Abstract
Background
The lack of standardized pre-hospital treatment is a weak link in the care of acute stroke patients.
Methods
Selective review of the literature on acute stroke, with consideration of current guidelines in Germany and other countries (DGN, ESO, AHA/ASA).
Results
The mandatory, immediate transfer of acute stroke patients to a specialized stroke unit is supported by high-level evidence. Simple, sensitive screening tests for the diagnosis of stroke are available that can be performed in the field by trained non-physician emergency medical personnel. With regard to pre-hospital treatment, adequate scientific evidence supports cardiopulmonary stabilization, as well as oxygen supplementation if there are signs of hypoxemia. The patient’s neurological findings, time of onset of symptoms, current medications, and past medical and surgical history must all be precisely and thoroughly documented. The receiving hospital must be informed of the patient’s impending arrival as early as possible, particularly in cases where recanalizing procedures are still a therapeutic option. Treatment with aspirin or heparin must not be started in situ, i.e. without prior cerebral imaging.
Conclusion
In the pre-hospital phase of stroke care delivery, the goal of a high capture rate can best be achieved through the use of appropriate diagnostic tests with maximal sensitivity. Patients with suspected acute stroke should be given the highest priority for transfer to a specialized stroke unit. Optimal pre-hospital care requires the smoothly functioning cooperation of all professionals involved, from the triaging and nursing personnel to the paramedics, dispatchers, emergency physicians in the field, and admitting physicians in the hospital.
Stroke is an important acute disease. Health economics require comprehensive care of stroke patients. In the acute phase of ischemic stroke, systemic thrombolysis currently represents the sole therapeutic option which is supported by evidence and meets both medical and drug-law requirements (1). This treatment requires a very tight time interval between onset of the incident and thrombolysis (“time is brain”) (2, 3). It was however also shown that even patients who do not undergo thombolysis benefit from treatment in a stroke unit (“competence is brain”) (4– 6). The German Society of Neurology (Deutsche Gesellschaft für Neurologie, DGN) and the German Stroke Society (Stiftung Deutsche Schlaganfallgesellschaft, DSG) have developed a guideline for the acute therapy of stroke (1). Institutions who wish to be recognized as certified stroke units by the German Stroke Society and the German Stroke Foundation (Deutsche Schlaganfallhilfe, SDSH) are required to have standard operation procedures (SOP) in place that have been adapted to local conditions. However, comparable guidelines for SOPs in the prehospital phase of stroke are currently still lacking.
The prehospital phase is defined as the interval between the onset of stroke and hospital admission. Successful acute therapy in a stroke unit is based on a structured approach which begins before the patient reaches the hospital. This structured approach ensures:
a shorter instruction period
optimal documentation of patient history, time of incident, concomitant medication, comorbidity, and surgical interventions.
In the following article, we present the findings of the working group “Prehospital Phase” of the German Stroke Society.
Methodology
A literature research of PubMed was conducted using the search terms “prehospital stroke”, “prehospital stroke care”, “stroke screening tool” and “emergency service stroke”. The authors reviewed the abstracts and, where relevant, analyzed the corresponding article. The analysis was confined to randomized controlled studies with clinically relevant endpoints (mortality, permanent disability, need for care, neurologically measurable changes) or, failing that, prospective observational studies with balanced cohorts. The key messages of these studies were assessed by grading them according to one of the four classes of evidence in compliance with the classification of the European Federation of Neurological Societies (EFNS) (Box) (7). The guidelines on ischemic stroke of the German Society of Neurology, the European Stroke Organisation and the American Stroke Association/American Heart Association were also taken into consideration in the phrasing of the clinical recommendations.
Box. Classification of evidence and strength of recommendation*1.
-
Evidence
Class I: adequately powered randomized controlled trial with blinded outcome assessment in a representative population, or an adequately powered systematic review of prospective, randomized, controlled clinical studies with blinded outcome assessment of representative populations
Class II: prospective matched cohort study in a representative population with blinded outcome assessment
Class III: controlled studies (incl. well defined controls with untreated course or with intra-individual comparison) in a representative population, in which outcome is assessed regardless of treatment
Class IV: evidence from uncontrolled studies, case series, single case reports, or expert opinion
-
strength of recommendation
A demonstrably effective, not effective or harmful
B probably effective, not effective or harmful
C possibly effective, not effective or harmful
GCP no class I to III scientific evidence or only inconsistent findings available; customary best medical practice, in consensus with experienced physicians
*1modified in accordance with the European Federation of Neurological Societies
Identification of a stroke patient by medical/non-medical staff
Both ambulance call center staff and the primary care team, either of whom might field the initial call, must be able to determine as accurately as possible whether the reported event represents a stroke with a high urgency for hospitalization (evidence class II) (8– 12). Table 1 shows the standardized filter questions that staff at the rescue center (dispatcher) must put to the caller. Staff must receive adequate training.
Table 1. Instructions for emergency staff and rescue center staff.
| Identifying a stroke patient | ||
| Staff at the hospital control center or GP office should suspect stroke when given the following statements: | ||
| Sudden onset of: | ||
| ||
| Always ask: When was the last point in time the patient did not exhibit the new symptoms? (time given should be as exact aspossible) | ||
| Questions | Answer | How to proceed |
| 1: Is the patient awake? | No | Immediate ambulance service with emergency doctor |
| Yes | Immediate ambulance service; whether or not an accompanying emergency physician is required is decided by the rescue service | |
| 2: Occurence of acute paralysis? (arms, legs, mouth droop) | If at least one question is answered with Yes: | Immediate ambulance service; whether or not an accompanying emergency physician is required is decided by the rescue service |
| 3. Occurence of acute speech disorders? (muffled speech, slurring, garbled speech, word finding difficulty, patient does not understand other people) | ||
| 4. Occurence of acute visual impairment? (double vision, hemilateral blindness, scotoma) | ||
| 5. If headache: has it appeared for the first time and is it acute and severe? | ||
| 6. Hemi- or unilateral sensory disturbances (face, arm, leg) | ||
| 7. Acute vertigo? | ||
Stroke patients must be transported to a stroke unit without delay (evidence class 1). Whether or not the individual situation requires an accompanying emergency physician is decided by the emergency medical service on the basis of the recommendations of the German Medical Association (13). The obligatory question is: When did the neurological symptoms set in, or when was the last point in time the patient remembers not having had the symptoms? The less time has passed since the onset of symptoms, the larger the potential therapeutic benefit (evidence class I) (2, 3), and the more urgent the need for hospitalization.
Keywords such as sudden-onset paralysis, acute impairment of speech or vision, severe, sudden-onset headache and loss of vigilance must be viewed as red flags that indicate immediate transfer to a hospital with a stroke unit (evidence class I), bypassing day time or after hour primary care services (3). This recommendation is based on consistent, reproducible findings from meta-analyses demonstrating the positive effect of care on a stroke unit. Several studies have shown significantly improved prognosis with shorter transfer time between onset of stroke symptoms and initiation of thrombolytic therapy (3, 12). A Cochrane meta-analysis from 2007 found
a 14% reduced chance of death after stroke (event rate 22.5% versus 26.6%; absolute risk reduction [ARR] 4,1%; odds ratio [OR] 0,83; 95% confidence interval [CI] 0.71–0.96; p = 0.01),
an 18% reduced chance of death or admission to care facility (event rate 36.4% versus 41.8%; ARR 5.4%; OR 0.80; 95% CI 0.70–0.90; p = 0.0005),
an 18% reduced chance of death or care dependence (event rate 53.0% versus 56.5%; ARR 3.5%; OR 0.83; 95% CI 0.72–0.96; p = 0.01) due to initial care on a stroke unit (8).
Subgroup analyses showed no age (</>75 years), sex or severity related differences in the effect of stroke unit treatment. The pooled analysis of thrombolysis studies showed a decrease of OR for a good functional result after three months from 2.81 (95% CI 1.75–4.50) in the group of patients thrombolyzed within 90 minutes for every 90-min latency to a non-significant 1.15 (95% CI 0.90–1.47) in the group treated within 271 and 360 minutes (14).
Check list and FAST test on the ground
Front line emergency staff should note the onset of symptoms and their development (spontaneous improvement, worsening, stability of symptoms) and also register potential contraindications to thrombolysis. This will facilitate the decision-making process of the specialist at the stroke unit regarding thrombolysis. Important contraindications include:
malignant diseases
surgery in the last three months
invasive procedures (punctures) in the last four weeks
earlier hemorrhagic events
existing anticoagulant therapy
known coagulation disorders.
Several validated tests are available for standard stroke screening, which can also be used by non-medical personnel (evidence class II) (10– 12, 15). As these tests have a false-negative rate of at least 5%, a negative test result does not exclude stroke with all certainty. In Europe the FAST test (face–arm–speech–test) is increasingly used, a variation of the Cincinnati Prehospital Stroke Scale (Table 2). A comparative study found this screening instrument to have the highest sensitivity of 95%, but with the disadvantage of a low specificity of 56% (15). However, since the goal is to administer thrombolysis to as many patients as possible, maximum sensitivity takes priority in this case.
Table 2. Stroke-checklist for front line emergency staff on the ground.
| Emergency care on the ground: three steps | |||
| 1. Securing and stabilizing vital functions | According to ABC | ||
| In addition in every patient: | |||
| |||
| 2. History | As reported by the patient and friends / relatives / other witnesses: | ||
| time of symptom onset or last point in time of being free of symptoms | |||
| |||
| |||
| 3. FAST | facial paralysis | both sides of the face move symmetrically | normal |
| a paralyzed face is droopy on one side | pathological | ||
| Arm drift | both arms move symmetrically or not at all | normal | |
| One arm drifts compared to the other | pathological | ||
| Speech / talking | Patient uses correct words, speech not slurred | normal | |
| Patient has slurred speech or uses wrong words or does not talk at all | pathological | ||
| If one of these points is pathological the presence of stroke is highly likely | |||
Specific training is required for the correct and reliable application of the FAST-test. The items of the stroke checklist for front line emergency staff are listed in Table 2.
Practical procedures before and during transport
Every stroke patient who can be treated within six hours after symptom onset at a hospital with stroke unit expertise is, in principle, a candidate for thrombolysis or any form of endovascular intervention, provided there are no obvious contraindications (evidence class II–III) (2, 16– 19). It is vital that the responsible stroke team is given notice by phone of the thrombolysis candidate; this also helps to save additional time (evidence class III) (20).
Regarding the actual time frame of the prehospital phase there are a number of studies covering various nations and regions with very different socioeconomic levels of development and a wide range of healthcare systems and structures. A comprehensive global meta-analysis showed a wide range of the share of patients with a prehospital phase of <3 hours between 6% and 92%, with a median latency of approximately 3 hours in the year 2005 compared to approximately 6 hours in the year 1995, before the approval of rt-PA (recombinant tissue plasminogen activator) in the US (21). German data show a median prehospital phase of 151 minutes and a share of 54% of patients reaching the hospital in <3 hours (22).
There is only little evidence based data on practical procedures before and during transport. Most of the information provided here is based on extrapolation from acute care in a stroke unit.
All patients require a peripheral venous catheter. If at all possible, the catheter should not be placed in the paretic arm, so as to avoid impaired fluid distribution and increased complications in the case of extravasation in the paretic extremity (evidence class IV).
Should signs of hypoxemia appear—i.e. clinical signs or peripheral oxygen saturation of <95%—the treatment consists of 4 L/min nasal oxygen (target value >95%, evidence class IV for prehospital application). According to pilot studies this approach ensures a survival benefit for patients treated with oxygen immediately after admission (OR 0.45, 95% CI 0.23–0.90; p = 0.023) (evidence class III) (23). Furthermore, patients treated with oxygen due to hypoxemia had a lower infarct volume in diffusion-weighted MRI (24). Routine oxygen administration is not indicated (evidence class IV).
The patient should recieve cardiovascular stablilization before transport. Hypertension should be treated if blood pressure exceeds 220 mm Hg systolic or 120 mm Hg diastolic (1) (evidence class IV). Electrolyte solutions or normal saline are indicated in arterial hypotension RR systolic below 120 mm Hg (evidence class IV) (25, e1), keeping in mind possible contraindications (severe heart failure). In hypoglycemia below 60 mg/dL (<3.3 mmol/L) the administration of 30 mL 20% to 40% glucose IV is recommended (evidence class IV). In hyperglycemic patients—above 200 mg/dL (>11mmol/L)—an adequate liquid intake is of particular importance (evidence class IV) (e2, e3). Note: No antithrombotic or platelet aggregation inhibitors must be administered until the results of cerebral imaging have differentiated between an ischemic infarction and cerebral hemorrhage.
Table 3 lists the most important therapeutic measures on the ground and during transport.
Table 3. Therapeutic measures on the ground and during transport.
| Emergency treatment on the ground | ||
| All patients |
|
|
| as contraindicated in intracranial hemorrhage or thrombolysis: no antithrombotics (heparin or aspirin lysine IV, no i.m. injections) | ||
| Arterial hypertension |
|
leave |
| Hypertensive values in 2 measurements of 5-min interval |
|
|
| Several antihypertensive drugs that are indicated in the treatment of other hypertensive decompensations should be avoided in stroke patients and/or only be used in refractory hypertension: nitrate SL, calcium channel blocker of the nifedipine-type SL or IV | ||
| Arterial hypotension |
|
500 mL electrolyte solution or NaCl 0.9% IV |
| Hypoglycemia | <60 mg/dl (<3.3 mmol/l) | 30 mL glucose 20% to 40% IV |
| Hyperglycemia | ≥ 200 mg/dL (11 mmol/L) | additional glucose-free fluid intake |
| Complications and underlying diseases |
|
Individual decision according to severity |
Handover at the hospital
It is the responsibility of the emergency team to hand over any information gathered in the prehospital phase to the hospital team providing further treatment (evidence class IV). The following information should be communicated in a structured, brief handover:
time of symptom onset or last point in time without the new deficit
type of complaint
relevant concomitant diseases
information regarding pre-morbid state
phone number of relatives for immediate contact regarding detailed patient history
current medication.
One criterion of a successful stroke unit is a well-structured prehospital phase. The main aim should be efficient organization so as to minimize the time window between stroke event and hospital admission.
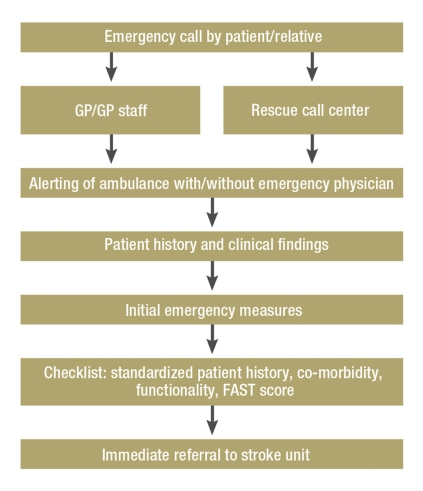
Figure.
Key points of prehospital stroke treatment
Key Messages.
The effective treatment of acute stroke within a stroke unit requires the minimization of the prehospital phase.
Clinical screening tests that are both easy to learn and to implement allow the selection of stroke patients. The next step is the immediate emergency transport to a stroke unit, where appropriate without an emergency physician.
The aim is initial care in a stroke unit. This has been shown to increase survival and reduce complications regarding nursing care.
The standard collection of basic data on the ground, if possible with the help of relatives / witnesses, is crucial for adequate further treatment in a stroke unit.
Unlike interventions in the acute hospital phase, few therapeutic and diagnostic interventions in prehospital care are as yet evidence-based. Further research in this area is urgently needed.
Acknowledgments
Translated from the original German by Lydia Unger-Hunt, MD.
Footnotes
Conflict of interest statement
Prof. Kessler has received reimbursement of participation fees as well as travel and accommodation expenses from Boehringer Ingelheim. He has received lecture fees from Boehringer Ingelheim, GlaxoSmithKline, Johnson-Johnson, Janssen-Cilag, Sanofi, and Pfizer. Servier, Paion, and Ferrer have paid clinical study fees onto a third party deposit account.
Dr. Khaw has received reimbursement for travel and accommodation expenses from Bristol-Myers Squibb. Boston Scientific, Cordis, and Bristol-Meyers Squibb have paid fees for the preparation of scientific training sessions. Paion Germany and Servier have paid expense allowances for clinical commission studies.
Prof. Navabi has received consultancy fees from Trommsdorff and Bristol-Myers Squibb. He has received reimbursement of congress participation fees and travel- and accommodation expenses from Boehringer Ingelheim. Sanofi, Boehringer Ingelheim, Pfizer, and Novartis have paid fees for the preparation of scientific training sessions.
Dr. Glahn has received reimbursements for congress participation fees as well as travel and accommodation expenses from Boehringer Ingelheim. Furthermore he has received lecture fees from Boehringer Ingelheim. He has received remuneration for conducting clinical commission studies from Boehringer, Cato Research, Schering-Plough, Trommsdorff, IntercoorNet, Sanofi, and PhotoThera.
Prof. Grond has received consultancy fees and reimbursement of congress participation fees from Boehringer Ingelheim, Bayer Vital, and Sanofi Aventis. Furthermore he has received reimbursement for travel- and accommodation expenses from Boehringer Ingelheim, Sanofi Aventis, Bayer Vital, and Lundbeck. He has received lecture fees from Boehringer Ingelheim, Sanofi Aventis, Bayer Vital, Lundbeck, Bristol-Myers Squibb, and Solvay. Boehringer Ingelheim has sponsored his research.
Prof. Busse has received lecture fees from Boehringer Ingelheim.
Prof. Hamann has received consultancy fees from Bristol-Myers Squibb, Boehringer Ingelheim, and Thrombogenics. He has received lecture fees from Boehringer Ingelheim, Sanofi-Aventis, GlaxoSmithKline, and Bayer Vital. Furthermore he has received study sponsoring from Servier Pharma.
Professor G. F. Hamann from the department of Neurology at the Dr.-Horst-Schmidt-Kliniken (HSK), Wiesbaden, has contributed to preparation of the Manuscript.
References
- 1.Deutsche Gesellschaft für Neurologie. Leitlinien; Akuttherapie des ischämischen Schlaganfalls. http://www.dgn.org/images/stories/dgn/leitlinien/LL2008/ll08kap_023.pdf. 2010. May 22, Last accessed on.
- 2.Hacke W, Donnan G, Fieschi C, et al. ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–774. doi: 10.1016/S0140-6736(04)15692-4. [DOI] [PubMed] [Google Scholar]
- 3.Marler JR, Tilley BC, Lu M, et al. Early stroke treatment associated with better outcome: the NINDS rt-PA stroke study. Neurology. 2000;55:1649–1655. doi: 10.1212/wnl.55.11.1649. [DOI] [PubMed] [Google Scholar]
- 4.Schwamm LH, Pancioli A, Acker JE, 3rd, et al. American Stroke Association’s Task Force on the Development of Stroke Systems. Recommendations for the establishment of stroke systems of care: recommendations from the American Stroke Association’s Task Force on the Development of Stroke Systems. Stroke. 2005;36:690–703. doi: 10.1161/01.STR.0000158165.42884.4F. [DOI] [PubMed] [Google Scholar]
- 5.Langhorne P. Do Stroke Units Improve Patient Outcomes? Stroke. 1997;28:2139–2144. doi: 10.1161/01.str.28.11.2139. [DOI] [PubMed] [Google Scholar]
- 6.Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2001;3 doi: 10.1002/14651858.CD000197.pub3. CD000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brainin M, Barnes M, Baron JC, et al. Guideline Standards Subcommittee of the EFNS Scientific Committee Guidance for the preparation of neurological management guidelines by EFNS scientific task forces–revised recommendations 2004. Eur J Neurol. 2004;11:577–581. doi: 10.1111/j.1468-1331.2004.00867.x. [DOI] [PubMed] [Google Scholar]
- 8.Ringleb P, Schellinger PD Hacke W für das ESO-Writing-Committee. Leitlinien zum Management von Patienten mit akutem Hirninfarkt oder TIA 2008 der Europäischen Schlaganfall Organisation (ESO) http://www.eso-stroke.org/pdf/ESO08_Guidelines_German.pdf Letzter Zugriff. 2010 May 25; [Google Scholar]
- 9.Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med. 1999;33:373–378. doi: 10.1016/s0196-0644(99)70299-4. [DOI] [PubMed] [Google Scholar]
- 10.Kidwell CS, Starkman S, Eckstein M, Weems K, Saver JL. Identifying stroke in the field. Prospective validation of the Los Angeles prehospital stroke screen (LAPSS) Stroke. 2000;31:71–76. doi: 10.1161/01.str.31.1.71. [DOI] [PubMed] [Google Scholar]
- 11.Porteous GH, Corry MD, Smith WS. Emergency medical services dispatcher identification of stroke and transient ischemic attack. Prehosp Emerg Care. 1999;3:211–216. doi: 10.1080/10903129908958939. [DOI] [PubMed] [Google Scholar]
- 12.Candelise L, Gattinoni M, Bersano A, et al. Stroke unit care for acute stroke patients: An observational follow-up study. Lancet. 2007;369:299–305. doi: 10.1016/S0140-6736(07)60152-4. [DOI] [PubMed] [Google Scholar]
- 13.Indikationskatalog für den Notarzteinsatz Handreichung für Telefondisponenten in Notdienstzentralen und Rettungsleitstellen. http://www.bundesaerztekammer.de/downloads/Notarzteinsatz.pdf. 2010. May 24, Last accessed on.
- 14.Stroke Unit Trialists’ Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database of Systematic Reviews. 2007;(Issue 4) doi: 10.1002/14651858.CD000197.pub2. Art. No.: CD000197. DOI: 10.1002/14651858.CD000197.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Smith WS, Corry MD, Fazackerley J, Isaacs SM. Improved paramedic sensitivity in identifying stroke victims in the prehospital setting. Prehosp Emerg Care. 1999;3:299–305. doi: 10.1080/10903129908958938. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Bluhmki E, et al. ECASS Investigators: Thrombolysis with alteplase 3 to 45 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 17.Lansberg MG, Bluhmki E, Thijs VN. Efficacy and safety of tissue plasminogen activator 3 to 45 hours after acute ischemic stroke: a metaanalysis. Stroke. 2009;40:2438–2441. doi: 10.1161/STROKEAHA.109.552547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 19.Nogueira RG, Smith WS. MERCI and Multi MERCI Writing Committee. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke. 2009;40:516–522. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 20.Belvís R, Cocho D, Martí-Fàbregas J, et al. Benefits of a prehospital stroke code system Feasibility and efficacy in the first year of clinical practice in Barcelona. Spain. Cerebrovasc Dis. 2005;19:96–101. doi: 10.1159/000082786. [DOI] [PubMed] [Google Scholar]
- 21.Evenson KR, Foraker RE, Morris DL, Rosamond WD. A comprehensive review of prehospital and in-hospital delay times in acute stroke care. Int J Stroke. 2009;4:187–199. doi: 10.1111/j.1747-4949.2009.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossnagel K, Jungehülsing GJ, Nolte CH, et al. Out-of-hospital delays in patients with acute stroke. Ann Emerg Med. 2004;44(5):476–483. doi: 10.1016/j.annemergmed.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Rønning OM, Guldvog B. Should stroke victims routinely receive supplemental oxygen? A quasi-randomized controlled trial. Stroke. 1999;30:2033–2037. doi: 10.1161/01.str.30.10.2033. [DOI] [PubMed] [Google Scholar]
- 24.Singhal AB, Benner T, Roccatagliata L, et al. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- 25.Castillo J, Leira R, García MM, Serena J, Blanco M, Dávalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520–526. doi: 10.1161/01.STR.0000109769.22917.B0. [DOI] [PubMed] [Google Scholar]
- e1.Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA. IST Collaborative Group. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- e2.Scott JF, Robinson GM, French JM, O’Connell JE, Alberti KG, Gray CS. Glucose potassium insulin infusions in the treatment of acute stroke patients with mild to moderate hyperglycemia: The Glucose Insulin in Stroke Trial (GIST) Stroke. 1999;30:793–799. doi: 10.1161/01.str.30.4.793. [DOI] [PubMed] [Google Scholar]
- e3.Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39:384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]