Abstract
Mesd is a specialized chaperone for Wnt co-receptor LRP5 and LRP6, which contain four β-propeller/EGF modules, named E1 to E4 from N- to C-terminal, in their extracellular domains. Herein, we demonstrated that recombinant Mesd protein is a general Wnt inhibitor that blocks Wnt/β-catenin signaling induced not only by LRP6 E1–E2-binding Wnts but also by LRP6 E3–E4-binding Wnts. We also found that Mesd suppressed Wnt/β-catenin signaling induced by Wnt1 in prostate cancer PC-3 cells, and inhibited tumor growth in PC-3 xenograft model. Our results indicate that Mesd is a universal inhibitor of Wnt/LRP signaling on the cell surface.
Keywords: Wnt, Mesd, LRP5/6, Wnt signaling, prostate cancer
1. Introduction
The low density lipoprotein receptor-related protein-5 (LRP5) and LRP6 are essential co-receptors for the Wnt/β-catenin signaling pathway [1]. In the absence of Wnt ligands, β-catenin is phosphorylated by a multi-protein complex that marks it for ubiquitination and degradation by the proteasome. The action of this complex is inhibited upon binding of Wnt to its cell-surface receptors Frizzled (Fz) and LRP. The LRP-Wnt-Fz binding results in stabilization of cytosolic β-catenin, which enters the nucleus to activate Wnt target genes by binding to transcription factors of the T-cell factor/lymphoid enhancing factor (TCF/LEF) family [1].
Mesd is a specialized molecular chaperone for the Wnt co-receptors LRP5 and LRP6 [2–7]. Both LRP5 and LRP6 contain four β-propeller/epidermal growth factor (EGF) modules, named E1 to E4 from N- to C-terminal. Mesd is specifically required for the maturation of these β-propeller/EGF modules through the secretory pathway [4]. In our previous studies, we found that recombinant Mesd protein binds to mature LRP5 and LRP6 on the cell surface, and is able to inhibit Wnt3a-induced Wnt/β-catenin signaling in LRP5/6 expressing cells [8]. All the identified LRP5/6 extracellular ligands including Mesd and Wnt proteins bind to the β-propeller/EGF repeat modules [1, 4, 9–12]. There are about 19 vertebrate members of the Wnt family. Recent studies indicated different Wnt proteins bind to distinct regions of LRP6. For example, Wnt 1, Wnt9a and Wnt10b bind exclusively within the LRP6 E1–E2 region in vitro, whereas Wnt3 and Wnt3a bind only to a fragment containing E3–E4 [10–12]. We have recently demonstrated that Mesd is able to block Wnt3a-induced Wnt/β-catenin signaling in LRP5- and LRP6-expressing cells, and inhibits Wnt/β-catenin signaling in prostate cancer PC-3 cells in vitro [8]. In this report, we demonstrated that Mesd is a general inhibitor of different Wnt proteins in Wnt/LRP signaling and suppresses PC-3 tumor growth in vivo.
2. Materials and Methods
2.1. Materials
Plasmid pcDNA3.1C-Myc-hLRP5 containing the full-length human LRP5 cDNA and plasmid pCS-Myc-hLRP6 containing the full-length human LRP6 cDNA were from Dr. Cindy Bartels (Case Western Reserve University, Cleveland) and Dr. Christof Niehrs (Deutsches Krebsforschungszentrum, Heidelberg, Germany), respectively. Plasmid pGST-E-cadherin was provided by Dr. Gail Johnson (University of Rochester, New York). Plasmid pUSEamp-Wnt1-HA containing the full-length mouse Wnt1 cDNA was purchased from Upstate. Plasmid pBS-Wnt3-HA containing full-length human Wnt3 and plasmid BA-Wnt10b containing full-length mouse Wnt10b were from Addgene. Plasmid pcDNA3-Wnt3-HA was constructed by ligating the EcoR1-Xba1 fragment containing the full-length Wnt3 cDNA with HA tag from pBS-Wnt3-HA into the EcoR1-Xba1 site of pcDNA3 vector. A β-galactosidase expressing vector and the luciferase and β-galactosidase assay systems were purchased from Promega. Preparation of recombinant mouse Mesd protein has been described before [5]. The monoclonal anti-HA antibody was generated as previously described [13]. The peroxidase-labeled anti-mouse antibody and ECL system were purchased from Amersham Life Science.
2.2. Cell Culture
HEK293 and PC-3 cells were purchased from American Type Culture Collection. HEK293 and PC-3 cells were maintained in Dulbecco’s modified Eagle’s medium and Roswell Park Memorial Institute medium-1640, respectively. Both media were supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin and 100 mg/ml streptomycin.
2.3. Luciferase Reporter Assay
Unlike our previous study (8), we used Super8XTOPFlash reporter (kindly provided by Dr. Randall T. Moon, University of Washington, Seattle) for the Wnt/β-catenin signaling reporter assay in the current study. Super8XTOPFlash reporter has a much higher signal/noise ratio than TOPFlash reporter (The Wnt homepage: http://www.stanford.edu/group/nusselab/cgi-bin/wnt/). HEK293 and PC-3 cells were plated into 24-well plates. After overnight culture, the cells were transiently transfected with various Wnt plasmids, LRP5 or LRP6 plasmid along with Super8XTOPFlash plasmid and β-galactosidase-expressing vector by FuGENE HD (Roche). After 24 h incubation, cells were treated with Mesd. Cells were then lysed 24 h later and both luciferase and β-galactosidase activities were determined. The luciferase activity was normalized to the β-galactosidase activity.
2.4. Western Blotting
Cells in 6-well plates were lysed in 0.5 ml of phosphate-buffered saline (PBS) containing 1% Triton X-100, 1 mM PMSF and 1 X protease inhibitor cocktail (lysis buffer) at 4°C for 10 min. Equal quantities of protein were subjected to SDS-PAGE under reducing conditions, and transferred to immobilon-P transfer membrane. Membranes were blocked with 5% nonfat milk in PBS containing 0.1% Tween 20 (blocking buffer) for 0.5 h at room temperature, and incubated overnight at 4 °C with anti-β-catenin (1:5000, BD Biosciences), anti-Wnt1 (1:3000, R&D Systems), anti-Wnt3 (1:3000, Sigma-Aldrich), anti-Wnt10b (1:8000, Sigma-Aldrich), anti-LRP5 (1:1000, MyBiosource), anti-LRP6 (1:500, Santa Cruz Biotechnology), or anti-actin (1:5000, Sigma-Aldrich) antibody diluted in the blocking buffer. After several washes with PBS containing 0.1% Tween 20, membranes were incubated in the blocking buffer with horseradish peroxide conjugated secondary antibody for 60 min at room temperature. After washing, the immunoreactive proteins were then detected using the ECL system. Films showing immunoreactive bands were scanned by Kodak Digital Science DC120 Zoom Digital Camera.
2.5. Cytosolic Free β-catenin Analysis with GST-E-cadherin Binding Assay
The GST-E-cadherin binding assay was carried out exactly as described [14]. Briefly, cells were lysed in 0.5 ml of lysis buffer at 4°C for 10 min, and extracts were clarified by centrifugation at 18,000 × g for 2 min. 100 μg of total cell extracts were incubated with Sepahrose beads bound to the GST-E-cadherin. The GST-E-cadherin Sepahrose beads were prepared as described [14]. After 1 h of incubation at 4°C, the Sepharose beads were collected by centrifugation at 10,000 × g for 1 min, washed 2 times with lysis buffer containing 0.1% SDS and 0.5 % bovine serum albumin and 2 times with PBS buffer, and boiled in SDS sample buffer containing β-mercaptoethanol. The supernatants were subjected to SDS-PAGE and Western blotting with β-catenin antibody.
2.6. In Vivo Tumor Suppression Study
The antitumor efficacy of Mesd was evaluated in a mouse model using human PC-3 tumor fragments (from an in vivo established line) that were implanted subcutaneously in 5–6 week old male NCr-nu/nu athymic mice. The day of tumor implantation was designated as day 0. Mesd at a dosage of 12.5 mg/kg/dose was given every other day by intraperitoneal injection. There were 10 mice in each group. Tumor dimensions and body weights were measured twice weekly. Tumor volume was calculated as x2y/2, where x = width, y = length, and x < y. This formula was also used to calculate tumor weight, assuming unit density (1 mm3 = 1 mg). All animal protocols were reviewed by the Southern Research IACUC prior to experimentation.
2.7. Immunohistochemistry Staining
The slides were prepared by the Pathology Core Facility at Southern Research Institute using a standard protocol. Immunohistochemistry for Ki67 (clone B56, BD Bioscience, 1:25 dilution) was carried out, with antigen retrieval using BD Bioscience Retrievagen solution, and detection via a BD Bioscience three-step staining procedure according to manufacturer-recommended protocol.
2.8. Statistics
Statistical analyses were performed using Student’s unpaired t-test for in vitro studies or one-way ANOVA analysis with Tukey post test for in vivo tumor suppression study with a significance level of ≤ 0.05.
3. Results
3.1. Mesd Suppresses Wnt/β-catenin Signaling Induced by Wnt1, Wnt3 and Wnt10b in HEK293 Cells
Recent studies indicated that different Wnt proteins bind to distinct regions of LRP5 and LRP6 [10–12]. In our previous study, we demonstrated that Mesd blocks Wnt3a-induced Wnt/β-catenin signaling [8]. Like Wnt3a, Wnt3 binds only to a fragment containing LRP6 E3–E4 region, whereas Wnt 1 and Wnt10b bind exclusively within the LRP6 E1–E2 region [10–12]. Initially, we performed Wnt/β-catenin signaling reporter assay to test whether Mesd blocks Wnt/β-catenin signaling induced by different Wnt proteins. As expected, Wnt1, Wnt3 or Wnt10b expression resulted in a significant increase of Super8XTOPFlash activity in HEK293 cells (Fig. 1A). Importantly, we also found that the Super8XTOPFlash activity induced by Wnt1, Wnt3 or Wnt10b was greatly inhibited by Mesd treatment (Fig. 1A). It is also interesting to note that the inhibitory effect of Mesd on the Wnt3-induced Super8XTOPFlash activity was not as obvious as on the Wnt1 or Wnt10b-induced Super8XTOPFlash activity, suggesting that Mesd has different levels of inhibition towards different Wnt proteins.
Fig. 1.
Mesd blocks Wnt/β-catenin signaling induced by Wnt1, Wnt3 and Wnt10b in HEK293 cells. (A) HEK293 cells in 24-well plates were transiently transfected with 0.1 μg of Wnt1, Wnt3, Wnt10b plasmid or the corresponding control vector, along with 0.05 μg of Super8XTOPFlash construct and 0.05 μg of β-galactosidase-expressing vector in each well. After being incubated for 24 h, cells were treated with 1 μM Mesd. The luciferase activity was then measured 24 h later with normalization to the activity of the β-galactosidase. Values are averages of three determinations with the standard deviations indicated by error bars. **P < 0.01 compared to the control cells without Mesd treatment. (B) HEK293 cells in 6-well plates were transiently transfected with 1 μg of Wnt1, Wnt3, Wnt10b plasmid or the corresponding control vector. After being incubated for 24 h, cells were treated with 1 μM Mesd. Twenty-four hours later, the levels of total β-catenin, free β-catenin, Wnt1, Wnt3, and Wnt10b were analyzed with Western blotting using a specific antibody against each protein. Samples were also probed with the anti-actin antibody to verify equal loading.
To confirm the inhibitory effects of Mesd on Wnt1, Wnt3 or Wnt10b-induced Wnt/β-catenin signaling, we examined the level of cytosolic free β-catenin after Mesd treatment. We found that Wnt1, Wnt3 or Wnt10b expression resulted in an increase of cytosolic free β-catenin levels in HEK293 cells, and that the increased cytosolic free β-catenin levels were significantly reduced after Mesd treatment (Fig. 1B). In addition, the increased total cellular β-catenin levels in Wnt1 or Wnt10b-expresssing cells were also decreased after Mesd treatment (Fig. 1B).
3.2. Mesd Suppresses Wnt/β-catenin Signaling Induced by Wnt1, Wnt3 and Wnt10b in LRP5 or LRP6 Expressing HEK293 Cells
LRP5 and LRP6 are essential Wnt co-receptors. LRP5 or LRP6 expression resulted in a significant increase of Super8XTOPFlash activity in HEK293 cells. Wnt1, Wnt3 or Wnt10b co-expression enhanced the Super8XTOPFlash activity in LRP5- and LRP6-expressing HEK293 cells. Importantly, the increased Super8XTOPFlash activity induced by LRP5, LRP6, Wnt1, Wnt3, Wnt10b, LRP5 plus Wnt, or LRP6 plus Wnt was blocked by Mesd treatment (Fig. 2 and Supplementary Fig. 1). The expression of LRP5, LRP6, LRP5 plus Wnt3 or Wnt10b, or LRP6 plus Wnt3 or Wnt10b also resulted in significant increases of cytosolic free β-catenin levels. As expected, the increased levels of cytosolic free β-catenin were suppressed by Mesd treatment (Fig. 3 and Supplementary Fig. 2). The levels of total cellular β-catenin were also reduced after Mesd treatment, although the changes were not as obvious as the levels of cytosolic free β-catenin (Fig. 3 and Supplementary Fig. 2).
Fig. 2.
Mesd blocks Wnt/β-catenin signaling reporter activities induced by LRP5 or LRP6 in combination with Wnt1, Wnt3 or Wnt10b in HEK293 cells. HEK293 cells in 24-well plates were transiently transfected with the LRP6 (A and C) or LRP5 (B and D) in combination with Wnt1 (A and B), Wnt10b (C and D) or the corresponding control vector, along with the Super8XTOPFlash construct and the β-galactosidase expressing vector in each well. After being incubated for 24 h, cells were treated with 1 μM Mesd. The luciferase activity was then measured 24 h later with normalization to the activity of the β-galactosidase. Values are averages of three determinations with the standard deviations indicated by error bars. **P < 0.01 compared to the control cells without Mesd treatment.
Fig. 3.
Mesd blocks cytosolic free β-catenin accumulation induced by LRP5 or LRP6 in combination with Wnt10b in HEK293 cells. HEK293 cells in 6-well plates were transiently transfected with LRP6 (A) or LRP5 (B) in combination with Wnt10b or the corresponding control vector. After being incubated for 24 h, cells were treated with 1.0 μM Mesd. Twenty-four hours later, the levels of cytosolic free β-catenin, LRP5, LRP6, and Wnt10b were analyzed with Western blotting using a specific antibody against each protein. Samples were also probed with the anti actin antibody to verify equal loading. The pixels for each cytosolic free β-catenin band were measured, normalized and plotted. Data are mean values of four independent experiments with the standard deviations indicated by error bars. *P < 0.05, **P < 0.01 versus corresponding control value.
3.3. Mesd Suppresses Wnt/β-catenin Signaling Induced by Wnt1 in Prostate Cancer PC-3 Cells
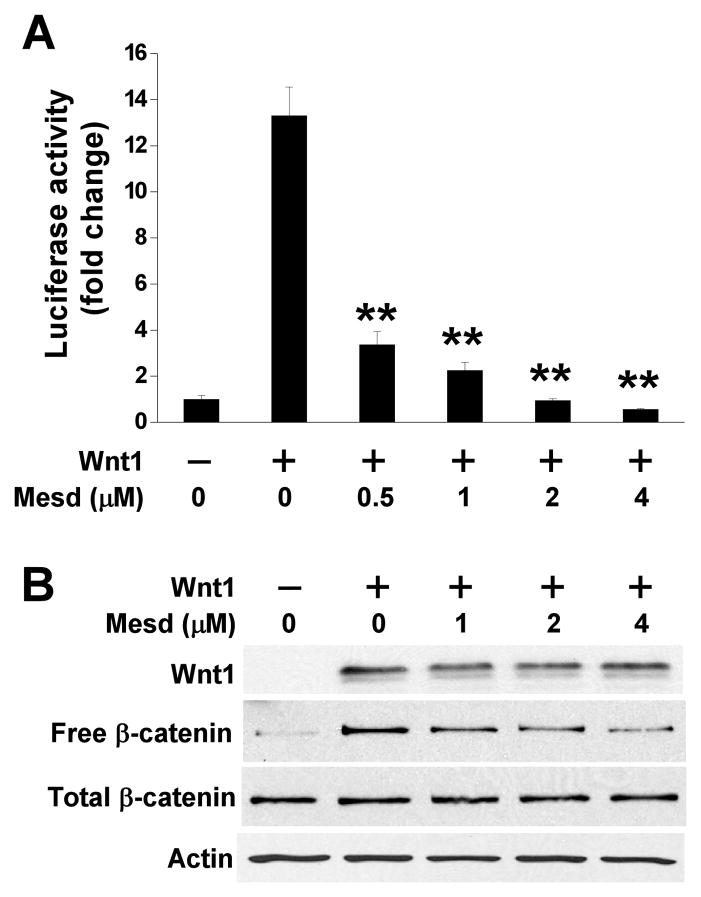
Overexpression of Wnt1 was found in the majority of prostate carcinoma specimens [15], suggesting that Wnt1 could play a direct role in promoting prostate carcinoma development and progression. In our previous study, we demonstrated that Mesd treatment inhibits Wnt/β-catenin signaling in prostate cancer PC-3 cells [8]. To test whether Mesd is able to suppress Wnt1-induced Wnt/β-catenin signaling in prostate cancer cells, we both transiently transfected PC-3 cells with Wnt1 and treated with Mesd. We found that Wnt1 expression resulted in an increase of Super8XTOPFlash activity in PC-3 cells, and that the increased Super8XTOPFlash activity was blocked by Mesd treatment (Fig. 4A). Accordingly, the increases of cytosolic free β-catenin level by Wnt1 were also suppressed by Mesd treatment (Fig. 4B).
Fig. 4.
Mesd blocks Wnt1-induced Wnt/β-catenin signaling in PC-3 cells. (A) PC-3 cells in 24-well plates were transiently transfected with 0.1 μg Wnt1 plasmid or the corresponding control vector, along with 0.05 μg Super8XTOPFlash construct and 0.05 μg β-galactosidase-expressing vector in each well. After being incubated for 24 h, cells were treated with Mesd at the indicated concentrations. The luciferase activity was then measured 24 h later with normalization to the activity of the β-galactosidase. Values are averages of three determinations with the standard deviations indicated by error bars. **P < 0.01 compared to the control cells without Mesd treatment. (B) PC-3 cells in 6-well plates were transiently transfected with 1.0 μg Wnt1 plasmid or the corresponding control vector. After being incubated for 24 h, cells were treated with Mesd at the indicated concentrations. Twenty-four hours later, the levels of total β-catenin, free β-catenin, and Wnt1 were analyzed with Western blotting using a specific anti-β-catenin or anti-Wnt1 antibody. Samples were also probed with the anti-actin antibody to verify equal loading.
3.4. Mesd Inhibits Tumor Growth in Prostate Cancer PC-3 Xenograft Model
In our previous study, we demonstrated that Mesd treatment suppress PC-3 cell proliferation in vitro [8]. Therefore, Mesd was further evaluated for in vivo antitumor efficacy in a mouse xenograft model using s.c. implanted human PC-3 tumor fragments. As shown in Fig. 5A, Mesd was tolerated for the duration of treatment. The body weight of the treatment group was comparable with the control group on any given day. The final body weight loss was 12% in the treatment group, while it was 17% in the control group. (The PC-3 model is cachexic.) On the other hand, Mesd treatment significantly attenuated PC-3 cell tumor growth in athymic mice (Fig. 5B). The mean tumor weight in treated mice was reduced by 42% (P<0.05) compared with the control group on day 21 (Fig. 5B). We also characterized tumors for expression of cell proliferation marker Ki67, and found that Ki67 expression was significantly decreased in Mesd-treated tumors (Figure 5C).
Fig. 5.
In vivo antitumor efficacy of Mesd with the human PC-3 prostate tumor xenograft mouse model. (A) Body weights of mice treated with vehicle (phosphate buffered saline) or Mesd given every other day by intraperitoneal injection for 11 treatments (days 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, and 21) at a dosage of 12.5 mg/kg/dose. (B) Tumor growth of mice treated with vehicle or Mesd at the dosage and schedule as described in (A). Values are averages of tumor weights from 10 mice in each group with the standard errors indicated by error bars. *P < 0.05 compared to the control mice without Mesd treatment. (C) Immunohistochemical staining with anti-Ki67 antibody of PC-3 tumors with or without Mesd treatment. Bar: 20μm.
4. Discussion
LRP5 and LRP6 are subjected to modulation by several secreted proteins which bind to the β-propeller/EGF repeat modules of LRP5/6 [1, 4, 9–12]. Different Wnt proteins require different β-propeller/EGF regions of LRP6 for Wnt/β-catenin signaling. Wnt 1 and Wnt10b specifically bind to the β-propeller/EGF region E1–E2 of LRP6 in vitro, whereas Wnt3 and Wnt3a bind only to a fragment containing the β-propeller/EGF region E3–E4 [10–12]. Interestingly, antibodies recognizing β-propeller/EGF region E1–E2 or E3–E4 of LRP6 specifically inhibit Wnt/β signaling induced by the Wnt proteins that bind these regions [11, 12]. Mesd binds to both the secreted mature β-propeller/EGF modules E1–E2 and E3–E4 of LRP6 [16], although it is still unclear whether a single molecule of Mesd is able to bind simultaneously to both E1–E2 and E3–E4 regions of LRP6. In our previous study, we reported that Mesd blocked Wnt3a-induced Wnt/β-catenin signaling in LRP5/6-expressing cells [8]. In the present study, we further demonstrated that Mesd was able to block Wnt1, Wnt3 and Wnt10b-induced Wnt/β-catenin signaling in LRP5/6-expressing cells, indicating that Mesd is a general Wnt inhibitor that blocks Wnt/β-catenin signaling induced not only by LRP6 E1–E2-binding Wnts but also by LRP6 E3–E4-binding Wnts. By performing coimmunoprecipitation experiments, we found that Mesd does not directly bind to β-catenin (data not shown), supporting the notion that the inhibitory effect of Mesd on Wnt/β-catenin signaling occurs on the cell surface.
While genetic mutations of certain intracellular components of the Wnt/β-catenin pathway, such as APC and CTNNB1, are significant contributing factors for colorectal cancers, dysregulation of cell surface Wnt/β-catenin signaling components could be an important mechanism associated with aberrant activation of this pathway in prostate cancer [17–19]. Overexpression of Wnt1 and accumulation of cytoplasmic/nuclear β-catenin were found in the majority of prostate carcinoma specimens, and were associated with advanced, metastatic, hormone-refractory prostate carcinoma [15]. ERG-mediated oncogenesis in prostate cancer was linked with activation of Wnt/β-catenin signaling through Wnt receptor Frizzled-4 [20]. In addition, gene silencing mediated through aberrant promoter methylation of upstream Wnt antagonist genes also resulted in constitutive activation of Wnt/β-catenin signaling in prostate cancer [21, 22]. As a correlate, treatment of prostate cancer LNCaP cells with Wnt3a significantly enhanced cell growth [23], whereas blocking Wnt/β-catenin signaling in prostate cancer cells either by the nonsteroidal anti-inflammatory drugs or small molecule Wnt/β-catenin signaling inhibitor PKF118–310 resulted in inhibition of prostate cancer cell proliferation [24]. Furthermore, the Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition [25]. In our recent study, we showed that recombinant Mesd protein suppressed Wnt/β-catenin signaling in prostate cancer PC-3 cells, in which Wnt/β-catenin signaling is highly activated, and inhibits PC-3 cell proliferation in vitro [8]. In the present study, we further demonstrated that recombinant Mesd protein was able to inhibit tumor growth in PC-3 xenograft model. Our results support the notion that the Wnt/β-catenin signaling pathway is a promising therapeutic target in prostate cancer treatment [6].
Both LRP5 and LRP6 are expressed in human cancer cell lines and human malignant tissues [26]. LRP5 deficient mammary glands were remarkably resistant to Wnt1-induced tumor development [27], whereas overexpression of LRP6 in the mouse mammary gland is sufficient to induce mammary hyperplasia [28]. Overexpression of a dominant-negative LRP5 mutant in osteosarcoma Saos-2 cells and prostate cancer PC-3 cells down-regulated Wnt/β-catenin signaling, and reduced cell invasion capacity and cell motility in vitro [29], and tumor growth in a xenograft mouse model [30]. In the present study, we demonstrated that recombinant Mesd protein was able to suppress PC-3 tumor growth in vivo. Consistent with our findings, it has been reported that in vivo administration of Mesd also suppressed growth of MMTV-Wnt1 tumors without causing undesirable side effects [31]. Altogether, these finding suggest that LRP5 and LRP6 are potential therapeutic targets for Wnt-dependent cancers.
In summary, we have demonstrated that recombinant Mesd protein was a general inhibitor for different Wnts in Wnt/LRP signaling activation, and was able to suppress the growth of PC3 prostate cancer xenografts in nude mice. Our results also suggest that Mesd is a useful research tool to study the function of Wnt/LRP signaling on the cell surface in various pathophysiological conditions such as bone metabolism, stem cells and cancer.
Supplementary Material
Highlights.
Mesd blocked Wnt/β-catenin signaling induced not only by LRP6 E1–E2-binding Wnts but also by LRP6 E3–E4-binding Wnts.
Mesd suppressed Wnt/β-catenin signaling induced by Wnt1 in prostate cancer PC-3 cells.
Mesd inhibited tumor growth in PC-3 xenograft model.
Acknowledgments
We are grateful to Drs. Christof Niehrs, Cindy Bartels, Gail Johnson, Randall T. Moon, Jan Kitajewski, Philip Leder for providing various plasmids and Gail Jones (Southern Research Institute) for help with statistical analysis. This work was supported by a grant from the National Institutes of Health RO1CA124531 (to Y.L.).
Abbreviations
- APC
adenomatous polyposis coli
- CM
conditioned medium
- EGF
epidermal growth factor
- ER
endoplasmic reticulum
- FBS
fetal bovine serum
- Fz
Frizzled
- GSK3β
glycogen synthase kinase 3β
- LDLR
low density lipoprotein receptor
- LRP5
low-density lipoprotein receptor-related protein-5
- RAP
receptor-associated protein
- TCF/LEF
T-cell factor/lymphoid enhancing factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Culi J, Mann RS. Boca, an endoplasmic reticulum protein required for wingless signaling and trafficking of LDL receptor family members in Drosophila. Cell. 2003;112:343–354. doi: 10.1016/s0092-8674(02)01279-5. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh JC, Lee L, Zhang L, Wefer S, Brown K, DeRossi C, Wines ME, Rosenquist T, Holdener BC. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell. 2003;112:355–367. doi: 10.1016/s0092-8674(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 4.Culi J, Springer TA, Mann RS. Boca-dependent maturation of beta-propeller/EGF modules in low-density lipoprotein receptor proteins. EMBO J. 2004;23:1372–1380. doi: 10.1038/sj.emboj.7600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Chen J, Lu W, McCormick LM, Wang J, Bu G. Mesd binds to mature LDL-receptor-related protein-6 and antagonizes ligand binding. J Cell Sci. 2005;118:5305–5314. doi: 10.1242/jcs.02651. [DOI] [PubMed] [Google Scholar]
- 6.Koduri V, Blacklow SC. Requirement for natively unstructured regions of mesoderm development candidate 2 in promoting low-density lipoprotein receptor-related protein 6 maturation. Biochemistry. 2007;46:6570–6577. doi: 10.1021/bi700049g. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Lu W, He X, Bu G. Modulation of LRP6-mediated Wnt signaling by molecular chaperone Mesd. FEBS Lett. 2006;580:5423–5428. doi: 10.1016/j.febslet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Lu W, Liu CC, Thottassery JV, Bu G, Li Y. Mesd is a universal inhibitor of Wnt coreceptors LRP5 and LRP6 and blocks Wnt/beta-catenin signaling in cancer cells. Biochemistry. 2010;49:4635–4643. doi: 10.1021/bi1001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, Costa M, Cochran AG, Hannoush RN. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem. 2010;285:9172–9179. doi: 10.1074/jbc.M109.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, Yu Q, Walsh R, Li S, Schmitz R, Heine H, Bilic S, Ostrom L, Mosher R, Hartlepp KF, Zhu Z, Fawell S, Yao YM, Stover D, Finan PM, Porter JA, Sellers WR, Klagge IM, Cong F. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci USA. 2010;107:15473–15478. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong Y, Bourhis E, Chiu C, Stawicki S, DeAlmeida VI, Liu BY, Phamluong K, Cao TC, Carano RA, Ernst JA, Solloway M, Rubinfeld B, Hannoush RN, Wu Y, Polakis P, Costa M. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One. 2010;5:e12682. doi: 10.1371/journal.pone.0012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obermoeller LM, Chen Z, Schwartz AL, Bu G. Ca2+ and receptor-associated protein are independently required for proper folding and disulfide bond formation of the low density lipoprotein receptor-related protein. J Biol Chem. 1998;273:22374–22381. doi: 10.1074/jbc.273.35.22374. [DOI] [PubMed] [Google Scholar]
- 14.Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, Rabbani SA. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 16.Liu CC, Pearson C, Bu G. Cooperative folding and ligand-binding properties of LRP6 beta-propeller domains. J Biol Chem. 2009;284:15299–15307. doi: 10.1074/jbc.M807285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wend P, Holland JD, Ziebold U, Birchmeier W. Wnt signaling in stem and cancer stem cells. Semin Cell Dev Biol. 2010;21:855–863. doi: 10.1016/j.semcdb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Verras M, Sun Z. Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, Rantala J, Alanen K, Nees M, Kallioniemi O. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70:6735–6745. doi: 10.1158/0008-5472.CAN-10-0244. [DOI] [PubMed] [Google Scholar]
- 21.Costa VL, Henrique R, Ribeiro FR, Carvalho JR, Oliveira J, Lobo F, Teixeira MR, Jerónimo C. Epigenetic regulation of Wnt signaling pathway in urological cancer. Epigenetics. 2010;16:343–351. doi: 10.4161/epi.5.4.11749. [DOI] [PubMed] [Google Scholar]
- 22.Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F, Hartmann A, Knuechel R, Rosenthal A, Pilarsky C. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003;201:204–212. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- 23.Verras M, Brown J, Li X, Nusse R, Sun Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004;64:8860–8866. doi: 10.1158/0008-5472.CAN-04-2370. [DOI] [PubMed] [Google Scholar]
- 24.Lu W, Tinsley HN, Keeton A, Qu Z, Piazza GA, Li Y. Suppression of Wnt/beta-catenin signaling inhibits prostate cancer cell proliferation. Eur J Pharmacol. 2009;602:8–14. doi: 10.1016/j.ejphar.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yee DS, Tang Y, Li X, Liu Z, Guo Y, Ghaffar S, McQueen P, Atreya D, Xie J, Simoneau AR, Hoang BH, Zi X. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol Cancer. 2010;23(9):162. doi: 10.1186/1476-4598-9-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Bu G. LRP5/6 in Wnt signaling and tumorigenesis. Future Oncol. 2005;1:673–81. doi: 10.2217/14796694.1.5.673. [DOI] [PubMed] [Google Scholar]
- 27.Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Li Y, Liu Q, Lu W, Bu G. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene. 2010;29:539–549. doi: 10.1038/onc.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- 30.Zi X, Guo Y, Simoneau AR, Hope C, Xie J, Holcombe RF, Hoang BH. Expression of Frzb/Secreted Frizzled-Related Protein 3, a Secreted Wnt Antagonist, in Human Androgen-Independent Prostate Cancer PC-3 Cells Suppresses Tumor Growth and Cellular Invasiveness. Cancer Res. 2005;65:9762–9770. doi: 10.1158/0008-5472.CAN-05-0103. [DOI] [PubMed] [Google Scholar]
- 31.Liu CC, Prior J, Piwnica-Worms D, Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci USA. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.