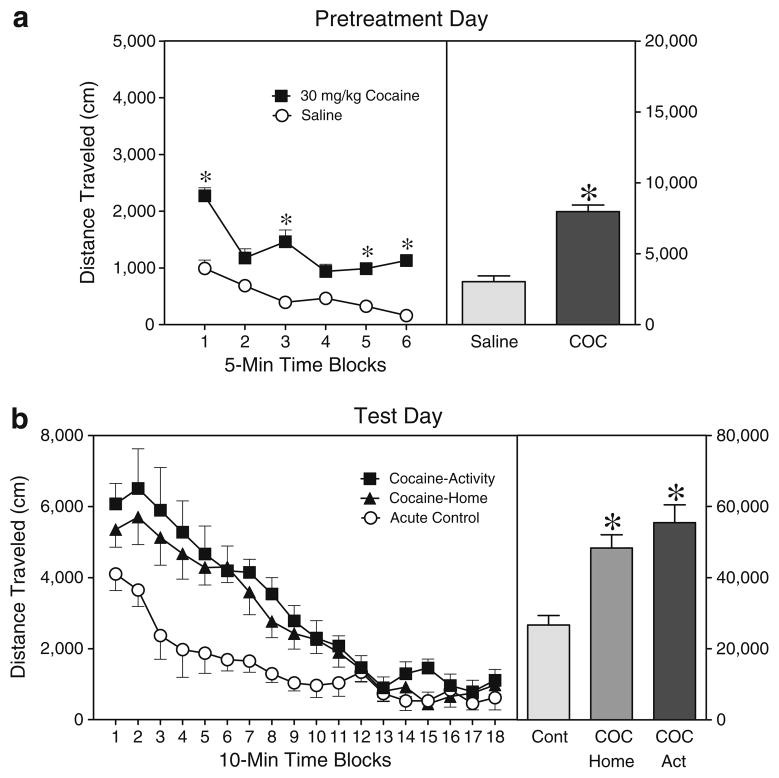
On the pretreatment day (i.e., PD 19), rats injected with 30 mg/kg cocaine had significantly greater distance traveled scores than saline controls (Fig. 1a) [Drug main effect, F1,7= 37.90, P<0.001]. This effect varied according to time block, because cocaine-treated rats exhibited greater locomotor activity than saline-treated rats on time blocks 1, 3, 5, and 6 [aDrug×Time interaction, F5,32=4.44, P<0.01 and Tukey tests]. On the test day (i.e., PD 21), behavioral sensitization was evident because rats in the Cocaine-Activity and Cocaine-Home groups had significantly greater distance traveled scores than rats given an acute injection of cocaine on the test day (Fig. 1b) [Condition main effect, F2,14=19.96, P<0.001 and Tukey tests]. Overall, distance traveled scores declined across the testing session until they stabilized on time block 13 [aTime main effect, F3,21=31.09, P<0.001 and Tukey tests].
Fig. 1.
a. Mean distance traveled scores (±SEM) of rats injected with saline or 30 mg/kg cocaine before a 30-min placement in the activity chambers on the pretreatment day (i.e., PD 19). b. Mean distance traveled scores of rats given a challenge injection of cocaine (20 mg/kg, IP) before the 180-min testing session on PD 21. Rats in the Cocaine-Activity group had been pretreated with cocaine (30 mg/kg, IP) before being placed in the activity chamber on PD 19, while rats in the Cocaine-Home group had been injected with cocaine 30 min after being returned to the home cage. The Acute Control group was injected with saline at both time points. Right panels show mean distance traveled collapsed across the testing sessions. *Significantly different from the control group (P<0.05)
Experiment 2: One- and four-trial methamphetamine sensitization
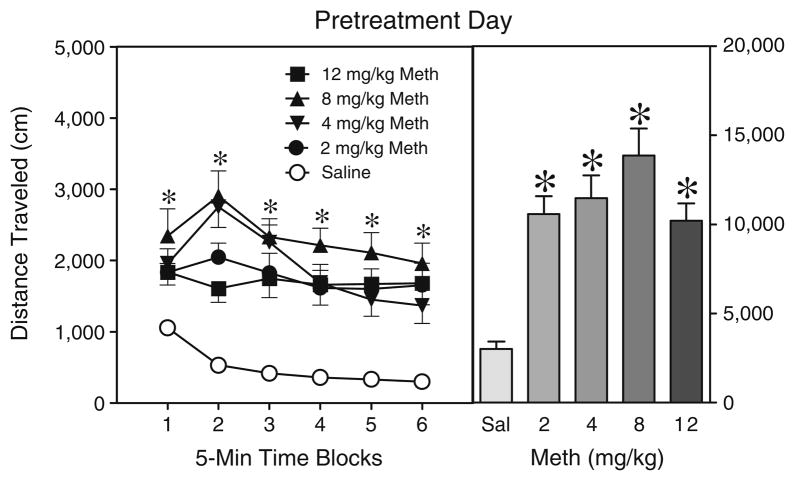
In Experiment 2a, rats injected with methamphetamine, regardless of dose (2, 4, 8, or 12 mg/kg), exhibited greater distance traveled scores on the pretreatment day (PD 19) than saline controls (Fig. 2) [Drug main effect, F4,25=20.86, P< 0.001 and Tukey tests]. The locomotor-enhancing effects of methamphetamine were evident on all time blocks. The distance traveled scores of methamphetamine-treated rats did not differ among themselves except for time block 2, when rats given 4 or 8 mg/kg methamphetamine exhibited more locomotor activity than rats treated with 12 mg/kg methamphetamine [aDrug×Time interaction, F8,55=2.32, P<0.05 and Tukey tests]. On the test day (PD 21), sensitized responding was not evident (Table 1) because rats pretreated and tested with methamphetamine did not exhibit greater locomotor activity than saline-pretreated rats acutely challenged with 2 mg/kg methamphetamine (all P>0.05).
Fig. 2.
Mean distance traveled scores (±SEM) of rats injected with saline or methamphetamine (Meth; 2, 4, 8, or 12 mg/kg, IP) before a 30-min placement in the activity chambers on the pretreatment day (i.e., PD 19). The right panel shows mean distance traveled collapsed across time blocks 1–6. *Significantly different from the saline group (P<0.05)
Table 1.
Mean distance traveled scores of rats given a challenge injection of 2 mg/kg methamphetamine (Meth) before the 180-min testing session on PD 21
| Treatment condition
|
Distance traveled (cm)
|
||
|---|---|---|---|
| Pretreatment day (PD 19) | Test day (PD 21) | Mean | SEM |
| Saline only (acute controls) | 2 mg/kg Meth | 67,127 | 5,683 |
| 2 mg/kg Meth-Home Cage | 2 mg/kg Meth | 67,019 | 5,873 |
| 2 mg/kg Meth-Activity Chamber | 2 mg/kg Meth | 64,333 | 5,524 |
| 4 mg/kg Meth-Home Cage | 2 mg/kg Meth | 59,631 | 3,124 |
| 4 mg/kg Meth-Activity Chamber | 2 mg/kg Meth | 61,169 | 3,827 |
| 8 mg/kg Meth-Home Cage | 2 mg/kg Meth | 63,932 | 6,028 |
| 8 mg/kg Meth-Activity Chamber | 2 mg/kg Meth | 57,367 | 4,383 |
| 12 mg/kg Meth-Home Cage | 2 mg/kg Meth | 61,802 | 6,807 |
| 12 mg/kg Meth-Activity Chamber | 2 mg/kg Meth | 53,231 | 5,299 |
Rats had been pretreated with saline or various doses of methamphetamine in either the home cage or activity chamber on PD 19
In Experiment 2b, rats injected with 4 mg/kg methamphetamine (M=10,175 cm; SEM=775) had greater distance traveled scores on the pretreatment day (PD 19) than rats injected with saline (M=2,867 cm; SEM=283) [Drug main effect, F1,7 =104.98, P< 0.001 and Tukey tests]. Behavioral sensitization was not evident when methamphetamine- and saline-pretreated rats were injected with various doses of methamphetamine on the test day (PD 21). Specifically, rats pretreated with 4 mg/kg methamphetamine and challenged with 1, 4, or 8 mg/kg methamphetamine did not exhibit significantly greater locomotor activity than their acutely challenged controls (Table 2) (all P>0.05).
Table 2.
Mean distance traveled scores of rats given a challenge injection of 1, 4, or 8 mg/kg methamphetamine (Meth) before the 180-min testing session on PD 21
| Treatment condition
|
Distance traveled (cm)
|
||
|---|---|---|---|
| Pretreatment day (PD 19) | Test day (PD 21) | Mean | SEM |
| Saline only (acute controls) | 1 mg/kg Meth | 44,387 | 6,343 |
| 4 mg/kg Meth-Home Cage | 1 mg/kg Meth | 38,767 | 6,873 |
| 4 mg/kg Meth-Activity Chamber | 1 mg/kg Meth | 45,110 | 4,603 |
| Saline only (acute controls) | 4 mg/kg Meth | 50,627 | 3,612 |
| 4 mg/kg Meth-Home Cage | 4 mg/kg Meth | 59,719 | 4,329 |
| 4 mg/kg Meth-Activity Chamber | 4 mg/kg Meth | 43,168 | 4,626 |
| Saline only (acute controls) | 8 mg/kg Meth | 33,596 | 3,186 |
| 4 mg/kg Meth-Home Cage | 8 mg/kg Meth | 38,220 | 4,586 |
| 4 mg/kg Meth-Activity Chamber | 8 mg/kg Meth | 31,496 | 2,561 |
Rats had been pretreated with saline or 4 mg/kg methamphetamine in either the home cage or activity chamber on PD 19
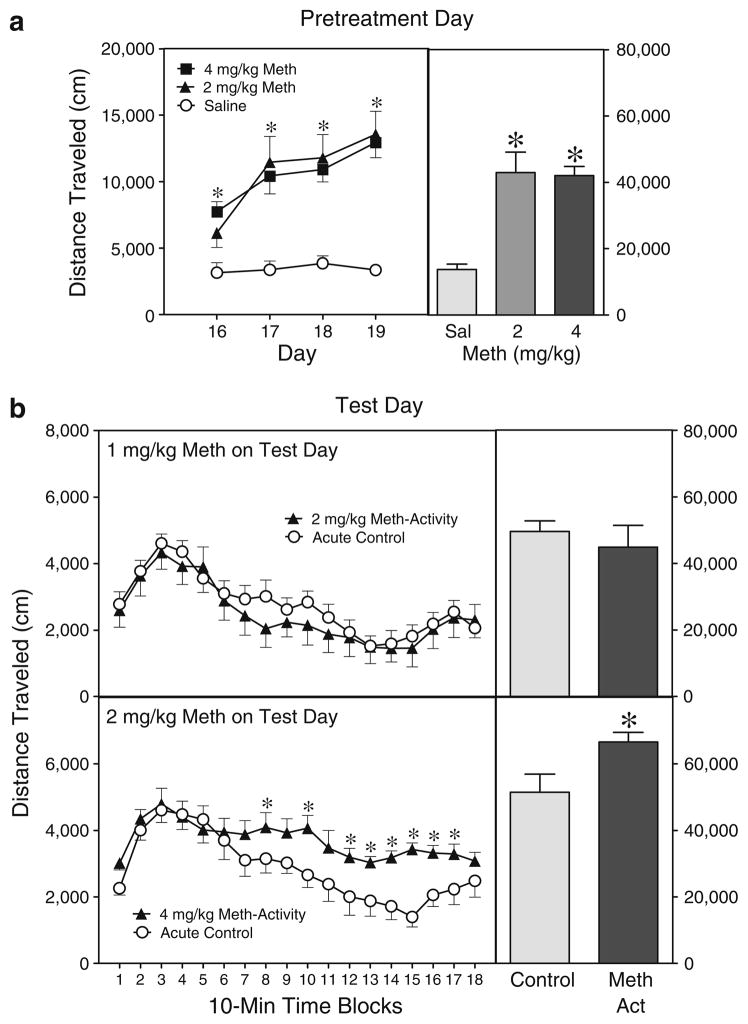
In Experiment 2c, the two methamphetamine (2 or 4 mg/kg) groups had greater distance traveled scores than saline controls when collapsed across the pretreatment phase (i.e., PD 16–19) (Fig. 3a) [Drug main effect, F2,14=19.08, P<0.001 and Tukey tests]. Differences between the methamphetamine and saline groups were statistically significant on all four pretreatment days [Drug×Day interaction, F6,42=23.28, P< 0.001 and Tukey tests]. Interestingly, repeated treatment with methamphetamine (2 or 4 mg/kg) caused progressively more locomotor activity from PD 16 to PD 19, while the saline controls evidenced a stable level of performance. On the test day (PD 21), rats pretreated with 2 mg/kg methamphetamine and tested with 1 mg/kg methamphetamine did not exhibit a sensitized locomotor response (upper graph, Fig. 3b) (all P> 0.05). Behavioral sensitization was evident if rats were pretreated and tested with higher doses of methamphetamine (lower graph, Fig. 3b). Specifically, rats pretreated with 4 mg/ kg methamphetamine on PD 16–19 and challenged with 2 mg/kg methamphetamine on PD 21 showed more test day locomotor activity than rats acutely challenged with 2 mg/kg methamphetamine [Condition main effect, F1,7=11.22, P< 0.05]. Differences between the Meth-Activity and Acute Control groups were statistically significant on time blocks 8, 10, and 12–17 [aCondition×Time interaction, F13,89=3.33, P<0.001 and Tukey tests].
Fig. 3.
a. Mean distance traveled scores (±SEM) of rats injected with saline or methamphetamine (Meth; 2 or 4 mg/kg, IP) before a 30-min placement in the activity chambers during the pretreatment phase (i.e., PD 16–19). b. Mean distance traveled scores of rats given a challenge injection of 1 mg/kg (upper graph) or 2 mg/kg (lower graph) Meth before the 180-min testing session on PD 21. Rats in the Meth-Activity groups had been pretreated with methamphetamine (2 or 4 mg/kg, IP) in the activity chambers on PD 16–19, while rats in the Acute Control groups were injected with saline. The right panels show mean distance traveled collapsed across the pretreatment phase and the test day. *Significantly different from the control group (P<0.05)
Experiment 3: One-trial methylphenidate sensitization
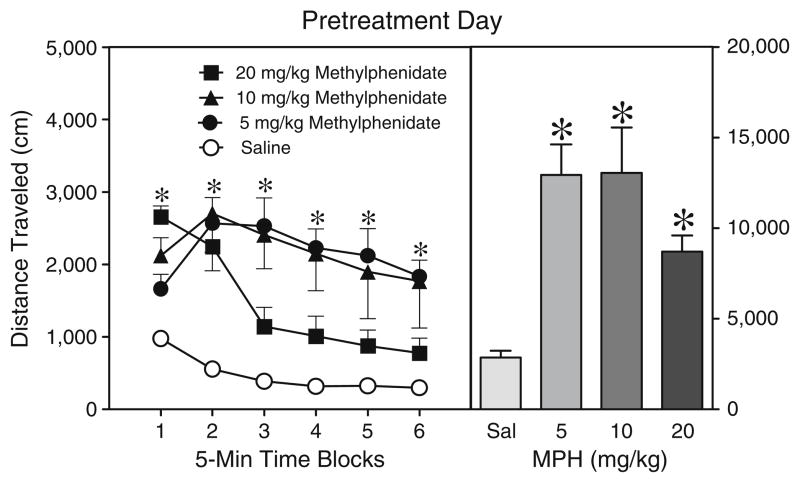
In Experiment 3a, rats injected with the two lower doses of methylphenidate (5 or 10 mg/kg) had greater distance traveled scores on the pretreatment day than saline controls (Fig. 4), with the 20 mg/kg methylphenidate group being intermediate between, and significantly different from, the other groups [Drug main effect, F3,21=10.76, P<0.001 and Tukey tests]. This effect varied across the pretreatment day, because all of the methylphenidate groups differed from the saline controls on time blocks 1 and 2; however, on time blocks 3–6 the distance traveled scores of rats given 20 mg/kg methylphenidate declined to a level that was not significantly different from the saline controls [aDrug×Time interaction, F4,26= 4.24, P<0.01 and Tukey tests]. Although all doses of methylphenidate increased locomotor activity on the pre-treatment day, behavioral sensitization was not apparent on the test day (Table 3). Specifically, the distance traveled scores of rats pretreated and tested with methylphenidate did not differ from acutely challenged controls (all P>0.05).
Fig. 4.
Mean distance traveled scores (±SEM) of rats injected with saline or methylphenidate (MPH; 5, 10, or 20 mg/kg, IP) before a 30-min placement in the activity chambers on the pretreatment day (i.e., PD 19). The right panel shows mean distance traveled collapsed across time blocks 1–6. *Significantly different from the saline group (P<0.05)
Table 3.
Mean distance traveled scores of rats given a challenge injection of 2.5 mg/kg methylphenidate (MPH) before the 180-min testing session on PD 21
| Treatment condition
|
Distance traveled (cm)
|
||
|---|---|---|---|
| Pretreatment day (PD 19) | Test day (PD 21) | Mean | SEM |
| Saline only (acute controls) | 2.5 mg/kg MPH | 25,621 | 3,572 |
| 5 mg/kg MPH-Home Cage | 2.5 mg/kg MPH | 18,026 | 2,417 |
| 5 mg/kg MPH-Activity Chamber | 2.5 mg/kg MPH | 28,062 | 4,041 |
| 10 mg/kg MPH-Home Cage | 2.5 mg/kg MPH | 29,415 | 3,189 |
| 10 mg/kg MPH-Activity Chamber | 2.5 mg/kg MPH | 24,940 | 4,706 |
| 20 mg/kg MPH-Home Cage | 2.5 mg/kg MPH | 18,463 | 3,290 |
| 20 mg/kg MPH-Activity Chamber | 2.5 mg/kg MPH | 28,346 | 4,883 |
Rats had been pretreated with saline or various doses of methylphenidate in either the home cage or activity chamber on PD 19
In Experiment 3b, the pretreatment day distance traveled scores of PD 19 rats injected with 10 mg/kg methylphenidate (M=9,295 cm; SEM=948) were significantly greater than rats injected with saline (M=2,762 cm; SEM=216) [Drug main effect, F1,7=40.78, P<0.001]. Even so, behavioral sensitization was not evident when the two pretreatment groups were challenged with 2.5, 5, or 7.5 mg/kg methylphenidate on PD 21 (Table 4) (all P>0.05).
Table 4.
Mean distance traveled scores of rats given a challenge injection of 2.5, 5, or 7.5 mg/kg methylphenidate (MPH) before the 180-min testing session on PD 21
| Treatment condition
|
Distance traveled (cm)
|
||
|---|---|---|---|
| Pretreatment day (PD 19) | Test Day (PD 21) | Mean | SEM |
| Saline only (acute controls) | 2.5 mg/kg MPH | 31,639 | 3,608 |
| 10 mg/kg MPH-Home Cage | 2.5 mg/kg MPH | 25,637 | 3,520 |
| 10 mg/kg MPH-Activity Chamber | 2.5 mg/kg MPH | 31,654 | 2,833 |
| Saline only (acute controls) | 5 mg/kg MPH | 46,636 | 5,034 |
| 10 mg/kg MPH-Home Cage | 5 mg/kg MPH | 43,801 | 2,431 |
| 10 mg/kg MPH-Activity Chamber | 5 mg/kg MPH | 42,965 | 4,471 |
| Saline only (acute controls) | 7.5 mg/kg MPH | 60,010 | 5,576 |
| 10 mg/kg MPH-Home Cage | 7.5 mg/kg MPH | 59,987 | 6,803 |
| 10 mg/kg MPH-Activity Chamber | 7.5 mg/kg MPH | 50,184 | 4,859 |
Rats had been pretreated with saline or 10 mg/kg methylphenidate in either the home cage or activity chamber on PD 19
Experiment 4: One-trial amphetamine sensitization
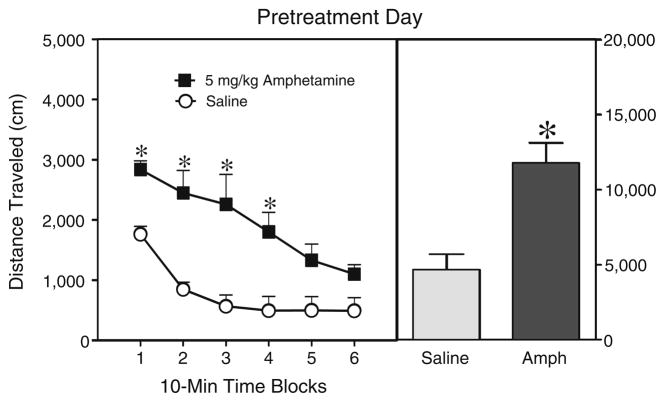
In Experiment 4, rats injected with 5 mg/kg amphetamine exhibited significantly greater distance traveled scores than their saline controls across the first 40 min (i.e., time blocks 1–4) of the pretreatment phase (Fig. 5) [Drug main effect, F1,6=187.95, P<0.001; Drug×Time interaction, F5,30= 2.88, P<0.05 and Tukey tests]. The locomotor activity of the amphetamine-pretreated rats was greatest on time block 1 and declined progressively across the session. On the test day (PD 21), rats pretreated with 5 mg/kg amphetamine in either the home cage or activity chambers did not have greater distance traveled scores than saline-pretreated rats given an acute challenge injection of 1 mg/kg amphetamine (Table 5). Locomotor sensitization was also not evident if rats were challenged with 2 mg/kg amphetamine (Table 5) (all P>0.05).
Fig. 5.
Mean distance traveled scores (±SEM) of rats injected with saline or 5 mg/kg amphetamine (AMPH) before a 60-min placement in the activity chambers on the pretreatment day (i.e., PD 19). The right panel shows mean distance traveled collapsed across time blocks 1–6. *Significantly different from the saline group (P<0.05)
Table 5.
Mean distance traveled scores of rats given a challenge injection of 1 or 2 mg/kg amphetamine (Amph) before the 180-min testing session on PD 21
| Treatment condition
|
Distance traveled (cm)
|
||
|---|---|---|---|
| Pretreatment day (PD 19) | Test day (PD 21) | Mean | SEM |
| Saline only (acute controls) | 1 mg/kg Amph | 31,049 | 5,811 |
| 5 mg/kg Amph-Home Cage | 1 mg/kg Amph | 23,102 | 5,259 |
| 5 mg/kg Amph-Activity Chamber | 1 mg/kg Amph | 35,812 | 5,465 |
| Saline only (acute controls) | 2 mg/kg Amph | 57,838 | 4,144 |
| 5 mg/kg Amph-Home Cage | 2 mg/kg Amph | 59,174 | 3,464 |
| 5 mg/kg Amph-Activity Chamber | 2 mg/kg Amph | 54,158 | 3,551 |
Rats had been pretreated with saline or 5 mg/kg amphetamine in either the home cage or activity chamber on PD 19
Discussion
It was originally hypothesized that all four psychostimulant drugs tested (i.e., cocaine, methamphetamine, methylphenidate, and amphetamine) would induce one-trial behavioral sensitization in preweanling rats. As predicted, robust locomotor sensitization was evident when cocaine-pretreated rats were given a challenge injection of cocaine on PD 21. Sensitized responding was observed regardless of whether cocaine pretreatment occurred in the test chamber (context-specific) or home cage (context-independent). This pattern of cocaine-induced effects has been observed multiple times in preweanling rats (McDougall et al. 2009b; Herbert et al. 2010). In contrast, various dose combinations of methamphetamine, methylphenidate, and amphetamine were unable to induce one-trial behavioral sensitization on PD 21. Although these data were presented collapsed across the 3-h testing session, repeated measures analyses showed that at no time during the testing session did rats pretreated and tested with methamphetamine, methylphenidate, and amphetamine respond differently than rats acutely challenged with the same psychostimulant drug. Statistical analysis of repetitive motor movements (i.e., a measure of stereotypy) indicated that sensitization was not manifested as an increase in stereotypy on the test day (data not shown). Therefore, the present results suggest that only cocaine, but not methamphetamine, methylphenidate, or amphetamine, is capable of inducing one-trial locomotor sensitization in preweanling rats. Of course, this conclusion is based on the specific treatment conditions utilized in the present study; moreover, it is uncertain whether the present results are characteristic of early ontogeny as a whole or are only representative of a restricted developmental period (i.e., the late preweanling period). It is clear, however, that a different pattern of behavioral effects is observed in adulthood, because amphetamine will produce one-trial context-specific sensitization in adult rats and mice (Drew and Glick 1989; Battisti et al. 2000).
As expected, all psychostimulants enhanced the locomotor activity of preweanling rats on the pretreatment day (as well as on the test day). Methamphetamine, regardless of dose (2–12 mg/kg), produced substantial locomotor activity on the single pretreatment day; whereas, methylphenidate stimulated greater locomotor activity when administered at the two lower doses (5 and 10 mg/kg) of the drug. Although not quantified, it is likely that the higher dose of methylphenidate (20 mg/kg) caused a pronounced stereotypic response that partially masked drug-induced locomotor activity (McDougall et al. 1999). Rats also exhibited elevated levels of locomotor activity when treated with 30 mg/kg cocaine and 5 mg/kg amphetamine, but dose–response relationships were not assessed using these compounds. Based on these data, it is clear that (a) all compounds significantly increased locomotor activity on the pretreatment day and (b) the subsequent lack of behavioral sensitization cannot be a consequence of using an ineffective dose of psychostimulant.
That being said, at least four potential explanations could account for the inability of methamphetamine, methylphenidate, and amphetamine to induce one-trial behavioral sensitization on PD 21: (1) methamphetamine, methylphenidate, and amphetamine may be unable to support any form of sensitization in preweanling rats; (2) the neural mechanisms underlying cocaine-induced behavioral sensitization may differ from the mechanisms mediating methamphetamine-, methylphenidate-, and amphetamine-induced sensitization; (3) among the various psychostimulants used, cocaine may be uniquely prepared to support the development of environment–drug (CS–US) associations; or (4) drug affinity and/or pharmacokinetic factors may make cocaine uniquely suited to induce one-trial behavioral sensitization. The first possibility (i.e., that methamphetamine, methylphenidate, and amphetamine are unable to induce behavioral sensitization in preweanling rats) can be discounted, because sensitized responding is evident if preweanling rats are given multiple pretreatment administrations of amphetamine (McDougall et al. 1994; Duke et al. 1997) or methylphenidate (McDougall et al. 1999). In the present study, we also showed that repeated treatment with 4 mg/kg methamphetamine on PD 16–19 would induce context-specific locomotor sensitization if rats were challenged with 2 mg/kg methamphetamine on PD 21. The somewhat modest sensitized response occurring on PD 21 is typical of behavioral sensitization exhibited during the preweanling period (Wood et al. 1998; Zavala et al. 2000).
A second explanation of our findings is that only the neural circuitry mediating cocaine sensitization is capable of becoming sensitized after a single pretreatment trial. This issue has not been addressed in the literature, although there is accumulating evidence that the neural mechanisms mediating amphetamine- and cocaine-induced behavioral sensitization are not identical (for reviews, see White et al. 1998; Vanderschuren and Kalivas 2000). For example, D1 receptor stimulation is unnecessary for the induction of cocaine sensitization (Mattingly et al. 1994; White et al. 1998), but is required for methamphetamine, methylphenidate, and amphetamine sensitization (Vezina and Stewart 1989; Kuribara 1995; Meririnne et al. 2001). In a similar vein, cocaine- but not amphetamine-induced behavioral sensitization is dependent on prefrontal glutamatergic transmission (Li and Wolf 1997; Pierce et al. 1998). Therefore, it is possible that the lack of methamphetamine-, methylphenidate-, and amphetamine-induced one-trial sensitization on PD 21 is due to the immaturity of underlying neural mechanisms. This phenomenon would have to be unique to young animals, however, because adult rats and mice exhibit one-trial sensitization to both amphetamine (Drew and Glick 1989; Battisti et al. 2000) and cocaine (Weiss et al. 1989; McDougall et al. 2009b).
A third explanation is that environment–drug associations may form more readily with cocaine than other psychostimulants. In preweanling rats, unlike adults, one-trial sensitization is evident if cocaine pretreatment occurs in either the home cage or a novel chamber distinct from the testing environment (present study; McDougall et al. 2009b; Herbert et al. 2010). These findings indicate that either associative processes do not modulate one-trial behavioral sensitization during the preweanling period or environment–drug (CS–US) pairings are processed differently in preweanling and adult rats. Consistent with the latter suggestion, adult rats treat multiple CSs as discrete and often competitive events (Spear and McKenzie 1994), whereas preweanling rats treat two distinguishable stimuli as if they were equivalent (i.e., components of a single event) as long as both stimuli were paired with the same US (Spear et al. 1988; Molina et al. 1991). Thus, preweanling rats may show context-independent behavioral sensitization because the different environmental contexts (i.e., the home cage and the activity chamber), although discriminable, are treated as components of a single CS (i.e., administering cocaine in the home cage on the pretreatment day has the same associative outcome as administering cocaine in the activity chamber). For this explanation to account for the present results, cocaine would have to be more “conditionable” than the other psychostimulants. In other words, it would have to be assumed that a single exposure to cocaine causes an environment–drug association to form (i.e., allowing one-trial behavioral sensitization), whereas multiple exposures to methamphetamine, methylphenidate, or amphetamine are required before the environmental context becomes associated with the drug.
Lastly, drug affinity and/or pharmacokinetic factors may explain why cocaine was the only psychostimulant capable of inducing one-trial behavioral sensitization in preweanling rats. More specifically, cocaine has approximately equal affinity at the dopamine, serotonin, and norepinephrine transporters, whereas the other three psychostimulants have relatively lower affinities for the serotonin transporter (for a review, see Howell and Kimmel 2008). Although cocaine-induced behavioral sensitization is often theorized to be primarily initiated through dopaminergic mechanisms (Robinson and Becker 1986; Pierce and Kalivas 1997), alterations in serotonergic functioning appear to modulate important components of the sensitization process (Szumlinski et al. 2004; Ago et al. 2006). In terms of the present study, it is possible that co-activation of dopaminergic and serotonergic pathways is necessary for the eventual expression of one-trial behavioral sensitization in preweanling rats. Pharmacokinetic factors may also be relevant, because cocaine penetrates the brain quickly and has a shorter half-life than the other psychostimulants tested (Lal and Feldmüller 1975; Brien et al. 1978; Benuck et al. 1987; Gerasimov et al. 2000). For example, the brain half-life of intraperitoneally administered cocaine is approximately 15–30 min (Benuck et al. 1987; Lau et al. 1991), while the half-life of amphetamine is on the order of 150 min (Lal and Feldmüller 1975). To compensate for amphetamine’s relatively longer half-life, a 60-min pretreatment session was used in the present study; even so, amphetamine did not induce one-trial behavioral sensitization in preweanling rats. Route of administration may also have been a factor influencing the results because preweanling rats exhibit greater cocaine-induced locomotor activity after subcutaneous, rather than intraperitoneal, injection (Smith and Morrell 2008). Thus, the possibility exists that methamphetamine, methylphenidate, and/or amphetamine would be able to induce one-trial behavioral sensitization if the drugs were administered subcutaneously. Of course, cocaine was injected intraperitoneally in the present study and strong one-trial behavioral sensitization was evident.
In all experiments, test day performance was assessed for an extended 3-h period in order to better describe the pattern of drug-induced responding. Smith and Morrell (2008) reported that preweanling rats repeatedly treated with cocaine initially exhibit a sensitization-like effect for 30 min followed by a tolerance-like response for the remainder of the testing session. We did not observe a tolerance-like effect after either a 2-day regimen of cocaine or a 5-day regimen of methamphetamine. Even so, various procedural differences do not allow for direct comparison between the two studies. First, Smith and Morrell (2008) did not see a tolerance-like effect until the third consecutive day of cocaine treatment (we only administered cocaine for 2 days). Second, tolerance was detected when the performance of the same group of cocaine-treated rats was compared across multiple days (i.e., their paradigm did not include a separate group of rats acutely treated with cocaine on the test day; Smith and Morrell 2008). Because our methamphetamine pretreatment sessions lasted only 30 min (instead of 180 min), we were unable to determine whether repeated methamphetamine treatment produced a behavioral profile that included a tolerance-like component. Certainly, tolerance was not evident when the Acute Control groups were compared to rats repeatedly treated with methamphetamine or cocaine.
As mentioned before, rats pretreated and tested with methamphetamine on PD 19 and PD 21 did not exhibit a sensitized response when compared to saline-pretreated rats challenged with methamphetamine on the test day. In contrast, sensitization-like responding (i.e., a progressive increase in methamphetamine-induced locomotor activity) was observed on the first 2 pretreatment days of Experiment 2c (i.e., PD 16 and PD 17). This day-dependent increase in locomotor activity may have been due to maturational changes in motoric ability or the transitory emergence of one-trial behavioral sensitization during a restricted developmental period. If the latter explanation is correct, then rats are capable of showing one-trial methamphetamine-induced behavioral sensitization both before and after the late preweanling period. Additional research will be required to determine the accuracy of this explanation because the design of Experiment 2c, which lacked an acutely challenged methamphetamine group on PD 17, made it impossible to disentangle potential drug effects from ontogenetic changes in motoric ability.
In summary, the present study was undertaken to determine whether psychostimulants other than cocaine would be capable of inducing one-trial behavioral sensitization in preweanling rats. Using the same methodology that produces robust cocaine-induced sensitization, we found that a single pretreatment exposure to methamphetamine, methylphenidate, or amphetamine did not produce a sensitized response when rats were challenged with the same psychostimulant on the test day. The reason for this effect is uncertain, but it is possible that: (a) the neural circuitry mediating behavioral sensitization differs depending on psychostimulant, (b) cocaine is more readily associated with environmental contexts than other psychostimulants, or (c) differences in affinity (e.g., relative activity at the serotonin transporter) and/or pharmacokinetics (e.g., a short half-life) may allow cocaine to induce one-trial behavioral sensitization in preweanling rats. It is also not known whether the pattern of results reported here (i.e., the apparent absence of one-trial methamphetamine sensitization) is restricted to the late preweanling period or is a more general phenomenon characteristic of early ontogeny.
Acknowledgments
This research was supported by NIDA research grant DA027985 (SAM) and NIGMS training grants GM083883 (KAC) and DA025319 (VYG).
References
- Ago Y, Nakamura S, Hayashi A, Itoh S, Baba A, Matsuda T. Effects of osemozotan, ritanserin and azasetron on cocaine-induced behavioral sensitization in mice. Pharmacol Biochem Behav. 2006;85:198–205. doi: 10.1016/j.pbb.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Kajita N, Uda M, Hashimoto H, Baba A, Matsuda T. Ritanserin reverses repeated methamphetamine-induced behavioral and neurochemical sensitization in mice. Synapse. 2007;61:757–763. doi: 10.1002/syn.20421. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Chang CH, Uretsky NJ, Wallace LJ. Sensitization of stereotyped behavior to amphetamine is context and response dependent. Pharmacol Biochem Behav. 1999a;63:263–269. doi: 10.1016/s0091-3057(98)00259-7. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Uretsky NJ, Wallace LJ. Sensitization of apomorphine-induced stereotyped behavior in mice is context dependent. Psychopharmacology (Berl) 1999b;146:42–48. doi: 10.1007/s002130051086. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Uretsky NJ, Wallace LJ. Importance of environmental context in the development of amphetamine- or apomorphine-induced stereotyped behavior after single and multiple doses. Pharmacol Biochem Behav. 2000;66:671–677. doi: 10.1016/s0091-3057(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Benuck M, Lajtha A, Reith ME. Pharmacokinetics of systemically administered cocaine and locomotor stimulation in mice. J Pharmacol Exp Ther. 1987;243:144–149. [PubMed] [Google Scholar]
- Brien JF, Kitney JC, Peachey JE, Rogers BJ. Methamphetamine-induced behavioural effects and brain concentrations of methamphetamine and its metabolite amphetamine in mice. Res Commun Chem Pathol Pharmacol. 1978;22:313–328. [PubMed] [Google Scholar]
- Browman KE, Badiani A, Robinson TE. Modulatory effect of environmental stimuli on the susceptibility to amphetamine sensitization: a dose–effect study in rats. J Pharmacol Exp Ther. 1998;287:1007–1014. [PubMed] [Google Scholar]
- Carey RJ, Gui J. Cocaine conditioning and cocaine sensitization: what is the relationship? Behav Brain Res. 1998;92:67–76. doi: 10.1016/s0166-4328(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Clifford PS, Hart N, Thompson J, Buckman S, Wellman PJ, Bratton GR, Nation JR. Prenatal lead exposure enhances methamphetamine sensitization in rats. Pharmacol Biochem Behav. 2009;93:165–169. doi: 10.1016/j.pbb.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew KL, Glick SD. Environment-dependent sensitization to amphetamine-induced circling behavior. Pharmacol Biochem Behav. 1989;31:705–708. doi: 10.1016/0091-3057(88)90251-1. [DOI] [PubMed] [Google Scholar]
- Duke MA, O’Neal J, McDougall SA. Ontogeny of dopamine agonist-induced sensitization: role of NMDA receptors. Psychopharmacology (Berl) 1997;129:153–160. doi: 10.1007/s002130050175. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Kazahaya Y, Nakashima M, Sato M, Otsuki S. Behavioral sensitization in the rat: an ontogenic study. Psychopharmacology (Berl) 1987;91:316–319. doi: 10.1007/BF00518183. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: a micro-dialysis and locomotor activity study. J Pharmacol Exp Ther. 2000;295:51–57. [PubMed] [Google Scholar]
- Herbert MS, Der-Ghazarian T, Palmer AG, McDougall SA. One-trial cocaine-induced behavioral sensitization in preweanling rats: role of contextual stimuli. Exp Clin Psychopharmacol. 2010;18:284–295. doi: 10.1037/a0019142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. J Educ Stat. 1976;1:69–82. [Google Scholar]
- Jackson HC, Nutt DJ. A single preexposure produces sensitization to the locomotor effects of cocaine in mice. Pharmacol Biochem Behav. 1993;45:733–735. doi: 10.1016/0091-3057(93)90533-y. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. NIH Publication No. 05–5726. National Institute on Drug Abuse; Bethesda: 2005. Monitoring the future national results on adolescent drug use: overview of key findings, 2004. [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, DuMars LA, Skinner C. Behavioral and neurochemical effects of acute and daily cocaine administration in rats. J Pharmacol Exp Ther. 1988;245:485–492. [PubMed] [Google Scholar]
- Kolta MG, Scalzo FM, Ali SF, Holson RR. Ontogeny of the enhanced behavioral response to amphetamine in amphetamine-pretreated rats. Psychopharmacology (Berl) 1990;100:377–182. doi: 10.1007/BF02244610. [DOI] [PubMed] [Google Scholar]
- Kuribara H. Dopamine D1 receptor antagonist SCH 23390 retards methamphetamine sensitization in both combined administration and early posttreatment schedules in mice. Pharmacol Biochem Behav. 1995;52:759–763. doi: 10.1016/0091-3057(95)00173-t. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Uchihashi Y. Effects of dopamine antagonism on methamphetamine sensitization: evaluation by ambulatory activity in mice. Pharmacol Biochem Behav. 1994;47:101–106. doi: 10.1016/0091-3057(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Lal S, Feldmüller F. Effect of amphetamine and apomorphine on brain monoamines and behaviour in the immature and young adult rat. Arch Int Pharmacodyn Ther. 1975;218:239–251. [PubMed] [Google Scholar]
- Lau CE, Imam A, Ma F, Falk JL. Acute effects of cocaine on spontaneous and discriminative motor functions: relation to route of administration and pharmacokinetics. J Pharmacol Exp Ther. 1991;257:444–456. [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Leith NJ, Kuczenski R. Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology (Berl) 1982;76:310–315. doi: 10.1007/BF00449116. [DOI] [PubMed] [Google Scholar]
- Li Y, Wolf ME. Ibotenic acid lesions of prefrontal cortex do not prevent expression of behavioral sensitization to amphetamine. Behav Brain Res. 1997;84:285–289. doi: 10.1016/s0166-4328(96)00158-1. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Hart TC, Lim K, Perkins C. Selective antagonism of dopamine D1 and D2 receptors does not block the development of behavioral sensitization to cocaine. Psychopharmacology (Berl) 1994;114:239–242. doi: 10.1007/BF02244843. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Duke MA, Bolanos CA, Crawford CA. Ontogeny of behavioral sensitization in the rat: effects of direct and indirect dopamine agonists. Psychopharmacology (Berl) 1994;116:483–490. doi: 10.1007/BF02247482. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharmacol. 1999;7:208–218. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Reichel CM, Cyr MC, Karper PE, Nazarian A, Crawford CA. Importance of D1 receptors for associative components of amphetamine-induced behavioral sensitization and conditioned activity: a study using D1 receptor knockout mice. Psychopharmacology (Berl) 2005;183:20–30. doi: 10.1007/s00213-005-0146-9. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Baella SA, Stuebner NM, Halladay LM, Crawford CA. Cocaine-induced behavioral sensitization in preweanling and adult rats: effects of a single drug–environment pairing. Psychopharmacology (Berl) 2007;193:323–332. doi: 10.1007/s00213-007-0788-x. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Charntikov S, Cortez AM, Amodeo DA, Martinez CE, Crawford CA. Persistence of one-trial cocaine-induced behavioral sensitization in young rats: regional differences in Fos immunoreactivity. Psychopharmacology (Berl) 2009a;203:617–628. doi: 10.1007/s00213-008-1407-1. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Cortez AM, Palmer AG, Herbert MS, Martinez CE, Charntikov S, Amodeo DA. Importance of environmental context for one- and three-trial cocaine-induced behavioral sensitization. Psychopharmacology (Berl) 2009b;206:377–388. doi: 10.1007/s00213-009-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meririnne E, Kankaanpää A, Seppälä T. Rewarding properties of methylphenidate: sensitization by prior exposure to the drug and effects of dopamine D1- and D2-receptor antagonists. J Pharmacol Exp Ther. 2001;298:539–550. [PubMed] [Google Scholar]
- Molina JC, Hoffmann H, Serwatka J, Spear NE. Establishing intermodal equivalence in preweanling and adult rats. J Exp Psychol Anim Behav Process. 1991;17:433–447. doi: 10.1037//0097-7403.17.4.433. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academy Press; Washington: 2003. [PubMed] [Google Scholar]
- Partridge B, Schenk S. Context-independent sensitization to the locomotor-activating effects of cocaine. Pharmacol Biochem Behav. 1999;63:543–548. doi: 10.1016/s0091-3057(99)00013-1. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology (Berl) 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert A, Post R, Weiss SR. Conditioning as a critical determinant of sensitization induced by psychomotor stimulants. NIDA Research Monographs. 1990;97:208–241. [PubMed] [Google Scholar]
- Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neuroscience. 1998;82:1103–1114. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR. Drug–environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sci. 1981;28:755–760. doi: 10.1016/0024-3205(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;571:330–337. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Individual differences in responsiveness to single and repeated amphetamine administration: behavioral characteristics and neurochemical correlates. J Pharm Exp Ther. 1987;242:917–926. [PubMed] [Google Scholar]
- Smith KS, Morrell JI. Behavioral responses during the initial exposures to a low dose of cocaine in late preweanling and adult rats. Neurotoxicol Teratol. 2008;30:202–212. doi: 10.1016/j.ntt.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Spear NE, McKenzie DL. Intersensory integration in the infant rat. In: Lewkowicz DJ, Terrace H, editors. The development of intersensory perception: comparative perspectives. Erlbaum; Hillsdale: 1994. pp. 133–161. [Google Scholar]
- Spear NE, Kraemer PJ, Molina JC, Smoller DE. Developmental change in learning and memory: infantile disposition for unitization. In: Delacour J, Levy JCS, editors. Systems with learning and memory abilities. Elsevier; New York: 1988. pp. 27–52. [Google Scholar]
- Suzuki H, Shishido T, Watanabe Y, Abe H, Shiragata M, Honda K, Horikoshi R, Niwa S. Changes of behavior and mono-amine metabolites in the rat brain after repeated methamphetamine administration: effects of duration of repeated administration. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:359–369. doi: 10.1016/s0278-5846(97)00006-7. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Frys KA, Kalivas PW. Dissociable roles for the dorsal and median raphé in the facilitatory effect of 5-HT1A receptor stimulation upon cocaine-induced locomotion and sensitization. Neuropsychopharmacology. 2004;29:1675–1687. doi: 10.1038/sj.npp.1300473. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: lack of conditioned effects. Brain Res. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. [DOI] [PubMed] [Google Scholar]
- Weiss SRB, Post RM, Pert A, Woodward R, Murman D. Context-dependent cocaine sensitization: differential effect of haloperidol on development versus expression. Pharmacol Biochem Behav. 1989;34:655–661. [PubMed] [Google Scholar]
- White FJ, Joshi A, Koeltzow TE, Hu X-T. Dopamine receptor antagonists fail to prevent induction of cocaine sensitization. Neuropsychopharmacology. 1998;18:26–40. doi: 10.1016/S0893-133X(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RD, Tirelli E, Snyder KJ, Heyser CJ, LaRocca TM, Spear LP. Evidence for behavioral sensitization to cocaine in preweanling rat pups. Psychopharmacology (Berl) 1998;138:114–123. doi: 10.1007/s002130050653. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Chronic administration of methylphenidate produces neurophysiological and behavioral sensitization. Brain Res. 2007;1145:66–80. doi: 10.1016/j.brainres.2007.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Nazarian A, Crawford CA, McDougall SA. Cocaine-induced behavioral sensitization in the young rat. Psychopharmacology (Berl) 2000;151:291–298. doi: 10.1007/s002130000377. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]