Abstract
In a continuing study of novel anti-HIV agents with drug-like structures and properties, 30 1′-O-, 1′-S-, 4′-O- and 4′-substituted-2′,3′-seco-3′-nor DCP and DCK analogues (8–37) were designed and synthesized. All newly synthesized seco-compounds were screened against HIV-1NL4-3 and a multiple reverse transcriptase (RT) inhibitor-resistant (RTMDR) strain in the TZM-bl cell line, using seco-DCK (7) and 2-ethyl-DCP (4) as controls. Several compounds (14, 18, 19, 22–24, and 32) exhibited potent anti-HIV activity with EC50 values ranging from 0.93 to 1.93 μM and therapeutic index (TI) values ranging from 20 to 39. 1′-O-Isopropoxy-2′,3′-seco-3′-nor-DCP (12) showed the greatest potency among the newly synthesized compounds with EC50 values of 0.47 and 0.88 μM, and TI of 96 and 51, respectively, against HIV-1NL4-3 and RTMDR strains. The seco-compounds exhibited better chemical stability in acidic conditions compared with DCP and DCK compounds. Overall, the results suggested that seco-DCP analogues with simplified structures may be more favorable for development as novel anti-HIV candidates.
Keywords: 2′, 3′-Seco-3′-nor-DCPs; Anti-HIV activity; Structure–activity relationship (SAR)
1. Introduction
Acquired immunodeficiency syndrome (AIDS), one of the most devastating diseases currently affecting mankind, is caused by infection with the human immunodeficiency virus (HIV). Although there are over 30 clinically used drugs [1], the fast emergence of drug resistance and toxicity problems due to long-term use and drug-drug interactions have limited their long-term drug effectiveness. Therefore, studies aimed at the discovery of new anti-viral agents with novel structures or targets are still needed.
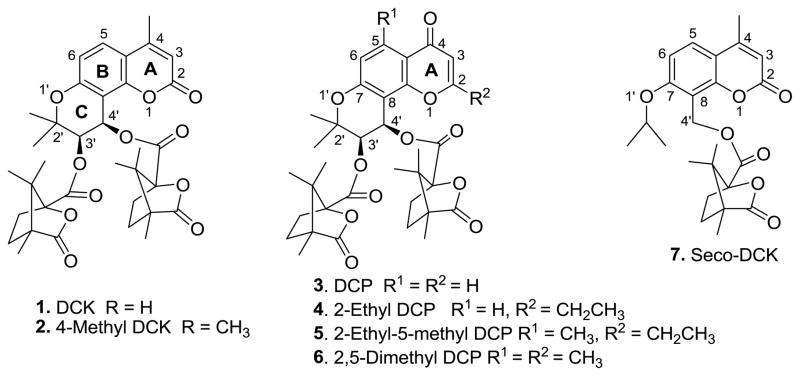
In our prior research, 3′R,4′R-di-O-(−)-camphanoyl-(+)-cis-khellactone (DCK, 1) and 4-methyl-DCK (2) exhibited high potency against HIV-1 replication in H9 lymphocytes (Figure 1) [2, 3]. Subsequently, diverse DCK analogues were designed and synthesized by modifications of DCK, particularly different coumarin substituents, ring isomers, and bioisosteric replacements [4–10]. Most of the new compounds showed promising in vitro inhibitory activity in anti-HIV replication assays, and a preliminary structure-activity relationship (SAR) was established. However, the problems of poor water solubility, low bioavailability and reduced potency against multiple reverse transcriptase (RT) inhibitor-resistant (RTMDR) strains have obstructed the further development of DCKs. Subsequently, 4H-chromone-4-one (DCP, 3) derivatives, which are positional isomers of DCK, were designed and synthesized [11, 12]. Among them, 2-ethyl DCP (4), 5-methyl-2-ethyl DCP (5), and 2,5-dimethyl DCP (6) not only exhibited high activity against wild-type HIV isolates, but also retained potency against RTMDR-1 (Figure 1).
Figure 1.
DCK & DCP analogues and seco-DCK
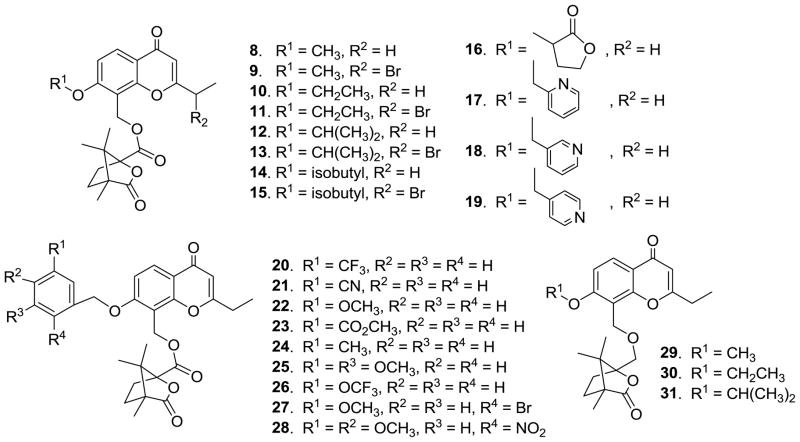
These favorable results led us to investigate whether the integrity of the tricyclic system (ring-A, -B and -C) was essential for the anti-HIV activity. While ring-A opened DCK analogues did not exhibit antiviral activity, ring-C opened DCK analogues (seco-DCKs) were active. In fact, compared with 2, seco-DCK analogue 7 showed better anti-HIV activity and increased sensitivity against RTMDR in anti-HIV replication assays using HIV-1IIIB in MT-2 cell lines, as well as HIV-1NL4-3 and RTMDR in MT-4 lymphocytes [13]. The seco-DCKs have a simplified skeleton, fewer hydrogen-bond acceptors and lower log P values, resulting in increased water solubility and better pharmacokinetic properties compared with DCKs. Our success with seco-DCKs prompted us to make corresponding modifications on DCP analogues in our search for new desirable anti-HIV inhibitors with better drug-like properties and inhibitory activity against RTMDR. Herein, we report the design, synthesis, biological evaluation and chemical stability of novel 2′,3′-seco-3′-nor DCP (8–31, 33–37) and DCK (32) analogues (Figures 2–4).
Figure 2.
Newly designed 1′-O-substituted 2′,3′-seco-3′-nor-DCP analogues (8–31)
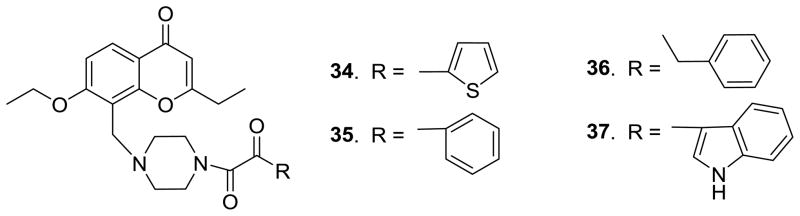
Figure 4.
Newly designed 4′-(N′-substituted piperidin-1-yl)-2′,3′-seco-3′-nor-DCP analogues (34–37)
2. Design
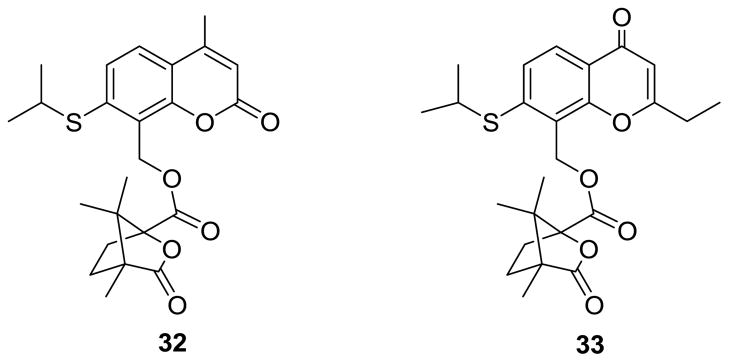
Previous research suggested that a 4′-camphanoyl moiety is preferred for anti-HIV activity of DCK/DCP analogues. Therefore, in our current study, we first retained the 4′-camphanoyl ester substitution, while focusing on diverse substituents at the 1′-O position of the new ring-C opened seco-DCP products. Eight 2′,3′-seco-3′-nor-2-ethyl DCP analogues (8–15) with different aliphatic groups, such as methyl, ethyl, isopropyl, isobutyl, and 2-α-bromoethyl, at the 1′-O position were synthesized. Aromatic and heterocyclic moieties, which were not introduced at the 1′-O position in our prior seco-DCK study, were also incorporated to examine the effects of 1′-O-aryl rings with electron-donating or -withdrawing groups and 1′-O-heterocyclic substituents on the antiviral replication activity of new compounds (16–28). In addition, because an ether bond usually has better chemical stability than an ester bond, the 4′-camphanoyl ester [-O(C=O)- linkage] was replaced with a 4′-camphanol ether [-O-CH2- linkage] with the aim of slowing down possible metabolism at this position. Accordingly, three 4′-camphanol ethers of 2′,3′-seco-3′-nor-2-ethyl-DCPs (29–31) were generated. Prior research on bioisosteric replacement showed that some 1-thia and/or 1′-thia DCKs exhibited comparable or better activity against HIV replication compared with 1-oxa and/or 1′-oxa DCKs. Consequently, we synthesized 1′-thia-2′,3′-seco-3′-nor-4-methyl-DCK (32) and 1′-thia-2′,3′-seco-3′-nor-2-ethyl-DCP (33) to verify if seco-series showed a similar trend. Finally, we replaced the 4′-O-camphanoyl ester with piperidinyl oxalamide moieties, and synthesized four 4′-(N′-substituted piperidin-1-yl)-2′,3′-seco-3′-nor DCP analogues (34–37). All new compounds were evaluated in an antiviral replication assay against wild-type virus, and 15 compounds were also assayed against RTMDR virus.
3. Chemistry
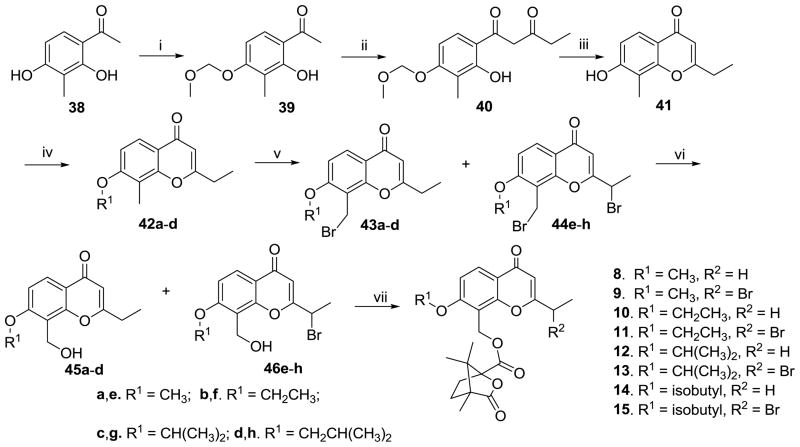
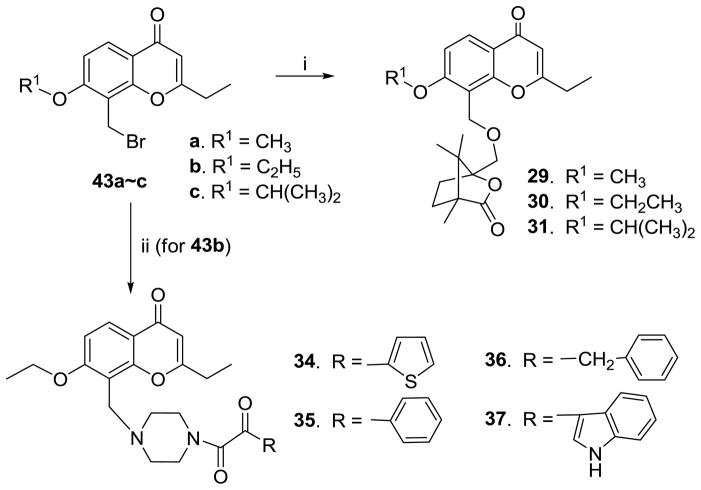
Scheme 1 shows the synthesis of 1′-O-alkyl-2′,3′-seco-3′-nor DCPs (8–15). Commercially available 2,4-dihydroxy-3-methylacetophenone (38) was selectively protected as the 4-methoxymethyl (MOM) ether (39), followed by condensation with ethyl propionate to afford 40, which was further reacted with concentrated hydrochloric acid in EtOH to provide bicyclic compound 41. Four 7-alkoxy ethers (42a–d) were synthesized by alkylation of 41 with corresponding bromides, and subsequent treatment with N-bromosuccinimide provided 8-bromomethylmonobromides (43a–d) and 2-α-bromoethyl-8-bromomethyldibromides (44e–h). A mixture of the mono- and di-bromides was first heated at reflux in a solution of NaOAc/Ac2O and then hydrolyzed with 2N HCl in EtOH to produce 8-hydroxymethyl compounds (45a–d) and 2-α-bromoethyl-8-hydroxymethyl compounds (46e–h), respectively. Subsequently, the desired esters 8–15 were prepared by esterification of 45a–d and 46e–h with camphanoyl chloride in DMAP and CH2Cl2 at room temperature.
Scheme 1.
The synthetic routes to 1′-O-alkyl-2′,3′-seco-3′-nor-DCPs (8–15). Reagents and conditions: (i). chloromethyl methyl ether/K2CO3/acetone/rt; (ii). ethyl propionate/NaH/THF/reflux; (iii). conc. HCl/EtOH/refux; (iv). variable bromides/K2CO3/DMF; (v). 1.2 equiv. NBS/CCl4/reflux; (vi). a. NaOAc/Ac2O/reflux; b. 2N HCl/EtOH/reflux; (vii). camphanoyl chloride/DMAP/CH2Cl2/rt.
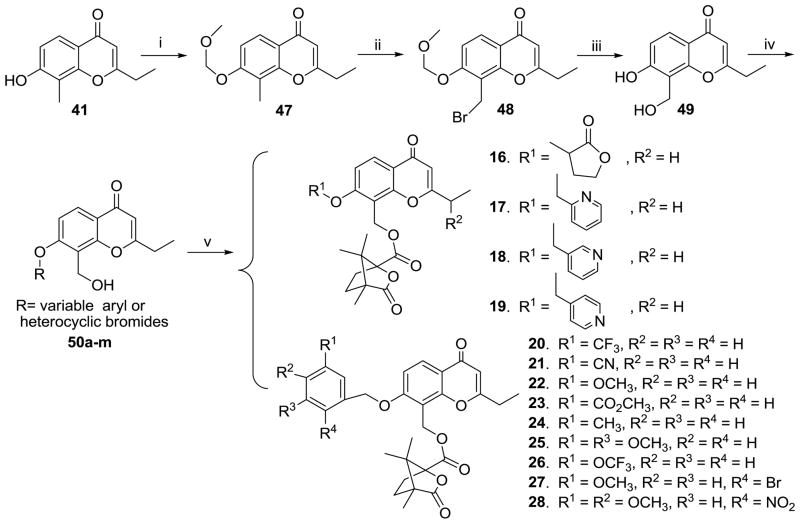
The synthesis of 1′-O-aryl or -heterocyclic substituted 2′,3′-seco-3′-nor-2-ethyl-DCPs (16–28) is depicted in Scheme 2. To avoid the reaction of the 7-OH group of 41 with NBS, the MOM ether 47 was prepared and subsequently brominated with NBS to yield the 8-bromomethyl compound 48, which was then treated as described above to afford 49. Thirteen 7-aromatic and -heterocyclic substituted ethers (50a–m) were prepared by alkylation of 49 with corresponding bromides in DMF in the presence of K2CO3 at room temperature. Compounds 50a–m were then converted into the target compounds (16–28) by acylation with camphanoyl chloride.
Scheme 2.
The synthesis of 1′-O-aryl or heterocyclic substituted 2′,3′-seco-3′-nor-2-ethyl-DCPs (16–28). Reaction Conditions: (i). chloromethyl methyl ether/K2CO3/DMF/rt; (ii). 1.2 equiv. NBS/CCl4/reflux; (iii). a. NaOAc/Ac2O/reflux; b. 2N HCl/EtOH/reflux; (iv). variable bromides/K2CO3/DMF/rt; (v). camphanoyl chloride/DMAP/CH2Cl2/rt.
As shown in Scheme 3, etherification of 7-alkyloxy-8-bromomethyl-2-ethyl-4H-chromen-4-ones (43a–c) with camphanol in toluene in presence of NaH afforded the three respective 4′-camphanol ether 2′,3′-seco-3′-nor-2-ethyl-DCPs (29–31). Four 4′-(N′-substituted piperidin-1-yl)-2′,3′-seco-3′-nor DCP analogues (34–37) were obtained from the amidation of 43b with the corresponding N′-substituted piperidine in THF in presence of DMAP at room temperature.
Scheme 3.
The synthetic routes to 4′-camphanol ethers of 2′,3′-seco-3′-nor-2-ethyl-DCPs (29–31) and 4′-(N′-substituted piperidin-1-yl)-2′,3′-seco-3′-nor=DCP analogues (36–39). Reaction Conditions: (i). camphanol/NaH/toluene/rt; (ii). variable N′-substituted piperidines/DMAP/THF/rt.
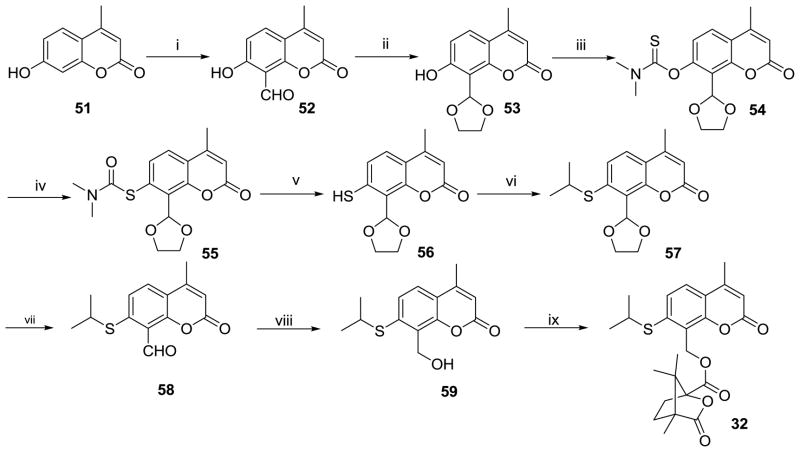
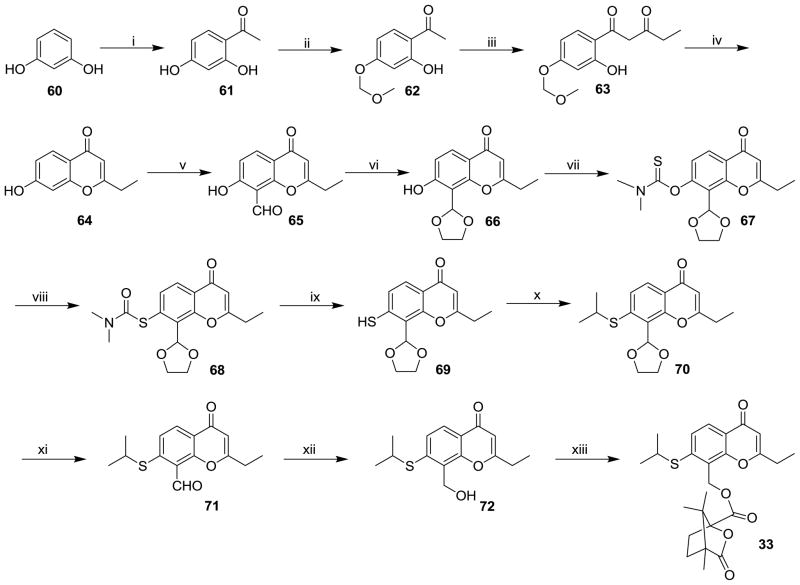
The syntheses of 1′-thia-2′,3′-seco-3′-nor-4-methyl-DCK (32) and 1′-thia-2′,3′-seco-3′-nor-2-ethyl-DCP (33) are depicted in Schemes 4 and 5, respectively. For 33, the mono-acetylation of commercially available resorcinol (60) in an HOAc solution containing zinc chloride gave 61. 2-Ethyl-7-hydroxy-4H-chromen-4-one (64) was then obtained from 61 via three steps, 4-OH protection with MOMCl (62), condensation with ethyl propionate (63), and ring-A closure. Compounds 52 (Scheme 4) and 65 (Scheme 5) were obtained by Duff formylation of 51 and 64, respectively, with hexamethylenamine in acetic acid solution. Because hydrogen bonding between the 7-hydroxy and 8-formyl groups could possibly interfere with the conversion of the 7-OH to 7-SH, the 8-formyl moiety in 52 and 65 was first protected as a cyclic acetal in intermediates 53 and 66 by reaction with ethylene glycol in the presence of p-toluenesulfonic acid in benzene under reflux-dehydration conditions. Subsequently, the 7-hydroxy compounds (53 and 66) were converted to the 7-mercapto compounds (56 and 69) in three steps, acylation with dimethylthiocarbamoyl chloride (54 and 67), Newman-Kwart rearrangement (55 and 68), and basic hydrolysis. The alkylation of 56 and 69 with isopropyl bromide yielded 57 and 70. Finally, target compounds 32 and 33 were prepared from 57 and 70 by the following reaction sequence, deprotection of the 8-acetal in 1N HCl, reduction of 58 and 71 with NaBH4 in MeOH, and esterification of 60 and 72 with camphanoyl chloride.
Scheme 4.
The synthetic route to 1′-thia-2′,3′-seco-3′-nor-4-methyl-DCK (32). Reaction Conditions: (i). hexamethylenamine/HOAc; (ii). HOCH2CH2OH/p-TSA/benzene/reflux; (iii). dimethylthiocarbamoyl chloride/DMF/K2CO3; (iv). heat at 195–205 ºC; (v). KOH/MeOH; (vi). isopropyl bromide/KOH/DMF; (vii). 1N HCl; (viii). NaBH4/MeOH; (ix). camphanoyl chloride/DMAP/CH2Cl2
Scheme 5.
The synthesis of 1′-thia-2′,3′-seco-3′-nor-2-ethyl-DCP (33). Reaction Conditions: (i). HOAc/ZnCl2/reflux; (ii). chloromethyl methyl ether/K2CO3/acetone/ice-water bath; (iii). ethyl propionate/NaH/THF/reflux; (iv). conc. HCl/EtOH/reflux; (v). a. urotropin/HOAc/reflux; b. HCl/reflux; (vi). ethylene glycol/benzene/p-TSA-H2O/reflux; (vii). dimethylthiocarbamoyl chloride/K2CO3/MeOH; (viii). N2/220 ºC; (ix). K2CO3/MeOH/reflux; (x). 2-bromopropane; (xi). 1N HCl; (xii). NaBH4/MeOH; (xiii). camphanoyl chloride/DMAP/CH2Cl2.
4. Results and discussion
All 30 newly synthesized seco-DCK and DCP analogues (8–37) were evaluated for in vitro suppression of HIV-1NL4-3 replication in a single cycle infection assay using the TZM-bl cell line with both 2-ethyl-DCP (4) and seco-DCK (7) as positive controls. Moreover, compounds 8–15, 29–31, and 34–37 were also screened for antiviral activity against HIV-1 RTMDR. The data are summarized in Tables 1 and 2.
Table 1.
Anti-HIV-1 NL4-3 and HIV-1 RTMDR results of seco-DCP analogues (8–15, 29–31, and 34–37) in TZM-bl cells a
| Compd | CC50 (μM) | HIV-1NL4-3 | RTMDR | ||
|---|---|---|---|---|---|
| EC50 (μM) | TI | EC50 (μM) | TI | ||
| 8 | >48.29 | 27.45 | >1.76 | 7.91 | >6.11 |
| 9 | >40.64 | 30.69 | >1.32 | 9.70 | >4.19 |
| 10 | >46.68 | 3.69 | >12.6 | 2.26 | >20.5 |
| 11 | 23.14 | 16.67 | 1.39 | 5.18 | 4.47 |
| 12 | >45.23 | 0.47 | >95.95 | 0.88 | >51.37 |
| 13 | 16.94 | 2.24 | 7.55 | 1.52 | 11.12 |
| 14 | 37.08 | 1.11 | 33.0 | 0.94 | 39.5 |
| 15 | >37.35 | 31.28 | >1.33 | 25.90 | >1.44 |
| 29 | >50 | >50 | NS | 23.3 | >2.14 |
| 30 | 36.72 | 18.36 | 2 | 9.28 | 3.96 |
| 31 | >47 | 2.5 | >18.75 | 1.49 | >31.45 |
| 34 | >22.00 | >22.00 | NS | 16.94 | 1.30 |
| 35 | >22.30 | 16.72 | >1.33 | 16.28 | >1.37 |
| 36 | >21.62 | 19.03 | >1.14 | 16.0 | >1.36 |
| 37 | >20.51 | >20.51 | NS | >20.51 | NS |
| 4 | 14.25 | 0.12 | 118.75 | 0.20 | 71.25 |
| 7 | 17.27 | 0.5 | 34.54 | 1.89 | 9.13 |
All data presented in this table were averaged from at least three independent experiments. Cytotoxicity was determined using a Promega CytoTox-Glo assay kit.
NS: no suppression at the highest tested concentration (20 μM).
Table 2.
Anti-HIV-1 NL4-3 data of seco-DCP and -DCK analogues (16–28, 32, and 33) in TZM-bl cells a
| Compd | CC50 (μM) | HIV-1NL4-3 | |
|---|---|---|---|
| EC50 (μM) | TI | ||
| 16 | - | - | NS |
| 17 | >40.69 | 11.00 | 3.70 |
| 18 | >40.69 | 1.26 | 32.29 |
| 19 | >40.69 | 1.93 | 21.08 |
| 20 | NS | ||
| 21 | >38.79 | 2.41 | >16.09 |
| 22 | >38.42 | 1.63 | 23.57 |
| 23 | >36.46 | 0.93 | 39.20 |
| 24 | >39.64 | 1.92 | 20.65 |
| 25 | >36.32 | 22.18 | 1.64 |
| 26 | >34.81 | 18.45 | 1.87 |
| 27 | - | - | NS |
| 28 | - | - | NS |
| 32 | 32.62 | 1.53 | 21.32 |
| 33 | 6.3 | 0.56 | 11.25 |
| 4 | 14.25 | 0.12 | 118.75 |
| 7 | 17.27 | 0.5 | 34.54 |
All data presented in this table were averaged from at least three independent experiments. Cytotoxicity was determined using a Promega CytoTox-Glo™ assay kit. NS: no suppression at the highest tested concentration (20 μM).
As shown in Table 1, compounds 8–15 exhibited varying degrees of potency against wild-type HIV-1NL4-3. Based on the size of the alkyl group at the 1′-O position, the rank order of antiviral activity was methyl < ethyl < isobutyl < isopropyl. 1′-O-Isopropyl substituted 12 showed the best antiviral activity against both HIV-1NL4-3 and RTMDR with EC50 values of 0.47 and 0.88 μM, and TI of 96 and 51, respectively, which were generally comparable or better, particularly against RTMDR, than those of prior hits 7 (EC50 0.5 and 1.89 μM; TI 34.54 and 9.13, respectively) and 4 (EC50 0.12 and 0.20 μM, TI 118.75 and 71.25, respectively). Compounds 12 and 14 (1′-O-isobutyl-2′,3′-seco-3′-nor-2-ethyl-DCP) had comparable anti-RTMDR potencies (EC50 0.88 and 0.94 μM, respectively), which were two-fold better than that of 7. Compounds with a bromine on the 2-ethyl group (9, 11, 13, and 15) exhibited much lower anti-HIV activity compared with the corresponding non-brominated compounds (8, 10, 12, and 14). This finding suggested that a 2-α-bromoethyl group was unfavorable. Overall, the results suggested that the size of an isopropyl group at the 1′-O position was more suitable to fit within the binding pocket of wild-type virus, while both isopropyl and isobutyl may fit into the slightly changed binding pocket of the RTMDR strain, due to possible mutations near the interaction site.
The three novel 4′-camphanol ether seco-DCP analogues (29–31) exhibited better inhibition activity against drug-resistant RTMDR than wild-type HIV-1NL4-3. 1′-O-Isopropyl-4′-camphanol ether 31 exhibited the best potency against RTMDR (EC50 1.49 μM, TI 31.45). However, the 4′-camphanol ether compounds (29–31) were less potent than their corresponding 4′-camphanoyl ester analogues (8, 10, 12, respectively). The four compounds (34–37) with a piperidinyl group rather than a camphanoyl ester or camphanol ether at the 4′-position showed decreased or completely abolished inhibitory activity against HIV, further confirming our prior finding that a 4′-camphanoyl group is very important for enhanced anti-HIV activity in the DCK/DCP series.
The anti-HIV-1NL4-3 results for 1′-O-aryl and -heterocyclic 2′,3′-seco-3′-nor-2-ethyl- DCPs (16–28) and 1′-thia compounds (32 and 33) are shown in Table 2. Among these analogues, 1′-O-3-methoxycarbonylbenzyl-2′,3′-seco-3′-nor-2-ethyl-DCP (23) (EC50 0.93 μM, TI 39.20) and the positive control (7) (EC50 0.5 μM, TI 34.54) exhibited comparable potency. 1′-O-Pyrid-3-yl-methyl, -pyrid-4-yl-methyl, 1′-O-3-cyanobenzyl, 1′-O-3-methoxybenzyl, and 1′-O-3-methylbenzyl analogues (18, 19, 21, 22, and 24, respectively) also showed moderate anti-HIV inhibitory activity with EC50 values ranging from 1.26 to 2.41 μM. The significantly reduced potency of 17, which contains a 1′-O-pyrid-2-yl-methyl substituent, compared with 18 and 19 suggested that a nitrogen atom at the 2-position of the aromatic ring is detrimental to the antiviral activity. Compounds 25 and 26 with 1′-O-3,5-dimethoxybenzyl and 1′-O-3-trifluoromethoxybenzyl substituents exhibited very weak anti-HIV activity, while compounds 16, 20, 27, and 28 with 1′-O-γ-butyrolactone, 1′-O-3-trifluoromethylbenzyl, 1′-O-2-bromo-5-methoxybenzyl, and 1′-O-2-nitro-4,5-dimethoxybenzyl substituents lost all activity. These results indicated that the 1′-O-substituents have a significant impact on the analogues’ antiviral activity. The 1′-O position in the seco-compounds, which corresponds to the 2′-postion of DCK and DCP analogues, should be interacting with the viral binding target, as consistent with our previous study in the DCK series. Finally, compound 32 (1′-thia-seco-DCK) showed moderate antiviral activity (EC50 1.53 μM, TI 21.32), while 33 (1′-thia-seco-DCP) was more active (EC50 0.56 μM, TI 11.25). Comparing the sulfur compounds with their oxygen counterparts, 32 was less potent than 12, while 33 was equipotent with 7.
Chemically, the seco-DCK/DCP analogues have lower molecular weights and should have reduced spatial strain, because they contain only one rather than two adjacent, extremely bulky cis-3′,4′-camphanoyl esters as found in the DCK and DCP series. Hypothetically, these changes could improve the chemical stability of the molecules and their drug-like properties. Consequently, the chemical stability of 4-Me-DCK (2), seco-DCK (7), 1′-thia-seco-DCK (32), 2-ethyl-DCP (4), and seco-DCP (12) were tested under acidic conditions with HPLC monitoring. The preliminary results are listed in Table 3. The results showed that, after 30 min, only 64% of compound 2 was detectable, while 77% of 7 and 96% of 32 were intact. Compound 12, which exhibited the greatest antiviral activity among the newly synthesized seco-DCK/DCP analogues, also showed the best chemical stability in this study (100% intact after 30 min). Overall, the seco-series of compounds showed good stability in acidic conditions, indicating that they should remain stable in the stomach via oral administration.
Table 3.
Chemical stability of 4-Me-DCK (2), seco-DCK (7), 1′-thia-seco-DCK (32), 2-ethyl-DCP (4), and seco-DCP (12) in acidic conditions a
| Purity (area %)
|
||
|---|---|---|
| Reaction Time = 1 min | Reaction Time = 30 min | |
| 2 | 100% | 64% |
| 7 | 100% | 77% |
| 32 | 100% | 96% |
| 4 | 100% | 91% |
| 12 | 100% | 100% |
Condition: 1% HCl/MeOH/rt; Compound purity: peak area percentage by HPLC determination.
5. Conclusions
In conclusion, most of the new 2′,3′-seco-3′-nor-2-ethyl-DCP analogues showed potent to moderate anti-HIV activity, and compounds 12 and 14 also showed promising activity against RTMDR. In addition, the chemical stability of seco-DCKs and seco-DCPs was improved in comparison with DCK and DCP analogues. Preliminary SAR conclusions were as follows, a) integrity of the ring-C is not essential for DCK and DCP analogues, and aliphatic alkyl substituents at 1′-O-position are better than aryl or heterocyclic groups, with isopropyl substitution being the most favorable for anti-HIV potency; b) converting the 4′-camphanoyl ester into a camphanol ether resulted in some potency decrease, but compounds remained more selective against RTMDR; c) 4′-camphanoyl is the most preferred moiety for anti-HIV activity of DCK and DCP analogues; and d) sulfur rather than oxygen at the 1′-position led to similar or slightly reduced anti-HIV activity.
6. Experimental
Melting points were measured with a Fisher Johns melting apparatus without correction. The proton nuclear magnetic resonance (1H NMR) spectra were measured on a Bruker-DPX 400 MHz spectrometer and 300 MHz Varian Gemini 2000 spectrometer using TMS as internal standard. The solvent used was CDCl3 unless indicated. Mass spectra were measured with HP5973N analytical mass spectrometers. High resolution mass spectra (HRMS) were measured on a Shimadzu LCMSIT-TOF with ESI interface. HPLC purity determinations were conducted using a Shimadzu LCMS-2010 with a Grace Alltima 2.1× 150 mm HP C18 3μM column and Shimadzu SPD-M20A detector at 254 nm wavelength. HPLC purity analyses were determined by using two different solvent conditions. The first was 70% MeCN as solvent A and 30% H2O as solvent B with 0.2 mL/min flow rate; the second was 70% MeOH as solvent A and 30% H2O as solvent B with 0.1 mL/min flow rate. The HPLC model was an isocratic system. All target compounds had purity greater than 95%. Commercially available silica gel H was used for column chromatography. Thin-layer chromatography (TLC) was performed on PLC silica gel 60 F254 plates.
6.1. Synthesis of 1′-alkoxy-seco-DCPs (8–15)
6.1.1. 1-(2-Hydroxy-4-(methoxymethoxy)-3-methylphenyl)ethanone (39)
Chloromethyl methyl ether (9.14 mL, 120 mmol) was added dropwise into a mixture of 38 (10.0 g, 60.2 mmol) and potassium carbonate (20.8 g, 150 mmol) in anhydrous acetone (60 mL) in an ice-bath. The reaction mixture was then allowed to warm to room temperature and stirred for 1.5 h. The mixture was then filtered, and the filtrate was dissolved in brine and extracted with EtOAc (35 mL). The organic layer was dried in vacuo to provide 39 (13.9 g) as brown oil: 91% yield. 1H NMR δ: 2.14 (3H, s, 3-CH3), 2.56 (3H, s, 1-COCH3), 3.50 (3H, s, 4-OCH2OCH3), 5.27 (2H, s, 4-OCH2OCH3), 6.65 (1H, d, J = 8.7 Hz, 6-H), 7.57 (1H, d, J = 8.7 Hz, 5-H), 12.80 (1H, s, 2-OH). ESI-MS m/z 209 (M+−1).
6.1.2. 2-Ethyl-7-hydroxy-8-methyl-4H-chromen-4-one (41)
Sodium hydride (60% in mineral oil, 9.52 g/15.9 g, 397 mmol) was added slowly to a mixture of 39 (13.9 mg, 66.1 mmol) and ethyl propionate (19.0 mL, 165 mmol) in absolute THF under nitrogen. Then, the mixture was warmed to reflux temperature for 2 h, cooled and neutralized to pH 8 with 37% HCl (25 mL). Water (60 mL) was added and the mixture was extracted with EtOAc (4 × 30 mL). The organic layer was collected and evaporated in vacuo to yield 40 as dark oil. This crude product and 37% HCl (3 mL) were dissolved in EtOH (60 mL) and refluxed for 45 min to give 41, which was used in the next reaction without further purification. mp 223–224 °C. 1H NMR (DMSO, δ): 1.23 (3H, t, J = 7.2 Hz, 2-CH2CH3), 2.20 (3H, s, 8-CH3), 2.65 (2H, q, J = 7.2 Hz, 2-CH2CH3), 6.06 (1H, s, 3-H), 6.98 (1H, d, J = 8.7 Hz, 6-H), 7.68 (1H, d, J = 8.7 Hz, 5-H). ESI-MS m/z 203 (M+−1).
6.1.3. Synthesis of 2-ethyl-7-alkoxychromones (42a–d)
A mixture of 41 (1 equiv), K2CO3 (3 equiv), and halogenated compounds (2 equiv) in DMF (5 mL) was stirred for 45 min at room temperature. After filtering the mixture and removing the solvent in vacuo, the residue was purified by column chromatography (eluent: hexane/EtOAc 95:5) to afford the desired compounds (42a–d) as white solids in 73–82% yields.
6.1.3.1. 2-Ethyl-7-methoxy-8-methyl-4H-chromen-4-one (42a)
75% yield, mp 104–106 °C. 1H NMR δ: 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.30 (3H, s, 8-CH3), 2.68 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.95 (3H, s, 7-OCH3), 6.11 (1H, s, 3-H), 6.96 (1H, d, J = 9.0 Hz, 6-H), 8.10 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 219 (M++1).
6.1.3.2. 7-Ethoxy-2-ethyl-8-methyl-4H-chromen-4-one (42b)
73% yield, mp 85–87 °C. 1H NMR δ: 1.33 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.48 (3H, t, J = 7.2 Hz, 7-OCH2CH3), 2.31 (3H, s, 8-CH3), 2.68 (2H, q, J = 7.5 Hz, 2-CH2CH3), 4.17 (2H, q, J = 7.2 Hz, 7-OCH2CH3), 6.11 (1H, s, 3-H), 6.93 (1H, d, J = 9.0 Hz, 6-H), 8.01 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 233 (M++1).
6.1.3.3. 2-Ethyl-7-isopropoxy-8-methyl-4H-chromen-4-one (42c)
79% yield, mp 99–101 °C. 1H NMR δ: 1.33 (3H, t, J = 6.9 Hz, 2-CH2CH3), 1.38 (6H, d, J = 6.0 Hz, 7-OCH(CH3)2), 2.29 (3H, s, 8-CH3), 2.68 (2H, J = 6.9 Hz, 2-CH2CH3), 4.70 (1H, m, J = 6.0 Hz, 7-OCH(CH3)2), 6.11 (1H, s, 3-H), 6.95 (1H, d, J = 9.0 Hz, 6-H), 8.01 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 247 (M++1).
6.1.3.4. 2-Ethyl-7-isobutoxy-8-methyl-4H-chromen-4-one (42d)
82% yield, mp 61–62 °C. 1H NMR δ: 1.07 (6H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 1.33 (3H, t, J = 7.2 Hz, 2-CH2CH3), 2.16 (1H, m, J = 6.6 Hz, 7-OCH2CH(CH3)2), 2.32 (3H, s, 8-CH3), 2.68 (2H, J = 7.2 Hz, 2-CH2CH3), 3.86 (2H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 6.11 (1H, s, 3-H), 6.93(1H, d, J = 9.0 Hz, 6-H), 8.01 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 261 (M++1).
6.1.4. Synthesis of bromides (43a–d and 44e–h)
A mixture of 42a–d (1 equiv) and NBS (1.2 equiv) in CCl4 was refluxed for 16 h. After filtration and removal of the solvent, the residue was purified by column chromatography with a gradient eluent of CH2Cl2/MeOH 40:1 to 30:1 to afford pure 43a–d and 44e–h in yields of 40–78% and 11–15%, respectively.
6.1.4.1. 8-(Bromomethyl)-2-ethyl-7-methoxy-4H-chromen-4-one (43a)
40% yield (starting with 300 mg of 42a): mp 162–164 °C. 1H NMR δ: 1.36 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.72 (2H, q, J = 7.5 Hz, 2-CH2CH3), 4.03 (3H, s, 7-OCH3), 4.78 (2H, s, 8-CH2Br), 6.15 (1H, s, 3-H), 6.99 (1H, d, J = 9.0 Hz, 6-H), 8.16 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 297 (M++1).
6.1.4.2. 8-(Bromomethyl)-7-ethoxy-2-ethyl-4H-chromen-4-one (43b)
58% yield (starting with 880 mg of 42b): mp 114–116 °C. 1H NMR δ: 1.36 (3H, t, J = 7.8 Hz, 2-CH2CH3), 1.52 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 2.72 (2H, q, J = 7.8 Hz, 2-CH2CH3), 4.25 (2H, q, J = 6.9 Hz, 7-OCH2CH3), 4.79 (2H, s, 8-CH2Br), 6.14 (1H, s, 3-H), 6.96 (1H, d, J = 9.0 Hz, 6-H), 8.13 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 311 (M++1).
6.1.4.3. 8-(Bromomethyl)-2-ethyl-7-isopropoxy-4H-chromen-4-one (43c)
45% yield (starting with 1.2 g of 42c): mp 66–68 °C. 1H NMR δ: 1.36 (3H, t, J = 7.8 Hz, 2-CH2CH3), 1.44 (6H, d, J = 6.0 Hz, 7-OCH(CH3)2), 2.71 (2H, q, J = 7.8 Hz, 2-CH2CH3), 4.72–4.81 (3H, m, J = 6.0 Hz, 7-OCH(CH3)2 & 8-CH2Br), 6.14 (1H, s, 3-H), 6.96 (1H, d, J = 9.0 Hz, 6-H), 8.12 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 325 (M++1).
6.1.4.4. 8-(Bromomethyl)-2-ethyl-7-isobutoxy-4H-chromen-4-one (43d)
78% yield (starting with 200 mg of 42d): mp 91–93 °C. 1H NMR δ: 1.25 (6H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 1.50 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.35 (1H, m, J = 6.6 Hz, 7-OCH2CH(CH3)2), 2.85 (2H, J = 7.5 Hz, 2-CH2CH3), 4.07 (2H, d, J =6.6 Hz, 7-OCH2CH(CH3)2), 4.93 (2H, s, 8-CH2Br), 6.28 (1H, s, 3-H), 7.09 (1H, d, J = 9.0 Hz, 6-H), 8.27 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 339 (M++1).
6.1.4.5. 2-(1-Bromoethyl)-8-(bromomethyl)-7-methoxy-4H-chromen-4-one (44e)
14% yield (starting with 300 mg of 42a): mp 153–156 °C. 1H NMR δ: 2.09 (3H, d, J = 7.2 Hz, 2-CHBr CH3), 4.04 (3H, s, 7-OCH3), 4.81 (2H, s, 8-CH2Br), 4.94 (1H, q, J = 7.2 Hz, 2-CHBrCH3), 6.33 (1H, s, 3-H), 7.02 (1H, d, J = 9.0 Hz, 6-H), 8.16 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 377 (100).
6.1.4.6. 2-(1-Bromoethyl)-8-(bromomethyl)-7-ethoxy-4H-chromen-4-one (44f)
15% yield (starting with 880 mg of 42b): mp 138–140 °C. 1H NMR δ: 1.53 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 2.09 (3H, d, J = 7.2 Hz, 2-CHBrCH3), 4.26 (2H, q, J = 6.9 Hz, 7-OCH2CH3), 4.82 (2H, s, 8-CH2Br), 4.95 (1H, q, J = 7.2 Hz, 2-CHBrCH3), 6.33 (1H, s, 3-H), 6.99 (1H, d, J = 9.0 Hz, 6-H), 8.12 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 384 (100).
6.1.4.7. 2-(1-Bromoethyl)-8-(bromomethyl)-7-isopropoxy-4H-chromen-4-one (44g)
15% yield (starting with 1.2 g of 42c): mp 95–97 °C. 1H NMR δ: 1.45 (6H, d, J = 6.0 Hz, 7-OCH(CH3)2), 2.85 (3H, d, J = 6.9 Hz, 2-CHBrCH3), 4.80 (3H, m, J = 6.0 Hz, 7-OCH(CH3)2 & 8-CH2Br), 4.94 (1H, q, J = 6.9 Hz, 2-CHBrCH3), 6.32 (1H, s, 3-H), 7.00 (1H, d, J = 9.0 Hz, 6-H), 8.12 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 405 (100).
6.1.4.8. 2-(1-Bromoethyl)-8-(bromomethyl)-7-isobutoxy-4H-chromen-4-one (44h)
11% yield (starting with 200 mg of 42d): mp 108–109 °C. 1H NMR δ: 1.11 (6H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 2.09 (3H, d, J = 6.9 Hz, 2-CHBrCH3), 2.22 (1H, m, J = 6.6 Hz, 7-OCH2CH(CH3)2), 3.95 (2H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 4.83 (2H, s, 8-CH2Br), 4.94 (1H, J = 6.9 Hz, 2-CHBrCH3), 6.33 (1H, s, 3-H), 7.0 (1H, d, J = 9.0 Hz, 6-H), 8.12 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 412 (100).
6.1.5. Synthesis of 8-hydroxymethyl compounds (45a–d and 46e–h)
A mixture of the above bromides [43a–d or 44e–h (1 equiv)] and NaOAc (10 equiv) in acetic anhydride (5 mL) was refluxed for 2 h and progress was monitored by TLC (CH2Cl2/MeOH 20:1). After removal of solvent in vacuo, EtOH (5 mL) and 2N HCl (2 mL) were added, and the mixture refluxed for 2 h. After the solvent was removed in vacuo, the crude product was purified by column chromatography (eluent: CH2Cl2/MeOH 99:1) to give 45a–d and 46e–h.
6.1.5.1. 2-EthyYl-8-(hydroxymethyl)-7-methoxy-4H-chromen-4-one (45a)
87% yield (starting with 300 mg of 43a): mp 139–140 °C. 1H NMR δ: 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.34 (1H, s, 8-CH2OH), 2.69 (2H, q, J = 7.5 Hz, 2-CH2CH3), 4.01 (3H, s, 7-OCH3), 4.97 (2H, s, 8-CH2OH), 6.13 (1H, s, 3-H), 7.02 (1H, d, J = 9.0 Hz, 6-H), 8.17 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 235 (M++1).
6.1.5.2. 7-Ethoxy-2-ethyl-8-(hydroxymethyl)-4H-chromen-4-one (45b)
42% yield (starting with 140 mg of 43b): mp 100–102 °C. 1H NMR δ: 1.32 (3H, t, J = 7.2 Hz, 2-CH2CH3), 1.51 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 2.44 (1H, s, 8-CH2OH), 2.68 (2H, q, J = 7.2 Hz, 2-CH2CH3), 4.24 (2H, q, J = 6.9 Hz, 7-OCH2CH3), 4.98 (2H, s, 8-CH2OH), 6.12 (1H, s, 3-H), 7.0 (1H, d, J = 9.0 Hz, 6-H), 8.13 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 249 (M++1).
6.1.5.3. 2-Ethyl-8-(hydroxymethyl)-7-isopropoxy-4H-chromen-4-one (45c)
35% yield (starting with 120 mg of 43c): mp 114–116 °C. 1H NMR δ: 1.32 (3H,t, J = 6.9 Hz, 2-CH2CH3), 1.43 (6H, d, J = 6.0 Hz, 7-OCH(CH3)2), 2.48 (1H, t, J = 6.4 Hz, 8-CH2OH), 2.68 (2H, q, J = 6.9 Hz, 2-CH2CH3), 4.78 (1H, m, J = 6.0 Hz, 7-OCH(CH3)2), 4.96 (2H, d, J = 6.4 Hz, 8-CH2OH), 6.12 (1H, s, 3-H), 7.0 (1H, d, J = 9.3 Hz, 6-H), 8.12 (1H, d, J = 9.3 Hz, 5-H). ESI-MS m/z 263 (M++1).
6.1.5.4. 2-Ethyl-8-(hydroxymethyl)-7-isobutoxy-4H-chromen-4-one (45d)
58% yield (starting with 200 mg of 43d): mp 120–122 °C. 1H NMR δ: 1.08 (6H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 1.32 (3H, t, J = 7.2 Hz, 2-CH2CH3), 2.19 (1H, m, J = 6.6 Hz, 7-OCH2CH(CH3)2), 2.35 (1H, s, 8-CH2OH), 2.68 (2H, J = 7.2 Hz, 2-CH2CH3), 3.93 (2H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 4.98 (2H, s, 8-CH2OH), 6.13 (1H, s, 3-H), 6.99 (1H, d, J = 9.0 Hz, 6-H), 8.13 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 277 (M++1).
6.1.5.5. 2-(1-Bromoethyl)-8-(hydroxymethyl)-7-methoxy-4H-chromen-4-one (46e)
38% yield (starting with 100 mg of 44e): colorless oil. 1H NMR δ: 1.97 (3H, d, J = 6.9 Hz, 2-CHBrCH3), 2.37 (1H, s, 8-CH2OH), 4.01 (3H, s, 7-OCH3), 4.90 (1H, q, J = 6.9 Hz, 2-CHBrCH3), 5.01 (2H, s, 8-CH2OH), 6.33 (1H, s, 3-H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 8.16 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 313 (M++1).
6.1.5.6. 2-(1-Bromoethyl)-7-ethoxy-8-(hydroxymethyl)-4H-chromen-4-one (46f)
34% yield (starting with 90 mg of 44f): colorless oil. 1H NMR δ: 1.50 (3H, t, J = 7.2 Hz, 7-OCH2CH3), 1.97 (3H, d, J = 6.9 Hz, 2-CHBrCH3), 2.27 (1H, s, 8-CH2OH), 4.25 (2H, q, J = 7.2 Hz, 7-OCH2CH3), 4.95 (1H, q, J = 6.9 Hz, 2-CHBrCH3), 5.01 (2H, s, 8-CH2OH), 6.33 (1H, s, 3-H), 7.02 (1H, d, J = 8.7 Hz, 6-H), 8.14 (1H, d, J = 8.7 Hz, 5-H). ESI-MS m/z 327 (M++1).
6.1.5.7. 2-(1-Bromoethyl)-8-(hydroxymethyl)-7-isopropoxy-4H-chromen-4-one (46g)
24% yield (starting with 120 mg of 44g): mp 63–65 °C. 1H NMR δ: 1.39 (6H, d, J = 6.0 Hz, 7-OCH(CH3)2), 1.97 (3H, d, J = 6.9 Hz, 2-CHBrCH3), 2.47 (1H, s, 8-CH2OH), 4.80 (1H, m, J = 6.0 Hz, 7-OCH(CH3)2), 4.91(1H, q, J =6.9 Hz, 2-CHBrCH3), 4.99 (2H, s, 8-CH2OH), 6.33 (1H, s, 3-H), 7.02 (1H, d, J = 9.3 Hz, 6-H), 8.12 (1H, d, J = 9.3 Hz, 5-H). ESI-MS m/z 341 (M++1).
6.1.5.8. 2-(1-Bromoethyl)-8-(hydroxymethyl)-7-isobutoxy-4H-chromen-4-one (46h)
14% yield (starting with 200 mg of 44h): mp 116–118 °C. 1H NMR δ:1.07 (6H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 1.58 (3H, d, J =6.6 Hz, 2-CHBrCH3), 2.18 (1H, m, J = 6.6 Hz, 7-OCH2CH(CH3)2), 3.10 (1H, s, 8-CH2OH), 3.93 (2H, d, J =6.6 Hz, 7-OCH2CH(CH3)2), 4.70 (1H, q, J = 6.6 Hz, 2-CHBrCH3), 5.49 (2H, s, J = 15.3 Hz, 8-CH2OH), 6.34 (1H,s, 3-H), 7.0 (1H, d, J = 9.0 Hz, 6-H), 8.17 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 355 (M++1).
6.1.6. Synthesis of 1′-alkoxy-seco-DCPs (8–15)
The substituted 8-hydroxymethylchromone (45a–d or 46e–h, 1 equiv), S-(−)-camphanic chloride (2.5 equiv), and DMAP (6 equiv) were stirred in CH2Cl2 (3 mL) for 1 h at room temperature, monitored by TLC (CH2Cl2/MeOH 20:1). At completion, the mixture was concentrated and the residue was purified by column chromatography with an eluent of hexane/EtOAc 3:1 to afford eight target compounds (8–15).
6.1.6.1. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-methoxy-4H-chromen-4-one (8)
78% yield (starting with 32 mg of 45a): mp 164–165 °C. 1H NMR δ 0.92, 1.00, 1.10 (9H, s, camphanoyl-CH3 × 3), 1.31 (3H, t, J = 7.8 Hz, 2-CH2CH3), 1.61–2.46 (4H, m, camphanoyl-CH2 × 2), 2.66 (2H, q, J = 7.8 Hz, 2-CH2CH3), 3.97 (3H, s, 7-OCH3), 5.54 (2H, t, J = 11.4 Hz, 4′-CH2O-), 6.14 (1H, s, 3-H), 7.02 (1H, d, J = 9.0 Hz, 6-H), 8.22 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 415 (M++1).
6.1.6.2. 2-(1-Bromoethyl)-4′-((−)-camphanoyloxymethyl)-7-methoxy-4H-chromen-4-one (9)
77% yield (starting with 33 mg of 46e): mp 211–213 °C. 1H NMR δ 0.92, 1.01, 1.09 (9H, s, camphanoyl-CH3 × 3), 1.62–2.43 (4H, m, camphanoyl-CH2 × 2), 1.88 (3H, d, J = 6.9 Hz, 2-CHBrCH3), 3.99 (3H, s, 7-OCH3), 4.84 (1H, q, J = 6.9 Hz, 2-CHBrCH3), 5.57 (2H, m, 4′-CH2O-), 6.36 (1H, s, 3-H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 8.23 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 493 (M++1).
6.1.6.3. 4′-((−)-Camphanoyloxymethyl)-7-ethoxy-2-ethyl-4H-chromen-4-one (10)
77% yield (starting with 24 mg of 45b): mp 117–118 °C. 1H NMR δ 0.93, 1.01, 1.10 (9H, s, camphanoyl-CH3 × 3), 1.31 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.46 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 1.64–2.46 (4H, m, camphanoyl-CH2 × 2), 2.66 (2H, q, J = 7.5 Hz, 2-CH2CH3), 4.21 (2H, q, J = 6.9 Hz, 7-OCH2CH3), 5.54 (2H, t, J = 11.4 Hz, 4′-CH2O-), 6.14 (1H, s, 3-H), 6.99 (1H, d, J = 9.0 Hz, 6-H), 8.20 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 429 (M++1).
6.1.6.4. 2-(1-Bromoethyl)-4′-((−)-camphanoyloxymethyl)-7-ethoxy-4H-chromen-4-one (11)
58% yield (starting with 19 mg of 46f): mp 151–152 °C. 1H NMR δ 0.93, 1.02, 1.09 (9H, s, camphanoyl-CH3 × 3), 1.47 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 1.62–2.43 (7H, m, camphanoyl-CH2 × 2 and 2-CHBrCH3), 4.23 (2H, q, J =6.9 Hz, 7-OCH2CH3), 4.88 (1H, q, 2-CHBrCH3), 5.58 (2H, m, 4′-CH2O-), 6.35 (1H, d, J = 8.4 Hz, 3-H), 7.02 (1H, d, J = 9.0 Hz, 6-H), 8.20 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 507 (M++1).
6.1.6.5. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-isopropoxy-4H-chromen-4-one (12)
70% yield (starting with 38 mg of 45c): mp 168–169 °C. 1H NMR δ 0.94, 1.01, 1.10 (9H, s, camphanoyl-CH3 × 3), 1.30 (3H, t, J = 7.2 Hz, 2-CH2CH3), 1.39 (6H, d, J = 6.0 Hz, 7-OCH(CH3)2), 1.63–2.45 (4H, m, camphanoyl-CH2 × 2), 2.66 (2H, q, J = 7.2 Hz, 2-CH2CH3), 4.76 (2H, m, J = 6.0 Hz, 7-OCH(CH3)2), 5.53 (2H, t, J = 11.4 Hz, 4′-CH2O-), 6.14 (1H, s, 3-H), 7.0 (1H, d, J = 9.0 Hz, 6-H), 8.20 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 443 (M++1).
6.1.6.6. 2-(1-Bromoethyl)-4′-((−)-camphanoyloxymethyl)-7-isopropoxy-4H-chromen-4-one (13)
92% yield (starting with 40 mg of 46g): mp 50–52 °C. 1H NMR δ 0.94, 1.01, 1.09 (9H, s, camphanoyl-CH3 × 3), 1.40 (6H, d, J = 6.0 Hz,7-OCH(CH3)2), 1.62–2.43 (7H, m, camphanoyl-CH2 × 2 and 2-CHBrCH3), 4.78 (1H, m, J = 6.0 Hz, 7-OCH(CH3)2), 4.88 (1H, m, 2-CHBrCH3), 5.55 (2H, m, 4′-CH2O-), 6.33 (1H, d, J = 7.5 Hz, 3-H), 7.02 (1H, d, J = 9.0 Hz, 6-H), 8.20 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 521 (M++1).
6.1.6.7. 4′-((−)-Camphanoyoxymethyl)-2-ethyl-7-isobutoxy-4H-chromen-4-one (14)
85% yield (starting with 30 mg of 45d): mp 161–162 °C. 1H NMR δ 0.92, 0.99, 1.04 (9H, s, camphanoyl-CH3 × 3), 1.07 (6H, d, J = 6.9 Hz, 7-OCH2CH(CH3)2), 1.31 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.60–2.45 (5H, m, camphanoyl-CH2 × 2 & 7-OCH2CH(CH3)2), 2.67 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.89 (2H, d, J = 6.9 Hz, 7-OCH2CH(CH3)2), 5.56 (2H, t, J = 17.4 Hz, 4′-CH2O-), 6.14 (1H, s, 3-H), 7.0 (1H, d, J = 9.0 Hz, 6-pH), 8.20 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 457 (M++1).
6.1.6.8. 2-(1-Bromoethyl)-4′-((−)-camphanoyloxymethyl)-7-isobutoxy-4H-chromen-4-one (15)
92% yield (starting with 23 mg of 46h): colorless oil. 1H NMR δ 0.98, 1.05, 1.08 (9H, s, camphanoyl-CH3 × 3), 1.12 (6H, d, J = 6.9 Hz, 7-OCH2CH(CH3)2), 1.68 (3H, d, J = 6.9 Hz, 2-CHBrCH3), 1.93–2.48 (5H, m, camphanoyl-CH2 × 2 & 7-OCH2CH(CH3)2), 3.91 (2H, d, J = 6.6 Hz, 7-OCH2CH(CH3)2), 5.40 (2H, s, 4′-CH2O-), 5.86 (1H, q, J = 6.9 Hz, 2-CHBrCH3), 6.33 (1H, s, 3-H), 7.02 (1H, d, J = 9.3 Hz, 6-H), 8.18 (1H, d, J = 9.3 Hz, 5-H). ESI-MS m/z 535 (M++1).
6.2. Synthesis of 1′-aryloxy-seco-DCPs (16–28)
6.2.1. 2-Ethyl-7-(methoxymethoxy)-8-methyl-4H-chromen-4-one (47)
The procedure was the same as that used for the preparation of 39. 60% yield [starting from 3.0 g of 41, but using DMF (20 mL) instead of acetone (60 mL)]: mp 93–94 °C. 1H NMR δ 1.33 (3H, d, J = 7.2 Hz, 2-CH2CH3), 2.34 (3H, s, 8-CH3), 2.68 (2H, q, J = 7.2 Hz, 2-CH2CH3), 3.51 (3H, s, 7-OCH2OCH3), 5.31 (2H, s, 7-OCH2OCH3), 6.13 (1H, s, 3-H), 7.15 (1H, d, J = 9.0 Hz, 6-H), 8.01 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 203 (M+-45).
6.2.2. 8-(Bromomethyl)-2-ethyl-7-(methoxymethoxy)-4H-chromen-4-one (48)
The procedure was the same as that used for the preparation of 43. 39% yield (starting from 1.5 g of 47): mp 61–64 °C. 1H NMR δ 1.36 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.72 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.55 (3H, s, 7-OCH2OCH3), 4.80 (2H, s, 8-CH2Br), 5.39 (2H, s, 7-OCH2OCH3), 6.16 (1H, s, 3-H), 7.18 (1H, d, J = 9.0 Hz, 6-H), 8.13 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 327 (M++1).
6.2.3. 2-Ethyl-7-hydroxy-8-(hydroxymethyl)-4H-chromen-4-one (49)
The procedure was the same as that used for the preparation of 45. 50% yield (starting from 100 mg of 48): mp 147–148 °C. 1H NMR δ 1.57 (3H, t, J = 7.8 Hz, 2-CH2CH3), 2.98 (2H, q, J = 7.8 Hz, 2-CH2CH3), 4.86 (2H, s, 8-CH2OH), 6.38 (1H, s, 3-H), 7.18 (1H, d, J = 9.0 Hz, 6-H), 8.14 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 243 (M++Na).
6.2.4. Synthesis of 7-aryloxy-subsituted-8-(hydroxymethyl)-4H-chromen-4-one (50a–m)
The procedure was identical to that used in the preparation of 42a–b.
6.2.4.1. 2-Ethyl-8-(hydroxymethyl)-7-(2-oxo-tetrahydrofuran-3-yloxy)-4H-chromen-4-one (50a)
70% yield (starting from 30 mg of 49): mp 210–212 °C. 1H NMR (CDCl3 + CD3OD) δ 1.33 (3H, t, J = 7.8 Hz, 2-CH2CH3), 2.54 (2H, m, J = 9.0 Hz, furanone 3-H2), 2.72 (2H, q, J = 7.8 Hz, 2-CH2CH3), 2.92 (1H, br s, 8-OCH2OH), 4.45, 4.62 (2H, m, J1=9.0 Hz, furanone 4-H2), 4.94 (1H, s, 8-CH2OH), 5.24 (1H, t, J=9.0 Hz, furanone 2-H), 6.19 (1H, s, 3-H), 7.17 (1H, d, J = 9.0 Hz, 6-H), 8.14 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 327 (M++Na).
6.2.4.2. 2-Ethyl-8-(hydroxymethyl)-7-(pyridin-2-ylmethoxy)-4H-chromen-4-one (50b)
30% yield (starting from 38 mg of 49): colorless oil. 1H NMR δ 1.36 (3H, t, J = 7.8 Hz, 2-CH2CH3), 2.20 (1H, br s, 8-CH2OH), 2.75 (2H, q, J = 7.8 Hz, 2-CH2CH3), 5.02 (2H, d, J = 6.9 Hz, 8-CH2OH), 5.39 (2H, s, 7-OCH2-), 6.17 (1H, s, 3-H), 7.10 (1H, d, J = 9.0 Hz, 6-H), 7.37 (1H, m, J = 7.2 Hz, pyridine H), 7.55, 7.84 (2H, d, J = 7.8 Hz, pyridine H), 8.10 (1H, d, J = 9.0 Hz, 5-H), 8.60 (1H, m, J = 7.8 Hz, pyridine H). ESI-MS m/z 334 (M++Na).
6.2.4.3. 2-Ethyl-8-(hydroxymethyl)-7-(pyridin-3-ylmethoxy)-4H-chromen-4-one (50c)
38% yield (starting from 30 mg of 49): mp 115–116 °C. 1H NMR δ 1.33 (3H, t, J = 7.8 Hz, 2-CH2CH3), 2.40 (1H, br s, 8-CH2OH), 2.70 (2H, q, J = 7.8 Hz, 2-CH2CH3), 5.00 (2H, s, 8-CH2OH), 5.27 (2H, s, 7-OCH2-), 6.14 (1H, s, 3-H), 7.07 (1H, d, J = 9.0 Hz, 6-H), 7.37 (1H, q, J = 7.2 Hz, pyridine H), 7.81 (1H, d, J = 7.8 Hz, pyridine H), 8.15 (1H, d, J = 9.0 Hz, 5-H), 8.62 (1H, d, J = 7.8 Hz, pyridine H), 8.72 (1H, s, pyridine H). ESI-MS m/z 334 (M++Na).
6.2.4.4. 2-Ethyl-8-(hydroxymethyl)-7-(pyridin-4-ylmethoxy)-4H-chromen-4-one (50d)
33% yield (starting from 30 mg of 49): mp 171–172 °C. 1H NMR δ 1.33 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.47 (1H, br s, 8-CH2OH), 2.70 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.06 (2H, d, J = 6.3 Hz, 8-CH2OH), 5.28 (2H, s, 7-OCH2-), 6.14 (1H, s, 3-H), 6.99 (1H, d, J = 9.0 Hz, 6-H), 7.37 (2H, d, J = 5.4 Hz, pyridine H), 8.14 (1H, d, J = 9.0 Hz, 5-H), 8.64 (2H, d, J = 4.8 Hz, pyridine H). ESI-MS m/z 312 (M++H).
6.2.4.5. 2-Ethyl-8-(hydroxymethyl)-7-(3-(trifluoromethyl)benzyloxy)-4H-chromen-4-one (50e)
29% yield (starting from 22 mg of 49): mp 171–172 °C. 1H NMR δ 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.32 (1H, br s, 8-CH2OH), 2.69 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.02 (2H, s, 8-CH2OH), 5.30 (2H, s, 7-OCH2-), 6.13 (1H, s, 3-H), 7.04 (1H, d, J = 9.0 Hz, 6-H), 7.53–7.71 (4H, m, aromatic H), 8.14 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 377 (M+−H).
6.2.4.6. 3-((2-Ethyl-8-(hydroxymethyl)-4-oxo-4H-chromen-7-yloxy)methyl)benzonitrile (50f)
44% yield (starting from 24 mg of 49): mp 201–202 °C. 1H NMR δ 1.35 (3H, t, J = 7.8 Hz, 2-CH2CH3), 2.74 (2H, q, J =7.8 Hz, 2-CH2CH3), 5.00 (2H, s, 8-CH2OH), 5.31 (2H, s, 7-OCH2-), 6.17 (1H, s, 3-H), 7.06 (1H, d, J = 9.0 Hz, 6-H), 7.56 (1H, t, J = 7.8 Hz, aromatic H), 7.68 (1H, d, J = 7.8 Hz, aromatic H), 7.74 (1H, d, J = 7.8 Hz, aromatic H), 7.81 (1H, s, aromatic H), 8.12 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 334 (M+−H).
6.2.4.7. 2-Ethyl-8-(hydroxymethyl)-7-(3-methoxybenzyloxy)-4H-chromen-4-one (50g)
34% yield (starting from 30 mg of 49): mp 108–110 °C. 1H NMR δ 1.33 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.38 (1H, br s, 8-CH2OH), 2.68 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.83 (3H, s, phenyl OCH3), 5.01 (2H, s, 8-CH2OH), 5.23 (2H, s, 7-OCH2-), 6.13 (1H,s, 3-H), 6.89–7.00 (3H, m, aromatic H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 7.33 (1H, t, J = 8.1 Hz, aromatic H), 8.12 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 339 (M+−H).
6.2.4.8. Methyl-3-(2-ethyl-8-(hydroxymethyl)-4-oxo-4H-chromen-7-yloxy)benzoate (50h)
40% yield (starting from 30 mg of 49): mp 153–155 °C. 1H NMR δ 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.33 (1H, br s, 8-CH2OH), 2.69 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.94 (3H, s, phenyl OCH3), 5.03 (2H, s, 8-CH2OH), 5.30 (2H, s, 7-OCH2-), 6.13 (1H, s, 3-H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 7.51 (1H, t, J = 7.8 Hz, aromatic H), 7.65 (1H, d, J = 7.8 Hz, aromatic H), 8.05 (1H, d, J = 7.8 Hz, aromatic H), 8.12 (1H, s, aromatic H), 8.14 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 367 (M+−H).
6.2.4.9. 2-Ethyl-8-(hydroxymethyl)-7-(3-methylbenzyloxy)-4H-chromen–4-one (50i)
48% yield (starting from 30 mg of 49): mp 147–149 °C. 1H NMR δ 1.32 (3H, t, J = 7.8 Hz, 2-CH2CH3), 2.36 (1H, br s, 8-CH2OH), 2.39 (3H, s, phenyl CH3), 2.68 (2H, q, J = 7.8 Hz, 2-CH2CH3), 5.00 (2H, br s, 8-CH2OH), 5.22 (2H, s, 7-OCH2-), 6.13 (1H, s, 3-H), 7.07 (1H, d, J = 9.0 Hz, 6-H), 7.12–7.34 (4H, m, aromatic H), 8.14 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 323 (M+−H).
6.2.4.10. 7-(3,5-Dimethoxybenzyloxy)-2-ethyl-8-(hydroxymethyl)-4H-chromen-4-one (50j)
44% yield (starting from 30 mg of 49): mp 154–155 °C. 1H NMR δ 1.32 (3H, t, J = 7.8 Hz, 2-CH2CH3), 2.37 (1H, t, J =6.9 Hz, 8-CH2OH), 2.68 (2H, q, J = 7.8 Hz, 2-CH2CH3), 3.81 (6H, s, 2 × OCH3), 5.01 (2H, d, J = 6.9 Hz, 8-CH2OH), 5.20 (2H, s, 7-OCH2-), 6.13 (1H, s, 3-H), 6.44 (1H, t, J = 2.4 Hz, aromatic H), 6.57 (2H, d, J = 2.4 Hz, aromatic H), 7.04 (1H, d, J = 9.0 Hz, 6-H), 8.13 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 369 (M+−H).
6.2.4.11. 2-Ethyl-8-(hydroxymethyl)-7-((3-trifluoromethoxy)benzyloxy)-4H-chromen-4-one (50k)
73% yield (starting from 30 mg of 49): mp 109–110 °C. 1H NMR δ 1.33 (3H, t, J = 7.5 Hz, 2-CH2CH3), 2.29 (1H, t, J = 6.6 Hz, 8-CH2OH), 2.69 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.02 (2H, d, J = 6.6 Hz, 8-CH2OH), 5.27 (2H, s, 7-OCH2-), 6.14 (1H, s, 3-H), 7.03 (1H, d, J = 9.0 Hz, 6-H), 7.23 (1H, d, J = 7.8 Hz, aromatic H), 7.31 (1H, s, aromatic H), 7.38 (1H, d, J = 7.8 Hz, aromatic H), 7.46 (1H, t, J = 7.8 Hz, aromatic H), 8.15 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 393 (M+−H).
6.2.4.12. 7-(2-Bromo-5-methoxybenzyloxy)-2-ethyl-8-(hydroxymethyl)-4H-chromen-4-one (50l)
39% yield (starting from 30 mg of 49): mp 132–134 °C. 1H NMR δ 1.32 (3H, t, J = 7.8 Hz, 2-CH2CH3), 1.83 (1H, br s, 8-CH2OH), 2.69 (2H, q, J = 7.8 Hz, 2-CH2CH3), 3.81 (3H, s, OCH3), 5.02 (2H, s, 8-CH2OH), 5.26 (2H, s, 7-OCH2-), 6.13 (1H, s, 3-H), 6.80 (1H, dd, J1=9.0 Hz, J2=3.3 Hz, aromatic H), 7.04 (1H, s, aromatic H), 7.07 (1H, d, J = 9.0 Hz, aromatic H), 7.51 (1H, d, J = 8.7 Hz, 6-H), 8.14 (1H, d, J = 8.7 Hz, 5-H). ESI-MS m/z 441 (M++Na).
6.2.4.13. 7-(4,5-Dimethoxy-2-nitrobenzyloxy)-2-ethyl-8-(hydroxymethyl)-4H-chromen-4-one (50m)
76% yield (starting from 30 mg of 49): mp 222–224 °C. 1H NMR δ 1.34 (3H, t, J = 7.8 Hz, 2-CH2CH3), 2.18 (1H, t, J = 6.0 Hz, 8-CH2OH), 2.70 (2H, q, J = 7.8 Hz, 2-CH2CH3), 3.99 (6H, s, 2 × OCH3), 5.07 (2H, d, J = 6.0 Hz, 8-CH2OH), 5.68 (2H, s, 7-OCH2-), 6.15 (1H, s, 3-H), 7.07 (1H, d, J = 8.7 Hz, 6-H), 7.37 (1H, s, aromatic H), 7.80 (1H, s, aromatic H), 8.16 (1H, d, J = 8.7 Hz, 5-H). ESI-MS m/z 438 (M++Na).
6.2.5. Synthesis of 1′-aryloxy-subsituted-seco-DCPs (16–28)
The procedure was the same as that used for the preparation of 8–15.
6.2.5.1. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(2-oxo-tetrahydrofuran-3-yloxy)-4H-chromen-4-one (16)
37% yield, white solid (starting from 29 mg of 50a): mp 211–212 °C. 1H NMR δ 0.88, 0.99, 1.07 (9H, s, camphanoyl-CH3 × 3), 1.32 (3H, t, J = 7.8 Hz, 2-CH2CH3), 1.68, 1.90, 2.00, 2.39 (4H, m, camphanoyl-CH2 × 2), 2.68 (2H, q, J = 7.8 Hz, 2-CH2CH3), 2.83 (2H, m, furanone 3-H2); 4.42, 4.58 (2H, m, J1=6.6 Hz, J2=1.5 Hz, furanone 4-H2), 5.19 (1H, t, J = 7.8 Hz, furanone 2-H), 5.58 (2H, m, J = 7.5 Hz, 4′-CH2-), 6.16 (1H, s, 3-H), 7.21 (1H, dd, J1=9.0 Hz, J2=3.3 Hz, 6-H), 8.22 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 507 (M++Na).
6.2.5.2. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(pyridin-2-ylmethoxy)-4H-chromen-4-one (17)
44% yield, white solid (starting from 16 mg of 50b): mp 169–170 °C. 1H NMR δ 0.86, 0.97, 1.08 (9H, s, camphanoyl-CH3 × 3), 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.66, 1.87, 2.01, 2.34 (4H, m, camphanoyl-CH2 × 2), 2.68 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.39 (2H, s, 7-OCH2-), 5.66 (2H, s, 4′-CH2-), 6.15 (1H, s, 3-H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 7.26, 7.55 (2H, m, J = 7.8 Hz, pyridine H), 7.78 (1H, m, J1=7.8 Hz, J2=1.8 Hz, pyridine H), 8.18 (1H, d, J = 9.0 Hz, 5-H), 8.61 (1H, m, J = 7.8 Hz, pyridine H). ESI-MS m/z 514 (M++Na).
6.2.5.3. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(pyridin-3-ylmethoxy)-4H-chromen-4-one (18)
83% yield, white solid (starting from 16 mg of 50c): mp 166–167 °C. 1H NMR δ 0.81, 0.95, 1.08 (9H, s, camphanoyl-CH3 × 3), 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.66, 1.87, 2.01, 2.34 (4H, m, camphanoyl-CH2 × 2), 2.67 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.27 (2H, s, 7-OCH2-), 5.57 (2H, q, J = 11.4 Hz, 4′-CH2-), 6.16 (1H, s, 3-H), 7.08 (1H, d, J = 8.7 Hz, 6-H), 7.39 (1H, dd, J1=7.8 Hz, J2=4.8 Hz, pyridine H), 7.84 (1H, d, J =7.8 Hz, pyridine H), 8.23 (1H, d, J = 8.7 Hz, 5-H), 7.78 (1H, dd, J1=6.3 Hz, J2=1.8 Hz, pyridine H), 8.69 (1H, d, J = 1.2 Hz, pyridine H). ESI-MS m/z 492 (M++H).
6.2.5.4. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(pyridin-4-ylmethoxy)-4H-chromen-4-one (19)
90% yield, white solid (starting from 14 mg of 50d): mp 162–163 °C. 1H NMR δ 0.84, 0.97, 1.08 (9H, s, camphanoyl-CH3 × 3), 1.33 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.66, 1.87, 2.01, 2.39 (4H, m, camphanoyl-CH2 × 2), 2.68 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.28 (2H, s, 7-OCH2-), 5.64 (2H, q, J = 11.4 Hz, 4′-CH2-), 6.16 (1H, s, 3-H), 7.00 (1H, d, J = 9.0 Hz, 6-H), 7.38 (2H, d, J = 6.0 Hz, pyridine H), 8.21 (1H, d, J = 9.0 Hz, 5-H), 8.65 (2H, d, J = 6.0 Hz, pyridine H). ESI-MS m/z 492 (M++H).
6.2.5.5. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(3-(trifluoromethyl)benzyloxy)-4H-chromen-4-one (20)
74% yield, white solid (starting from 11 mg of 50e): mp 156–157 °C. 1H NMR δ 0.81, 0.94, 1.07 (9H, s, camphanoyl-CH3 × 3), 1.32 (3H, t, J = 7.8 Hz, 2-CH2CH3), 1.66, 1.86, 1.98, 2.35 (4H, m, camphanoyl-CH2 × 2), 2.70 (2H, q, J = 7.8 Hz, 2-CH2CH3), 5.29 (2H, s, 7-OCH2-), 5.60 (2H, q, J = 11.4 Hz, 4′-CH2-), 6.16 (1H, s, 3-H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 7.54–7.69 (4H, m, aromatic H), 8.22 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 559 (M++H).
6.2.5.6. 4′-((−)-Camphanoyloxymethyl)-7-(3-cyanobenzyloxy)-2-ethyl-4H-chromen-4-one (21)
65% yield, white solid (starting from 16 mg of 50f): mp 175–176 °C. 1H NMR δ 0.84, 0.98, 1.08 (9H, s, camphanoyl-CH3 × 3), 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.67, 1.90, 1.99, 2.40 (4H, m, camphanoyl-CH2 × 2), 2.70 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.28 (2H, s, 7-OCH2-), 5.61 (2H, q, J = 11.4 Hz, 4′-CH2-), 6.16 (1H,s, 3-H), 7.02 (1H, d, J = 9.0 Hz, 6-H), 7.54–7.75 (4H, m, aromatic H), 8.22 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 516 (M++H).
6.2.5.7. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(3-methoxybenzyloxy)-4H-chromen-4-one (22)
57% yield, white solid (starting from 16 mg of 50g): mp 126–127 °C. 1H NMR δ 0.83, 0.94, 1.07 (9H, s, camphanoyl-CH3 × 3), 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.67, 1.87, 1.98, 2.37 (4H, m, camphanoyl-CH2 × 2), 2.69 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.23 (2H, s, 7-OCH2-), 3.82 (3H, s, OCH3), 5.60 (2H, q, J = 11.4 Hz, 4′-CH2-), 6.14 (1H, s, 3-H), 6.87, 6.97 (2H, dd, J1=8.1 Hz, J2=2.1 Hz, aromatic H), 7.00 (1H, s, aromatic H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 7.31 (1H, t, J = 8.1 Hz, aromatic H), 8.18 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 543 (M++Na).
6.2.5.8. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(3-methoxycarbonyl)benzyloxy)-4H-chromen-4-one (23)
97% yield, white solid (starting from 20 mg of 50h): mp 190–191 °C. 1H NMR δ 0.82, 0.94, 1.07 (9H, s, camphanoyl-CH3 × 3), 1.31 (3H, t, J = 7.8 Hz, 2-CH2CH3), 1.66, 1.87, 1.99, 2.37 (4H, m, camphanoyl-CH2 × 2), 2.67 (2H, q, J = 7.8 Hz, 2-CH2CH3), 3.94 (3H, s, -OCOCH3), 5.29 (2H, s, 7-OCH2-), 5.60 (2H, q, J = 11.7 Hz, 4′-CH2O-), 6.15 (1H, s, 3-H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 7.51,7.67, 8.03 (3H, d, J = 7.8 Hz, aromatic H), 8.09 (1H, s, aromatic H), 8.21 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 571 (M++Na).
6.2.5.9. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(3-methylbenzyloxy)-4H-chromen-4-one (24)
64% yield, white solid (starting from 21 mg of 50i): mp 172–173 °C. 1H NMR δ 0.83, 0.94, 1.07 (9H, s, camphanoyl-CH3 × 3), 1.31 (3H, t, J = 7.8 Hz, 2-CH2CH3), 1.66, 1.87, 1.98, 2.37 (4H, m, camphanoyl-CH2 × 2), 2.37 (3H, s, CH3 on phenyl), 2.67 (2H,q, J = 7.8 Hz, 2-CH2CH3), 5.22 (2H, s, 7-OCH2-), 5.59 (2H, q, J =11.1 Hz, 4′-CH2O-), 6.14 (1H, s, 3-H), 7.06 (1H, d, J = 9.0 Hz, 6-H), 7.14–7.31 (4H, m, aromatic H), 8.19 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 527 (M++Na).
6.2.5.10. 4′-((−)-Camphanoyloxymethyl)-7-(3,5-dimethoxybenzyloxy)-2-ethyl-4H-chromen-4-one (25)
65% yield, white solid (starting from 30 mg of 50j): mp 159–160 °C. 1H NMR δ 0.84, 0.95, 1.07 (9H, s, camphanoyl-CH3 × 3), 1.31 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.66, 1.87, 1.99, 2.37 (4H, m, camphanoyl-CH2 × 2), 2.67 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.80 (6H, s, 2 × CH3 on phenyl), 5.19 (2H, s, 7-OCH2-), 5.60 (2H, q, J = 11.4 Hz, 4′-CH2O-), 6.15 (1H, s, 3-H), 6.42 (1H, d, J = 2.4 Hz, aromatic H), 6.56 (2H, d, J = 8.4 Hz, aromatic H), 7.04 (1H, d, J = 9.0 Hz, 6-H), 8.18 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 573 (M++Na).
6.2.5.11. 4′-((−)-Camphanoyloxymethyl)-2-ethyl-7-(3-trifluoromethoxybenzyloxy)-4H-chromen-4-one (26)
79% yield, white solid (starting from 39 mg of 50k): mp 133–134 °C. 1H NMR δ 0.82, 0.95, 1.07 (9H, s, camphanoyl-CH3 × 3), 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.66, 1.87, 1.99, 2.37 (4H, m, camphanoyl-CH2 × 2), 2.67 (2H, q, J = 7.5 Hz, 2-CH2CH3), 5.26 (2H, s, 7-OCH2-), 5.60 (2H, q, J = 11.1 Hz, 4′-CH2O-), 6.15 (1H, s, 3-H), 7.04 (1H, d, J = 9.0 Hz, 6-H), 7.22, 7.39 (2H, d, J = 7.8 Hz, aromatic H), 7.29 (1H, s, aromatic H), 7.46 (1H, t, J = 7.8 Hz, aromatic H), 8.21 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 597 (M++Na).
6.2.5.12. 7-(2-Bromo-5-methoxybenzyloxy)-4′-((−)-camphanoyloxymethyl)-2-ethyl-4H-chromen-4-one (27)
54% yield, white solid (starting from 22 mg of 50l): mp 158–159 °C. 1H NMR δ 0.85, 0.95, 1.07 (9H, s, camphanoyl-CH3 × 3), 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.66, 1.87, 1.99, 2.37 (4H, m, camphanoyl-CH2 × 2), 2.67 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.80 (3H, s, CH3 on phenyl), 5.25 (2H, s, 7-OCH2-), 5.63 (2H, q, J = 11.7 Hz, 4′-CH2O-), 6.16 (1H, s, 3-H), 6.77 (1H, dd, J1=8.7 Hz, J2=3.0 Hz, aromatic H), 7.05 (1H, d, J = 9.0 Hz, 6-H), 7.08 (1H, d, J = 3.0 Hz, aromatic H), 7.48 (1H, d, J = 8.7 Hz, aromatic H), 8.22 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 623 (M++Na).
6.2.5.13. 4′-((−)-Camphanoyloxymethyl)-7-(4,5-dimethoxy-2-nitrobenzyloxy)-2-ethyl-4H-chromen-4-one (28)
79% yield, light yellow solid (starting from 43 mg of 50m): mp 211–212 °C. 1H NMR δ 0.81, 0.94, 1.06 (9H, s, camphanoyl-CH3 × 3), 1.34 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.66, 1.87, 1.96, 2.37 (4H, m, camphanoyl-CH2 × 2), 2.69 (2H, q, J = 7.5 Hz, 2-CH2CH3), 4.00 (6H, s, 2 × OCH3), 5.66 (4H, s, 7-OCH2- & 4′-CH2O-), 6.17 (1H, s, 3-H), 7.09 (1H, d, J = 9.0 Hz, 6-H), 7.37 (1H, s, aromatic H), 7.81 (1H, s, aromatic H), 8.24 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 618 (M++Na).
6.3. Synthesis of 4′-camphanol-seco-DCPs (29–31)
A mixture of 43a–c (1 equiv), camphanol (1.2 equiv), and NaH (6 equiv) in toluene (6 mL) was stirred for 4 h at room temperature and monitored by TLC (CH2Cl2/MeOH 20:1). Ice-water (10 mL) was then added to stop the reaction. Following extraction with EtOAc (10 mL × 3), the organic layer was dried over Na2SO4. After removal of solvent in vacuo, the residue was purified by PTLC (eluent: CH2Cl2/MeOH 40:1 and 20:1) to give 29–31.
6.3.1. 2-Ethyl-7-methoxy-8-(((4,7,7-trimethyl-3-oxo-2-oxabicyclo[2.2.1]heptan-1-yl)methoxy)methyl)-4H-chromen-4-one (29)
12% yield, white solid (starting from 50 mg of 43a): mp 127–128 °C. 1H NMR δ 0.87 (6H, s, camphanol-CH3 × 2), 1.07 (3H, s, camphanol-CH3), 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.58–2.10 (4H, m, camphanol-CH2 × 2), 2.68 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.84 (2H, q, J = 11.4 Hz, camphanol-CH2), 3.97 (3H, s, 7-OCH3), 4.86 (2H, q, J1=11.4 Hz, J2=13.8 Hz, 4′-CH2O-), 6.14 (1H, s, 3-H), 7.02 (1H, d, J = 9.0 Hz, 6-H), 8.18 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 423 (M++Na).
6.3.2. 2-Ethyl-7-ethoxy-8-(((4,7,7-trimethyl-3-oxo-2-oxabicyclo[2.2.1]heptan-1-yl)methoxy)methyl)-4H-chro men-4-one (30)
9% yield, white solid (starting from 100 mg of 43b): mp 60–62 °C. 1H NMR δ 0.87 (6H, s, camphanol-CH3 × 2), 1.07 (3H, s, camphanol-CH3), 1.32 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.48 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 1.61–2.04 (4H, m, camphanol-CH2 × 2), 2.67 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.80 (2H, q, J = 13.8 Hz, camphanol-CH2), 4.20 (2H, q, J = 6.9 Hz, 7-OCH2CH3), 4.87 (2H, q, J1=10.5 Hz, J2=24 Hz, 4′-CH2O-), 6.13 (1H, s, 3-H), 6.99 (1H, d, J = 9.0 Hz, 6-H), 8.16 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 437 (M++Na).
6.3.3. 2-Ethyl-7-isopropoxy-8-(((4,7,7-trimethyl-3-oxo-2-oxabicyclo[2.2.1]heptan-1-yl)methoxy)methyl)-4H-c hromen-4-one (31)
5% yield, colorless oil (starting from 200 mg of 43c). 1H NMR δ 0.87 (6H, s, camphanol-CH3 × 2), 1.06 (3H, s, camphanol-CH3), 1.32 (3H, t, J = 7.2 Hz, 2-CH2CH3), 1.40 (6H, d, J = 6.0 Hz, 7-OCH(CH3)2), 1.57–2.03 (4H, m, camphanol-CH2 × 2), 2.67 (2H, q, J = 7.2 Hz, 2-CH2CH3), 3.78 (2H, q, J = 14.4 Hz, camphanol-CH2), 4.74 (1H, m, J = 6.0 Hz, 7-OCH(CH3)2), 4.84 (2H, q, J1=11.4 Hz, J2=10.8 Hz, 4′-CH2O-), 6.13 (1H, s, 3-H), 7.0 (1H, d, J = 9.0 Hz, 6-H), 8.13 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 429 (M++1).
6.4. Synthesis of 4′-piperidinyl oxalamide-seco-DCPs (34–37)
A mixture of 43b (1 equiv), piperidinyl oxalamide (1.2 equiv), and DMAP (2.5 equiv) in THF (5 mL) was stirred for 24 h at room temperature and monitored by TLC (CH2Cl2/MeOH 20:1). After removal of solvent in vacuo, the residue was purified by column chromatography (eluent: CH2Cl2/MeOH 99:1) to give 34–37.
6.4.1. 7-Ethoxy-2-ethyl-8-((4-(2-oxo-2-(2-thienyl)acetyl)piperazin-1-yl)methyl)-4H-chromen-4-one (34)
27% yield, white solid (starting from 50 mg of 43b): mp 62–64 °C. 1H NMR δ 1.31 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.46 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 2.58 (2H, t, J = 5.1 Hz, piperazine H2), 2.66 (4H, m, 2-CH2CH3 & piperazine H2), 3.44, 3.72 (4H, t, J = 5.1 Hz, piperazine H2 × 2), 3.87 (2H, s, 8-CH2-), 4.17 (2H, q, J = 6.9 Hz, 7-OCH2CH3), 6.12 (1H, s, 3-H), 6.98 (1H, d, J = 9.0 Hz, 6-H), 7.18, 7.80 (3H, d, J = 3.9 Hz, thiophene H), 8.12 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 455 (M++1).
6.4.2. 7-Ethoxy-2-ethyl-8-((4-(2-oxo-2-phenylacetyl)piperazin-1-yl)methyl)-4H-chromen-4-one (35)
11% yield, white solid (starting from 50 mg of 43b): mp 61–62 °C. 1H NMR δ 1.31 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.45 (3H, t, J = 7.2 Hz, 7-OCH2CH3), 2.53 (2H, t, J = 5.1 Hz, piperazine H2), 2.64 (2H, q, J = 7.5 Hz, 2-CH2CH3), 2.68, 3.33, 3.75 (6H, t, J = 5.1 Hz, piperazine H2 × 3), 3.87 (2H, s, 8-CH2-), 4.17 (2H, q, J = 7.2 Hz, 7-OCH2CH3), 6.12 (1H, s, 3-H), 6.98 (1H, d, J = 9.0 Hz, 6-H), 7.51 (2H, t, J = 7.8 Hz, aromatic H), 7.65 (1H, t, J = 6.6 Hz, aromatic H), 7.94 (1H, d, J = 7.4 Hz, aromatic H), 8.13 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 449 (M++1).
6.4.3. 7-Ethoxy-2-ethyl-8-((4-(2-oxo-3-phenylpropanoyl)piperazin-1-yl)methyl)-4H-chromen-4-one (36)
10% yield, white solid (starting from 50 mg of 43b): mp 54–56 °C. 1H NMR δ 1.30 (3H, t, J = 7.2 Hz, 2-CH2CH3), 1.45 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 2.06, 2.44, 3.08, 3.52 (8H, t, J = 5.1 Hz, piperazine H2 × 4), 2.63 (2H, q, J = 7.2 Hz, 2-CH2CH3), 3.78 (2H, s, 8-CH2-), 4.02 (2H, s, -CH2-C6H5), 4.16 (2H, q, J = 6.9 Hz, 7-OCH2CH3), 6.13 (1H, s, 3-H), 6.97 (1H, d, J = 9.0 Hz, 6-H), 7.17–7.27 (1H × 5, m, J1 = 7.2 Hz, J2 = 6.6 Hz, aromatic H), 8.13 (1H, d, J = 9.0 Hz, 5-H). ESI-MS m/z 463 (M++H).
6.4.4. 4′-((4-(2-(1H-Indol-3-yl)-2-oxoacetyl)piperazin-1-yl)methyl)-2-ethyl-7-ethoxy-4H-chromen-4-one (37)
29% yield, white solid (starting from 50 mg of 43b): mp 122–124 °C. 1H NMR δ 1.30 (3H, t, J = 7.5 Hz, 2-CH2CH3), 1.44 (3H, t, J = 6.9 Hz, 7-OCH2CH3), 2.53, 2.66, 3.48, 3.73 (8H, t, J = 5.1 Hz, piperazine H2 × 4), 2.64 (2H, q, J = 7.5 Hz, 2-CH2CH3), 3.90 (2H, s, 8-CH2-), 4.15 (2H, q, J = 6.9 Hz, 7-OCH2CH3), 6.12 (1H, s, 3-H), 6.97 (1H, d, J = 9.0 Hz, 6-H), 7.24–7.41 (3H, m, aromatic H), 7.85 (1H, br s, aromatic H), 8.13 (1H, d, J = 9.0 Hz, 5-H), 8.31 (1H, br s, pyrrole H), 10.09 (1H, br s, pyrrole NH). ESI-MS m/z 488 (M++H).
6.5. Synthesis of 1′-thia -seco-DCK (32)
6.5.1. 7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde (52)
A reaction mixture of 51 (20.0 g, 114 mmol) and hexamethylenetetramine (40.0 g, 285 mmol) in HOAc (150 mL) was stirred for 5.5 h at 80–90 °C. Aq. HCl (300 mL, conc. HCl/H2O 84:100, v/v) was then added, and the reaction mixture was stirred for 0.5 h at 70 °C. After cooling, the reaction mixture was poured into ice-water (1.5 L) and extracted with EtOAc (500 mL×3). The combined organic fraction was dried over Na2SO4, and the solvent was removed in vacuo. The residue was recrystallized from EtOAc to provide 52 as a light yellow solid (2.4 g, 11% yield): mp 120–122 °C.
6.5.2. 8-(1,3-Dioxolan-2-yl)-7-hydroxy-4-methyl-2H-chromene-2-one (53)
A mixture of 52 (2.13 g, 10.4 mmol), ethane-1,2-diol (3.0 mL) and p-toluenesulfonic acid (120 mg) in toluene (25 mL) was refluxed for 2 h with removal of water by a water separator. After removing solvent in vacuo, the residue was dissolved in CH2Cl2 and filtered. The filtrate was washed with saturated NaHCO3, and the organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure to afford 53 as a yellow solid (1.96 g, 76% yield): mp 215–217 °C. 1H NMR δ 2.39 (3H, s, 4-CH3), 4.11–4.61 (4H, m, 2 × CH2 of 1,3-dioxolane), 6.12 (1H, s, 3-H), 6.39 (1H, s, 8-CH-), 6.84 (1H, d, J = 9.1 Hz, 6-H), 7.50 (1H, d, J = 9.1 Hz, 5-H).
6.5.3. O-(8-(1,3-Dioxolan-2-yl)-4-methyl-2-oxo-2H-chromen-7-yl)-N,N-dimethylcarbamothioate (54)
Compound 53 (1.50 g, 6.05 mmol) and KOH (2.0 g, 36 mmol) were dissolved in MeOH (30 mL) and reacted with dimethylthiocarbamoyl chloride (2.5 g, 20 mmol) for 3 h at rt. The mixture was poured into saturated NH4Cl (150 mL) and filtered to afford crude product, which was purified by column chromatography (eluent: petroleum ether/EtOAc 4:1) to give 54 as a white solid (667 mg, 33% yield): mp 190–192 °C. 1H NMR δ 2.36 (3H, s, 4-CH3), 3.38, 3.47 (6H, s, -N(CH3)2), 4.05–4.40 (4H, m, 2×CH2 of 1,3-dioxolane), 6.27 (2H, s, 3-H & 8-CH-), 7.02 (1H, d, J = 8.6 Hz, 6-H), 7.62 (1H, d, J = 8.6 Hz, 5-H). ESI-MS m/z 336 (M+H).
6.5.4. S-(8-(1,3-Dioxolan-2-yl)-4-methyl-2-oxo-2H-chromen-7-yl)-N,N-dimethylcarbamothioate (55)
Compound 54 (1.50 g, 5.70 mmol) was heated to 200 °C with stirring for 1 h. The crude product was purified by column chromatography (eluent: petroleum ether/EtOAc 4:1) to give 55 as light yellow crystals (235 mg, 47% yield): mp 138–140 °C. 1H NMR δ 2.42 (3H, s, 4-CH3), 3.08 (6H, s, -N(CH3)2), 4.05–4.48 (4H, m, 2×CH2 of 1,3-dioxolane), 6.36 (1H, s, 3-H), 6.65 (1H, s, 8-CH-), 7.46 (1H, d, J = 8.29 Hz, 6-H), 7.59 (1H, d, J = 8.29 Hz, 5-H). ESI-MS m/z 336 (M+H).
6.5.5. 7-(Isopropylthio)-4-methyl-2-oxo-2H-chromene-8-carbaldehyde (58)
Under nitrogen and in the dark, compound 55 (200 mg, 0.6 mmol) was hydrolyzed in the presence of KOH (500 mg) in MeOH (20 mL) for 2 h at reflux temperature. At completion, 2-bromopropane (2.0 mL) was added, and the reaction continued for another 30 min. The reaction solution was then poured into cool 1N HCl (100 mL) and filtered to give 58 as a light yellow solid (12 mg, 8% yield): mp 159–162 °C.1H NMR δ 1.44 (6H, d, J = 6.7 Hz, 7-SCH(CH3)2), 2.45 (3H, s, 4-CH3), 3.62 (1H, m, J = 6.7 Hz, 7-SCH(CH3)2), 6.15 (1H, s, 3-H), 7.30 (1H, d, J = 9.0 Hz, 6-H), 7.67 (1H, d, J = 9.0 Hz, 5-H), 10.84 (1H, s, 8-CHO). ESI-MS m/z 263 (M+H).
6.5.6. 4′-((−)-Camphanoyloxymethyl)-7-(isopropylthio)-4-methyl-2H-chromen-2-one (32)
A mixture of 58 (9 mg, 0.034 mmol) and NaBH4 (3 mg) in MeOH (2 mL) was stirred for 1 h at room temperature, then acidified to pH 3–4 with 1N HCl and extracted with CH2Cl2 (10 mL×3). The organic layer was dried over anhydrous Na2SO4, and most of the solvent was removed under reduced pressure to give crude 59. Then, 32 was synthesized following the same synthetic procedure as for 16~28, but starting from 59. 52% yield, White solid: mp 146–147 °C. 1H NMR δ × 3), 1.34 (6H, d, J = 6.7 1.00, 1.05, 1.09 (9H, s, camphanoyl-CH3 Hz, 7-SCH(CH3)2), 1.67–2.46 (4H, m, camphanoyl-CH2 × 2), 2.47 (3H, s, 4-CH3), 3.57 (1H, m, J = 6.7 Hz, 7-SCH(CH3)2), 5.65 (2H, s, 4′-CH2O-), 6.27 (1H, s, 3-H), 7.36 (1H, d, J = 8.4 Hz, 6-H), 7.57 (1H, d, J = 8.4 Hz, 5-H). ESI-MS m/z 445 (M+H).
6.6. Synthesis of 1′-thia-seco-DCP (33)
6.6.1. 2-Ethyl-7-hydroxy-4H-chromen-4-one (64)
The procedure was the same as that used for the preparation of 41. 52% yield, white crystals (starting from 10 g of 60): mp 188–189 °C.
6.6.2. 2-Ethyl-7-hydroxy-4-oxo-4H-chromen-8-carbaldehyde (65)
The procedure was the same as that used for the preparation of 52. 44% yield, white crystals (starting from 650 mg of 64): mp 179–181 °C.
6.6.3. O-(8-(1,3-Dioxolan-2-yl)-2-ethyl-4-oxo-4H-chromen-7-yl)-N,N-dimethylcarbamothioate (67)
Compound 66 was prepared by the same procedure as used for the preparation of 52. However, due to its instability, 66 was not purified, but was reacted directly with dimethylthiocarbamoyl chloride (1.0 g, 8.09 mmol) to give compound 67 following the same method used for the synthesis of 54. 31% yield, white solid (starting from 600 mg of 66): mp 132–134 °C. 1H NMR δ 1.31 (3H, t, J = 7.43 Hz, 2-CH2CH3), 2.66 (2H, q, J = 7.43 Hz, 2-CH2CH3), 3.43 (6H, s, -N(CH3)2), 4.08, 4.24 (4H, m, 2 × CH2 of 1,3-dioxolane), 6.19 (1H, s, 3-H), 6.35 (1H, s, 8-CH-), 7.06 (2H, d, J = 8.6 Hz, 6-H), 8.22 (2H, d, J = 8.6 Hz, 5-H). ESI-MS m/z 350 (M+H)
6.6.4. S-(8-(1,3-Dioxolan-2-yl)-2-ethyl-4-oxo-4H-chromen-7-yl)-N,N-dimethylcarbamothioate (68)
The procedure was the same as that used for the preparation of 55. 34% yield, light yellow solid (starting from 450 mg of 67): mp 132–135 °C. 1H NMR δ 1.32 (3H, t, J = 7.4 Hz, 2-CH2CH3), 2.66 (2H, q, J = 7.4 Hz, 2-CH2CH3), 3.13 (6H, s, -N(CH3)2), 4.08, 4.27 (4H, m, 2×CH2 of 1,3-dioxolane), 6.21 (1H, s, 3-H), 6.59 (1H, s, 8-CH-), 7.52 (1H, d, J = 8.6 Hz, 6-H), 8.20 (2H, d, J = 8.6 Hz, 5-H). ESI-MS m/z 350 (M+H).
6.6.5. 2-Ethyl-7-(isopropylthio)-4-oxo-4H-chromene-8-carbaldehyde (71)
The procedure was the same as that used for the preparation of 58. 52% yield, light yellow solid (starting from 70 mg of 68): mp 152–154 °C. 1H NMR δ 1.32 (3H, t, J = 7.3 Hz, 2-CH2CH3), 1.45 (6H, d, J = 6.7 Hz, 7-SCH(CH3)2), 2.72 (2H, q, J = 7.3 Hz, 2-CH2CH3), 3.67 (1H, m, J = 6.7 Hz, 7-SCH(CH3)2), 6.23 (1H, s, 3-CH), 7.40 (1H, d, J = 9.0 Hz, 6-H), 8.24 (1H, d, J = 9.0 Hz, 5-H), 10.81 (1H, s, 8-CHO). ESI-MS m/z 277 (M+H).
6.6.6. 4′-((−)-Camphanoyloxymethyl)-7-(isopropylthio)-2-ethyl-4H-chromene-2-one (33)
The procedure was the same as that used for the preparation of 32. 48% yield, white solid (starting from 27 mg of 71): mp 92–94 °C. 1H NMR δ 0.95, 1.05, 1.00, 1.09 (9H, s, camphanoyl-CH3 × 3), 1.31 (3H, t, J = 7.3 Hz, 2-CH2CH3), 1.35 (6H, d, J = 6.7 Hz, 7-SCH(CH3)2), 1.89–2.40 (4H, m, camphanoyl-CH2 × 2), 2.67 (2H, q, J =7.3 Hz, 2-CH2CH3), 3.62 (1H, m, J = 6.7 Hz, 7-SCH(CH3)2), 5.66 (2H, s, 4′-CH2O-), 6.18 (1H, s, 3-H), 7.42 (1H, d, J = 8.6 Hz, 6-H), 8.14 (1H, d, J = 8.6 Hz, 5-H). ESI-MS m/z 459 (M+H).
6.7. HIV-1 infectivity assay
Anti-HIV-1 activity was measured as reductions in Luc reporter gene expression after a single round of virus infection of TZM-bl cells. HIV-1 at 200 TCID50 and various dilutions of test samples (eight dilutions, fourfold stepwise) were mixed in a total volume of 100 μL growth medium in 96-well black solid plates (Corning-Costar). After 48-h incubation, culture medium was removed from each well and 100 μL of Bright Glo luciferase reagent was added to each culture well. The luciferase activity in the assay wells was measured using a Victor 2 luminometer. The 50% inhibitory dose (EC50) was defined as the sample concentration that caused a 50% reduction in Relative Luminescence Units (RLU) compared to virus control wells after subtraction of background RLU.
6.8. Cytotoxicity assay
Compounds were tested for cytotoxicity against TZM-bl cells. The cells at 1 × 105 cells/mL were added to each well in a 96-well plate in the presence of various concentrations of the tested compounds for an indicated period parallel to the antiviral assays. Cell viability was determined by using a Promega cytotoxicity assay kit, CellTiter-Glo® Luminescent Cell Viability Assay, following the manufacturer’s instruction. The drug concentration that resulted in a 50% decrease in viable cells was defined as the IC50 of the compound.
6.9. Chemical stability analysis
Each tested compound (1 mg) was dissolved in MeOH (0.5 mL) in centrifuge tubes. After adding 1% HCl (0.2 mL) into the tubes, each mixture was shaken at room temperature. The amount of compound in acidic solution was measured by HPLC (Column: Hypersil ODS2 5μm, 4.6 mm G250 mm; a mobile phase of 30% water and 70% MeOH) at 1 min and 30 min.
Supplementary Material
Figure 3.
Newly designed 1′-thia-2′,3′-seco-3′-nor-DCK (32) and -DCP (33) analogues
Research Highlights.
Thirty 2′,3′-seco-3′-nor DCP and DCK analogs (8–37) were designed and synthesized.
Several seco-compounds showed potent anti-HIV activity against HIV-1NL4-3 and RTMDR strains.
1′-O-Isopropoxy-2′,3′-seco-3′-nor-DCP (12) showed the greatest potency among the newly synthesized compounds.
The seco-analogues exhibited better chemical stability in acidic conditions than DCP/DCK compounds.
Seco-DCP analogues may be favorable for development as novel anti-HIV candidates.
Acknowledgments
This research was supported by the grants from the National Natural Science Foundation of China awarded to P.X. (No. 20272010) and Y.C. (No. 30200348 and 30873164, respectively), and Grant AI-33066 from the National Institute of Allergies and Infectious Diseases awarded to K.H.L. Thanks are also due to the National Drug Innovative Program (Grant No. 2009ZX09301-011) for partial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kilmarx PH. Curr Opin HIV AIDS. 2009;4:240–246. doi: 10.1097/COH.0b013e32832c06db. [DOI] [PubMed] [Google Scholar]
- 2.Huang L, Kashiwada Y, Cosentino LM, Fan S, Chen CH, McPhail AT, Fujioka T, Mihashi K, Lee KH. J Med Chem. 1994;37:3947–3955. doi: 10.1021/jm00049a014. [DOI] [PubMed] [Google Scholar]
- 3.Xie L, Takeuchi Y, Cosentino LM, Lee KH. J Med Chem. 1999;42:2662–2672. doi: 10.1021/jm9900624. [DOI] [PubMed] [Google Scholar]
- 4.Yang ZY, Xia Y, Xia P, Brossi A, Cosentino LM, Lee KH. Bioorg Med Chem Lett. 2000;10:1003–1005. doi: 10.1016/s0960-894x(00)00126-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Zhang Q, Zhang B, Xia P, Xia Y, Yang ZY, Kilgore N, Wild C, Morris-Natschke SL, Lee KH. Bioorg Med Chem. 2004;12:6383–6387. doi: 10.1016/j.bmc.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 6.Xia P, Yin ZJ, Chen Y, Zhang Q, Zhang B, Xia Y, Yang ZY, Kilgore N, Wild C, Morris-Natschke SL, Lee KH. Bioorg Med Chem Lett. 2004;14:3341–3343. doi: 10.1016/j.bmcl.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Xie L, Yu D, Wild C, Allaway G, Turpin J, Smith PC, Lee KH. J Med Chem. 2004;47:756–760. doi: 10.1021/jm030416y. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Chen Y, Xia P, Xia Y, Yang ZY, Yu D, Morris-Natschke SL, Lee KH. Bioorg Med Chem Lett. 2004;14:5855–5857. doi: 10.1016/j.bmcl.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Huang SX, Xia P, Xia Y, Yang ZY, Kilgore N, Morris-Natschke SL, Lee KH. Bioorg Med Chem Lett. 2007;17:4316–4319. doi: 10.1016/j.bmcl.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu SQ, Yan X, Chen Y, Xia P, Qian K, Yu D, Xia Y, Yang ZY, Morris-Natschke SL, Lee KH. Bioorg Med Chem. 2010;18:7203–7211. doi: 10.1016/j.bmc.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D, Brossi A, Kilgore N, Wild C, Allaway G, Lee KH. Bioorg Med Chem Lett. 2003;13:1575–1576. doi: 10.1016/s0960-894x(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 12.Zhou T, Shi Q, Chen CH, Zhu H, Huang L, Ho P, Lee KH. Bioorg Med Chem. 2010;18:6678–6689. doi: 10.1016/j.bmc.2010.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang J, Qian K, Zhang BN, Chen Y, Xia P, Yu D, Xia Y, Yang ZY, Chen CH, Morris-Natschke SL, Lee KH. Bioorg Med Chem. 2010;18:4363–4373. doi: 10.1016/j.bmc.2010.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.