Introduction
There are approximately one million glomeruli in each human kidney. Each glomerulus is composed of a tuft of capillary loops supported by the mesangium and enclosed in a pouch-like extension of the renal tubule of the nephron known as Bowman’s capsule. The glomerulus consists of four resident cell types, the mesangial cell, the glomerular endothelial cell, the visceral epithelial cell (podocyte), and the parietal epithelial cell lining Bowman’s basement membrane. Recent experimental and clinical advances have identified the podocyte as the predominant cell of injury in glomerular diseases typified by heavy proteinuria, which is the focus of this article.
Structure, Function, and Injury of the Podocyte
Normal Structure of the Podocyte
-
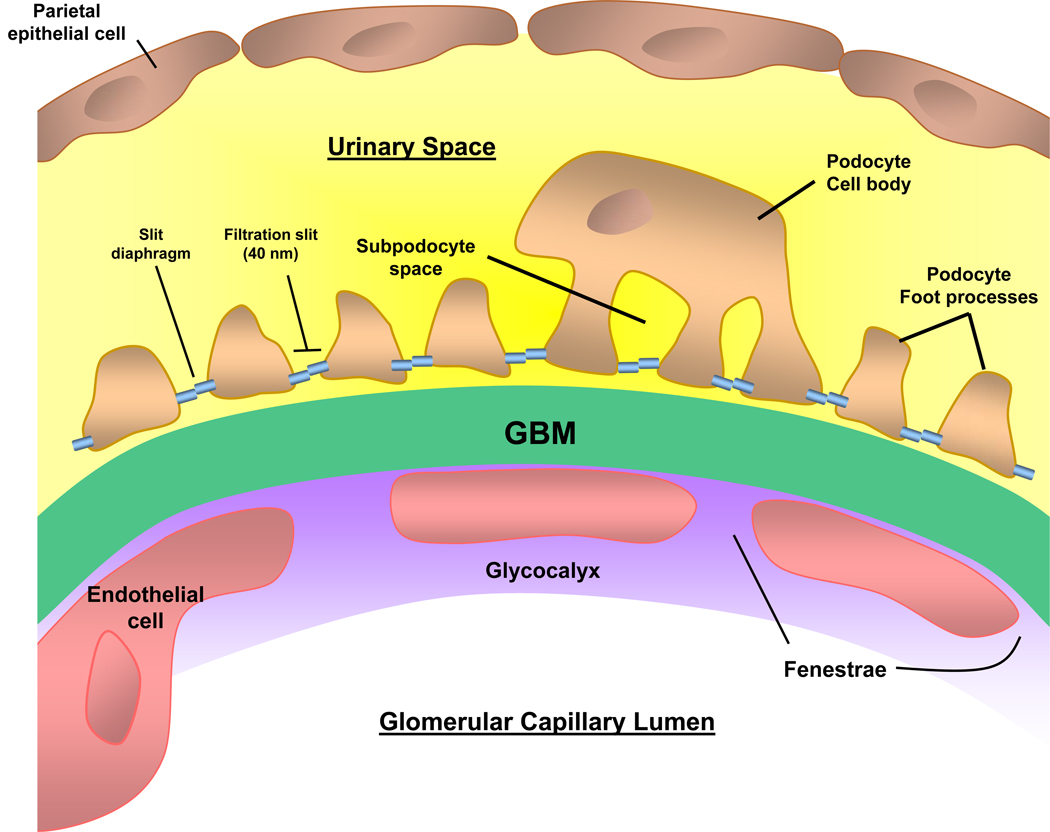
The podocyte is a highly differentiated epithelial cell sitting on the outside of the glomerular capillary loop
Consists of a large cell body (soma) in the urinary space
Connects to the underlying glomerular basement membrane (GBM) of the capillary loop by major cellular extensions from the soma
Extensions terminate as foot processes on the GBM that interdigitate with those from adjacent podocytes (Fig 1)
Podocyte foot processes are anchored to the GBM by α3β1 integrins and α- and β-dystroglycans
-
Between the foot processes, the filtration slit is bridged by a 40-nm wide zipper-like slit diaphragm
Slit diaphragm highly permeable to water and small solutes
Small pore size (5–15 nm) of slit diaphragm limits the passage of larger proteins, including albumin
Nephrin is the major component of the slit diaphragm, and is linked to the actin cytoskeleton by CD2AP (CD2-associated protein), podocin, and others
-
Roughly 500–600 podocytes per glomerular tuft in the adult human kidney
Rate of turnover is very slow
Very limited ability to proliferate
-
An extensive actin cytoskeleton
Allows dynamic contraction to support the glomerular capillary
Counteracts glomerular capillary hydrostatic pressure (~60 mm Hg), which is much greater than other capillary beds
Figure 1. Glomerular capillary wall.
The 3 layers of the capillary wall (glomerular endothelial cell, glomerular basement membrane (GBM), and podocyte) act as the glomerular filtration barrier (GFB) preventing proteins and large molecules from passing from the capillary lumen into the urinary space. The podocyte cell body lies with the urinary space, and the cell is attached to the GBM via the foot processes. Adjacent foot processes are separated by the filtration slit, bridged by the slit diaphragm. Disruption of the GFB leads the passage of protein across the capillary wall leading to proteinuria.
Major Functions of the Podocyte
Structural support of the capillary loop
Major component of glomerular filtration barrier (GFB) to proteins
Synthesis and repair of the GBM
-
Production of growth factors
-
Vascular endothelial growth factor (VEGF) traverses the GBM against the flow of glomerular filtration
Acts on VEGF receptors on the glomerular endothelial cells
Effect is to maintain a healthy fenestrated endothelium
Platelet derived growth factors (PDGFs) critical for development and migration of mesangial cells into the mesangium
-
-
Immunological function
Podocytes may be a component of the innate immune system
Possibly play a surveillance role for pathogens or abnormal proteins in Bowman’s space
Glomerular Filtration Barrier
Glomerular Filtration of Plasma Water
-
Occurs across the glomerular capillary walls into the urinary (Bowman’s) space
Approximately 180 L/day filtered
-
A portion of the glomerular ultrafiltrate is not filtered directly into the urinary space
Instead, goes first to a space underneath the podocyte cell body (subpodocyte space)
Subpodocyte space may play a role in restricting hydraulic permeability
-
GFB limits the passage of larger molecules such as albumin
Small amounts of protein (~4g/day) are normally filtered across the GFB into the urinary (Bowman’s) space
Vast majority of protein is reabsorbed in the proximal tubule via megalin/cubulin coreceptor
Structure of GFB
Composed of three layers (Fig 1); damage to one or more layers leads to proteinuria
-
Layer closest to lumen: fenestrated endothelial cells coated with glycocalyx
Fenestrations facilitate hydraulic permeability
Overlying glycocalyx (composed of a network of proteoglycans with negatively charged glycosaminoglycan side chains) limits the passage of albumin and larger molecules
-
Middle layer: GBM
-
Major component is type IV collagen
Early α1α2α1 collagen network secreted by the glomerular endothelial cell during fetal development is replaced by the more robust α3α4α5 collagen network secreted by the podocyte
Failure to secrete this network results in a range of hereditary nephropathies, the Type IV collagenopathies
Type IV collagenopathies include Alport syndrome, nail patella syndrome, thin basement membrane disease, and can all be considered podocyte disorders
-
Other GBM components include the glycoproteins laminin, entactin, and nidogen, and heparan-sulfate proteoglycans
Laminin serves as the predominant cell attachment ligand for podocyte and endothelial integrins
Heparan-sulfate proteoglycans confer an overall anionic charge
-
-
Layer closest to urinary space: podocytes
Multiple examples of both inherited and acquired podocyte injury, especially to proteins comprising the slit diaphragm domain, demonstrate the critical role of the podocyte in the prevention of proteinuria
Podocytes also maintain the GFB by removing protein and immunoglobulins that may clog the filter
Although injury to any layer may lead to proteinuria, nephrotic-range proteinuria is most typically due to diseases of podocytes
Podocyte Responses to Injury in Disease
Overview
Glomerular diseases comprise a wide range of immune and non-immune insults that may target, and thus injure, the podocyte
In many of these conditions, the podocytes respond to injury along defined pathways, which may explain the resultant clinical and histological changes
Reduction in Podocyte Number (Podocytopenia)
-
Potential causes (can occur in combination)
Detachment: podocytes may lose their ability to anchor to the GBM, detach into Bowman’s space, and shed into the urine
Apoptosis: podocytes may undergo programmed cell death
-
Inability to proliferate
Characteristic response of differentiated podocytes to most insults
Podocytes lost by detachment or apoptosis are not replaced by adjacent viable podocytes, leading to podocytopenia
Ultimate result is a leaky GFB
-
Consequences of podocytopenia
Glomerular capillaries denuded of podocytes balloon and form synechial attachments to Bowman’s capsule
Kriz hypothesis: these attachments can lead to the development of focal segmental glomerulosclerosis (FSGS)
Recent evidence suggests that parietal epithelial cell precursors on Bowman’s basement membrane may serve as a source for podocyte replacement
Clinical studies in diabetic kidney disease have suggested that the degree of podocytopenia predicts progression of kidney disease
Podocyte Proliferation
May be rarely seen in de-differentiated podocytes
Feature of collapsing glomerulopathy
Foot Process effacement
-
Characteristic feature of proteinuric diseases
Readily seen on electron microscopy as flattening of foot processes
The only pathological abnormality seen in minimal change disease (MCD)
An active process induced by changes in the actin cytoskeleton
The flattened foot processes, which should not be considered as cells adherent to one another, severely disrupt in the normal shape and integrity of these cells
Other morphological changes characteristic of podocyte injury include microvillus transformation and the presence of protein reabsorption droplets
It is unclear if effacement alone may cause proteinuria, or if effacement is simply a manifestation of podocyte injury
Altered Slit Diaphragm Integrity
The slit diaphragm between adjacent podocyte foot processes is one of the major impediments to protein permeability across the glomerular capillary wall
Alterations in cytoskeletal architecture and/or expression of slit diaphragm proteins can be demonstrated in most nephrotic disorders
Production of Inflammatory Mediators
-
Podocytes may respond to immune complex-mediated injury by producing inflammatory mediators
Examples are oxidative radicals, proteases, eicosanoids, and chemokines, growth factors
Inflammatory mediators may amplify the initial podocyte injury
Oxidative injury is a prominent feature in membranous nephropathy
Suggested Reading
≫ Haraldsson B, Jeansson M. Glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18(4):331–335.
≫ Jefferson JA, Shankland SJ, Pichler RH. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 2008;74(1):22–36.
≫ Kriz W. The pathogenesis of 'classic' focal segmental glomerulosclerosis-lessons from rat models. Nephrol Dial Transplant. 2003;18(Suppl 6):vi39–44.
≫ Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5(8):463–468.
Nephrotic Syndrome
Classical Features of Nephrotic Syndrome
Heavy proteinuria (> 3.5 g/24 hr - also called nephrotic-range proteinuria)
Hypoalbuminemia (< 3 g/dl)
Peripheral edema
Hyperlipidemia (elevated total and low-density lipoprotein cholesterol)
Lipiduria
Pathophysiology of Nephrotic Syndrome
-
Proteinuria and nephrotic syndrome are the clinical signatures of podocyte injury
Podocytes lie on the outside of the glomerular capillary, and are therefore separated from the circulation by the GBM
-
Subepithelial immune complexes (as in membranous nephropathy) or podocyte injury usually do not lead to leukocyte recruitment and inflammation, but rather disrupt the GFB
Typically urine sediment is devoid of leucocytes and erythrocytes
Disruption of GFB leads to proteinuria
By contrast, injury to mesangial or endothelial cells, which are in direct contact with the blood (containing leukocytes, complement, inflammatory proteins), typically leads to an inflammatory kidney disease (nephritis) with an active urine sediment
Clinical Manifestations and Complications of Nephrotic Syndrome
Hypoalbuminemia and Edema
Hypoalbuminemia may decrease the plasma oncotic pressure resulting in a decrease in effective circulating volume and activation of the renin angiotensin system leading to sodium retention (underfill theory)
-
In most cases however, edema appears to result from a primary defect in sodium excretion (ie, glomerular disease inhibits sodium excretion)
Leads to an expanded plasma volume
Followed by transudation of fluid in the setting of low oncotic pressure (overfill theory)
Hyperlipidemia
Hepatic cholesterol and lipoprotein synthesis are increased in nephrotic patients, probably in response to decreased oncotic pressure
There is also decreased catabolism, partly explaining the increase in levels of very low density lipoprotein
Lipiduria
Following glomerular filtration of lipoproteins, lipids may be taken up by proximal epithelial tubular cells
Desquamated proximal epithelial tubular cells containing lipid may be seen in the urine as oval fat bodies, or as lipid-containing granular casts (fatty casts)
Thrombosis
Hypercoagulability from increased hepatic synthesis of coagulation factors (eg, fibrinogen) and loss of regulatory factors (antithrombin III, protein C and protein S) in the urine
-
Kidney vein thrombosis complicates all forms of the nephrotic syndrome (especially membranous nephropathy)
May be asymptomatic
May present acutely as a sudden decrease in kidney function, loin pain, hematuria, or even systemic emboli
Infection
-
Increased susceptibility to infection
Particular vulnerability to Gram-positive bacteria
Caused by urinary losses of IgG and complement, plus impaired cellular immunity
Bone disease
Loss of vitamin D binding protein in the urine may lead to vitamin D deficiency
Also, treatment with steroids may exacerbate bone loss
Common Causes of Nephrotic Syndrome
-
Two categories of nephrotic syndrome etiology
Major pathology limited to, or predominantly, in the glomerulus
-
Systemic disorders, in which glomerular disease is a component of systemic manifestations (Box 1)
Systemic disorders do not manifest an idiopathic form limited to the glomerulus
Diabetic kidney disease is the most common systemic cause of nephrotic syndrome
Although mesangial cell injury is prominent in diabetic kidney disease, the proteinuria is likely a manifestation of podocyte injury
Each of the glomerular disorders may be idiopathic, or associated with other secondary causes (eg, membranous nephropathy secondary to lupus)
Box 1: Common Causes of Nephrotic Syndrome.
Predominant Glomerular Disease
Systemic Disorders with Glomerular Component
Diabetic kidney disease
Amyloidosis
Note: Podocyte injury is prominent in each of these conditions. Note that nephritic glomerular disorders [eg, IgA nephropathy] may also present with nephrotic-range proteinuria. Rare causes of nephrotic syndrome include fibrillary glomerulopathy, immunotactoid glomerulopathy, collagen III glomerulopathy, lipoprotein glomerulopathy, fibronectin glomerulopathy.
Abbreviations: FSGS, Focal Segmental Glomerulosclerosis; MPGN, Membranoproliferative Glomerulonephritis
General Therapeutic Strategies for Nephrotic Syndrome
-
Reduce proteinuria (to < 1 g/24 hr)
Use combination therapy with angiotensin-converting enzyme inhibitors and diuretics [+/− angiotensin receptor blocker, spironolactone]
Proteinuria reduction may slow the progression of kidney disease by ameliorating the tubular toxicity of filtered proteins
-
Treat any complications
Volume overload: salt restriction, diuretics
Hypertension: Blood pressure goal < 125/75 mm Hg.
Hyperlipidemia: statins
Thromboembolism: Aspirin; anticoagulation therapy for patients at high risk for venous thrombosis (eg, those with a serum albumin level < 2.0 g)
Bone disease: calcium and vitamin D supplementation
Treat any underlying secondary cause (eg, Hepatitis B in membranous nephropathy)
Provide disease-specific therapy (typically immunosuppression)
Suggested Reading
Clinical Podocyte Disorders
Minimal Change Disease
Epidemiology
Most common cause of nephrotic syndrome in children
Vast majority (90%) of cases occur in children less than 10 years of age
Therefore, most young children with nephrotic syndrome are treated empirically with steroids without kidney biopsy
Causes 10%-15% of adult nephrotic syndrome
Etiology and Pathogenesis
Podocyte injury typified by diffuse foot process effacement on electron microscopy
-
Evidence for a possible T-cell mediated cytokine leading to podocyte injury (Box 2)
-
Interleukin-13 (IL-13) is a recent candidate
Serum IL-13 levels are increased in patients with MCD
Rats overexpressing IL-13 develop minimal change type lesions
Angiopoietin-like 4 (ANGPTL4): overexpression in rat podocytes leads to a steroid-sensitive nephrotic syndrome
-
Proteinuria likely secondary to loss of slit diaphragm integrity and podocyte effacement; some evidence for decrease in glomerular charge barrier
Box 2: Secondary Causes of Minimal Change Disease.
Tumors (often T cell related)
Hodgkin’s lymphoma
Thymoma
Drugs and toxins
NSAIDs
Lithium
Bisphosphonate
Rarely: tiopronin, ampicillin, rifampicin, interferon
Other
Atopy/eczema
Chronic graft versus host disease
Abbreviations: NSAID, nonsteroidal anti-inflammatory drug
Pathology
Light microscopy: unremarkable
Immunofluorescence: unremarkable (rarely, C1q or IgM staining, which may herald a worse prognosis)
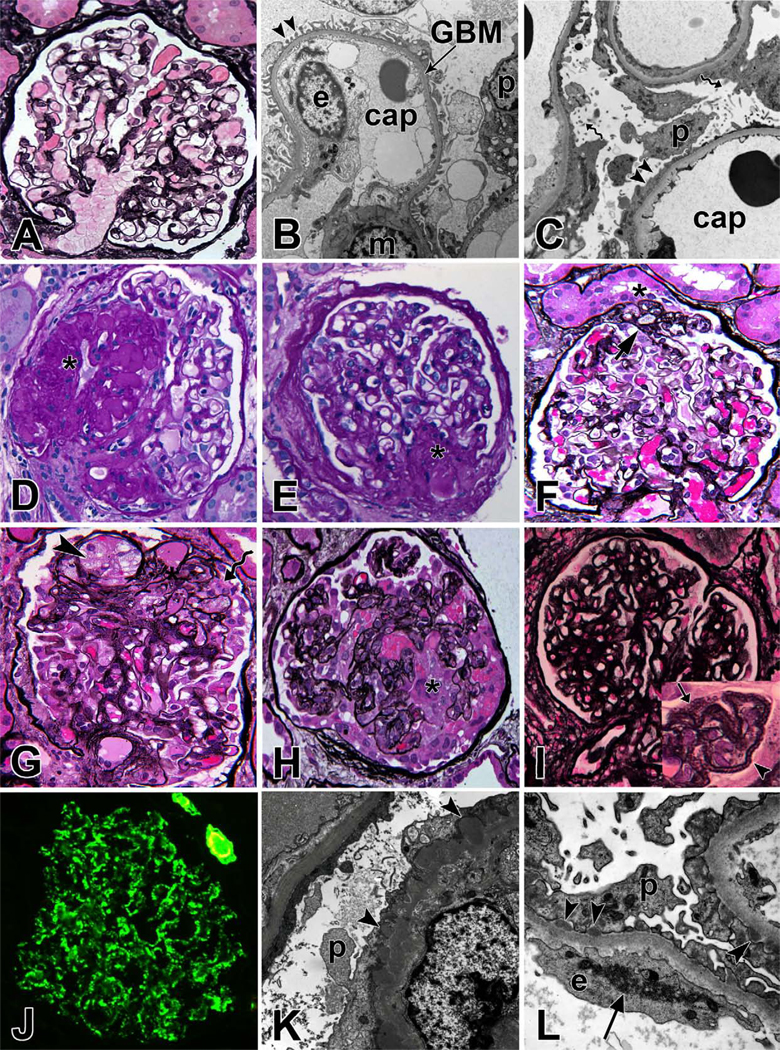
Electron microscopy shows characteristic diffuse effacement of podocyte foot processes (Fig 2C)
Figure 2. Renal Pathology of Clinical Podocyte Disorders.
(A) Light microscopy image of a normal glomerulus, Jones methenamine silver (JMS) stain; (B) Electron micrograph of a capillary loop from a normal glomerulus. Arrow heads point to regularly arranged intact foot processes. cap = capillary lumen, GBM = glomerular basement membrane, p = podocyte, e = endothelial cell; (C) Extensive effacement of foot processes (arrowheads) in minimal change disease. Spiral arrows point to microvillus transformation of podocytes; (D) Focal segmental glomerulosclerosis (FSGS), not otherwise specified (NOS) with obliterated capillary loops (*), hyalin deposition and adhesion of tuft to Bowman's capsule, periodic acid Schiff (PAS) stain; (E) FSGS, perihilar variant with segmental sclerosis at the vascular pole (*), PAS; (F) FSGS, tip variant with segmental sclerosis (arrow) located at the glomerulotubular junction (*), JMS; (G) FSGS, cellular variant with foam cells (arrowhead) infiltrating capillary loops of sclerotic segment and prominent overlying podocytes (spiral arrow), but no collapse of capillary loops, JMS; (H) FSGS, collapsing variant with collapse of capillary loops and podocyte proliferation (*), JMS; (I) Membranous nephropathy with thickened glomerular basement membrane. The inset shows a magnified view of capillary loops with frequent GBM holes (arrow) and spikes (arrowhead); (J) Immunofluorescent staining for IgG in membranous nephropathy shows global fine granular peripheral capillary wall staining pattern; (K) Electron micrograph of membranous nephropathy with subepithelial immune complex deposits (arrowhead) and extensive effacement of foot processes; (L) Electron micrograph of a case of membranous nephropathy secondary to lupus erythematosus. Arrow heads show subepithelial deposits and arrow shows an endothelial tubuloreticular inclusion, a common finding in lupus nephritis.
Clinical Features
Presents with acute-onset nephrotic syndrome (may be very heavy proteinuria (>10g/24h))
-
Associated features in adults
Include hematuria (~30%); hypertension (~40%); thrombosis (5%)
Acute kidney injury (AKI) occurs in 10%-25% (mostly older, severe nephrotic syndrome)
In children, hypertension less common, AKI may occur
Treatment
-
For adults, prednisone 1mg/kg daily (or 2mg/kg alternate days)
High dose until 2 weeks after complete remission (minimum 8 weeks)
Then taper over 2–4 months
-
Relapse rate is approximately 50%
Steroid dependent/multiply relapsing: each flare responds to steroid
Prolonged remission may be achieved with 3 month course cyclophosphamide (60%-70%) or with prolonged course of mycophenolate
-
Steroid resistant form occurs in 25%
failure to enter remission after 16 weeks high dose steroid
may respond to cyclosporin or mycophenolate
Steroid resistance suggests the possibility of not having identified FSGS on the biopsy specimen, due to sampling phenomenon
Children are typically more steroid sensitive, but high relapse rate (~70%) and 30%-40% will have multiple relapses
Focal Segmental Glomerulosclerosis
Overview
FSGS describes a histological pattern rather than a specific disease
Can be idiopathic or due to secondary causes from a variety of underlying disorders (Table 1)
“Focal” defines that less than 50% of glomeruli in the sample are affected
“Segmental” defines that only a portion of the effected glomerulus is sclerosed (scarred), while other portions of the glomerular tuft look normal by light microscopy
Table 1.
Etiological Classification of FSGS
| Classification/Etiology | Causes |
|---|---|
| Primary | |
| ? Circulating permeability factor |
|
| Secondary | |
| Glomerular hyperfiltration |
|
| Viral infection |
|
| Drugs & toxins |
|
| Familial | |
| Podocyte gene disorder |
|
Abbreviations: HIV, human immunodeficiency virus; CMV, cytomegalovirus; FSGS, Focal Segmental Glomerulosclerosis; INF2, inverted formin 2; CD2AP, CDs-associated protein; WT1, Wilms tumor 1; TRPC6, transient receptor potential cation channel 6;
Epidemiology
-
Increasing in prevalence
Has become the most common cause of nephrotic syndrome in adults
Higher prevalence in Black and Hispanic races
Most common cause of primary glomerular disease leading to end-stage renal disease (ESRD) in the US
Although often considered a more advanced manifestation of MCD, many clinico-pathological features suggest FSGS is a completely separate group of diseases
FSGS often responds poorly to steroid therapy and commonly progresses to kidney failure
Pathology
Light microscopy
-
Lesion is defined by the early presence of an adhesion between a peripheral capillary loop and Bowman’s capsule
Progressive obliteration of the glomerular capillary lumen by acellular matrix-like material (Fig 2D)
Leads to a segmental scarring of the glomerular tuft
Uninvolved areas of the glomerular tuft are relatively normal
In addition to the clinical / etiological classification (Table 1), FSGS may be classified by histological features (Box 3)
Box 3: Columbia Pathological Classification of FSGS.
Not otherwise specified (NOS)
Classic FSGS
Perihilar variant
Exemplified in Figure 2E
More common in FSGS second to hyperfiltration as glomerular pressure highest closer to afferent arteriole (ie perihilar)
Tip variant
Exemplified in Figure 2F
Tuft adhesion at glomerular tip (the area adjacent to the origin of the proximal tubule, opposite the vascular pole)
Usually idiopathic, may be more steroid responsive
Cellular variant
Exemplified in Figure 2G
Segmental endocapillary hypercellularity
Intermediate prognosis between NOS and collapsing
Collapsing variant
Exemplified in Figure 2H
Tuft collapse with proliferation of overlying epithelial cells
Worst prognosis
Many consider this a separate disorder (collapsing glomerulopathy)
Abbreviations: NOS, not otherwise specified; FSGS, Focal Segmental Glomerulosclerosis
Immunofluorescence
C3, IgM, and fibrin staining in the sclerotic regions; otherwise unremarkable
Electron microscopy
Diffuse effacement of podocyte foot processes even in glomeruli seemingly uninvolved on light microscopy
Pathogenesis
Proteinuria due to an alteration in glomerular perm-selectivity in a similar manner to MCD (may be the glomeruli that appear normal on light microscopy that are mostly responsible for the proteinuria)
Ultrastructural examination of the podocyte shows evidence of cell injury with foot process effacement, cell hypertrophy, and the formation of pseudocysts
-
Podocyte detachment and apoptosis
Reduction in podocyte number
Loss of structural support to the capillary loop
Areas of denuded GBM, which can attach to the overlying parietal epithelial cells on Bowman’s basement membrane, forming synechiae
Capillary loops within the adhesion may deliver filtrate into interstitial areas rather than Bowman’s space, but ultimately collapse with thrombosis and hyalinosis
Primary FSGS
Immunological injury to the podocyte; exact mechanisms remain unclear
-
Circulating Permeability Factor
The rapid recurrence of primary FSGS after kidney transplant, sometimes as early as the first week, suggests a circulating host factor leads to podocyte injury
Cardiotrophin-like cytokine 1 (CLC1) is a recently proposed candidate
Secondary FSGS
Glomerular hyperfiltration: Loss of nephrons (reduced nephron mass) or dilation of the afferent arteriole (eg, obesity) may lead to glomerular hypertension and hyperfiltration
Chronic glomerular hypertension promotes podocyte injury and distension of the glomerular capillary
Glomerulomegaly (larger glomeruli may be more vulnerable to hyperfiltration injury and it is often the larger juxtamedullary glomeruli that develop glomerulosclerosis)
Black individuals have fewer, and larger glomeruli than Caucasians, which may partly explain the greater prevalence of FSGS
-
Nephron endowment
New nephrons continue to develop in the third trimester
Children born prematurely may have a decreased nephron number
Could predispose to glomerular hyperfiltration, with increased kidney disease and hypertension in later life
Clinical Features
Primary FSGS
Typically presents with severe nephrotic syndrome, which may be of acute onset
Associated with hematuria (~50%), hypertension (~60%) and reduced kidney function (25%-50%)
Prognosis heavily dependent on achievement of partial/complete remission with immunosuppression
Nonresponders have only 40% chance of a ten-year kidney survival
Secondary FSGS
Typically slower onset, less proteinuria
Serum albumin often preserved, less edema
Does not respond to immunosuppression, but overall prognosis much better
Treatment
-
Differentiate primary from secondary FSGS, as the latter are typically not steroid responsive
Clinical: assess for secondary causes, acuteness, and severity of nephrotic syndrome
Pathological: secondary FSGS suggested by glomerulomegaly, perihilar variant, and focal (<50%) effacement of foot processes
General therapy for nephrotic syndrome
-
Immunosuppression (for primary FSGS only) (Table 2)
Prednisone 1mg/kg (or 2mg/kg alternate days); prolonged course (up to 4 months) may be required before taper
Steroid resistant (50%): Consider cyclosporin 3–6mg/kg/day or mycophenolate mofetil 1g-1.5g bid
Table 2.
Immunosuppressive Treatment for Adult MCD and primary FSGS
| Initial Approach | Prednisone Duration |
Second-line Agents | |
|---|---|---|---|
| Minimal Change Disease | |||
| Initial Therapy | Prednisone (1 mg/kg) (max of 80 mg/d) | Until 2 wk post complete remission (min of 8 wk) Taper over 2–4 mo | NA |
| Steroid Resistant | Prolonged high-dose steroid course | Discontinue after 4–6 mo if no response | MMF; cyclosporine; tacrolimus |
| Relapsing / steroid dependent | Try to detect early Repeat prednisone (1 mg/kg) Consider MMF or cyclosporin for induction | Shorter steroid course (4 wk high dose, taper 1–2 mo), then second-line agent | Oral cyclophosphamide (2 mg/kg for 12 wk); MMF; calcineurin inhibitors; rituximab |
| Focal Segmental Glomerulosclerosis | |||
| Initial Therapy | Prednisone (1 mg/kg) (max 80 mg/d) | Until 2 wk post complete remission (min 8 wk), then taper 2–4 mo | n/a |
| Partial Remission | Prolonged steroid course, as late complete remissions seen | High-dose steroid for 3–4 mo, then slow taper over 6–9 mo | Calcineurin inhibitors; MMF |
| Steroid Resistant | Prolonged steroid course | High dose for 4 mo Add second-line agent with taper | Calcineurin inhibitors; MMF |
| Relapsing / steroid dependent | Treat as MCD (above) | NA | NA |
Abbreviations: FSGS, focal segmental glomerulosclerosis; MMF, mycophenolate mofetil; MCD, Minimal Change Disease; max, maximum; min, minimum; NA, not applicable
Special Considerations
Collapsing Glomerulopathy
Classified as a pathological variant of FSGS, but many consider this a separate disease entity
Most commonly described secondary to HIV, but other secondary causes noted (Box 4)
Characteristic feature is the extracapillary proliferation of glomerular epithelial cells with collapse of glomerular tuft
Recent evidence suggests that podocyte injury results in de-differentiation and a renewed ability to proliferate and/or induction of aberrant hyperplastic repair by the parietal epithelial cells
-
HIV-associated nephropathy (HIVAN)
Almost exclusively in patients of African descent; associated with low CD4 counts and more advanced HIV infection
Typically presents with severe nephrotic syndrome, often progresses rapidly to ESRD (< 12months)
Surprisingly, patients are often normotensive
Evidence for direct infection of podocytes by HIV; tubular cell infection may account for the prominent tubular microcystic changes often found
Treatment with highly active antiretroviral therapy has dramatically changed the prevalence and prognosis for this condition
-
Non-HIV collapsing glomerulopathy
Predominately in patients of African descent, but more whites noted than in HIVAN
Clinical features and pathology similar to HIVAN; tubuloreticular structures are not typically found in non-HIV collapsing glomerulopathy
Box 4: Causes of Collapsing Glomerulopathy.
Infection
HIV
CMV
Parvovirus B19
Tuberculosis
Malignancy
Myeloma
Hemophagocytic syndrome
Acute leukemia
Drugs
Bisphosphonates
Interferons
Anabolic steroids
Autoimmune
Adult Still’s disease
Lupus
Mixed connective tissue disease
Abbreviations: HIV, human immunodeficiency virus; CMV, cytomegalovirus
Familial FSGS
Present at different ages with different modes of inheritance (Table 3)
Genetic testing is clinically available for most of these conditions
Establishing diagnosis may alter therapy as these disorders are typically resistant to immunosuppression
Familial FSGS is less likely to recur post-transplant
Sequence variants in the APOL1 (aloplipoprotein L-I) gene have been identified in African American patients with sporadic FSGS and hypertensive nephrosclerosis, which partly accounts for the increased prevalence in this group
Table 3.
Common Forms of Familial FSGS
| Gene (protein effected) |
Inheritance | Typical Age of Onset |
Distinguishing Clinical Features |
|---|---|---|---|
| NPHS1 (nephrin) | AR | infancy | Congenital nephrotic syndrome (Finnish type); severe nephrosis leading to ESRD |
| NPHS2 (podocin) | AR | 3 mo-5 y | 10–20% of SRNS in children |
| WT1 (Wilms tumor 1) | AD | child | Diffuse mesangial sclerosis/FSGS +/− Wilms tumor or urogenital lesions |
| PLCε1 (phospholipase Cε1) | AR | 4 mo-12 y | Diffuse mesangial sclerosis/FSGS |
| CD2AP (CD2- associated protein) | AR | <6 y | Rre, progresses to ESRD |
| INF2 (inverted formin 2) | AD | Teen/young adult | Mild nephrotic syndrome, but progressive CKD |
| ACTN4 (α- actinin 4) | AD | Any age | Mild nephrotic syndrome, may develop progressive CKD |
| TRPC6 | AD | Adult (age 20- 35) | Nephrotic, progressive CKD |
| tRNALeu(UUR) gene | Mitochondrial DNA | Adult | May be associated deafness, diabetes, muscle problems, retinopathy (maternal inheritance) |
Abbreviations: AR, autosomal recessive; AD, autosomal dominant; CKD, Chronic Kidney Disease; ESRD, End-Stage Renal Disease; FSGS, Focal Segmental Glomerulosclerosis; tRNA, transfer RNA; Leu, leucine; TRPC6, transient receptor potential cation channel 6;
Recurrent FSGS Posttransplant
-
Primary FSGS recurs in 20%-30% of patients
Typically within the first month, but can occur later
Early recurrence supports theory of circulating permeability factor
Graft loss 40%-50% without plasmapheresis
Treatment: plasmapheresis for 2–3 weeks, longer in some; cyclophosphamide may be appropriate
-
Risk Factors for recurrence
Young Age(< 15yrs)
Aggressive Course (< 3yrs from diagnosis to ESRD)
Race (less common in African Americans)
Living Donor (some recommend avoiding living donors in those at high risk for recurrence, but data not clear)
Membranous Nephropathy
Epidemiology
Membranous nephropathy (MN) is most common cause of nephrotic syndrome in Caucasians and older adults
Seen more often in males, rare in children
Mostly primary (idiopathic), although about 20% of cases are associated with clinical conditions such as cancer, infections, autoimmune disease and drugs (Box 5)
Familial membranous nephropathy has been described, but is rare
Box 5: Secondary Causes of Membranous Nephropathy.
Tumors
Carcinoma (lung, colon, rectum, stomach, breast, kidney), melanoma, leukemia/lymphoma
Infections
Hepatitis B, hepatitis C, syphilis, quartan Malaria, schistosomiasis, filariasis, hydatid disease, leprosy, scabies, tuberculosis
Drugs and Toxins
Gold, penicillamine, bucillamine, captopril, probenecid, NSAIDs, tiopronin, lithium, mercury, formaldehyde, hydrocarbons
Autoimmune Diseases
Systemic lupus erythematosis, rheumatoid arthritis, mixed connective tissue disease, Sjogren syndrome, Graves disease, Hashimoto thyroiditis, dermatomyositis, primary biliary cirrhosis, bullous pemphigoid, dermatitis herpetiformis, ankylosing spondylitis, Guillain-Barre syndrome, myasthenia gravis
Miscellaneous
Diabetes mellitus, sarcoidosis, sickle cell anemia, kimura disease, sclerosing cholangitis, systemic mastocytosis, Gardner-Diamond syndrome
Abbreviations: NSAID, nonsteroidal anti-inflammatory drug
Etiology and Pathogenesis
Characterized by the development of immune complexes in the subepithelial (subpodocyte) space
In primary MN, immune deposits likely develop in situ, due to the passage of preformed antibodies across the capillary wall targeting a specific podocyte antigen
-
The immune deposits consist of immunoglobulin (IgG, predominantly IgG4), complement components (C3, C5b-9), and antigen
Leads to podocyte damage, which causes increased production of extracellular matrix proteins along the GBM
Results in the characteristic thickening of the GBM, from which the name of the disease derives
-
Antigens in MN
-
M-type phospholipase A2 receptor (PLA2R)
Antibodies to PLA2R have been identified in 70% of patients with idiopathic MN
Antibody levels may correlate with disease activity and help identify patients suitable for immunosuppression
Anti-PLA2R antibodies not found in secondary forms of MN
Neutral endopeptidase: identified as the antigen in alloimmune neonatal MN occurring in newborns from neutral endopeptidase-deficient mothers
-
Subepithelial deposits of secondary MN
Believed to derive from circulating pre-formed immune complexes that dissociate and reform in the subepithelial space, or by the deposition of antigen alone (planted antigen), followed by antibody response
Range of antigens have been detected, including tumor antigens (carcinoembryonic antigen, prostate specific antigen), thyroglobulin, infection antigens (hepatitis B, hepatitis C, helicobacter pylori, syphilis) and DNA associated antigens (double-stranded DNA, histones, nucleosomes)
Unclear if antigens are causal or epiphenomena
-
Heymann nephritis model
A rat model of MN that has played a key role in identifying many of the pathogenic mechanisms in MN
Pathogenic antigen is megalin, but this is not expressed by human podocytes
-
-
Complement activation occurs, likely via the alternate pathway
C5b-9 is generated and inserts into podocyte membrane
Instead of cell lysis, a series of signaling events result in cell activation (release of reactive oxygen species, proteases and eicosanoids) and changes in podocyte structure
Pathology
Light Microscopy
At early stages, the glomeruli and interstitium look essentially normal
-
With disease progression, the pathognomonic thickening of capillary loops becomes evident
Accumulation of sub-epithelial immune complexes
Deposition of new basement membrane material by the podocyte
Staining with silver methenamine may reveal spikes representing new basement membrane material projecting between the immune deposits (Fig 2I)
Glomerular cellularity is typically normal
Immunofluorescence
Granular deposits of IgG in a subepithelial distribution (Fig 2J)
C1q, IgA, and IgM undetectable
Complement C3 present in about 50% of adult patients
Electron Microscopy
-
Characteristic subepithelial immune deposits
Initially small without a prominent basement membrane response
With time, basement membrane material projects around and encloses the immune deposits (Fig 2K)
Effacement of podocyte foot processes is found overlying the areas of electron dense deposits
Biopsy features suggestive of secondary MN include mesangial hypercellularity, leukocyte infiltration, the presence of C1q, IgA or IgM by immunofluoresence, or the presence of mesangial/subendothelial immune deposits or tubuloreticular structures by electron microscopy (Fig 2L)
Clinical Features of Idiopathic MN
Typically presents as nephrotic syndrome (80%), onset more gradual than MCD or primary FSGS
-
Associated features
Microhematuria is common (50%)
Blood pressure and kidney function typically normal at presentation.
Less severe disease in younger females and Asian race
Risk of kidney vein thrombosis higher than other forms of nephrotic syndrome
Natural History and Prognosis of Idiopathic MN
Course in adults is variable, but about 30%-40% develop progressive disease
30% undergo spontaneous remission (especially in younger females)
-
Prognostic risk factors for progression include:
Greater degree and duration of proteinuria
Impaired kidney function at presentation
Hypertension
Male sex and age older than 50 years
Non-Asian race
-
Biopsy features
glomerulosclerosis, FSGS, Stage III/IV disease, tubulointerstitial fibrosis
Has been argued that the pathological features on kidney biopsy do not give further prognostic risk stratification independent of the clinical variables
The urinary excretion of biomarkers such as β2-microglobulin and/or IgG may be more accurate prognostic indicators than total urinary protein excretion, although these assays are not widely available
Treatment of Idiopathic MN
-
Exclusion of Secondary Causes
Thorough history and examination
Check of antinuclear antibody, complement levels, Hepatitis B and C
-
Malignancy Screen
In general, the risk of malignancy is greatest in males and increases with age
Rare in those less than 40 years of age
Investigations may include stool guaics, colonoscopy, chest radiography, mammography and prostate specific antigen measurements
Screening recommendations are similar to the age appropriate cancer screening investigations for the general population
-
Assessment of Prognosis
Treatment is individualized based on the prognostic risk factors
Almost all patients are treated with the general measures outlined in the section on treatment of nephrotic syndrome
-
Immunosuppression is considered for patients at higher risk of progression (Table 4)
If the nephrotic syndrome is not too severe, 6 months’ close observation is often employed to determine if there is any evidence of a spontaneous remission (occurs in ~30% patients)
Cyclophosphamide or calcineurin inhibitor with steroid is usual first line therapy
Steroids alone are typically ineffective
Emerging data on rituximab is promising
Table 4.
Treatment of Membranous Nephropathy
| Risk Level | Approach | Immunosuppression |
|---|---|---|
| Low Risk (proteinuria <4 g/d, normal kidney function) | General measures* | None |
| Moderate risk (proteinuria 4- 8 g/d, normal kidney function) | General measures; observe for 6 mo | Cyclophosphamide + steroid (alternative is cyclosporin / tacrolimus) |
| High risk (proteinuria >8 g/d +/− reduced kidney function) | General measures; consider early immunosuppression | Cyclophosphamide + steroid (alternative is Cyclosporin / tacrolimus) |
see general measures for treatment of nephrotic syndrome.
Suggested Reading
≫ Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol. 2007;2(3):445–453.
≫ Cattran DC, Alexopoulos E, Heering P, et al. Cyclosporin in idiopathic glomerular disease associated with the nephrotic syndrome: workshop recommendations. Kidney Int. 2007;72(12):1429–1447.
≫ D'Agati VD. The spectrum of focal segmental glomerulosclerosis: new insights. Curr Opin Nephrol Hypertens. 2008;17(3):271–281.
≫ Albaqumi M, Barisoni L. Current views on collapsing glomerulopathy. J Am Soc Nephrol. 2008;19(7):1276–1281.
≫ Machuca E, Benoit G, Antignac C. Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum Mol Genet. 2009;18(R2):R185–194.
≫ Ulinski T. Recurrence of focal segmental glomerulosclerosis after kidney transplantation: strategies and outcome. Curr Opin Organ Transplant. 2010;15(5):628–632
≫ Beck LH, Jr., Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361(1):11–21.
≫ Glassock RJ. The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am J Kidney Dis. 2010;56(1):157–167.
≫ Waldman M, Austin HA, 3rd. Controversies in the treatment of idiopathic membranous nephropathy. Nat Rev Nephrol. 2009;5(8):469–479.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Statement: The authors declare that they have no relevant financial interests.