Abstract
Background
To evaluate risk factors that predict brain metastasis in epithelial ovarian, fallopian tube, and peritoneal cancer.
Methods
All patients with FIGO stage I to IV who underwent initial cytoreductive surgery between January 1995 and January 2009 were evaluated. The tumor samples were evaluated for 7 markers including multi-drug resistance gene (MDR-1), DNA aneuploidity and S-phase fraction, human epidermal growth factor receptor 2, estrogen receptor, progesterone receptor, p53 mutation, epidermal growth factor receptor, and CD31. Biomarker expression was evaluated as a predictor of hematogenous metastasis to the following locations: (i) liver and spleen, (ii) lung, and (iii) brain.
Results
There were 309 cases identified during the period. Of those, five (1.6%, 95%CI 0.2-3.0%) women developed brain metastasis. Time to onset of brain metastasis was significantly longer than for other recurrent sites (median time to recurrence after initial cytoreduction, brain vs lung vs liver, 21.4 vs 12.6 vs 11.0 months, p<0.05). Significantly increased expression of MDR-1 was seen in tumors from women who developed brain metastasis (brain vs non-brain sites, 80% vs 4.2-24.3%, p=0.004). In multivariate analysis, MDR-1 was the only significant variable associated with the risk of brain metastasis. MDR-1 expression predicted brain metastasis (Receiver-operator-characteristic curve analysis, AUC 0.808, p=0.018), and with a 10% positive expression of MDR-1 as the cutoff value, sensitivity, specificity, positive predictive value, negative predictive value, accuracy of prediction of brain metastasis were 80%, 86.1%, 15.4%, 99.3%, and 85.9%, respectively (odds ratio 24.7, 95%CI 2.64-232, p=0.002).
Conclusions
Increased expression of MDR-1 in the tumor tissue obtained at initial cytoreduction is associated with increased risk of developing brain metastases in women with epithelial ovarian, fallopian tube, or peritoneal cancer.
Keywords: ovarian cancer, brain metastasis, multi-drug resistance gene, MDR-1, biomarker
INTRODUCTION
There are approximately 25,000 women diagnosed annually with primary ovarian, fallopian tube, and primary peritoneal cancers in the United States.1 More than 75% of these women present with advanced stage disease. This is illustrated by the overall poor prognosis of women with ovarian cancer with a 5-year overall survival rate of around 29% for stage IIIc and 13% for stage IV ovarian cancer.2 The standard management is maximal cytoreductive surgery followed by systemic combination platinum and taxane chemotherapy.3 The response rate to primary chemotherapy in advanced disease is up to 80%. The majority of patients enter remission, but unfortunately, 75% of these patients relapse.4 The majority of recurrences are loco-regional in the abdomen and pelvis, and distant metastasis via hematogenous pathway is rare (16%) demonstrating a late manifestation in the clinical course.5 The liver and lung account for 26% and 3%, respectively, for the sites of metastasis, and brain is uncommon location for metastatic disease.5
In previous studies (published in 2000 or later), the average incidence of brain metastasis was 0.86% (range 0.5-3), seen in 125 among 14533 cases of ovarian cancer.5-10 A published literature review of autopsies in ovarian cancer patients showed a brain metastasis incidence of 4%.11 Late recurrence was one of the characteristics of brain metastasis in ovarian cancer patients and median time to onset of brain metastasis was 19.5 (range 15-46) months from the initial diagnosis.5,7-9,12-14 Patients with brain metastasis often appear well until they develop symptoms, such as neurologic deficits, headaches, or seizures.7-10 Median survival time after the diagnosis of brain metastasis was only 6.27 (3-19.5) months.5,7-10,12,13 While previous research has mainly focused on prognosis after brain metastasis,5-10,12-15 identifying risk factors for developing brain metastasis may be of great value, given the late onset of recurrence and delayed clinical manifestations.7-10 The aim of the current study was to evaluate the risk factors of brain metastasis in epithelial ovarian, fallopian tube, and primary peritoneal cancers.
STUDY DESIGN
A retrospective study was conducted using the database for in-vitro drug resistance assay (EDR Assay®, Oncotech, Inc., Tustin, CA)16 for specimens obtained at Mercy Medical Center in Baltimore, Maryland between January 1995 and January 2009. Biomarker analyses are included as a part of the standard package of the assay. Inclusion criteria were cases with FIGO Stage I to IV underwent initial cytoreductive surgery. Exclusion criteria were neo-adjuvant chemotherapy, co-incidence of other cancers, and low malignancy potential. Patient demographics, clinico-pathologic data, biomarker assay results, and site of metastasis during follow-up were obtained from medical records and pathology reports. Risk analysis for brain metastasis was performed. The study protocol was approved by the Institutional Review Board (IRB) at Mercy Medical Center, Baltimore, Maryland.
Location of metastasis was categorized into four types in our study: abdomen and pelvis; liver or spleen parenchyma; lung; and brain. Cancer recurrence in the abdomen and pelvis represents local metastasis, and recurrence in liver and/or spleen was not categorized in this group. The remaining three groups represent distant metastasis (hematogenous dissemination). As the control group, cases with recurrence-free were evaluated for expression of biomarkers.
Expression of the following biomarkers was evaluated in fresh tumor tissues obtained at the time of primary cytoreduction: multi-drug resistance gene product (MDR-1), p53 mutation (m-p53), human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), CD31 for angiogenesis index (per x200 field), estrogen receptor (ER), and progesterone receptor (PR). Percentage of positive expression of biomarker (%) and histoscore were evaluated. DNA profiles with presence of aneuploidity, DNA index, and S-phase fraction (%) were also evaluated. These biomarker assays are in the standard package for EDR Assay®.
MDR-1 IHC performed by Oncotech, Inc. as part of routine clinical testing was as follows. The IHC assay for MDR-1 (clone JSB-1, Chemicon International, lot#0505000300) was previously designed and validated to be compatible with CLIA guidelines for a “homebrew” class I test validation. Immunohistochemical analysis of MDR-1 (clone JSB-1) was performed using the Biogenex Super Sensitive Detection Kit (Cat #AP900-204M, Biogenex Laboratories, San Ramon, CA) on the BioGenex i6000 Autostainer. Specimens were sectioned at 4-5 micron thickness, mounted on positive-charged glass slides, deparaffinized, and rehydrated.
Immunohistochemistry was performed with the following procedure at room temperature. Tissue sections of paraffin specimens underwent pretreatment using a one step, heat-induced epitope retrieval method (Reveal 1X, pH 6.0, Cat# RV1000M, Biocare Medical, Walnut Creek, CA) for 3 (±1) minutes at 120°C. Slides were allowed to cool for 20 (±1) minutes at room temperature followed by a deionized water rinse for 5 (±1) minutes. Slides were then placed in Isoton II Diluent (Cat#8456719, Beckman Coulter/Kuehne & Nagel, Ontario, CA). Next, the slides were placed in the BioGenex chamber, rinsed with Reaction Buffer (Cat#950-300 2L, Ventana Medical Systems, Tucson, AZ), and incubated with 3% H2O2 for 10 (±1) minutes. The slides were rinsed three times with Reaction Buffer and incubated with avidin (Cat#HK102-20X, Biogenex, San Ramon, CA) for 15 (±1) minutes followed by another three rinses with Reaction Buffer. Slides were then incubated with biotin (Cat#HK102-20X, Biogenex, San Ramon, CA) for 15 (±1) minutes, rinsed three times with Reaction Buffer, and incubated with protein block (Cat#HK112-9K, BioGenex, San Ramon, Ca.) for 10 (±1) minutes. Next, the slides were incubated with primary anti-MDR-1 (clone JSB-1) antibody (Cat# MAB4120, Clone JSB-1, 9.38 μg/ml, Mouse IgG1, Chemicon International, Inc. Temecula, CA) or the isotype (Cat#X0931, Mouse IgG1, DakoCytomation, Carpenteria, CA) for 60 (±1) minutes. The slides were rinsed three times with Reaction Buffer and incubated with biotinylated goat anti-mouse secondary antibody (Biogenex Super Sensitive Detection Kit) for 20 (±1) minutes. Slides were again rinsed three times with Reaction Buffer and incubated with peroxidase conjugated streptavidin (Biogenex Super Sensitive Detection Kit) for 20 (±1) minutes followed by five rinses with Reaction Buffer. The slides were then incubated with DAB Substrate (Cat#HK153-5K, Biogenex, San Ramon, CA) for 5 (±1) minutes, rinsed five times with Reaction Buffer, and placed in a plastic slide basket submerged in deionized water. The stained slides were counterstained with Hematoxylin, dehydrated through graded alcohols and xylene, and coverslipped.
Optimal cytoreductive surgery was defined as < 1 cm residual disease in maximal dimension at the end of the surgical procedure. Date of progression was determined by clinical examination, imaging study with CT scan, and/or CA-125 levels. Response to chemotherapy was defined by the new Response Evaluation Criteria in Solid Tumours (RECIST) guidelines.17 Progression-free survival (PFS) was defined as the time interval from the date of initial cytoreductive surgery to the date of documented first recurrence or progression of disease. If there was no recurrence, PFS was determined as the date of last the follow-up. Overall survival (OS) was defined as the interval between the initial surgery and the date of death or last follow-up visit.
Continuous variables were assessed for the normal distribution by utilizing the Kolmogorov-Smirnov test and for the statistical significance by utilizing Mann-Whitney U test, expressed either by mean (±SD) or median (range) as appropriate. Categorical variables were evaluated with Fisher’s exact test with odds ratio and 95% confidence interval (95%CI). Univariate analysis with linear regression test, Mann-Whitney U test, and Fisher’s exact test was used to assess all the corrected variables for brain metastasis. For the significant variables in univariate analyses, multivariate logistic regression test was further performed to determine the difference in significance. Cox log rank test was performed to determine the difference in survival. Kaplan-Meier test was used to estimate survival curves. Receiver-operator-characteristics (ROC) analysis was performed to predict brain metastasis determined area under the curve (AUC). All statistical tests were two-tailed, and p-values of less than 0.05 were considered statistically significant. The statistical significance of the data was determined by using the Statistical Package for Social Scientists software (SPSS, Inc., version 12.0, Chicago, IL).
RESULTS
There were 309 cases identified for epithelial ovarian, fallopian tube, and primary peritoneal cancers. Brain metastasis was noted in 5 (1.6%, 95%CI, 0.2-3.0%) patients. Patient demographics were shown in Table 1. Mean age was 61.7 (±11.4) years. The majority of patients had epithelial ovarian cancer (90.6%), FIGO Stage III (74.1%), serous histology (72.2%), and high grade tumor (73.1%). Median PFS was 12.6 months and there were 193 (62.5%) women that developed recurrence or progression of disease. Among patients with recurrent cancer, the most common recurrent site was abdomen and/or pelvis (63.7%), followed by liver and/or spleen (24.4%), lung (13.5%), and then brain (2.5%). With median follow-up of 24.8 months, 138 (44.7%) women died during the study period. Univariate analysis showed a statistically significant relationship between brain metastasis and type of cancer (p=0.002) and also suboptimal surgery (p=0.043).
Table 1.
Patient demographics
| Cases | Brain mets† | P-value | |
|---|---|---|---|
| Subjects | n=309 | n=5 (1.6%) | |
| Age | 61.7 ± 11.4 | 0.75 | |
|
| |||
| Type of cancer | 0.002 | ||
| Epithelial ovarian cancer | 280 (90.6%) | 1.7% | |
| Primary peritoneal cancer | 24 (7.8%) | 6.3% | |
| Fallopian cancer | 5 (1.6%) | 33.3% | |
|
| |||
| Lymph nodes metastasis | 101 (32.7%) | 2.9% | 0.62 |
| Lympho-vascular invasion | 95 (30.7%) | 3.4% | 0.49 |
|
| |||
| FIGO Stage | 0.88* | ||
| I | 22 (7.1%) | 0% | |
| II | 23 (7.4%) | 0% | |
| III | 229 (74.1%) | 2.5% | |
| IV | 35 (11.3%) | 4.5% | |
|
| |||
| Histologic type | 0.96 | ||
| Serous | 223 (72.2%) | 2.4% | |
| Endometrioid | 19 (6.1%) | 0% | |
| Clear cell | 17 (5.5%) | 0% | |
| Mucinous | 13 (4.2%) | 0% | |
| Undifferentiated | 9 (2.9%) | 0% | |
| Transitional cell | 3 (1.0%) | 0% | |
| Mixed | 25 (8.1%) | 0% | |
|
| |||
| Tumor size | 9 cm (1-27) | ||
| High grade tumor | 226 (73.1%) | 2.2% | 0.36 |
| Preoperative CA-125 | 587.8 (8-26000) | 0.98 | |
|
| |||
| Optimal surgery | 152 (49.2%) | 0% | 0.043 |
| Bowel resection | 152 (49.2%) | 3.4% | 0.51 |
|
| |||
| Type of chemotherapy | 0.99 | ||
| Carboplatin + Paclitaxel | 141 (45.6%) | 3.3% | |
| Carboplatin + Docetaxel | 89 (28.8%) | 3.5% | |
| Cisplatin + Paclitaxel | 42 (13.6%) | 0% | |
|
| |||
| Chemotherapy response | 226 (84.3%) | 3.4% | 0.32 |
|
| |||
| Site of recurrence | |||
| Abdomen, Pelvis** | 123 (63.7%) | ||
| Liver, Spleen | 47 (24.4%) | ||
| Lung | 26 (13.5%) | ||
| Brain | 5 (2.5%) | ||
Number (%), mean (±SD), or median (range) is shown. Univariate analysis with linear regression test or Fisher’s exact test.
proportion of cases with brain metastasis among all recurrent cases.
p=0.7 (Stage I+II vs III+IV).
not include liver and spleen parenchyma metastasis.
Among the five patients with brain metastasis, all had FIGO Stage IIIC or greater disease at the time of initial diagnosis, had serous histology, suboptimal surgery, and received at least 6 cycles of postoperative combination chemotherapy with platinum and taxane with showed complete response. At the time of brain metastasis, all patients did not develop concomitant abdominal or lung recurrence. Multiple brain lesions were documented in two cases. All received chemotherapy for brain metastasis: median 3 (range 2-4) regimens. The chemotherapy regimen included topotecan (n=3), carboplatin with gemcitabine (n=1), and paclitaxel (n=1). External radiation therapy was performed in four cases. One patient underwent craniotomy for an isolated brain lesion. Median PFS and OS were 21.4 (20-27.7) months and 52.9 (30.8-71) months, respectively, and three patients died of disease. Median survival time after the diagnosis of brain metastasis was 31.5 (9.9-51.1) months. Time interval between the initial surgery and brain metastasis was significantly longer than the time interval for development of lung metastasis (median PFS, 21.4 vs. 12.6 months, p=0.016). Survival time after brain metastasis was significantly longer than lung metastasis (median OS, 52.9 vs. 29.9 months, p=0.013) Similar results were noted for liver metastasis case (median PFS, 21.4 vs. 11.0 months, p=0.02; median OS, 52.9 vs. 23.7 months, p=0.04).
Biomarker expression was evaluated for the type of metastatic site (Table 2). Expression of MDR-1 in tumor from women who developed brain metastasis counted in 80% of the cases, and this proportion was significantly higher than tumor from women who developed recurrence in abdomen or pelvis (24.3%), liver or spleen (18.4%), and lung (4.2%) metastasis (p=0.004; Figure 1). Percentage of positive MDR-1 cells as well as staining intensity were also significantly elevated among tumor from women who developed brain metastasis compared to tumor from women developed other locations of recurrence (p=0.001, 0.003, respectively; Table 2). Among tumors from women who developed recurrence, DNA S-phase fraction showed a statistical significance (DNA S-phase fraction ≥ 11%, p=0.031) and an increasing trend of DNA S-phase fraction was observed as remote from abdomen to cephalad: DNA S-phase fraction, liver and/or spleen 8.2%, lung 11.4%, and brain 14.9% (Table 2 and Figure 2). There was no statistical significance in the remaining six tested biomarkers.
Table 2.
Biomarker expressions and risk of distant metastasis
| Non-recurrent | Abdomen, pelvis | Liver, Spleen | Lung | Brain | P-value | |
|---|---|---|---|---|---|---|
| Cases | n=64 (20.7%) | n=123 (39.8%) | n=47 (15.2%) | n=26 (8.4%) | n=5 (1.6%) | |
| Multi-drug Resistance Gene | ||||||
| MDR-1 % Cells + | 0% (0-90) | 0% (0-90) | 0% (0-30) | 0% (0-5) | 20% (0-30) | |
| MDR-1 Staining Intensity | 0 (0-3) | 0 (0-3) | 0 (0-3) | 0 (0-1) | 1+ (0-2) | |
| MDR-1 Result | 19% | 24.3% | 18.4% | 4.2% | 80% | 0.004 |
| DNA Profiles | ||||||
| DNA Aneuploidity | 70% | 76.7% | 75.6% | 87.5% | 100% | |
| DNA Index | 1.2 (1-3.3) | 1.5 (0.8-2.7) | 1.5 (1-2.9) | 1.5 (1.0-2.3) | 1.4 (1.1-2.9) | |
| DNA S-Phase Fraction | 8.0% (0.3-29.7) | 8.0% (0-30.4) | 8.2% (0.6-21.4) | 11.4% (2.6-22.2) | 14.9% (6.8-30.6) | |
| S-phase Fraction ≥11% | 37.3% | 34.6% | 31.7% | 54.1% | 80% | 0.031† |
| Human Epidermal Growth Factor Receptor 2 | ||||||
| HER2 % Cells + | 20% (0-90) | 10% (0-90) | 10% (0-90) | 5% (0-95) | 20% (0-90) | |
| HER2 Staining Intensity | 1+ (0-3) | 1+ (0-3) | 1+ (0-3) | 1+ (0-3) | 1+ (0-2) | |
| HER2 Result | 22.2% | 20.4% | 21.9% | 30% | 20% | 0.9 |
| Estrogen Receptor | ||||||
| ER % Cells + | 30% (0-90) | 40% (0-100) | 30% (0-90) | 50% (0-90%) | 55% (30-70) | |
| ER Staining Intensity | 2+ (0-3) | 2+ (0-3) | 1+ (0-3) | 2+ (0-3) | 2+ (1-3) | |
| ER Histoscore | 120 (0-360) | 120 (0-400) | 80 (0-360) | 125 (0-360) | 180 (60-240) | |
| ER Result | 76% | 83.6% | 72.4% | 81% | 100% | 0.58 |
| Progesterone Receptor | ||||||
| PR % Cells + | 0% (0-80) | 0% (0-70) | 0% (0-5) | 0% (0-50) | 0% | |
| PR Staining Intensity | 0 (0-4) | 0 (0-3) | 0 (0-3) | 0 (0-4) | 0 | |
| PR Histoscore | 0 (0-320) | 0 (0-280) | 0 (0-20) | 0 (0-250) | 0 | |
| PR Result | 22% | 10.9% | 3.4% | 4.8% | 0% | 0.11 |
| Mutation of p-53 | ||||||
| m-p53 % Cells + | 60% (0-100) | 70% (0-100) | 50% (0-100) | 60% (0-100) | 80% (0-90) | |
| m-p53 Staining Intensity | 3+ (0-4) | 3+ (0-4) | 3+ (0-4) | 3 + (0-3) | 2+ (0-3) | |
| m-p53 Result | 73.8% | 73.1% | 62.5% | 41.7% | 60% | 0.26 |
| Epidermal Growth Factor Receptor | ||||||
| EGFR % Cells + | 0% (0-90) | 10% (0-90) | 10% (0-90) | 0% (0-90) | 20% (0-50) | |
| EGFR Staining Intensity | 0 (0-3) | 1+ (0-3) | 1+ (0-3) | 0 (0-3) | 1+ (0-2) | |
| EGFR Result | 46.5% | 62% | 66.7% | 40% | 66.7% | 0.18 |
| Angiogenesis | ||||||
| CD31 | 20 (10-30) | 48 (5-125) | 30 (10-40) | 40 (20-60) | 32 | |
comparison between recurrent cases.
Figure 1.
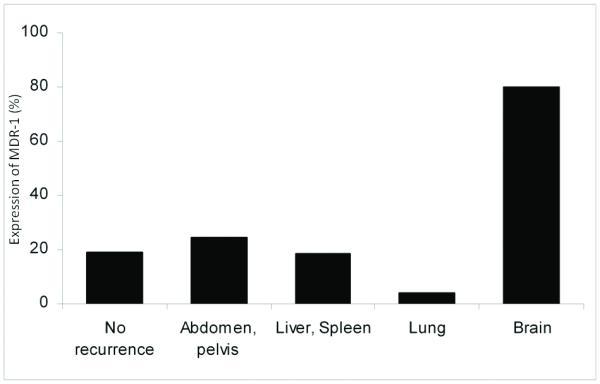
MDR-1 expression and risk of distant metastasis.
Percentage is shown. p=0.004 (all cases).
Figure 2.
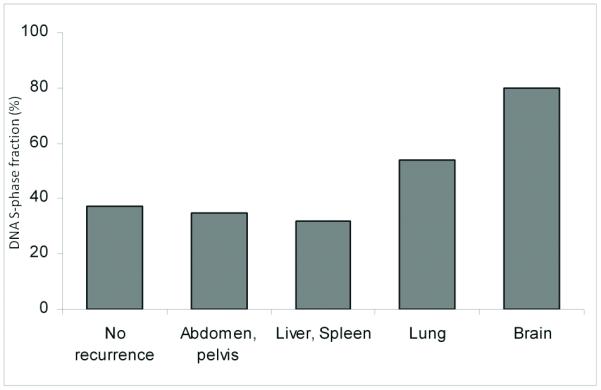
DNA S-phase fraction in tumors from patients with distant metastasis.
Percentage is shown. p=0.031 (among metastatic cases).
Multivariate analysis was performed for the significant variables in univariate analyses for brain metastasis. Among the variables such as optimal surgery, type of chemotherapy, and MDR-1, only MDR-1 was statistically significant for brain metastasis (p=0.045). ROC curve analysis for extent of expression of MDR-1 was then performed to evaluate the prediction of brain metastasis among 309 cases (Figure 3). Increased expression of MDR-1 in the tumor tissue obtained at the time of initial cytoreductive surgery was a significant predictor for brain metastasis (percentage of positive expression of MDR-1, AUC 0.808, p=0.018). Various cutoffs for percentage of positive expression of MDR-1 are shown in Table 3. The value that maximized the prediction of brain metastasis was 10% (odds ratio, 24.7, 95%CI 2.64-232, p=0.002). Sensitivity, specificity, positive predictive value, negative predictive value, accuracy of prediction of brain metastasis at the 10% cutoff value were 80%, 86.1%, 15.4%, 99.3%, and 85.9%, respectively. Expression of MDR-1 in tumor obtained at the primary cytoreduction was not associated with progression-free survival (p=0.79) or overall survival (p=0.97).
Figure 3.
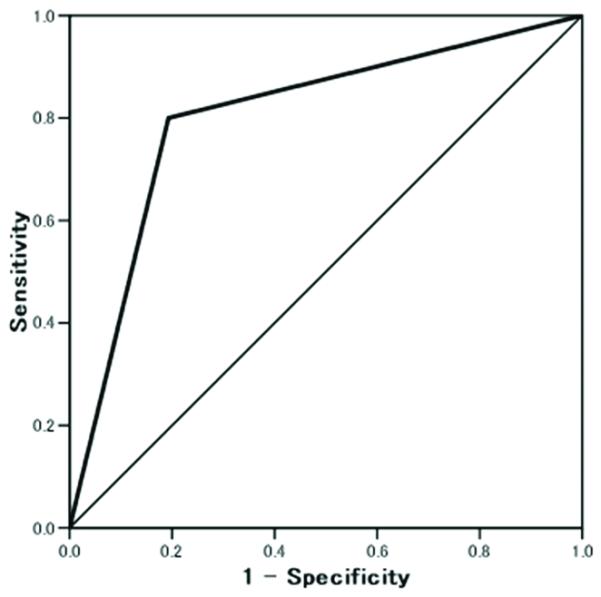
Extent of expression of MDR-1 for prediction of brain metastasis.
Receiver-operator characteristic analysis. AUC 0.808, p=0.018.
Table 3.
Various cutoff values for MDR-1 and risk of brain metastasis
| Cutoff | 5% | 10% | 20% | 30% |
|---|---|---|---|---|
| Sensitivity | 80 | 80 | 60 | 40 |
| Specificity | 79.7 | 86.1 | 90.5 | 91.1 |
| PPV | 11.1 | 15.4 | 16.7 | 12.5 |
| NPV | 99.2 | 99.3 | 98.6 | 98 |
| Accuracy | 79.8 | 85.9 | 89.6 | 89.6 |
| Odds ratio | 15.8 | 24.7 | 14.3 | 6.9 |
| 95%CI | 1.70-146 | 2.64-232 | 2.21-92.5 | 1.06-44.6 |
| P-value | 0.009 | 0.002 | 0.01 | 0.076 |
Tested for all 309 cases. Abbreviations: PPV, positive predictive value; NPV, negative predictive value.
DISCUSSION
In our analysis of 309 cases of epithelial ovarian, fallopian tube, and peritoneal cancer, increased expression of MDR-1 in the tumor tissue obtained at the initial cytoreductive surgery was significantly associated with the risk of developing brain metastasis in the late time of follow-up. This information may be useful to identify patients at risk for developing brain metastasis. Expression of MDR-1 of 10% or less provides a high negative predictive value for developing brain metastasis (99.3%).
Previous studies have mainly investigated the predictors of survival outcome after brain metastasis was diagnosed.5-10,12-15 Multivariate analysis showed the following variables as having significant predictive value for survival: presence of extra-cranial lesion, number of lesion (single or multiple), histology, and treatment modality including chemotherapy, radiation, and surgery.5-10,12-15 Metastasis to only the brain (12.5 to 40.9%),7,8,10,14 and single brain lesions (43 to 75%),8,13 were associated with comparable survival outcome (median survival time, 10 months).5,6 Presence of extra-cranial lesions was significantly associated with worse survival outcome (hazard ratio, 6.207).15 Serous histology type was associated with improved survival (hazard ratio, 0.42).15
Treatment modality was the strongest predictor of survival. Patients who received any treatment after diagnosis of brain metastasis had a significantly longer survival time compared to patients without treatment (7 vs. 2 months, p<0.001; 6 vs 0.5 months).9,13 External radiation was the most common treatment modality (range, 33-77.2%) followed by chemotherapy (22.7%) and surgery (17%).5,13,14 Combination of these modalities was common and showed better survival outcome: radiation with surgery (median survival, 12 months); radiation with surgery with or without chemotherapy (median survival, 20 months); and radiation with surgery vs. radiation alone vs. surgery alone, 23.07 vs. 5.33 vs. 6.9 months, p<0.01.8-10 One report concluded that gamma-knife radiosurgery was the most significant variable for survival.12 Our results supported these previous studies, and brain metastasis occurred later than lung or liver metastasis (median 21.4, 12.6, and 11.0 months, respectively, p<0.05). Survival time after the diagnosis of brain metastasis in our patients was longer than seen in previous reports. Relatively favorable prognosis in our patients was most likely due to having isolated brain lesions without evidence of extra-cranial lesions, having serous histology, undergoing chemotherapy, surgery, radiation, and combination therapy.
The increasing incidence of brain metastasis in ovarian cancer over time deserves special attention (1980-1984, 0.2%; 1985-1989, 0%; 1990-1994, 0.3%; and 1995-1999, 1.3%, p<0.001).5 The authors suggested that presence of effective chemotherapy was paradoxically associated with increased risk of brain metastasis in ovarian cancer. Isolated central nervous system lesion, long treatment-free time in patients receiving multiple chemotherapy regimens and late time to relapse all suggest that the brain may serve as a “sanctuary” site from chemotherapy in ovarian caner.5 Similar observations have been noted in acute lymphocytic leukemia, where increased brain metastasis was seen as a results of more effective chemotherapy achieving more complete remission.5 In their study, however, there was no explanation of the mechanism for effective chemotherapy increasing the incidence of brain metastasis. In this particular setting, increased expression of MDR-1 may fulfill the role for predicting brain metastasis in ovarian cancer as demonstrated in our results. MDR-1 encodes P-glycoprotein, a 170 kDa plasma membrane protein that functions as an ATP-driven drug export pump.18 Overexpression of P-glycoprotein on tumor tissue is suggested as the mechanism for acquiring resistance to drugs such as paclitaxel.19,20 Ovarian cancer patients who did not show response to postoperative combination chemotherapy with carboplatin and paclitaxel had higher expression of MDR-1 in primary tumor compared to patients who showed response.21 While some investigators have suggested that there is no correlation between expression of MDR-1 and survival outcomes of ovarian cancer patients,22,23 others have found that MDR-1 gene polymorphism G2677T/A is associated with survival in ovarian cancer patients.24,25 Our results did not show statistical significance for overall survival based on MDR-1 expression in tumor obtained from primary surgery.
Paclitaxel has been used as the first line postoperative chemotherapy in combination with platinum agents for mullerian cancer. Recent increase in the incidence of brain metastasis in ovarian cancer may thus partly be associated with the use of paclitaxel, which is associated with acquired drug resistance via the MDR-1 pathway.
P-glycoprotein is also expressed naturally in the blood brain barrier, influencing the pharmacokinetics and distribution of various drugs.26 Given these two facts (physiologic expression of MDR-1 in blood brain barrier and acquired expression of MDR-1 after chemotherapy exposure), once the tumor cells reach and invade inside the brain tissue, chemotherapeutic agents are less likely to be effective. This most likely explains the phenomenon clinically named the “sanctuary” effect in the previous study.5 Our results further showed increased expression of MDR-1 at the time of initial cytoreductive surgery, which represents an intrinsic gene expression. The increased baseline activity of MDR-1 may synergistically contribute to decrease the effectiveness of chemotherapy at the “sanctuary” site.
DNA S-phase fraction, which is an index of cell proliferation, is associated with ovarian cancer survival.27 Our results suggest that DNA S-phase fraction may be associated with the potency of hematogenous spread of tumor cells. The level of DNA S-phase fraction seen at the site of recurrence increased in a caudal to cephalic direction (DNA S-phase fraction, liver and/or spleen 8.2%, lung 11.4%, and brain 14.9%, respectively. This indicates that the anatomic sites of recurrence are related to the tumor cells’ ability to spread and survive.
A strength of our study is that we evaluated risk factors predicting the future development of brain metastasis using information obtained at the time of initial cytoreduction. Potential weaknesses of the study are that this is a retrospective study that may miss confounding factors, and that the study size is relatively small with heterogeneity including fallopian tube and peritoneal cancers. Another limitation of the study is that tumor biology may possibly change after initial cytoreductive surgery and exposure to chemotherapy.28 We evaluated the MDR-1 expression using tissue samples obtained at the initial surgeries. We did not evaluate the MDR-1 expression from the tissue obtained from brain metastasis, and thus, do not know if the tumor biology of the two samples was the same, or whether the tumor acquired drug resistance during the time interval. It is possible that increased MDR-1 expression may serve as a predictive factor and could also be a potential therapeutic target. Experiments targeting this gene pathway with modalities such as RNA interference may be instructive for determining the biological role(s) of MDR-1 in affecting metastatic potential.29,30
The small numbers limit our ability to determine anything but the largest differences between patient groups. For example, we would have between 70% and 80% power to detect a difference in proportions of 66% between patients with brain metastasis and the other groups presented in the paper. This power estimate is based on a 2-sided Fisher’s exact test with 5% statistical significance. The detectable difference in means for any comparisons between the group with brain metastases and the other groups ranges between 1.3 and 1.4 standard units at 80% power. This estimate was calculated for a 2-sided t-test with equal variances at 5% statistical significance.
In summary, brain metastasis in ovarian cancer patients is a rare and late manifestation. Increased expression of the MDR-1 gene in the tumor tissue obtained at the initial cytoreductive surgery is significantly associated with the risk of developing brain metastasis in epithelial ovarian, fallopian, and peritoneal cancer. Further investigation to evaluate the expression of MDR-1 in brain metastases is of great interest.
Acknowledgements
KM is supported by an award from the Meyer and Ida Gordon Foundation #2 and GCF/OCRF Ann Schreiber Ovarian Cancer Research Grant. MMS is supported by the GCF-Molly Cade ovarian cancer research grant and the NIH/NICHD Baylor WRHR scholarship grant (HD050128). Portions of this work were supported by the U. T. M. D. Anderson Cancer Center SPORE (P50CA083639), the Marcus Foundation, the Entertainment Industry Foundation, the Blanton-Davis Ovarian Cancer Research Program, and the Betty Anne Asche Murray Distinguished Professorship.
Footnotes
Abstract is accepted and scheduled for presenting at 41st Annual Meeting of Society of Gynecologic Oncologists, San Francisco, March 14-17, 2010.
The authors declare that there are no conflicts of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Heintz AP, Odicino F, Maisonneuve P, et al. Carcinoma of the ovary. Int J Gynaecol Obstet. 2003;83S:135–66. doi: 10.1016/s0020-7292(03)90118-4. [DOI] [PubMed] [Google Scholar]
- 3.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 4.Loizzi V, Chan JK, Osann K, et al. Survival outcomes in patients with recurrent ovarian cancer who were treated with chemoresistance assay-guided chemotherapy. Am J Obstet Gynecol. 2003;189:1301–7. doi: 10.1067/s0002-9378(03)00629-x. [DOI] [PubMed] [Google Scholar]
- 5.Kolomainen DF, Larkin JM, Badran M, et al. Epithelial ovarian cancer metastasizing to the brain: a late manifestation of the disease with an increasing incidence. J Clin Oncol. 2002;20:982–6. doi: 10.1200/JCO.2002.20.4.982. [DOI] [PubMed] [Google Scholar]
- 6.Kastritis E, Efstathiou E, Gika D, et al. Brain metastases as isolated site of relapse in patients with epithelial ovarian cancer previously treated with platinum and paclitaxel-based chemotherapy. Int J Gynecol Cancer. 2006;16:994–9. doi: 10.1111/j.1525-1438.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 7.Tay SK, Rajesh H. Brain metastases from epithelial ovarian cancer. Int J Gynecol Cancer. 2005;15:824–9. doi: 10.1111/j.1525-1438.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaminsky-Forrett MC, Weber B, Conroy T, et al. Brain metastases from epithelial ovarian carcinoma. Int J Gynecol Cancer. 2000;10:366–371. doi: 10.1046/j.1525-1438.2000.010005366.x. [DOI] [PubMed] [Google Scholar]
- 9.Anupol N, Ghamande S, Odunsi K, et al. Evaluation of prognostic factors and treatment modalities in ovarian cancer patients with brain metastases. Gynecol Oncol. 2002;85:487–92. doi: 10.1006/gyno.2002.6653. [DOI] [PubMed] [Google Scholar]
- 10.Cohen ZR, Suki D, Weinberg JS, et al. Brain metastases in patients with ovarian carcinoma: prognostic factors and outcome. J Neurooncol. 2004;66:313–25. doi: 10.1023/b:neon.0000014516.04943.38. [DOI] [PubMed] [Google Scholar]
- 11.Porzio G, Ronzino G, Farina E, et al. Cerebral metastasis from ovarian cancer treated with a multidisciplinary approach. Case report and review of literature. Eur J Gynaecol Oncol. 2003;24:563–4. [PubMed] [Google Scholar]
- 12.Kim TJ, Song S, Kim CK, et al. Prognostic factors associated with brain metastases from epithelial ovarian carcinoma. Int J Gynecol Cancer. 2007;17:1252–7. doi: 10.1111/j.1525-1438.2007.00941.x. [DOI] [PubMed] [Google Scholar]
- 13.McMeekin DS, Kamelle SA, Vasilev SA, et al. Ovarian cancer metastatic to the brain: what is the optimal management? J Surg Oncol. 2001;78:194–200. doi: 10.1002/jso.1149. [DOI] [PubMed] [Google Scholar]
- 14.Cormio G, Maneo A, Colamaria A, et al. Surgical resection of solitary brain metastasis from ovarian carcinoma: an analysis of 22 cases. Gynecol Oncol. 2003;89:116–9. doi: 10.1016/s0090-8258(03)00060-x. [DOI] [PubMed] [Google Scholar]
- 15.Growdon WB, Lopez-Varela E, Littell R, et al. Extent of extracranial disease is a powerful predictor of survival in patients with brain metastases from gynecological cancer. Int J Gynecol Cancer. 2008;18:262–8. doi: 10.1111/j.1525-1438.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- 16. [cited October 1, 2008];EDR Assay®. www.oncotech.com.
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Germann UA. P-glycoprotein--a mediator of multidrug resistance in tumour cells. Eur J Cancer. 1996;32A:927–44. doi: 10.1016/0959-8049(96)00057-3. [DOI] [PubMed] [Google Scholar]
- 19.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–27. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 20.Kamazawa S, Kigawa J, Kanamori Y, et al. Multidrug resistance gene-1 is a useful predictor of Paclitaxel-based chemotherapy for patients with ovarian cancer. Gynecol Oncol. 2002;86:171–6. doi: 10.1006/gyno.2002.6738. [DOI] [PubMed] [Google Scholar]
- 21.Naniwa J, Kigawa J, Kanamori Y, et al. Genetic diagnosis for chemosensitivity with drug-resistance genes in epithelial ovarian cancer. Int J Gynecol Cancer. 2007;17:76–82. doi: 10.1111/j.1525-1438.2006.00752.x. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K, Sakai K, Yamamoto R, et al. Multivariate analysis for prognostic significance of histologic subtype, GST-pi, MDR-1, and p53 in stages II-IV ovarian cancer. Int J Gynecol Cancer. 2003;13:776–84. doi: 10.1111/j.1525-1438.2003.13381.x. [DOI] [PubMed] [Google Scholar]
- 23.Matsuo K, Bond VK, Eno ML, et al. Low drug resistance to both platinum and taxane chemotherapy on an in vitro drug resistance assay predicts improved survival in patients with advanced epithelial ovarian, fallopian and peritoneal cancer. Int J Cancer. 2009;125:2721–7. doi: 10.1002/ijc.24654. 2009. [DOI] [PubMed] [Google Scholar]
- 24.Gréen H, Söderkvist P, Rosenberg P, et al. mdr-1 single nucleotide polymorphisms in ovarian cancer tissue: G2677T/A correlates with response to paclitaxel chemotherapy. Clin Cancer Res. 2006;12:854–9. doi: 10.1158/1078-0432.CCR-05-0950. [DOI] [PubMed] [Google Scholar]
- 25.Johnatty SE, Beesley J, Paul J, et al. ABCB1 (MDR 1) polymorphisms and progression-free survival among women with ovarian cancer following paclitaxel/carboplatin chemotherapy. Clin Cancer Res. 2008;14:5594–601. doi: 10.1158/1078-0432.CCR-08-0606. 2008. [DOI] [PubMed] [Google Scholar]
- 26.Sun H, Dai H, Shaik N, et al. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 2003;55:83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 27.Kallioniemi OP, Punnonen R, Mattila J, et al. Prognostic significance of DNA index, multiploidy, and S-phase fraction in ovarian cancer. Cancer. 1988;61:334–9. doi: 10.1002/1097-0142(19880115)61:2<334::aid-cncr2820610224>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Matsuo K, Eno ML, Im DD, et al. Chemotherapy time interval and development of platinum and taxane resistance in ovarian, fallopian, and peritoneal carcinomas. Arch Gynecol Obstet. 2010;281:325–8. doi: 10.1007/s00404-009-1121-1. [DOI] [PubMed] [Google Scholar]
- 29.Hua J, Mutch DG, Herzog TJ. Stable suppression of MDR-1 gene using siRNA expression vector to reverse drug resistance in a human uterine sarcoma cell line. Gynecol Oncol. 2005;98:31–8. doi: 10.1016/j.ygyno.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 30.Yadav S, van Vlerken LE, Little SR, et al. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol. 2009;63:711–22. doi: 10.1007/s00280-008-0790-y. 2009. [DOI] [PubMed] [Google Scholar]


