Abstract
Background and Purpose
The migratory efficiency of mesenchymal stem cells (MSC) toward cerebral infarct after transplantation is limited. Valproate (VPA) and lithium enhance in vitro migration of MSC by upregulating CXC chemokine receptor 4 and matrix metalloproteinase-9, respectively. Ability of VPA and lithium to promote MSC homing and to improve functional recovery was assessed in a rat model of cerebral ischemia.
Methods
MSC primed with VPA (2.5 mmol/L, 3 hours) and/or lithium chloride (2.5 mmol/L, 24 hours) were transplanted into rats 24 hours after transient middle cerebral artery occlusion (MCAO). Neurological function was assessed via rotarod test, Neurological Severity Score, and body asymmetry test for 2 weeks. Infarct volume was analyzed by MRI. The number of homing MSC and microvessel density in the infarcted regions were measured 15 days after MCAO using immunohistochemistry.
Results
Priming with VPA or lithium increased the number of MSC homing to the cerebral infarcted regions, and copriming with VPA and lithium further enhanced this effect. MCAO rats receiving VPA-primed and/or lithium-primed MSC showed improved functional recovery, reduced infarct volume, and enhanced angiogenesis in the infarcted penumbra regions. These beneficial effects of VPA or lithium priming were reversed by AMD3100, a CXC chemokine receptor 4 antagonist, and GM6001, a matrix metalloproteinase inhibitor, respectively.
Conclusions
Priming with VPA and/or lithium promoted the homing and migration ability of MSC, improved functional recovery, reduced brain infarct volume, and enhanced angiogenesis in a rat MCAO model. These effects were likely mediated by VPA-induced CXC chemokine receptor 4 overexpression and lithium-induced matrix metalloproteinase-9 upregulation.
Keywords: cerebral ischemia, lithium, mesenchymal stem cells, transplantation, valproate, MRI
For the past decade, stem cell therapy has been investigated as a potential treatment for stroke. Many studies have demonstrated the beneficial effects of stem cell therapy, particularly in rodent models.1 One major advantage of using mesenchymal stem cells (MSC) from bone marrow is that the preparation allows for autologous transplantation, thus avoiding tissue rejection responses.2 Recently, 2 small-scale clinical trials in ischemic stroke patients showed that intravenous autologous MSC transplantation improves functional recovery without adverse effects such as seizures or tumor formation.3,4 Nevertheless, MSC transplantation still requires extensive standardization and optimization before it can be deemed a feasible therapy for stroke and other central nervous system disorders.5
One key feature of MSC-based therapy is that MSC must migrate to disease target sites after transplantation. However, the homing of MSC to brain infarcted sites is far from ideal.6,7 In cerebral ischemia, stromal cell-derived factor-1α, secreted by astrocytes and endothelial cells around the infarcted region, attracts stem cells.8 CXC chemokine receptor 4 (CXCR4) is a α-chemokine receptor-specific for stromal cell-derived factor-1α; pretreatment of MSC with its antagonist inhibits MSC homing into the infarcted area.9 In addition, matrix metalloproteinase-9 (MMP-9) is involved in the motility of stem cells by degrading components of the extracellular matrix.10 It has been reported that MMP-9 suppression attenuated stem cells migration in the infarcted brain.11 Therefore, both stromal cell-derived factor-1α/CXCR4 interaction and MMP-9 expression appear to be essential for the homing ability of stem cells.
For decades, the mood stabilizers valproate (VPA) and lithium have been used to treat bipolar disorder.12,13 VPA inhibits histone deacetylases, which play a prominent role in transcriptional regulation,14 and lithium activates the Wnt downstream signaling pathway by inhibiting glycogen synthase kinase-3.15 We recently demonstrated that treatment with VPA or lithium enhances MSC migration by increasing CXCR4 expression via histone deaceteylase inhibition or by elevating MMP-9 levels through glycogen synthase kinase-3β inhibition, respectively. 16 This current study investigated whether priming MSC with VPA and/or lithium would enhance the homing ability of MSC and improve functional recovery after transplantation into rats in an experimental stroke model.
Materials and Methods
Cells and Chemicals
Cryopreserved rat MSC were purchased from Cell Applications. MSC used in this study were from the fifth passage of cultivation. MSC were plated at a density of 20 cells/mm2 and expanded in rat MSC culture medium (Cell Applications) at 37°C in a humidified atmosphere containing 95% air and 5% CO2, according to the manufacturer’s instructions. Chemicals used in the experiments included sodium VPA, lithium chloride, AMD3100 (Sigma-Aldrich), and GM6001 (Millipore).
Animal Middle Cerebral Artery Occlusion Model
All animal experiments were performed according to protocols approved by the National Institute of Mental Health Animal Care and Use Committee. Male Sprague-Dawley rats (190 to 230 g; Charles River Laboratories, Wilmington, MA) underwent middle cerebral artery occlusion (MCAO) as previously described.17 Briefly, rats were anesthetized with isoflurane (1.5% in a mixture of 70% N2O and 30% O2). A 4-0 nylon suture with heat-produced round tip was inserted from the right common carotid artery into the right internal carotid artery and then to the circle of Willis to occlude the origin of the right middle cerebral artery. One hour after MCAO, the nylon suture was withdrawn. Sham-operated rats underwent the same procedure without the nylon suture.
Study Design
Rats were divided into 9 groups (Table; n = 8 rats in each group except for group 1): (1) sham: sham-operated rats with (n = 4) or without (n = 8) MSC transplantation; (2) no treatment: rats received MCAO without MSC transplantation; (3) MSC: rats received naive MSC after MCAO; (4) MSC/VPA: rats received VPA-primed MSC after MCAO; (5) MSC/VPA/AMD: rats received VPA/AMD3100-primed MSC after MCAO; (6) MSC/Li-Li: rats received lithium-primed MSC after MCAO followed by 1 daily lithium injection for 14 days; (7) MSC/Li/GM-Li: rats received lithium/GM6001-primed MSC after MCAO followed by 1 daily lithium injection for 14 days; (8) Li: rats received 1 daily lithium injection for 14 days, beginning 24 hours after MCAO; and (9) MSC/VPA/Li-Li: rats received VPA/Li-primed MSC after MCAO followed by 1 daily lithium injection for 14 days.
Table.
Rat Grouping Information
| MSC Priming
|
Rat Treatment
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | LiCl 0 to 1 (Day) |
GM6001 0 to 1 (Day) |
VPA 18 to 21 (Hour) |
AMD3100 18 to 24 (Hour) |
MCAO 0 (Day) |
MSC 1 (Day) |
LiCl 1 to 14 (Day) |
|
| 1 | Sham | − | − | − | − | − | +/− | − |
| 2 | No treatment | − | − | − | − | + | − | − |
| 3 | MSC | − | − | − | − | + | + | − |
| 4 | MSC/VPA | − | − | + | − | + | + | − |
| 5 | MSC/VPA/AMD | − | − | + | + | + | + | − |
| 6 | MSC/Li-Li | + | − | − | − | + | + | + |
| 7 | MSC/Li/GM-Li | + | + | − | − | + | + | + |
| 8 | Li | − | − | − | − | + | − | + |
| 9 | MSC/VPA/Li-Li | + | − | + | − | + | + | + |
AMD indicates AMD3100; GM, GM6001; Li, lithium; MSC, mesenchymal stem cells; VPA, valproate.
Before transplantation, MSC were incubated with 100 μmol/L BrdU (Invitrogen) for 72 hours. MSC were primed with 2.5 mmol/L VPA for three hours, 20 μmol/L AMD3100 (a CXCR4 antagonist) for six hours, 2.5 mmol/L lithium for 24 hours, and/or 15 μmol/L GM6001 (an MMP inhibitor) for 24 hours before transplantation (Figure 1). At 24 hours after MCAO (Day 1), approximately 1×106 MSC in 1 mL PBS were injected into a rat through the tail vein. Rats receiving continuous lithium received one daily subcutaneous injection (2 mEq/kg) for 14 days.
Figure 1.
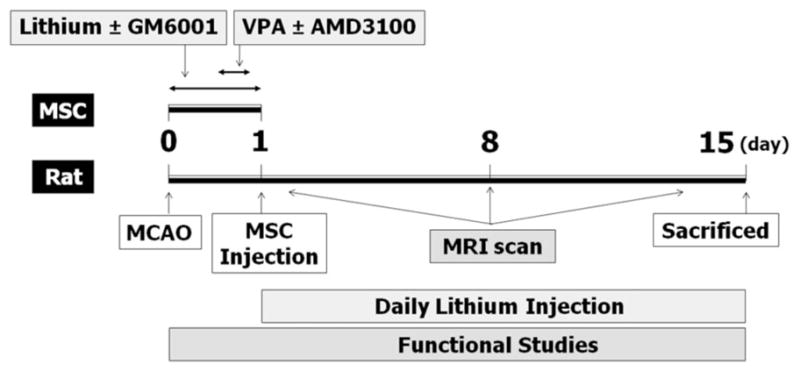
Schematic representation of the time course of experimental design.
Immunohistochemistry
The number of BrdU-labeled homing MSC and microvessel density in the penumbra cortex and striatum were detected by immunohistochemistry using antibodies against BrdU and RECA-1 (a pan-endothelium marker, Abcam), respectively. Detailed procedures are available online (Supplemental Methods, http://stroke.ahajournals.org).
Behavioral Testing
The rotarod test was performed 3 consecutive days before and on days 1, 4, 7, 11, and 15 after MCAO. Neurological Severity Score, 18 body asymmetry, 19 and body weight were evaluated immediately before and every day after MCAO. Detailed procedures are available online (Supplemental Methods).
MRI
All MRI experiments were performed on a 7-T (Bruker Avance), 210-mm horizontal scanner. The infarct volume on days 1, 8, and 15 after MCAO was evaluated using diffusion- or T2-weighted images. Detailed procedures are available online (Supplemental Methods).
Statistical Analyses
Values are expressed as mean ± standard error of the mean. For behavioral data, 2-way repeated measures analysis of variance was performed to analyze the overall difference between treatment groups over time, and then Bonferroni-corrected post hoc comparisons were used to analyze the difference between treatment groups at each time point. MRI results were analyzed by 2-way analysis of variance, followed by Bonferroni post-tests. Other data were analyzed by 1-way analysis of variance, followed by Turkey post hoc comparisons. Two-tailed P<0.05 were considered statistically significant.
Results
Optimization of VPA-Priming and Lithium-Priming Conditions
MSC were primed with 2.5 mmol/L VPA for 3 hours, followed by drug washout. Western blotting showed that CXCR4 in MSC was increased at 3 hours, reaching a peak of 2.9-fold compared to control at 6 hours after VPA washout (Supplemental Figure I). Acetylated histone H3 elevations were observed 1 hour after washout, indicating rapid histone deaceteylase inhibition. In contrast, MMP-9 levels remained unchanged. Therefore, VPA-primed MSC were transplanted 3 hours after drug washout to achieve the highest CXCR4 elevation.
MMP-9 protein levels in MSC were slightly increased after priming with 2.5 mmol/L lithium for 24 hours. However, continuous treatment with 1 mmol/L lithium after 24-hour lithium (2.5 mmol/L) priming markedly and time-dependently enhanced MMP-9 expression in MSC with a 2.4-fold increase after 2 days (Supplemental Figure IB). Glycogen synthase kinase-3β phosphorylation at Ser9 was elevated >2-fold 12 hours after continuous treatment, indicating glycogen synthase kinase-3β inhibition. These results are consistent with our recent report. 16 Hence, MSC primed with 2.5 mmol/L lithium for 24 hours were transplanted to MCAO rats, followed by 1 daily injection of lithium (2 mEq/kg) for 14 days to maintain MMP-9 elevations.
Priming With VPA and/or Lithium Enhances MSC Homing Into the Infarcted Brain
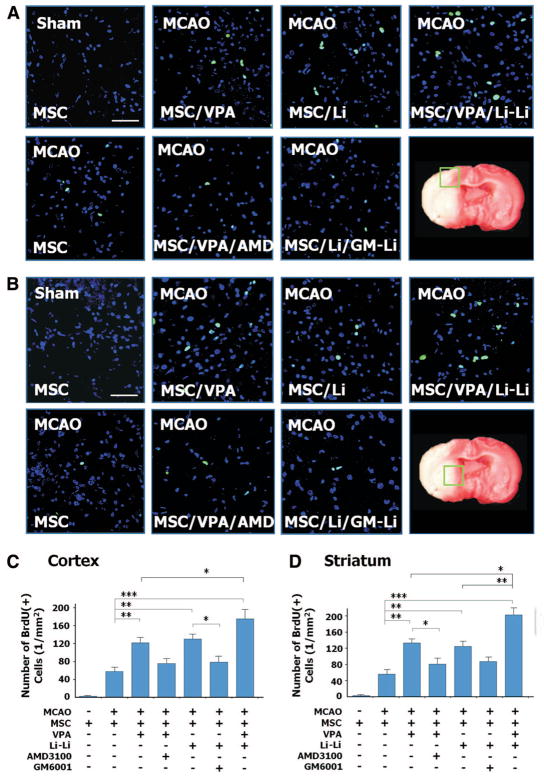
To detect the homing efficiency of injected MSC, the numbers of BrdU-positive MSC were counted in ischemic brain regions. In the penumbra cortex, priming with VPA or lithium increased MSC homing efficiency >2-fold compared to the unprimed MSC group (from 58 to 122/mm2 for VPA and to 130/mm2 for lithium; Figure 2A, C). Copriming with the CXCR4 antagonist AMD3100 or the MMP-9 inhibitor GM6001 largely inhibited the effects of VPA or lithium, respectively. Moreover, copriming with VPA and lithium further enhanced the number of the BrdU-positive MSC to 175/mm2. MSC primed with VPA and/or lithium had similar effects in the penumbra striatum (Figure 2B, D).
Figure 2.
Priming with valproate (VPA) and/or lithium promotes mesenchymal stem cells (MSC) homing into infarcted brain regions. MSC were labeled with BrdU before transplantation. Homing MSC (green) were detected using immunohistochemistry. In the ischemic frontal cortex (A) and striatum (B), the number of BrdU-positive cells increased when MSC were primed with sodium VPA (2.5 mmol/L) and/or lithium chloride (2.5 mmol/L) before transplantation. The homing-promoting effects of VPA and lithium were eliminated by AMD3100 (20 μmol/L) and GM6001 (15 μmol/L), respectively. Blue, DAPI. Bar, 100 μm. Quantitative data are shown in C and D. N = 8 per group except sham control (n = 4); *P<0.05; **P<0.01; ***P<0.001.
Immunohistochemistry staining showed that most of the homing MSC prelabeled with BrdU were well-colocalized with MSC markers, CD54 and fibronectin, in the infarcted brain in all treatment groups on day 15 after MCAO (Supplemental Figure IIA and B). A small fraction of MSC differentiated into astrocytes (GFAP staining; 1.5%, 1.3%, 1.6%, 1.5%) and endothelial cells (RECA-1 staining; 0.8%, 0.8%, 0.7%, 0.8%) in MSC, MSC/VPA, MSC/Li-Li, and MSC/VPA/Li-Li groups, respectively (Supplemental Figure IIC and D). These results suggested that the differentiating ability of MSC was similar between treatment groups. No BrdU-positive cells colocalized with the neuronal marker (NeuN, Supplemental Figure IIE) or microglial cell marker (Ibal, data not shown). Furthermore, there was no colocalization of BrdU and a proliferation marker Ki67 in any of the treatment groups (Supplemental Figure IIF). Additionally, no positive staining of osteocalin (an osteoblast marker) was detected in the ischemic brain (data not shown).
Transplantation of MSC Coprimed With VPA and Lithium Facilitates Functional Recovery
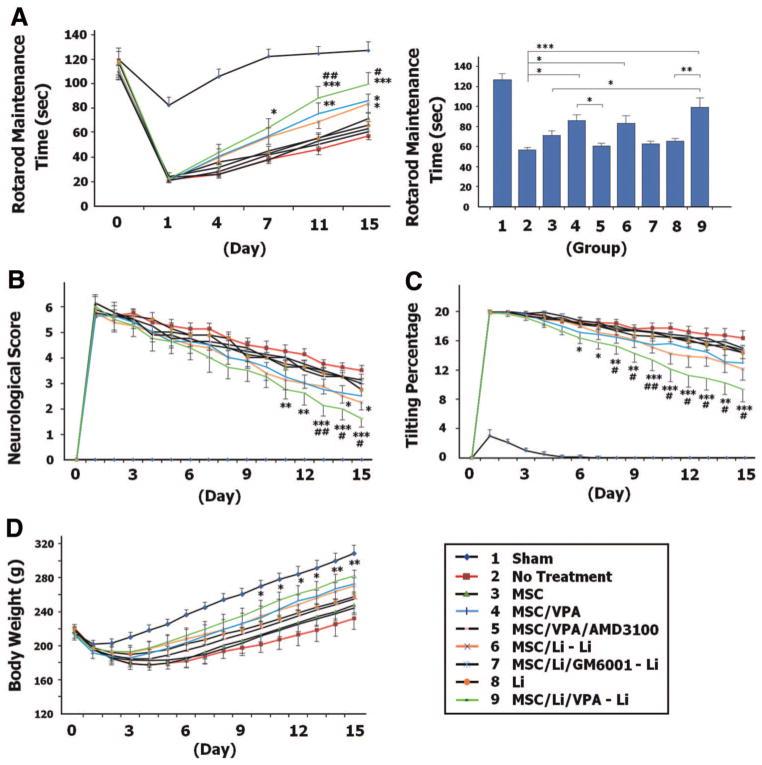
In all groups, the time that rats were able to stay on an accelerating rotarod declined sharply 1 day after MCAO and then gradually increased toward the control level (Figure 3A). Rats in the naive MSC transplantation group (group 3) did not improve their rotarod performance compared with untreated animals (group 2). However, compared with rats receiving no treatment, MCAO rats transplanted with either VPA-primed or lithium-primed MSC (groups 4 and 6) had notable increases in the amount of time that they stayed on the rotarod on day 15 after MCAO (see bar in Figure 3A). These beneficial effects of VPA or lithium priming were diminished by AMD3100 or GM6001, respectively. Furthermore, transplantation of MSC coprimed with VPA and lithium (group 9) robustly prolonged the time that MCAO rats remained on the rotarod compared with the groups of no treatment and naive MSC transplantation.
Figure 3.
Mesenchymal stem cells (MSC) primed with valproate (VPA) and/or lithium facilitate functional recovery in middle cerebral artery occlusion (MCAO) rats. A, MCAO rats that received MSC primed with VPA and/or lithium remained on the rotarod longer than rats in the untreated MCAO group (treatment F8,315 = 34.24, P<0.0001; time F5,315 = 307.67, P<0.0001; interaction F40,315 = 15.69, P<0.0001). The bar graph shows the results on day 15 after MCAO. The neurological deficit score (B; treatment F8,945 = 44.57, P<0.0001; time F15,945 = 535.21, P<0.0001; interaction F120,945 = 9.99, P<0.0001), body tilting percentage (C; treatment F8,945 = 143.37, P<0.0001; time F15,945 = 699.33, P<0.0001; interaction F120,945 = 11.95, P<0.0001), and body weight loss (D; treatment F8,945 = 4.74, P<0.0001; time F15,945 = 337.85, P<0.0001; interaction F120,945 = 3.94, P<0.0001) were improved in MCAO rats that received MSC primed with VPA and/or lithium, compared with untreated MCAO rats. N38 per group; * or #P<0.05; ** or ##P<0.01; ***P<0.001. *Comparison with the untreated group. #Comparison with the MSC group.
Similarly, neurological deficits, increased body tilting, and weight loss were observed 1 day after MCAO, but these changes gradually approached control conditions in all MCAO groups (Figure 3B–D). Transplantation of MSC coprimed with VPA and lithium markedly enhanced the recovery on neurological score, body tilting, and body weight loss starting from 11, 6, and 10 days after MCAO, respectively. Naive MSC transplantation did not show any benefits, whereas lithium-primed MSC significantly ameliorated the neurological severity score of MCAO rats on days 14 and 15. Functional performance was also significantly improved in the MSC/VPA/Li-Li group compared with the MSC group, as assessed by neurological score and body tilting (Figure 3B, C).
Transplantation of MSC Coprimed With VPA and Lithium Reduces Infarct Volume
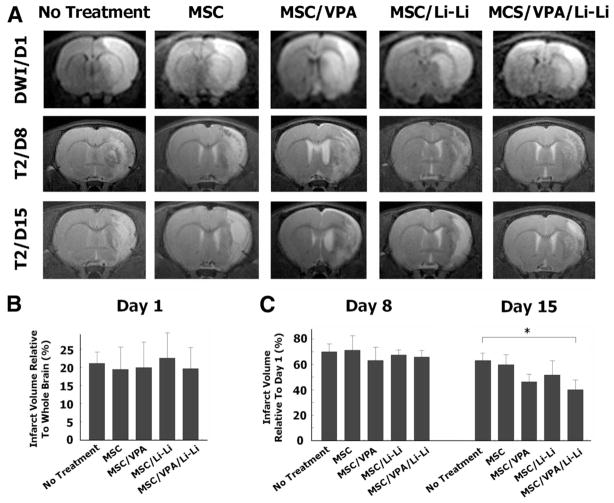
MRI sequences of diffusion-weighted images were used to determine the size of acute cerebral infarct 1 day after MCAO, and T2-weighted imaging was applied to analyze the residual infarct volume on days 8 and 15 after MCAO. Before MSC transplantation, diffusion-weighted images showed that infarct size did not differ between the MCAO groups (Figure 4A, B). On day 8, infarct size as measured by T2-weighted imaging decreased to 60% to 70% of the initial day 1 infarct volume without significant differences between groups (Figure 4A, C). On day 15, the mean infarct volume was significantly reduced in MSC/VPA/Li-Li rats (group 9) compared to MCAO rats (40% versus 63%; Figure 4A, C). To confirm this finding, we further measured the infarct area using hematoxylin and eosin staining in brain slices derived from untreated MCAO and MSC/VPA/Li-Li groups on day 15. There was a significant reduction in infarct area in MSC/VPA/Li-Li rats compared with untreated MCAO rats (5.2 versus 16.4 mm2; Supplemental Figure III).
Figure 4.
Mesenchymal stem cells (MSC) primed with valproate (VPA) and/or lithium reduce infarct volume in middle cerebral artery occlusion (MCAO) rats. A, MRI sequences of diffusion-weighted images (DWI) were used to observe the size of acute cerebral infarct on day 1. T2-weighted imaging was applied to analyze the residual infarct volume on days 8 and 15 after MCAO under experimental conditions as indicated. B, One day after MCAO, and immediately before drug treatment, cerebral infarct volume did not differ between the 5 experimental groups. C, On day 8 after MCAO, the infarct size decreased to 60% to 70% of the initial infarct volume detected on day 1. There was no statistical difference between groups. On day 15, the infarct volume in the MSC/VPA/Li-Li group was significantly smaller than that in the MCAO control group. N = 5 per group; *P<0.05.
Transplantation of MSC Coprimed With VPA and Lithium Increases Microvessel Density in Infarcted Brain
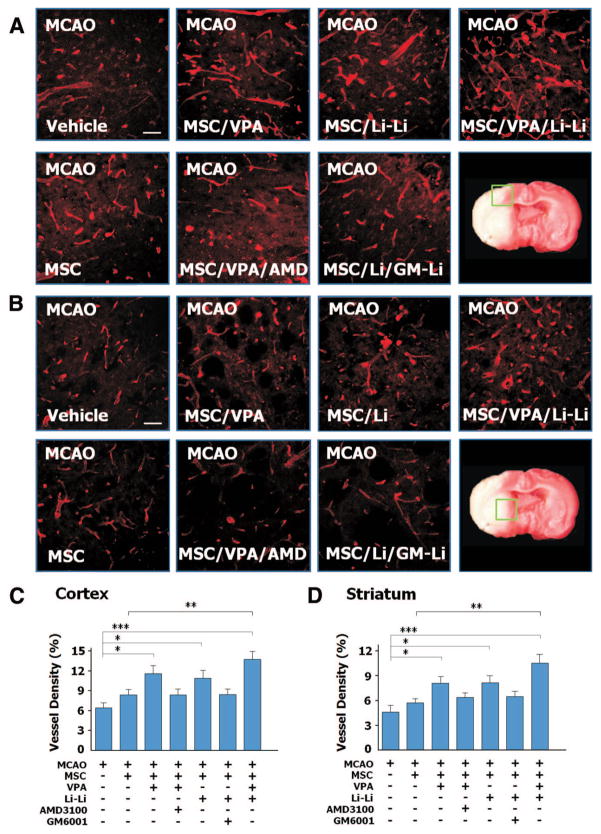
Microvessel density was analyzed in the penumbra cortex and striatum on day 15 after MCAO. In the cortex, naive MSC transplantation did not significantly affect cortical microves-sel density in MCAO rats compared with the MCAO group (6.4%). Priming with VPA or lithium significantly increased microvessel density compared with the MCAO group (11.5% for VPA and 10.9% for lithium). Moreover, MCAO rats transplanted with MSC coprimed with VPA and lithium (group 9) had the highest microvessel density (13.7%). Copriming with AMD3100 or GM6001 partially eliminated the effects of VPA or lithium, respectively. Similar patterns were observed in the penumbra striatum (Figure 5B, D).
Figure 5.
Mesenchymal stem cells (MSC) primed with valproate (VPA) and/or lithium increased microvessel density in the ischemic brain regions after transplantation. In the ischemic frontal cortex (A) and striatum (B), microvessel density increased when middle cerebral artery occlusion (MCAO) rats were transplanted with VPA-primed and/or lithium-primed MSC, and this effect of VPA or lithium was largely suppressed by AMD3100 and GM6001, respectively, as determined by immuno-histochemistry. Bar = 50 μm. Quantitative data are shown in C and D. N = 8 per group; *P<0.05; **P<0.01; ***P<0.001.
Discussion
MSC-based therapy has been shown to improve neurological outcome after ischemic stroke in rodent models and in some patients.1–4 Because the homing ability of MSC toward a brain infarcted region is restricted, 6,7 enhancing the migration of MSC is key to optimize MSC therapy in stroke. Here, we demonstrated for the first time to our knowledge the utility of priming with mood stabilizers before MSC transplantation in an experimental ischemic model of stroke.
Specifically, this study demonstrated that priming with VPA or lithium before transplantation enhanced the migration of MSC toward brain infarcted areas in rats that underwent MCAO. Copriming with VPA and lithium further promoted this homing efficiency. MSC coprimed with VPA and/or lithium significantly reduced infarct volume and improved functional recovery and angiogenesis in the penumbra cortex and striatum. Notably, VPA and lithium copriming produced the most advantageous effects. These observations echo previous work showing that cotreatment with VPA and lithium synergistically protected against glutamate-induced excitotoxicity in cultured neurons, 20 a mechanism that has been implicated in the pathophysiology of stroke. In contrast, naive MSC transplantation showed no beneficial effect compared with the MCAO rats. These results are consistent with those in previous reports showing that transplantation of the same amount (1×106) of MSC fails to produce protective effects after either intravenous or intracerebral injection in MCAO rats. 7,21 Previous study from our laboratory demonstrated that lithium administration at 3 hours, but not 24 hours, after MCAO reduces brain damage and facilitates neurological recovery. 22 In this study, daily lithium treatment starting at 24 hours after MCAO also did not improve performance on rotarod, tilting, and neurological tests. These findings strongly suggest that the beneficial effects observed in the MSC/Li-Li group were mainly attributable to lithium priming rather than direct injection of this drug into animals after MSC transplantation.
CXCR4 and MMP-9 are 2 major molecules known to facilitate MSC homing and migration in cerebral ischemia.9,11 A recent study found that upregulation of CXCR4 and MMP-9 promotes the mobility of transplanted MSC toward the cerebral infarcted site and improves functional outcome in a rodent stroke model.23 Our recent in vitro study showed that VPA and lithium enhance MSC migration by respective upregulation of the histone deaceteylase-CXCR4 and glycogen synthase kinase-3β – MMP-9 signaling pathways.16 Therefore, we optimized the priming conditions of VPA and lithium by detecting the ability of these drugs to upregulate CXCR4 and MMP-9 in MSC. Transient treatment of MSC with VPA for 3 hours followed by 3 hours of drug washout ensured high levels of CXCR4 protein expression. Hence, we used this VPA priming method to improve migration to the ischemia-injured brain sites. With regard to lithium, continuous treatment after priming robustly enhanced MMP-9 expression in MSC. Therefore, MCAO rats transplanted with lithium-primed MSC also received 1 daily subcutaneous lithium injection for 14 days to sustain MMP-9 upregulation and facilitate migration toward infarcted regions. We found that copriming with AMD3100 for VPA or GM6001 for lithium largely suppressed the homing ability of MSC and diminished their beneficial effects on functional recovery and angiogenesis. Because GM6001 is not a specific MMP-9 inhibitor, other MMP could also be involved. However, our in vitro study demonstrated that lithium enhances the expression and activity of MMP-9 in MSC without affecting mRNA levels of MMP-2, MMP-3, and membrane-type MMP.16 Thus, the reverse effect of GM6001 here is most likely attributable to MMP-9 inhibition. Taken together, the results of the present study confirm and extend our previous in vitro work and suggest that CXCR4 and MMP-9 are key players in mediating the priming effects of VPA and lithium in MSC transplanted into ischemic rats. Accordingly, both CXCR4 and MMP-9 are rational targets for MSC-based therapy in future studies aimed at improving the clinical efficacy of MSC transplantation.
After transplantation, the majority of MSC continued to express MSC markers, CD54 and fibronectin, in the ischemic brain. A small number of MSC differentiated into astrocytes and endothelial cells. The differentiating ability was similar between naive and VPA-primed and/or lithium-primed MSC groups, suggesting that VPA and lithium priming does not affect MSC differentiating ability in the ischemic brain. We did not detect colocalization of BrdU with the neuronal or microglial cell marker. Thus, we tend to exclude the possibility of neuronal differentiation or microglial differentiation/phagocytosis. Long-term VPA treatment has been reported to enhance osteogenic differentiation in MSC cultured in osteogenic differentiation medium, but 1-day VPA exposure has no such effect.24 In our study, MSC were cultured in growth medium and were exposed to VPA for only 3 hours before transplantation. Therefore, short-term VPA priming and lack of osteogenic differentiation conditions may have excluded the possibility of osteogenic differentiation in VPA-primed MSC in the ischemic brain. It has been reported that lithium enhances MSC proliferation through the Wnt signaling pathway,25 whereas VPA at high concentrations reduces human MSC proliferation.26 Our in vitro study found that treatment with 2.5 mmol/L lithium for 24 hours increases MSC proliferation from 33.4% to 46.9% (unpublished data), and 2.5 to 10 mmol/L VPA treatment for 3 hours followed by drug washout does not affect MSC proliferation.16 In the current study, we failed to detect colocalization of BrdU and Ki67, a proliferation marker, in the ischemic brain in either naive or drug-primed MSC groups on day 15 after MCAO, suggesting that VPA priming and lithium priming do not affect MSC proliferation in the ischemic brain. MSC used in this study were from the fifth passage of cultivation. It is conceivable that these comparatively aged MSC may have largely lost their proliferative ability in the ischemic brain after transplantation.
The potential effects of MSC in stroke therapy include immunomodulation, angiogenesis, neurogenesis, neurotrophism, and neuroprotection.5,6 There is a possibility that MSC integrate into the brain, differentiate into brain cells, and replace damaged tissue. However, our results showed that the majority of MSC still retained their undifferentiated character in the infarcted brain, and only a small number of MSC differentiated into astrocytes and endothelial cells with no neuronal or microglial differentiation detected. In addition, we did not find MSC proliferation in our experimental paradigm. Furthermore, MSC coprimed with VPA and lithium facilitated functional recovery as early as 6 to 11 days after MCAO, a time period that is likely too short for cell replacement to occur. Therefore, cell replacement may not be involved in the beneficial effects. Accumulating reports demonstrate that transplanted MSC produce neurotrophic factors and promote angiogenesis in the ischemic brain.27,28 In the present study, VPA and/or lithium priming significantly promoted angiogenesis in the infarcted brain. Moreover, the improvement in neurological performance and reduction in the infarct size were well-correlated with increased microvessel density in penumbra regions. Hence, our findings suggest that production of neurotrophic factors and promotion of angiogenesis may underlie the beneficial effects of MSC primed with VPA and lithium.
It should be noted that intravascular injection of MSC poses a risk of flow disturbance in cerebral arteries, which might exacerbate infarct status.29 Previous clinical trials also found that to obtain sufficient MSC before transplantation, autologous MSC need to be cultured for >1 month.3,4 Because VPA/lithium priming robustly improved the homing efficacy of MSC, it is likely that fewer MSC would be required to achieve clinical efficacy, thus reducing the risk of cerebral flow interruption and curtailing the time necessary to cultivate MSC for transplantation.
Conclusions
MSC coprimed with VPA and lithium showed remarkable improvement in homing ability and contributed significantly to functional recovery after transplantation into MCAO rats. The underlying mechanisms likely involve CXCR4 overexpression by VPA and MMP-9 upregulation by lithium. Both VPA and lithium have long been used in the treatment of bipolar disorder, and their safety profile, clinical features, and potential adverse effects are well-characterized. Thus, future clinical trials using both of these drugs to prime MSC before transplantation into stroke patients are warranted.
Supplementary Material
Supplemental Figure S1. Treatment of MSCs with VPA and lithium causes increases in CXCR4 and MMP-9, respectively. (A) MSCs were treated with 2.5 mmol/L sodium VPA for three hours and then cultured with fresh medium; cells were harvested at different time points after VPA washout. Levels of acetylated histone-H3 and CXCR4 protein peaked at one and six hours after VPA washout, respectively, as determined by Western blotting. (B) When MSCs were treated with 2.5 mmol/L lithium for 24 hours followed by drug washout, MMP-9 levels did not significantly increase. However, when MSCs were treated with 2.5 mmol/L lithium for 24 hours followed by continuous treatment with 1 mmol/L lithium, levels of MMP-9 gradually increased,
Supplemental Figure S2. VPA and/or lithium priming does not affect the differentiation and proliferation of MSCs in the infarcted brain. (A and B) Most of BrdU-labeled MSCs (green) expressed MSC markers CD54 (red) and fibronectin (red). Enlarged colocalization is shown in the upper right insert. (C and D) A few BrdU-labeled MSCs (green) expressed the astrocyte marker GFAP (red) and endothelial cell marker RECA-1 (red). (E) No BrdU-labeled MSCs (green) colocalized with the neuronal marker NeuN (red). NeuN staining in the infarcted cortex is shown in the upper right insert as the positive control. (F) No BrdU-labeled MSCs (green) colocalized with a proliferation marker Ki67 (red). Ki67 staining in the infarcted cortex is shown in the upper right insert as the positive control. Blue, DAPI; arrow head, BrdU-positive cell; arrow, NeuN staining; Bar = 50 μm.
Supplemental Figure S3. Transplantation of MSCs primed with VPA and lithium reduces infarct area in MCAO rats. H&E staining was used to measure brain infarct area on Day 15 after MCAO. MCAO rats that received transplanted VPA-and lithium-primed MSCs had significantly smaller infarct area than rats in the untreated MCAO group. (A) Representative brain slices. (B) Quantified results. N = 4–6 per group; **p < 0.01.
Acknowledgments
The authors thank Dr Alan Koretsky of NINDS, NIH for his support and discussions in the MRI studies, Dave Lukenbaugh and Dr Chi-Tso Chiu for their assistance in statistical analysis, and Dr Fengshan Yu for his assistance in hematoxylin and eosin staining. The authors also thank Ioline Henter, Emily Fessler, and Dr Joshua Hunsberger for their assistance in the preparation of this paper.
Sources of Funding
This research was supported by the Intramural Research Program of the National Institute of Mental Health, NIH, the Hsu family gift fund, and National Taiwan University Hospital (100-N1673).
Footnotes
The online-only Data Supplement is available at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.111.612788/-/DC1.
Disclosures
None.
References
- 1.Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, et al. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66:757–772. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 4.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TM, Andres RH, Steinberg GK. Optimizing the success of cell transplantation therapy for stroke. Neurobiol Dis. 2010;37:275–283. doi: 10.1016/j.nbd.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87–117. doi: 10.1146/annurev-bioeng-070909-105309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 8.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Deng Y, Zhou GQ. SDF-1alpha/CXCR4-mediated migration of systemically transplanted bone marrow stromal cells towards ischemic brain lesion in a rat model. Brain Res. 2008;1195:104–112. doi: 10.1016/j.brainres.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 10.Starckx S, Van den Steen PE, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B and chemokines in leukocytosis and stem cell mobilization. Leuk Lymphoma. 2002;43:233–241. doi: 10.1080/10428190290005982. [DOI] [PubMed] [Google Scholar]
- 11.Kang SS, Kook JH, Hwang S, Park SH, Nam SC, Kim JK. Inhibition of matrix metalloproteinase-9 attenuated neural progenitor cell migration after photothrombotic ischemia. Brain Res. 2008;1228:20–26. doi: 10.1016/j.brainres.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Koch-Weser J, Browne TR. Drug therapy: Valproic acid. N Engl J Med. 1980;302:661–666. doi: 10.1056/NEJM198003203021204. [DOI] [PubMed] [Google Scholar]
- 13.Price LH, Heninger GR. Lithium in the treatment of mood disorders. N Engl J Med. 1994;331:591–598. doi: 10.1056/NEJM199409013310907. [DOI] [PubMed] [Google Scholar]
- 14.Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu CT, Chuang DM. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol Ther. 2010;128:281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai LK, Leng Y, Wang Z, Leeds P, Chuang DM. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology. 2010;35:2225–2237. doi: 10.1038/npp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: The roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31:52–57. doi: 10.1038/jcbfm.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, et al. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- 20.Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: Roles of glycogen synthase kinase-3 inhibition. J Neurosci. 2008;28:2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikeda N, Nonoguchi N, Zhao MZ, Watanabe T, Kajimoto Y, Furutama D, et al. Bone marrow stromal cells that enhanced fibroblast growth factor-2 secretion by herpes simplex virus vector improve neurological outcome after transient focal cerebral ischemia in rats. Stroke. 2005;36:2725–2730. doi: 10.1161/01.STR.0000190006.88896.d3. [DOI] [PubMed] [Google Scholar]
- 22.Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci U S A. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, et al. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25:2777–2785. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho HH, Park HT, Kim YJ, Bae YC, Suh KT, Jung JS. Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. J Cell Biochem. 2005;96:533–542. doi: 10.1002/jcb.20544. [DOI] [PubMed] [Google Scholar]
- 25.Neth P, Ciccarella M, Egea V, Hoelters J, Jochum M, Ries C. Wnt signaling regulates the invasion capacity of human mesenchymal stem cells. Stem Cells. 2006;24:1892–1903. doi: 10.1634/stemcells.2005-0503. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Park JR, Seo MS, Roh KH, Park SB, Hwang JW, et al. Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Prolif. 2009;42:711–720. doi: 10.1111/j.1365-2184.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- 29.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Treatment of MSCs with VPA and lithium causes increases in CXCR4 and MMP-9, respectively. (A) MSCs were treated with 2.5 mmol/L sodium VPA for three hours and then cultured with fresh medium; cells were harvested at different time points after VPA washout. Levels of acetylated histone-H3 and CXCR4 protein peaked at one and six hours after VPA washout, respectively, as determined by Western blotting. (B) When MSCs were treated with 2.5 mmol/L lithium for 24 hours followed by drug washout, MMP-9 levels did not significantly increase. However, when MSCs were treated with 2.5 mmol/L lithium for 24 hours followed by continuous treatment with 1 mmol/L lithium, levels of MMP-9 gradually increased,
Supplemental Figure S2. VPA and/or lithium priming does not affect the differentiation and proliferation of MSCs in the infarcted brain. (A and B) Most of BrdU-labeled MSCs (green) expressed MSC markers CD54 (red) and fibronectin (red). Enlarged colocalization is shown in the upper right insert. (C and D) A few BrdU-labeled MSCs (green) expressed the astrocyte marker GFAP (red) and endothelial cell marker RECA-1 (red). (E) No BrdU-labeled MSCs (green) colocalized with the neuronal marker NeuN (red). NeuN staining in the infarcted cortex is shown in the upper right insert as the positive control. (F) No BrdU-labeled MSCs (green) colocalized with a proliferation marker Ki67 (red). Ki67 staining in the infarcted cortex is shown in the upper right insert as the positive control. Blue, DAPI; arrow head, BrdU-positive cell; arrow, NeuN staining; Bar = 50 μm.
Supplemental Figure S3. Transplantation of MSCs primed with VPA and lithium reduces infarct area in MCAO rats. H&E staining was used to measure brain infarct area on Day 15 after MCAO. MCAO rats that received transplanted VPA-and lithium-primed MSCs had significantly smaller infarct area than rats in the untreated MCAO group. (A) Representative brain slices. (B) Quantified results. N = 4–6 per group; **p < 0.01.