Abstract
Background
Abnormalities of ventricular repolarization as well as depolarization have been associated with increased risk of ventricular arrhythmias.
Objective
We evaluated the relative contribution of these predictors to risk of sudden cardiac death (SCD) among patients with coronary artery disease (CAD).
Methods
In the ongoing Oregon Sudden Unexpected Death Study (Oregon SUDS), adult residents of Portland, OR metropolitan area (population ~1 million) who suffered SCD were identified prospectively (2002-2007). Of these, we analyzed the subgroup of SCDs that had a resting 12-lead ECG prior to SCD and also had associated CAD. Comparisons were conducted with a control group of subjects with known CAD, but no history of SCD from the same geographic region. Corrected QT interval (QTc), JT interval (JTc), QRS duration (QRSd) and other parameters were measured from ECG prior and unrelated to SCD. Analysis of LV function was limited to those subjects that had echocardiography performed prior to and remote from SCD.
Results
A total of 642 SCD cases (71±13 yrs, 62% male) were compared to 450 controls (66±12 yrs, 64% male). SCD cases had significantly longer QRSd (102±25 vs. 97±20 ms, p=0.0008) as well as JTc (348±44 vs. 339±34 ms, p=0.0006) vs. controls. In cases with prolonged QRSd, 38% had severe LV systolic dysfunction (LVSD) and 62% had normal, mild or moderately decreased LV systolic function. In a multivariable model, QRSd, JTc, age and severe LVSD were independent predictors. There was minimal overlap between prolonged QRSd and JTc in both case and control groups (3% and 4%, respectively).
Conclusions
Prolonged QRSd, JTc and severe LVSD had independent contributions to risk of SCD in coronary disease, in this community-based setting.
Keywords: Epidemiology, risk, stratification, predictor, population, sudden cardiac death, electrocardiogram, ventricular fibrillation, prevention
INTRODUCTION
The annual incidence of sudden cardiac death (SCD) in the US ranges between 200,000 - 300,000 and the vast majority occur due to fatal arrhythmia (1,2). The majority of SCD cases have associated coronary artery disease (CAD) (3). Based on the current eligibility criterion of severe left ventricular systolic dysfunction (LVSD), the prophylactic implantable cardioverter-defibrillator (ICD) has been a useful preventive intervention (4,5). However, there is increasing recognition that a significant proportion of patients that suffer SCD may have risk predictors other than severe LVSD. In two large population-based evaluations of SCD, severe LVSD was a significant predictor but was found to affect only 25-30% of all SCD cases in the community (6,7). Therefore at least 70-75% of overall SCD cases that occur in the community would not meet criteria for prophylactic ICDs by the current guidelines. A consensus has emerged in the field for delineation of multiple predictors that could be combined as a risk score to enhance SCD risk stratification (8).
The Oregon Sudden Unexpected Death Study (Oregon SUDS (1,7,9,10) is a prospective community-based case-control study with the overall goal of identifying risk predictors for SCD other than severe LVSD. We and others have reported that another ECG variable, prolonged ventricular repolarization measured as the corrected QT or JT interval (↑QTc, ↑JTc), independently increases SCD risk in the general population (9,11) and that genetic variants associated with ↑QTc also increase SCD risk (12,13). Increased QRS duration (↑QRSd) has long been recognized as a predictor of overall mortality among patients with ischemic cardiomyopathy (14) and myocardial infarction (15). Several studies have reported that the presence of an intraventricular conduction delay or left bundle branch block, but not right bundle branch block, can be associated with an increase in arrhythmic death and overall mortality (16-18). However, these findings have largely been reported in subgroups of patients with congested heart failure (CHF) or other selected patient populations. The potential overlap with prolonged ventricular repolarization has not been well studied. We evaluated the relative contribution of prolonged ventricular depolarization and repolarization to the ventricular arrhythmia substrate in coronary artery disease (CAD), from a community-based study.
METHODS
Ascertainment of subjects
Detailed methods of the Oregon SUDS have been published earlier (1,7) and a brief description follows. Cases of sudden cardiac death (SCD) from the Portland, Oregon metropolitan area were identified prospectively from the general population using the emergency medical system, local area hospitals and physicians, and medical examiners. After a review of available medical records and the circumstances of arrest, and a process of in-house adjudication conducted by three physicians, subjects who suffered SCD were included in the study. SCD was defined as a sudden unexpected pulseless condition within one hour of symptom onset if witnessed, and within 24 hours of being seen alive and in usual state of health if unwitnessed. Subjects with chronic terminal illnesses (e.g. cancer), known non-cardiac causes of sudden death (e.g. pulmonary embolism, cerebral vascular accident), traumatic deaths and overdoses were excluded. Cases with documented or probable (≥ 50 years of age) (19,20) coronary artery disease (CAD) were compared to controls with CAD but no cardiac arrest from the same geographic region. Controls were patients transported by the emergency medical system for complaints suggestive of ongoing coronary ischemia, were recruited from clinics of participating health systems, or had received a coronary angiogram revealing significant CAD. Documented CAD was defined as history of myocardial infarction, coronary revascularization, or at least 50% stenosis on coronary angiography.
ECG inclusion criteria
All subjects were required to have a 12-lead ECG with QRS measurements available. Sinus rhythm, sinus arrhythmia, atrial fibrillation and atrial flutter rhythms were included. For cases, the closest ECG prior and unrelated to the cardiac arrest was used. For controls, ECGs were obtained at the time of the study enrollment visit or from clinic or hospital visits unrelated to the study. We used standard 12-lead ECG tracing at 25-mm/s paper speed and 10-mm/mV amplitude received from participating hospitals. QRSd and heart rate were as reported on the ECG recording while QT and JT intervals were manually measured from the lead with the longest complex. For QT and JT interval measurements only patients with sinus rhythm were included and these were manually-measured and corrected (QTc and JTc) using Bazett’s formula (21). Findings of QTc and JTc were also validated using Fridericia’s and Sagie’s formulae (22,23).The QTc interval was considered missing for subjects with QRS ≥120 ms. Prolonged JTc was defined as an interval greater than 75th percentile of JTc in the control subjects (359ms). Two trained personnel performed separate, blinded measurements on a subset of ECGs (n=473). The intraclass correlation coefficient comparing the first and second readers for the QT interval was 0.92 (95% CI,0.90-0.93); for the RR interval, it was 0.94 (95% CI, 0.93-0.95).
Subject characteristics
Demographic and clinical characteristics were recorded from first responder and medical examiner reports as well as medical records. The analysis of clinical variable is therefore conducted retrospectively. For a subset of patients (49.1%), left ventricular systolic function was assessed by LV ejection fraction (EF) from echocardiogram, angiogram or multigated acquisition SCDn. Left ventricular mass (LVM) was calculated from quantitative values on echocardiograms using the American Society of Echocardiography modified equation, indexed to body surface area (BSA) and LV hypertrophy was defined as LVM/BSA >134 g/m2 for men and >110 g/m2 for women (24). Echocardiograms prior and unrelated to cardiac arrest were used for cases and up to 5 days post-ascertainment for control subjects.
This study was approved by the Institutional Review Boards of Cedars-Sinai Medical Center, Oregon Health and Science University and of participating hospitals.
Statistical analysis
Independent samples t-tests and Pearson’s chi-square tests were used for case-control comparisons of continuous and categorical variables, respectively. A multiple logistic regression model was used to estimate odds ratios for SCD associated with one SD increase in QRS duration (20 ms) or corrected JT interval (34 ms). The JT interval was used to represent ventricular repolarization. Using JTc interval instead of QTc allowed subjects with QRS ≥120 ms to be included in the multivariate model. Multiple logistic regression models were also run for a subgroup of patients with LV systolic function assessed and in subjects without conduction blocks. In addition to the ECG parameters, all logistic regression models include age, gender, diabetes and hypertension. Values are presented as n (%) or mean ± SD and a p value ≤ 0.05 was considered significant for all analyses.
RESULTS
Demographic and clinical characteristics
From Feb 1st 2002 to Jan 31st 2007, 1435 cases and 473 controls were enrolled. Of these, a total of 642 cases and 450 controls met ECG inclusion criteria for analysis. Demographics and clinical characteristics of the subjects are shown in Table 1. Cases were older compared to controls (71 yrs vs 66 yrs, p < 0.0001). Cases were more likely to have diabetes (41% vs. 32%, p = 0.002) and hypertension (76% vs. 71%, p = 0.06). Severe LV systolic dysfunction (LVSD) and LV hypertrophy (LVH) assessed by echocardiography were significantly more common among cases than controls (p < 0.0001).
Table 1.
Demographic and clinical characteristics of subjects ≥ 35 years of age, Oregon SUDS 2002 – 2007 (n = 1092)
| Case (n = 642) |
Control (n = 450) |
P value* | |
|---|---|---|---|
| Age (mean SD) | 71.2 13.1 (n = 642) |
66.5 12.2 (n = 450) |
<0.0001 |
| Male | 398 (62.0%) | 290 (64.4%) | 0.41 |
| BMI (mean SD) | 29.3 9.1 (n = 514) |
29.4 6.5 (n = 414) |
0.88 |
| Diabetes | 265 (41.3%) | 145 (32.2%) | 0.002 |
| Hypertension | 489 (76.2%) | 320 (71.1%) | 0.06 |
| LV systolic dysfunction‡ Normal function Mild-moderate dysfunction Severe dysfunction Unknown/not evaluated† |
142 (40.2%) 112 (31.7%) 99 (28.0%) 289 |
122 (66.7%) 42 (22.9%) 19 (10.4%) 267 |
<0.0001 |
| LVH by echocardiography Unknown/not evaluated † |
111 (44.0%) 390 |
42 (24.3%) 277 |
<0.0001 |
P value from Pearson chi-square test for categorical variables and t-test for continuous variables.
For variables with missing values, proportions and p-values are calculated using the non-missing data as the denominator.
LV systolic dysfunction defined as: normal (EF ≥ 55%), mild-moderate dysfunction (EF = 36-54%), severe dysfunction (EF ≤ 35%).
BMI, body mass index; LVH, left ventricular hypertrophy; SD, standard deviation.
Findings on the resting 12-lead electrocardiogram
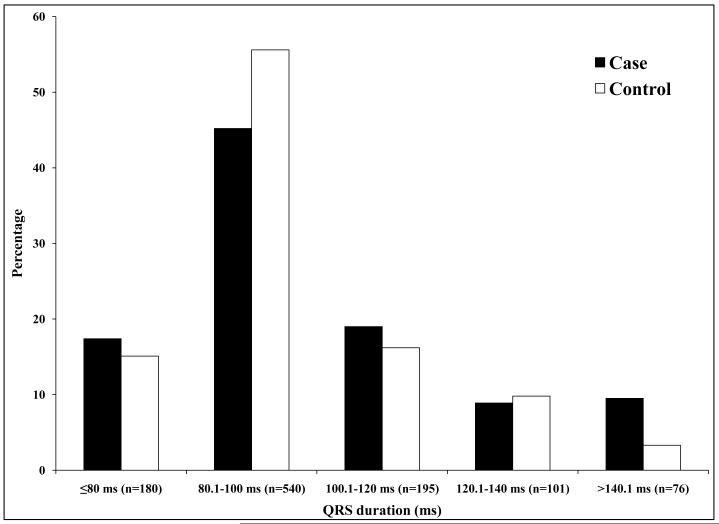
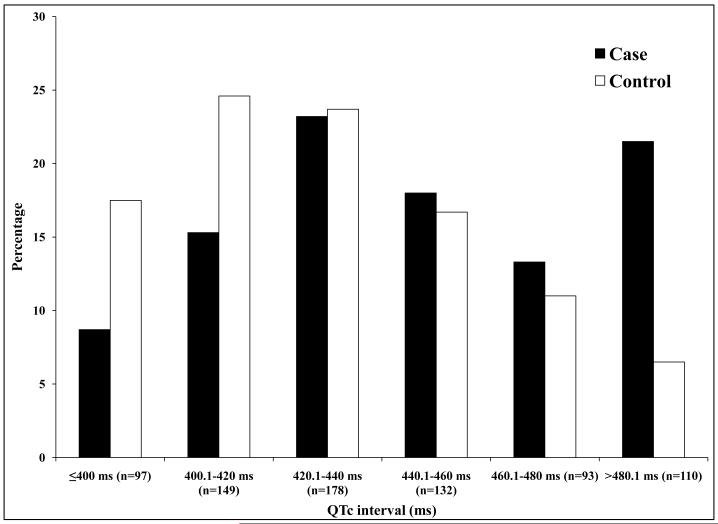
For SCD cases, ECGs analyzed were performed a median of 10.4 months prior to SCD. For controls, ECGs were obtained more than 14 days prior to the ascertainment day in 32% (n = 162 controls), more than 14 days post ascertainment in 38% (n = 198) and within 14 days of ascertainment in 30% (n = 158). QRS duration was significantly longer in cases versus controls (102 25 ms vs. 97 20 ms, p = 0.0008) (Table 2). Cases were also more likely to have QRSd 120 ms or greater (p = 0.03). QRS duration was significantly higher in cases compared to controls (105.7 26 ms vs 97.2 19 ms, p<0.0001) after adjustment for age, gender, heart rate, hypertension, diabetes and LV systolic function. There was no difference in qualitative conduction system disease findings from the ECG, between cases and controls (p = 0.59). Measures of repolarization (QTc and JTc) were significantly more prolonged in cases vs. controls (Table 2). Heart rate was higher in cases (79 19 bpm) compared to controls (71 17 bpm, p < 0.0001). Among cases, 88% of ECGs were in sinus rhythm and 12% in atrial fibrillation/flutter (Table 2). Atrial fibrillation was more common among cases (p = 0.0003). QRSd remained significantly longer in cases than controls when only ECGs in sinus rhythm were used (p = 0.001). Distribution of ECG intervals is presented in Figure 1.
Table 2.
Findings on resting 12-lead electrocardiogram, age ≥ 35 yrs, Oregon SUDS 2002–2007 (n=1092)
| Case (n = 642) |
Control (n = 450) |
P value* | |
|---|---|---|---|
| QRS (mean in ms SD) | 101.7 25.0 (n = 642) |
97.1 19.7 (n = 450) |
0.0008 |
| Prolonged QRSd (≥ 120 ms) | 123 (19.2%) | 64 (14.2%) | 0.03 |
| QRS Morphology Normal IVCD LBBB RBBB LAFB LPFB Bifascicular block IRBBB/ILBBB 1st degree block |
435 (67.8%) 64 (10.0%) 21 (3.3%) 23 (3.6%) 22 (3.4%) 3 (0.5%) 15 (2.3%) 14 (2.2%) 45(7.0%) |
308(68.4%) 54 (12.0%) 8 (1.8%) 16 (3.6%) 15 (3.3%) 0 (0.0%) 7 (1.6%) 8 (1.8%) 34 (7.6%) |
0.59 |
| JTc (mean in ms SD) | 348.2 44.1 (n = 495) |
339.2 34.4 (n = 407) |
0.0006 |
| Prolonged JTc Not evaluated† |
167 (33.7%) 147 |
101 (24.8%) 43 |
0.004 |
| QTc (mean in ms SD) | 449.9 43.4 (n = 405) |
428.9 32.8 (n = 354) |
<0.0001 |
| QT (mean in ms SD) | 404.0 54.2 (n = 579) |
406.5 42.8 (n = 435) |
0.41 |
| RR (mean in ms SD) | 799.1 192.7 (n = 578) |
892.2 194.5 (n = 435) |
<0.0001 |
| Rate (mean bpm SD) | 78.8 18.7 (n = 637) |
70.7 17.2 (n = 450) |
<0.0001 |
| Rhythm Sinus rhythm Sinus arrhythmia Atrial fibrillation Atrial flutter |
552 (86.0%) 11 (1.7%) 70 (10.9%) 9 (1.4%) |
422 (93.8%) 7 (1.5%) 18 (4.0%) 3 (0.7%) |
0.0003 |
P value from Pearson chi-square test for categorical variables and t-test for continuous variables.
For variables with missing values, proportions and p-values are calculated using the non-missing data as the denominator.
IVCD, intraventricular conduction delay; LBBB, left bundle branch block; RBBB, right bundle branch block; LAFB, left anterior fascicular block; LPFB, left posterior fascicular block; IRBBB/ILBBB, incomplete right/left bundle branch block.
Figure 1.
Case-control distribution of QRSd (Fig 1A; p=0.0002), JTc (Fig 1B; p=0.01) and QTc (Fig 1C; p<0.0001).
Overall, in this study population, only 34% of subjects with prolonged QRSd had severe LVSD. Severe LVSD was present in 38.0% of cases with prolonged QRS and in 25.2% of cases with normal QRS (p = 0.03) (Figure 2). A similar trend was found in controls (23.3% vs. 7.8%, p = 0.02).
Figure 2.
The relationship between LV systolic function and QRS duration among SCD (sudden cardiac death) cases and controls. In either group, only a minority of subjects with prolonged QRS duration had severe LV systolic dysfunction (EF≤ 35%).
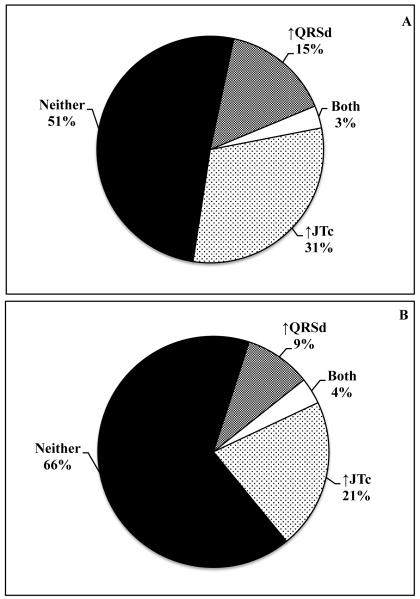
Relationship between ventricular depolarization and repolarization (QRSd vs. JTc)
Among 495 cases with information on QRSd and JTc, only 3.2% had prolonged JTc in addition to prolonged QRSd. Similarly, among 407 controls, both QRSd and JTc were prolonged in only 3.7% of subjects (Figure 3).
Figure 3.
Distribution of QRSd and JTc prolongation among cases (Panel A) and controls (Panel B).
In a multivariable logistic regression model adjusted for age and gender, both QRS and JTc intervals were significantly associated with SCD (Table 3). In a subset of patients with information on LV systolic dysfunction, when LV systolic dysfunction was added to the logistic model, QRS duration and JTc remained significant independent predictors of SCD (Table 3). In addition, the presence of LV dysfunction almost tripled the odds of SCD (OR, 2.50; 95% CI, 1.35 to 4.62). Among subjects with preserved LV function (EF > 35%) (n = 432), QRSd was a significant predictor of SCD (OR, 1.40; 95% CI, 1.10 – 1.78) independent of JTc. QRSd remained independently associated with SCD in the subset of patients with numerically available LVEF (n = 353) (OR, 1.31; 95% CI, 1.03 – 1.66). While QTc was also independently associated with increased risk of SCD, the insertion of QTc in the model rendered the association of QRSd with risk of SCD to be non-significant.
Table 3.
Factors independently associated with SCD from the multivariate analysis
| Model with JTc (n =900) |
Model with JTc, LV dysfunction (n = 432) |
|
|---|---|---|
| Age Male gender QRS (1SD increase) JTc (1SD increase) Rate (1SD increase) Diabetes Hypertension Severe LV dysfunction |
1.03(1.01 – 1.04) 1.12 (0.822 – 1.52) 1.24 (1.07 – 1.42) 1.22 (1.07 – 1.38) 1.74 (1.49 – 2.03) 1.32 (0.98 – 1.79) 1.35 (0.98 – 1.86) - |
1.03 (1.01 – 1.05) 1.13 (0.72 – 1.78) 1.37 (1.11 – 1.70) 1.33 (1.10 – 1.61) 1.66 (1.31 – 2.12) 1.54 (0.98 – 2.41) 1.14 (0.66 – 1.95) 2.50 (1.35 – 4.62) |
When the analysis was repeated using the same model, but with exclusion of all patients with LBBB or RBBB on the 12-lead ECG (n = 848 remaining after exclusions), QRSd remained significantly associated with SCD, independent of JTc (OR, 1.32; 95% CI, 1.12 – 1.56). When severe LV dysfunction was added to this latter model (n = 403), both QRSd (OR, 1.50; CI, 1.16 – 1.94) and severe LV dysfunction (OR, 2.69; CI, 1.38 – 5.-23) remained significantly and independently associated with SCD.
DISCUSSION
This study has confirmed the association between prolonged QRSd and SCD among patients with CAD in the general population; reported earlier in studies of defibrillator patients and hospital-based subjects. When measured from resting 12-lead ECGs performed prior (and remote from) the SCD event, ↑QRSd is associated with SCD risk among patients with CAD; and was independent of the effects of age, gender, severe LVSD and prolonged ventricular repolarization (↑JTc). In addition, there was minimal overlap between SCD cases or controls that manifested with both ↑QRSd and ↑JTc. While ↑QTc was also independently associated with increased risk of SCD, QRSd was not a significant predictor when included with QTc in multivariate analyses. Likely due to the fact that QTc forms a portion of the QRSd itself, JTc will be more useful in the context of 12-lead ECG intervals and risk of SCD. Whether QRSd specifically predicts fatal tachycardia or bradycardia or a combination of both, needs further evaluation. For example, in the CARISMA trial (25) high degree atrioventricular block was the most powerful determinant of cardiac death among patients with acute myocardial infarction and reduced ejection fraction.
These findings have implications for prediction and prevention of SCD. Only 28% of all cases and 10% of all controls had severe LVSD, highlighting the fact that the use of the LV ejection fraction as a risk stratification tool is effective for only a subgroup of subjects with coronary disease who will suffer future SCD. Given the complex and multifactorial nature of SCD mechanisms, it is likely that QRSd and JTc may help to enhance risk prediction in other subsets of SCD cases. There has been a recent consensus around the need for a “risk score” for SCD (8) and it remains to be seen whether these intervals from the resting ECG could contribute to such a score. Given the wide availability, non-invasiveness and low expense of the 12-lead ECG it would be particularly interesting to perform genotype-phenotype correlations with recently published (26,27) (as well as ongoing) genome wide association studies of SCD.
The minimal overlap observed between QRSd and JTc prolongation in this study merits attention. There are clearly a variety of factors and pathways that could lead to either abnormality but genetic variations in both known and novel genes have been recently identified as determinants. It is of significant interest that the vast majority of these DNA variants are distinct for both ↑QRSd (28,29) and ↑QTc (30,31). What are other possible determinants of QRS and JTc/QTc prolongation among patients with coronary disease? There are likely to be other shared but also distinct mechanistic pathways. It has long been established that approximately 20% of patients with severe LVSD will have prolonged ventricular depolarization (largely due to remodeling of Na ion channels) manifesting as QRSd, and in our study, 31% of subjects with severe LVSD had prolonged QRSd. Similarly the remodeling of myocardial repolarizing ion channels is known to prolong myocardial repolarization. In the current study, QRSd prolongation occurred more often in the absence of severe LVSD. QRSd may also reflect the presence of increased fibrosis and interstitial remodeling of the myocardium in the absence of severe LV dysfunction. An early study analyzed the effects of heart weights and myocardial fibrosis upon the duration of the QRS complex and found a positive correlation between presence or absence of fibrosis and duration of the QRS complex (32). Increased QRS duration was reported in a population with LVH from the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) study (33). In the Framingham Heart Study, wide QRS was associated with greater LV mass (34). The relationship of abnormal repolarization, specifically QTc prolongation, to SCD in patients that suffered acute myocardial infarction was observed over thirty years ago by Schwartz and Wolf (35).
There are limitations inherent in community-based studies that should be considered while interpreting the findings of this study. The same clinical information is not uniformly available for all subjects ascertained in the study. An important reason is that 40% of subjects that suffer SCD may have this condition as the initial manifestation of heart disease, with no opportunity for clinical evaluations to be performed. However any differences identified using a case-control design where all subjects are from the same geographic area and all have probable or definite coronary artery disease, are likely to be highly specific for SCD. Additional limitations include the availability of EF for a subgroup only, and using automated measures of QRSd from the standard 12-lead ECG. Although the association of QRSd and SCD is not novel, the finding that prolonged depolarization and repolarization have independent effects on risk of SCD in the community is unique to this study.
Conclusions
Among patients with coronary artery disease ascertained from the community, both prolonged ventricular depolarization and repolarization are associated with sudden death, but the risk conferred by each is independent of the other. These effects remain significant when adjusted for age, sex and severe LV systolic function. These findings could be confirmed in prospective studies of ICD patients using appropriate therapies as a surrogate for SCD, as well as in larger populations with diverse ethnicities. Given the wide applicability and relatively low cost of the 12-lead ECG, these markers have potential for contributing to SCD risk stratification in patients with coronary artery disease.
Acknowledgments
Funded by National Heart Lung and Blood Institute HL105170 and HL088416 to Dr Chugh. Dr Chugh is the Pauline and Harold Price Professor of Cardiac Electrophysiology at the Cedars-Sinai Medical Center, Los Angeles, CA.
Abbreviations
- BMI
body mass index
- BSA
body surface area
- CAD
coronary artery disease
- CHF
congested heart failure
- EF
ejection fraction
- ICD
implantable cardioverter-defibrillator
- IRBBB/ILBBB
incomplete right/left bundle branch block
- IVCD
intraventricular conduction delay
- LBBB
left bundle branch block
- LAFB
left anterior fascicular block
- LPFB
left posterior fascicular block
- LVH
left ventricular hypertrophy
- LVM
left ventricular mass
- LVSD
left ventricular systolic dysfunction
- RBBB
right bundle branch block
- SCD
sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none
REFERENCES
- 1.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–75. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 2.Myerburg RJ. Scientific gaps in the prediction and prevention of sudden cardiac death. J Cardiovasc Electrophysiol. 2002;13:709–23. doi: 10.1046/j.1540-8167.2002.00709.x. [DOI] [PubMed] [Google Scholar]
- 3.Myerburg RJ, Interian A, Jr., Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F–19F. doi: 10.1016/s0002-9149(97)00477-3. [DOI] [PubMed] [Google Scholar]
- 4.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.de Vreede-Swagemakers JJ, Gorgels AP, Dubois-Arbouw WI, et al. Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. J Am Coll Cardiol. 1997;30:1500–5. doi: 10.1016/s0735-1097(97)00355-0. [DOI] [PubMed] [Google Scholar]
- 7.Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161–6. doi: 10.1016/j.jacc.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 8.Fishman GI, Chugh SS, Dimarco JP, et al. Sudden cardiac death prediction and prevention: report from a National Heart, Lung, and Blood Institute and Heart Rhythm Society Workshop. Circulation. 122:2335–48. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chugh SS, Reinier K, Singh T, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: the Oregon Sudden Unexpected Death Study. Circulation. 2009;119:663–70. doi: 10.1161/CIRCULATIONAHA.108.797035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chugh SS, Reinier K, Teodorescu C, et al. Epidemiology of sudden cardiac death: clinical and research implications. Prog Cardiovasc Dis. 2008;51:213–28. doi: 10.1016/j.pcad.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 12.Eijgelsheim M, Newton-Cheh C, Aarnoudse AL, et al. Genetic variation in NOS1AP is associated with sudden cardiac death: evidence from the Rotterdam Study. Hum Mol Genet. 2009;18:4213–8. doi: 10.1093/hmg/ddp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao WH, Arking DE, Post W, et al. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation. 2009;119:940–51. doi: 10.1161/CIRCULATIONAHA.108.791723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iuliano S, Fisher SG, Karasik PE, Fletcher RD, Singh SN. QRS duration and mortality in patients with congestive heart failure. Am Heart J. 2002;143:1085–91. doi: 10.1067/mhj.2002.122516. [DOI] [PubMed] [Google Scholar]
- 15.Fosbol EL, Seibaek M, Brendorp B, et al. Differential prognostic importance of QRS duration in heart failure and acute myocardial infarction associated with left ventricular dysfunction. Eur J Heart Fail. 2007;9:814–9. doi: 10.1016/j.ejheart.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Freedman RA, Alderman EL, Sheffield LT, Saporito M, Fisher LD. Bundle branch block in patients with chronic coronary artery disease: angiographic correlates and prognostic significance. J Am Coll Cardiol. 1987;10:73–80. doi: 10.1016/s0735-1097(87)80162-6. [DOI] [PubMed] [Google Scholar]
- 17.Kalahasti V, Nambi V, Martin DO, et al. QRS duration and prediction of mortality in patients undergoing risk stratification for ventricular arrhythmias. Am J Cardiol. 2003;92:798–803. doi: 10.1016/s0002-9149(03)00886-5. [DOI] [PubMed] [Google Scholar]
- 18.Zimetbaum PJ, Buxton AE, Batsford W, et al. Electrocardiographic predictors of arrhythmic death and total mortality in the multicenter unsustained tachycardia trial. Circulation. 2004;110:766–9. doi: 10.1161/01.CIR.0000139311.32278.32. [DOI] [PubMed] [Google Scholar]
- 19.Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. J Am Coll Cardiol. 1985;5:141B–149B. doi: 10.1016/s0735-1097(85)80545-3. [DOI] [PubMed] [Google Scholar]
- 20.Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159:33–9. doi: 10.1016/j.ahj.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 22.Fridericia LS. Die Systolendauer im Elektrokardiogramm bei normalen Menchen und bei Herzkranken. Acta Med Scand. 1920;53:469–486. [Google Scholar]
- 23.Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study) Am J Cardiol. 1992;70:797–801. doi: 10.1016/0002-9149(92)90562-d. [DOI] [PubMed] [Google Scholar]
- 24.Devereux RB, Lutas EM, Casale PN, et al. Standardization of M-mode echocardiographic left ventricular anatomic measurements. J Am Coll Cardiol. 1984;4:1222–30. doi: 10.1016/s0735-1097(84)80141-2. [DOI] [PubMed] [Google Scholar]
- 25.Bloch Thomsen PE, Jons C, Raatikainen MJ, et al. Long-term recording of cardiac arrhythmias with an implantable cardiac monitor in patients with reduced ejection fraction after acute myocardial infarction: the Cardiac Arrhythmias and Risk Stratification After Acute Myocardial Infarction (CARISMA) study. Circulation. 2010;122:1258–64. doi: 10.1161/CIRCULATIONAHA.109.902148. [DOI] [PubMed] [Google Scholar]
- 26.Arking DE, Reinier K, Post W, et al. Genome-Wide Association Study Identifies GPC5 as a Novel Genetic Locus Protective Against Sudden Cardiac Arrest. PLoS One. 2010:5. doi: 10.1371/journal.pone.0009879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bezzina CR, Pazoki R, Bardai A, et al. Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nat Genet. 42:688–91. doi: 10.1038/ng.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holm H, Gudbjartsson DF, Arnar DO, et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet. 42:117–22. doi: 10.1038/ng.511. [DOI] [PubMed] [Google Scholar]
- 29.Sotoodehnia N, Isaacs A, de Bakker PI, et al. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet. 42:1068–76. doi: 10.1038/ng.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arking DE, Pfeufer A, Post W, et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–51. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 31.Newton-Cheh C, Eijgelsheim M, Rice KM, et al. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat Genet. 2009;41:399–406. doi: 10.1038/ng.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzoleni A, Curtin ME, Wolff R, Reiner L, Somes G. On the relationship between heart weights, fibrosis, and QRS duration. J Electrocardiol. 1975;8:233–6. doi: 10.1016/s0022-0736(75)80050-1. [DOI] [PubMed] [Google Scholar]
- 33.Oikarinen L, Nieminen MS, Viitasalo M, et al. QRS duration and QT interval predict mortality in hypertensive patients with left ventricular hypertrophy: the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension. 2004;43:1029–34. doi: 10.1161/01.HYP.0000125230.46080.c6. [DOI] [PubMed] [Google Scholar]
- 34.Dhingra R, Ho Nam B, Benjamin EJ, et al. Cross-sectional relations of electrocardiographic QRS duration to left ventricular dimensions: the Framingham Heart Study. J Am Coll Cardiol. 2005;45:685–9. doi: 10.1016/j.jacc.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz PJ, Wolf S. QT interval prolongation as predictor of sudden death in patients with myocardial infarction. Circulation. 1978;57:1074–7. doi: 10.1161/01.cir.57.6.1074. [DOI] [PubMed] [Google Scholar]