Abstract
Stro-1 is the best-known mesenchymal stem cell (MSC) marker. However, previous studies have observed its expression in the endothelium. In the present study we performed immunofluorescence (IF) staining for Stro-1, using endothelial marker vWF as reference. In the liver, both proteins were expressed in the endothelium of the central veins and hepatic sinusoids. In the lung, both were expressed in the endothelium of pulmonary blood vessels, but while vWF was absent in the alveolar capillaries, Stro-1 was present. In the kidney, both were expressed in the endothelium of renal arterial branches, but while vWF was strongly expressed in the glomeruli, Stro-1 only scantly. IF staining in cultured endothelial cells also showed extensive overlaps between Stro-1 and vWF. Western blot analysis with Stro-1 antibody detected a single protein band of 75kd in endothelial cells but not smooth muscle cells, fibroblasts, or B cells. Cancer cell lines PC3, DU145, MCF7, and K562 were also positive. Adipose-derived stem cells (ADSCs) expressed higher levels of Stro-1 when cultured beyond the first passage or when induced to differentiate into endothelial cells. These data, together with previous studies, indicate that Stro-1 is intrinsically an endothelial antigen, and its expression in MSC is probably an induced event.
Keywords: Stro-1, vWF, endothelial, mesenchymal stem cells, liver, lung, kidney
1. Introduction
Since their discovery in the early 1970s, mesenchymal stromal/stem cells (MSC) have been extensively studied, including numerous efforts to find a reliable marker to facilitate their identification and isolation. One of these efforts was to use CD34+ bone marrow cells as immunogen that eventually led to the production of a monoclonal antibody named Stro-1 [1]. Since then, Stro-1 antibody has been heavily relied upon for the recognition and isolation of various types of MSC, particularly those in dental tissues. Thus, while its identity remained completely unknown, Stro-1 was considered the best-known MSC marker [2]. However, despite such an important position in MSC research and being in existence for more than 20 years, Stro-1 antibody continues to be employed in a large number of studies with very little knowledge about the identity of its cognate antigen.
The predominant usage of Stro-1 antibody has been for FACS analysis and staining of putative MSC. However, disagreements over whether a particular MSC type is Stro-1 positive are commonplace [3; 4; 5] (see Discussion for possible explanations). For western blotting, it is surprising that so far only one study has shown such data (see Discussion for more details). For tissue staining, despite its bone marrow origin, Stro-1 antibody was never used to stain the bone marrow stroma until recently when we reported Stro-1’s tissue distribution [6]. In that study and in a previous study [5] we found that Stro-1 reactivity occurred most commonly in the endothelium of blood vessels or sinusoids in tissues such as the adipose and penis. In the literature we found that three earlier studies have mentioned Stro-1’s endothelial or perivascular reactivities [7; 8; 9]; in particular, an image in the 2008 paper clearly shows endothelial expression of Stro-1. Thus, our suspicion of Stro-1’s endothelial connection led us to conduct the present study. We performed immunofluorescence (IF) staining and western blotting to examine Stro-1 expression in cultured endothelial cells, cancer cells, and non-endothelial cells. We also performed IF staining on kidney, liver, and lung, focusing on the blood vessels. By using endothelial marker vWF as a reference, our data provide evidence that Stro-1 is a 75kd endothelial antigen.
2. Materials and methods
2.1 Cell cultures
Human umbilical vein endothelial cell strain, HUVEC, was purchased from Lonza Biologics Inc. (Portsmouth, NH) and cultured in EGM2 medium (Lonza Biologics Inc.). Human penile endothelial cells (HPEC-7 and HPEC-11) were isolated using Dynabeads CD31 (Invitrogen, Carlsbad, CA) and cultured in EGM2. Human and rat ADSCs were isolated as described previously [10; 11]. Human fibroblasts PT and NT were isolated from the penile albuginea tunica of patients with and without Peyronie’s disease, respectively [12]. Human penile foreskin fibroblasts (HFF) were isolated using the same procedure. Rat aorta smooth muscle cells (rVSMC) were described previously [13]. Human and rat cavernous smooth muscle cells (hCSMC and rCSMC) were isolated as described previously [14]. Rat urethral smooth muscle cells (rUSMC) were isolated as described previously [15]. Rat bladder smooth muscle cells (rBSMC) were isolated using the same procedure. All smooth muscle cells and fibroblasts were cultured in DMEM supplemented with 10% FBS, 1% nonessential amino acid, 10,000 units/mL penicillin, 10,000 mcg/mL streptomycin SO4, 0.025 mg/mL fungizone, and 110 mg/mL sodium pyruvate. Cancer cell lines DU145, PC3, MCF7, and K562 were from American Type Culture Collection (Manassas, VA). B-cell line 721.221 was from International Histocompatibility Working Group (Seattle, WA). DU145, PC3, and 721.221 were cultured in RPMI1640 medium supplemented with 10% FBS, MCF7 in DMEM medium supplemented with 10% FBS.
2.2. Western blot analysis
Cells were lysed in a lysis buffer containing 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, aprotinin (10 µg/ml), leupeptin (10 µg/ml), and PBS. Cell lysates containing 20 µg of protein were electrophoresed in SDS-PAGE and then transferred onto a PVDF membrane (Millipore Corp., Bedford, MA). The membrane was stained with Ponceau S to verify the integrity of the transferred proteins and to monitor the unbiased transfer of all protein samples. Detection of protein on the membrane was performed with the ECL kit (Amersham Life Sciences Inc., Arlington Heights, IL) using anti-Stro-1 (MAB1038, BD Biosciences, Bedford, MA), or anti-β-actin antibody (A5441, Sigma-Aldrich, St. Louis, MO). Before re-probing with anti-β-actin antibody, the membrane was stripped in 62.5 mM Tris-HCl, pH 6.7, 2% SDS, 10 mM 2-mercaptoethanol at 56 °C for 30 min and then washed four times in 1xTBST. The resulting images were analyzed with ChemiImager 4000 (Alpha Innotech Corporation, San Leandro, CA) to determine the integrated density value (IDV) of each protein band normalized to the IDV of β-actin.
2.3. Preparation of tissue sections
Freshly dissected rat liver, lung, and kidney were fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 M phosphate buffer, pH 8.0, for 4 hours followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetek USA, Torrance, CA) and stored at −80 °C until use. Fixed frozen tissue specimens were cut at 10 microns, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA) and air dried for 5 min.
2.4. Immunofluorescence staining
HUVEC, HPEC-7, and HPEC-11 (1 × 105 cells/well) were cultured on coverslips placed in 6-well plates with 3 ml/well of EGM2 and 20% FBS. After 24 h, the cells were fixed in cold methanol for 5 min at 4 °C, permeabilized with 0.05% Triton X-100 for 5 min, and blocked with 5% normal equine serum in PBS for 1 h at room temperature. The cells were then incubated with anti-vWF (ab6994, Abcam, Cambridge, MA) and anti-Stro-1 antibodies for 1 h at room temperature. After washing with PBS three times, the cells were incubated with Texas Red–conjugated anti-rabbit IgG and FITC-conjugated anti-mouse IgM antibody for 1 h at room temperature. After three washes with PBS, the cells were further stained with 4-,6- diamidino-2-phenylindole (DAPI, for nuclear staining) for 5 min. The stained cells were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software (Nikon Instruments Inc., Melville, NY).
Frozen tissue sections were placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining solution from the tissue section, the tissue was incubated with rabbit anti-vWF and mouse anti-Stro-1 for overnight at 4°C. After rinses with PBS, the sections were incubated with Texas Red–conjugated anti-rabbit IgG and FITC- conjugated anti-mouse IgM antibody for 1 h at room temperature. After three washes with PBS, the sections were further stained with DAPI for 5 min. The stained tissues were examined with Nikon Eclipse E600 fluorescence microscope and photographed with Retiga 1300 Q-imaging camera using the ACT-1 software.
2.5. Statistics
All the statistical data were analyzed with Prism 4 (GraphPad Software Inc., San Diego, CA). T-test was used to compare each pair of treatment. P value <0.05 was considered significant.
3. Results
3.1. Stro-1 and vWF expression in liver, lung, and kidney
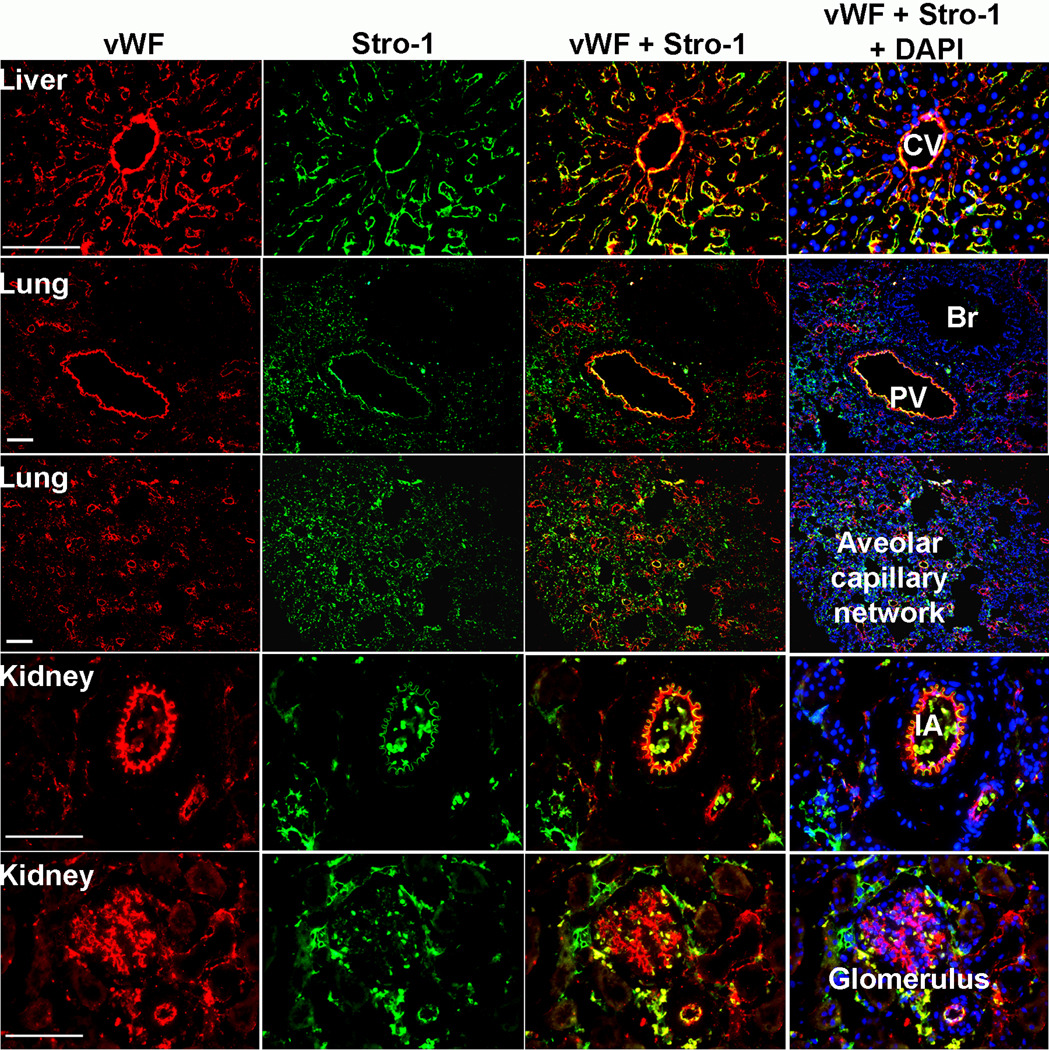
In the liver, both Stro-1 and vWF antibodies reacted with the central vein in each hepatic lobule and with the sinusoidal linings that radiate outward from the central vein (Fig. 1). However, these two antibodies produced slightly different staining patterns in the sinusoidal linings: vWF evenly distributed while Stro-1 more intense in the outer zone than in the inner zone.
Fig. 1.
Immunostaining of Stro-1 and vWF in tissues. Frozen sections of rat liver, lung, and kidney were stained with Stro-1 (green), vWF (red), and DAPI (blue). CV: Central vein; Br: Bronchiole; PV: Pulmonary vein; IA: Interlobular artery. Scale bar = 50 µ.
In the lung, both Stro-1 and vWF antibodies reacted with the endothelium in the branches of pulmonary arteries and veins (Fig. 1). However, these two antibodies produced clearly distinguishable staining patterns in the alveolar capillary network: vWF was essentially absent while Stro-1 highly represented.
In the kidney, both Stro-1 and vWF antibodies reacted with the endothelium in the branches of renal arteries and veins (Fig. 1). However, these two antibodies produced clearly distinguishable staining patterns in the renal corpuscle: vWF was strongly present in the glomeruli while Stro-1 scantly. On the other hand, vWF was largely absent in the Bowman’s capsule while Stro-1 more represented.
3.2. Stro-1 and vWF expression in cultured endothelial cells
HUVEC is a widely used endothelial cell strain from human umbilical vein. HPEC-7 and HPEC-11 are two independent endothelial cell strains isolated from human penile biopsies. All three cell strains were stained positive for Stro-1 although individually some cells were negative (Supplemental Fig. 1). Double staining showed large degrees of overlap between Stro-1 and vWF in all three cell strains.
3.3. Stro-1 is a 75kd protein in endothelial cells but not smooth muscle cells or fibroblasts
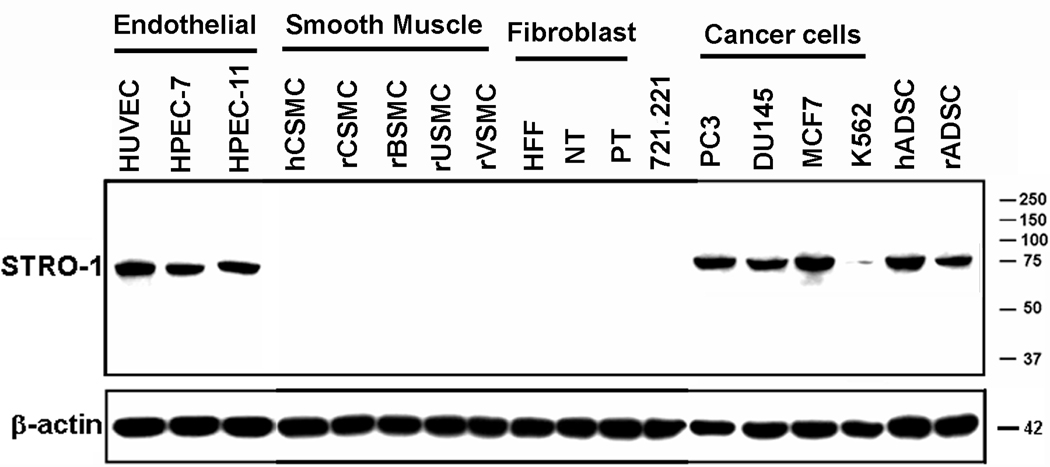
Western blot analysis with Stro-1 antibody detected a single protein band of 75kd in endothelial cells HUVEC, HPEC-7, and HPEC-11 (Fig. 2). The same protein band was also detected in human and rat ADSC, and in several cancer cell lines, including prostate (PC3 and DU145), breast (MCF7), and erythroleukemia (K562). No such a protein band was detected in immortalized human B cell line, 721.221; smooth muscle cells from rat aorta, bladder, urethra, and penis; or fibroblasts from human foreskin (HFF) and penile tunica albuginea (NT and PT).
Fig. 2.
Western blot analysis of Stro-1 expression in various cell strains and lines. The cells are arranged in groups according to cell types. Each lane contains 20 µg of cellular protein. Size markers in kd are shown on the right. β–actin is used for normalization.
3.4. Increased Stro-1 expression in adipose-derived stem cells after first passage
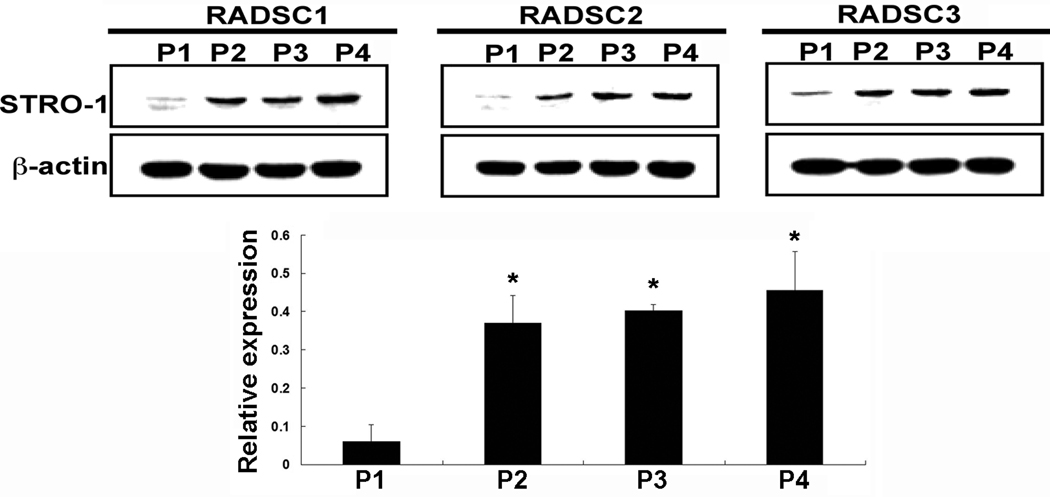
ADSCs were isolated from three rats, propagated to passage 4, and subjected to western blot analysis for Stro-1 expression. The results show that in each ADSC strain, Stro-1 expression was significantly higher in longer passaged cells (P2, P3, and P4) than in first-passaged cells (P1) (Fig. 3).
Fig. 3.
Western blot analysis of Stro-1 expression in adipose-derived stem cells from 1st to 4th passage. Each lane contains 20 µg of cellular protein. Relative expression is the ratio between Stro-1 and β–actin in band intensity as determined by densitometry. *P<0.05 compared to P1.
3.5. Increased Stro-1 expression in endothelially induced adipose-derived stem cells
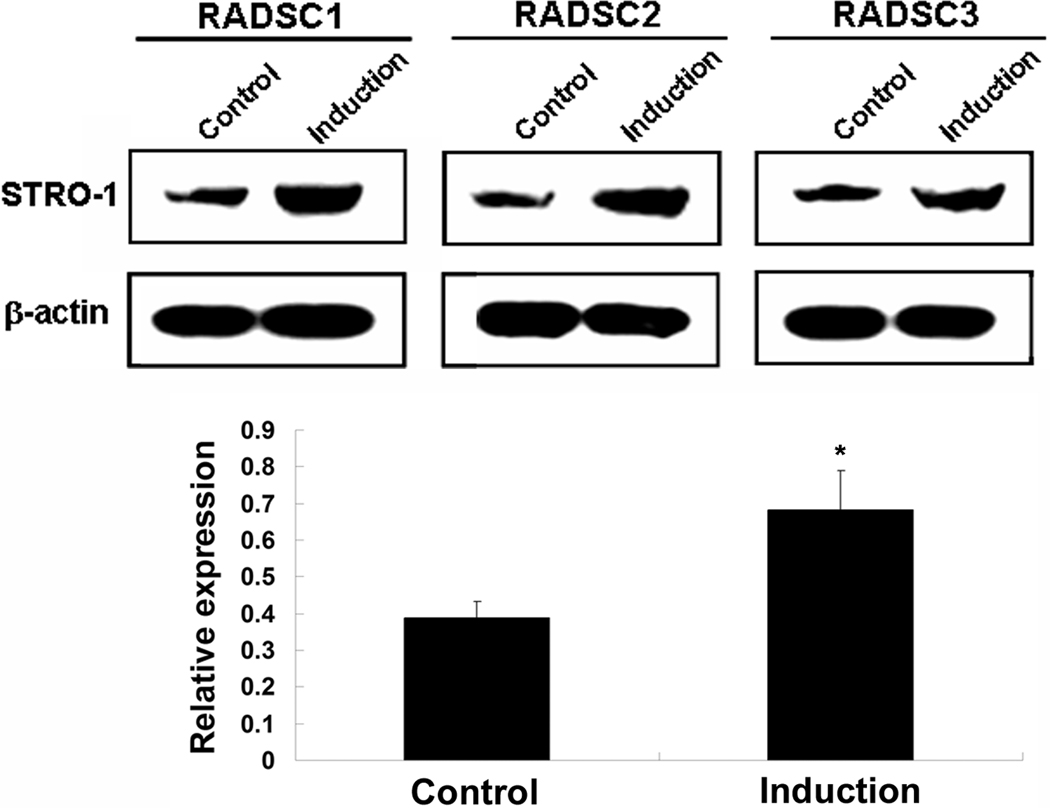
ADSCs at passage 3 were cultured in DMEM (uninduced control) or EGM2 (endothelial induction), and then analyzed for Stro-1 expression. The results show that in each ADSC strain, Stro-1 expression was significantly higher in induced than in uninduced cells (Fig. 4).
Fig. 4.
Western blot analysis of Stro-1 expression in endothelially induced adipose-derived stem cells. P3 ADSCs were grown in DMEM (Control) or EGM2 (Induction) for 7 days. Each lane contains 20 µg of cellular protein. Relative expression is the ratio between Stro-1 and β–actin in band intensity as determined by densitometry. *P<0.05 compared to Control.
4. Discussion
We previously presented histological data of Stro-1 expression in the heart, aorta, rectum, bladder, urethra, prostate, penis, testis, leg muscle, and adipose tissue [6]. In the present study we chose to further examine Stro-1 expression in liver, lung, and kidney. These choices were made with consideration that these three organs harbor specialized blood vessels within their functional units – specifically, the hepatic sinusoids, the alveolar capillary networks, and the glomeruli. Another improvement over our previous studies was the addition of endothelial marker vWF as a reference.
Previous studies have shown positive vWF staining in the endothelium of hepatic central veins and sinusoids [16; 17]. Data in the present study are consistent with these findings; but more importantly, we show that Stro-1 expression largely overlapped with vWF expression, except that in the sinusoids Stro-1 was more strongly expressed in the outer zone than in the inner zone. The significance of this differential Stro-1 expression is unknown. In regard to lungs, it has been shown that vWF is highly expressed in the endothelium of pulmonary blood vessels but only sporadically in the endothelium of alveolar capillaries [18; 19; 20]. Data in the present study are consistent with these findings. However, while Stro-1 expression largely overlapped with vWF expression in pulmonary blood vessels, it was also highly visible in the alveolar capillaries. In the kidney, vWF is expressed in the endothelium of large and small branches of renal artery and in the glomeruli [17; 21; 22]. Data in the present study agree with these findings, but in regard to Stro-1 expression, similarity with vWF expression existed only in the renal arterial branches, not the renal corpuscles. Specifically, while vWF is highly expressed in the glomeruli, Stro-1 only scantly. In addition, Stro-1 staining was much more extensive in the Bowman’s capsule. Whether Stro-1 is expressed in some special cell types, such as podocytes, which do not express vWF [23] requires further investigation.
Although not completely identical, the staining patterns of vWF and Stro-1 in the liver, lung, and kidney as discussed above reinforced our hypothesis that Stro-1 is an endothelial antigen. To further test this hypothesis, we performed double staining for vWF and Stro-1 in a widely used human umbilical vein endothelial cell strain HUVEC and in two human penile endothelial strains HPEC-7 and HPEC-11, together with several non-endothelial cell strains and lines. The results show that endothelial cells, but not smooth muscle cells, fibroblasts, or B-cells, expressed a Stro-1 positive protein at approximately 75kd. This protein size is much larger than the 27kd reported in a recent study, in which Stro-1 antibody detected several fuzzy and diffused bands ranging from 18 to 50kd [24]. Since the protein band in our western blots were singular, clear, and discrete, we believe that it represents the authentic Stro-1 antigen.
In addition to endothelial cells, our western blot analysis also detected Stro-1 expression in cancer cell lines PC3, DU145, MCF7, and K562. The significance of this finding is presently unknown. In primary cells that we have tested, only ADSCs isolated from either humans or rats were positive in Stro-1 expression. As discussed in our previous publications, whether Stro-1 is expressed in ADSCs has been controversial, and for which there has been no convincing explanation [4; 5]. In the present study we found that cells at P1 expressed Stro-1 at much lower levels than cells at P2, P3, or P4. Thus, it is possible that this differential Stro-1 expression in P1 versus later passages might explain why different studies detected different Stro-1 expression levels. In particular, we have shown that in adipose tissue putative ADSCs (CD34+CD31−) do not express Stro-1 [5]. Therefore, Stro-1 expression appears to be induced in ADSCs under cell culture conditions. This consideration extends to other types of MSC as their Stro-1 expression has also been shown in culture but not in tissue.
We have previously shown that ADSCs can acquire endothelial properties (e.g., LDL-uptake, tube formation, and CD31 expression) when cultured in endothelial growth medium EGM2 [25]. In the present study we found that ADSCs cultured in EGM2 expressed higher levels of Stro-1, thus further reinforcing Stro-1’s endothelial connection.
While our data clearly show that Stro-1 is an endothelial protein, we consider that Stro-1 can still be a bona fide MSC marker because, under cell culture conditions, MSCs such as ADSC are Stro-1+CD31− while endothelial cells are Stro-1+CD31+. Thus, while MSCs may not express Stro-1 in vivo, they can still be distinguished from non-MSCs in vitro by being Stro-1+CD31−. The question is, other than being an MSC marker and an endothelial antigen, what is Stro-1? This and related questions such as how its expression is induced in cultured cells and what relationship it has with CD34 need to be investigated further.
Highlights.
Stro-1 is identified in the vasculature of liver, lung and kidney.
Stro-1 is co-localized with endothelial marker vWF.
Western blot analysis identifies Stro-1 as a 75kd protein in endothelial cells.
Stro-1 is expressed in cultured adipose-derived stem cells (ADSC).
Stro-1 expression is higher in endothelially induced ADSC.
Supplementary Material
Acknowledgments
This work was supported by the Rock Foundation and the National Institutes of Health (DK64538, DK045370, and DK069655).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 2.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF. Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol. 2010;25:807–815. doi: 10.14670/HH-25.807. [DOI] [PubMed] [Google Scholar]
- 5.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin G, Liu G, Banie L, Wang G, Ning H, Lue TF, Lin CS. Tissue Distribution of Mesenchymal Stem Cell Marker Stro-1. Stem Cells Dev. doi: 10.1089/scd.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 8.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 9.Rolf HJ, Kierdorf U, Kierdorf H, Schulz J, Seymour N, Schliephake H, Napp J, Niebert S, Wolfel H, Wiese KG. Localization and characterization of STRO-1 cells in the deer pedicle and regenerating antler. PLoS One. 2008;3:e2064. doi: 10.1371/journal.pone.0002064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–518. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 11.Ning H, Liu G, Lin G, Garcia M, Li LC, Lue TF, Lin CS. Identification of an aberrant cell line among human adipose tissue-derived stem cell isolates. Differentiation. 2009;77:172–180. doi: 10.1016/j.diff.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CS, Lin G, Wang Z, Maddah SA, Lue TF. Upregulation of monocyte chemoattractant protein 1 and effects of transforming growth factor-beta 1 in Peyronie's disease. Biochem Biophys Res Commun. 2002;295:1014–1019. doi: 10.1016/s0006-291x(02)00765-9. [DOI] [PubMed] [Google Scholar]
- 13.Lin G, Chow S, Lin J, Wang G, Lue TF, Lin CS. Effect of cell passage and density on protein kinase G expression and activation in vascular smooth muscle cells. J Cell Biochem. 2004;92:104–112. doi: 10.1002/jcb.20043. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Lin CS, Spencer EM, Lue TF. Insulin-like growth factor-I promotes proliferation and migration of cavernous smooth muscle cells. Biochem Biophys Res Commun. 2001;280:1307–1315. doi: 10.1006/bbrc.2001.4285. [DOI] [PubMed] [Google Scholar]
- 15.Ning N, Lin G, Lue TF, Lin CS. Effects of estrogen, raloxifene, and levormeloxifene on the expression of Rho-kinase signaling molecules in urethral smooth muscle cells. Urology. 2011;76(1517):e6–e11. doi: 10.1016/j.urology.2010.07.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudas J, Mansuroglu T, Batusic D, Ramadori G. Thy-1 is expressed in myofibroblasts but not found in hepatic stellate cells following liver injury. Histochem Cell Biol. 2009;131:115–127. doi: 10.1007/s00418-008-0503-y. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto K, de Waard V, Fearns C, Loskutoff DJ. Tissue distribution and regulation of murine von Willebrand factor gene expression in vivo. Blood. 1998;92:2791–2801. [PubMed] [Google Scholar]
- 18.Kawanami O, Jin E, Ghazizadeh M, Fujiwara M, Jiang L, Ohaki Y, Gomibuchi M, Takemura T. Mosaic-like distribution of endothelial cell antigens in capillaries and juxta-alveolar microvessels in the normal human lung. Pathol Int. 2000;50:136–141. doi: 10.1046/j.1440-1827.2000.01006.x. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Shimokata K, Nagura H. An immunohistochemical study on phenotypic heterogeneity of human pulmonary vascular endothelial cells. Virchows Arch A Pathol Anat Histopathol. 1988;412:479–486. doi: 10.1007/BF00750582. [DOI] [PubMed] [Google Scholar]
- 20.Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54:385–395. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- 21.Kwon O, Hong SM, Sutton TA, Temm CJ. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F351–F359. doi: 10.1152/ajprenal.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama T, Sato W, Yoshimura A, Zhang L, Kosugi T, Campbell-Thompson M, Kojima H, Croker BP, Nakagawa T. Endothelial von Willebrand factor release due to eNOS deficiency predisposes to thrombotic microangiopathy in mouse aging kidney. Am J Pathol. 2010;176:2198–2208. doi: 10.2353/ajpath.2010.090316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pavenstadt H. Roles of the podocyte in glomerular function. Am J Physiol Renal Physiol. 2000;278:F173–F179. doi: 10.1152/ajprenal.2000.278.2.F173. [DOI] [PubMed] [Google Scholar]
- 24.Castrechini NM, Murthi P, Gude NM, Erwich JJ, Gronthos S, Zannettino A, Brennecke SP, Kalionis B. Mesenchymal stem cells in human placental chorionic villi reside in a vascular Niche. Placenta. 2010;31:203–212. doi: 10.1016/j.placenta.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Ning H, Liu G, Lin G, Yang R, Lue TF, Lin CS. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.