Abstract
Background
Chronic obstructive pulmonary disease (COPD) is associated with an increased risk for thromboembolic events. We investigated thrombin generation profiles in COPD patients and their dependence on plasma factor/inhibitor composition.
Methods
Factors (f) (fII, fV, fVII, fVIII, fIX, fX), antithrombin, protein C (PC) and free tissue factor pathway inhibitor (fTFPI) from 60 COPD patients (aged 64.2±10.1 years; a mean forced expiratory volume in 1 second [FEV1], 55.6 ± 15.8% of predicted values) were compared with those for 43 controls matched for age, sex, weight and smoking. Patients receiving anticoagulation were excluded. Using each individual’s plasma coagulation protein composition, tissue factor-initiated thrombin generation was assessed computationally.
Results
COPD patients had higher fII (115±16 vs 102±10%, p<0.0001), fV (114±19 vs 102±12%, p=0.0002), fVII (111±15 vs 102±17%, p=0.002), fVIII (170±34 vs 115±27%, p<0.0001), and fIX (119±21 vs 107±17%, p=0.003), and lower fTFPI (17.7±3.2 vs 18.9±3.2 ng/ml, p=0.047) compared with controls, while fX, antithrombin, and PC were similar in both groups. Computational thrombin generation profiles showed that compared with controls, COPD patients had higher maximum thrombin levels (+28.3%, p<0.0001), rates of thrombin generation (+46.1%, p<0.0001) and total thrombin formation (+14.4%, p<0.001), together with shorter initiation phase of thrombin generation (p<0.0001) and the time to maximum thrombin levels (p<0.0001). Thrombin generation profiles in COPD patients can be normalized via correction of fII, fVIII, fIX and TFPI. The severity of COPD and inflammatory markers were not associated with thrombin generation profiles.
Conclusions
Prothrombotic phenotype in COPD patients is largely driven by increased prothrombin, fVIII, fIX, and lower fTFPI.
Keywords: coagulation factors, COPD, thrombin
Introduction
The prevalence of chronic obstructive pulmonary disease (COPD) rises in the majority of countries. Growing evidence indicates that COPD is associated with an increased risk for myocardial infarction and two to four times higher risk for CV mortality, mostly due to ischemic heart disease [1]. The Lung Health Study investigators reported that in subjects with mild to moderate airways obstruction, every 10% decrease in forced expiratory volume in one second (FEV1) is associated with 28% increase in CV mortality and 20% increase in the risk for nonfatal coronary event [2]. Moreover, CV disease-related mortality represents 27% of all deaths observed in the COPD patients [3].
Potential mechanisms underlying the association between COPD and CV disease are unclear. Evidence points to four major mechanisms, namely systemic inflammation, prothrombotic state, platelet activation, and oxidative stress [4]. Increased thrombin formation, reflected by elevated thrombin antithrombin complexes [5,6], tissue factor (Tf) procoagulant activity and activated fXI in the blood of stable COPD [7,8] support the concept that a hypercoagulable state occurs in patients with COPD and might contribute to the occurrence of atherothrombotic events and venous thrombosis.
The aim of the current study was to investigate whether coagulation factors and inhibitors differ in patients with COPD from those found in healthy individuals and which factors influence thrombin generation in this chronic disease.
Materials and Methods
Patients
Sixty white patients with documented COPD not requiring oral corticosteroids on a regular basis were recruited in an outpatient clinic at a tertiary reference center. Patients were eligible if they were 40 years or more and had stable disease (defined as the lack of exacerbations [9] within the previous month) with post-bronchodilatator FEV1 <80% predicted in the presence of FEV1/forced vital capacity (FVC) ratio <70% for at least 2 months. The exclusion criteria were as follows: any acute illness, congestive heart failure (NYHA class III or IV), left ventricular ejection fraction <40%, known cancer, hepatic injury, renal insufficiency, a history of venous thromboembolism, current anticoagulant therapy, statin administration, previous acute coronary event. Patients performed spirometric tests following ATS standards. Age-, sex-, weight- and smoking-matched volunteers from the hospital personnel served as controls. They had no history of COPD, confirmed by normal results of standard spirometry. Subjects were eligible if they had mild arterial hypertension and did not take any medication on a regular basis.
The University Ethical Committee at the Jagiellonian University approved the study. All participants provided written, informed consent.
Blood collection
Fasting blood samples were taken into 0.1 volume of 3.2% trisodium citrate from an antecubital vein with minimal stasis on the same day that clinical data were recorded. Citrated blood samples were centrifuged within 15 minutes of collection and stored in aliquots at −80°C until further use. Glucose and C-reactive protein were assayed by routine laboratory techniques.
Coagulation Protein Analyses
Fibrinogen was determined using the Clauss method. Factors II, V, VII, VIII, IX, and X were measured by one-stage clotting assays using factor-deficient plasmas (Dade Behring, Liederbach, Germany). Antithrombin activity was measured using Berichrom (Dade Behring). Protein C (PC) activity was measured using a chromogenic substate assay (Dade Behring). Immunoenzymatic assays were used to determine plasma free TFPI (Diagnostica Stago, Asnieres, France), serum interleukin-6 (IL-6) and serum tumor necrosis factor-α (TNFα), both from R&D Systems, Abingdon, Great Britain. All measurements were performed by technicians blinded to the origin of the samples. The intra-assay and inter-assay coefficients of variation for all the data were <7% (n=24).
Computational Model
The mathematical model used in the current study yields concentration versus time profiles for selected species when electronic mixtures of fII, fV, fVII/fVIIa, fVIII, fIX and fX, and anticoagulants, free TFPI and antithrombin are exposed to 5 pM Tf as previously described [11,12]. Factor levels expressed as a percentage were translated into molar concentrations by using literature values for mean plasma concentrations. Each individual’s plasma factor concentration was entered into the computer database, and simulated reactions were initiated with Tf and solved for active thrombin over 1200 s. The outputs of these active thrombin curves were assessed using a set of parameters that describe the initiation, propagation and termination phases of thrombin generation: maximum level of thrombin generation, maximum rate of thrombin generated, time to 10 nM thrombin (clot time), time to maximum level of thrombin generated and total thrombin generated (area under the curve, AUC) [11,12]. A systematic analyses of the contribution of the plasma factors to the thrombin generation output was conducted on the populations as previously described [12] by adjusting the factors to mean physiologic concentrations and reevaluating the thrombin generation profiles.
Statistical analysis
Data are expressed as the mean ±SD or median (interquartile range). The Kolmogorov-Smirnov test was used to assess conformity with a normal distribution. Categoric values were analyzed using the χ2 test or Fisher’s exact test as appropriate. Continuous variables were compared by Student t test when normally distributed or by the Mann-Whitney U test for non-normally distributed variables. Correlations between the individual parameters were calculated using the Pearson or Spearman rank correlation as appropriate. A p-value <0.05 was considered statistically significant.
Results
A total of 60 COPD patients comprised subjects with advanced disease having a mean FEV1, 55.6 ± 15.8% of the predicted values. Proportions of the patients classified according to the GOLD stages [9] were as follows: II - 35 (58.3%), III - 22 (36.7%), IV - 3 (5.0%).
They used inhaled long and short acting β2-agonists (88% and 7%, respectively), short acting inhaled anticholinergics (75%), inhaled corticosteroids (53%) and theophylline (28%). Nine (15%) COPD patients had diabetes. Acetylsalicylic acid was used by 10% of COPD patients; other cardiovascular medication included angiotensin-converting enzyme inhibitors (23%), diuretics (15%) and cardioselective β-blockers (11.6%). Enhanced inflammatory state in COPD patients was reflected by elevated concentrations of fibrinogen, CRP, IL-6, and TNFα (Table 1).
Table 1.
Comparison of COPD patients and controls.
| COPD patients (n=60) | Healthy controls (n=43) | P | ||||
|---|---|---|---|---|---|---|
| Mean ± SD or median [IQR] | Range | Mean ± SD or median [IQR] | Range | |||
| Age, years | 64.2 ± 10.1 | 44–81 | 61.6 ± 6.0 | 50–75 | 0.13 | |
| Females, n (%) | 5 (8.3) | - | 6 (13.9) | - | 0.52 | |
| Current smokers, n (%) | 22 (36.7) | - | 18 (41.8) | - | 0.85 | |
| Hypertension, n (%) | 28 (46.7) | - | 26 (60.5) | - | 0.24 | |
| BMI, kg/m2 | 25.4 ± 4.6 | 17.8–38.5 | 25.7±2.5 | 20.9–30.4 | 0.79 | |
| Coagulation factors and inhibitors | ||||||
| Factor II, % | 115 ± 16 | 88–152 | 102 ± 10 | 85–137 | 0.00003 | |
| Factor V, % | 114 ± 19 | 83–169 | 102 ± 12 | 86–128 | 0.0002 | |
| Factor VII, % | 111 ± 15 | 85–147 | 102 ± 17 | 76–151 | 0.002 | |
| Factor VIII, % | 170 ± 34 | 109–238 | 115 ± 27 | 69–180 | <0.000001 | |
| Factor IX, % | 119 ± 21 | 79–194 | 107 ± 17 | 80–158 | 0.003 | |
| Factor X, % | 117 ± 21 | 83–174 | 110 ± 19 | 85–164 | 0.08 | |
| Antithrombin, % | 104 ± 13 | 77–139 | 100 ± 11 | 79–131 | 0.21 | |
| Free TFPI, ng 3 mL−1 | 17.7 ± 3.2 | 11.7–22.9 | 18.95 ± 3.17 | 12.9–25 | 0.047 | |
| Protein C, % | 107 ± 14 | 77–133 | 102 ± 10* | 84–124* | 0.14 | |
| Fibrinogen, g/l | 4.14±1.60 | 1.27–7.92 | 2.85 ± 0.55 | 2.05–4.01 | 0.000002 | |
| Other laboratory tests | ||||||
| Glucose, mM | 4.70 [1.00] | 2.60–8.60 | 4.80 [0.60] | 4.00–6.20 | 0.41 | |
| CRP, mg/l | 2.87 [5.16] | 0.21–25.3 | 1.81 [2.03] | 0.54–5.78 | 0.04 | |
| IL-6, pg/ml | 3.07 [3.32] | 0.85–15.34 | 1.52 [1.15] | 0.66–6.62 | 0.000001 | |
| TNFα, pg/ml | 1.93 [0.85] | 0.76–51.68 | 1.26 [0.54] | 0.66–3.02 | <0.000001 | |
COPD, chronic obstructive pulmonary disease; BMI, body mass index; CRP, C-reactive protein; IL-6, interleukin 6; IQR, interquartile range; SD, standard deviation; TFPI, tissue factor pathway inhibitor; TNFα, tumor necrosis factor α
data for 27 subjects
Normally distributed continuous variables were compared using t test for independent variables; otherwise Mann-Whitney U-test was employed. Categorical variables were compared by two-sided Fisher exact test.
Coagulation factors and inhibitors
Compared with controls, COPD patients had significantly higher prothrombin (by 12.7%), fV (by 11.8%), fVII (by 8.8%), fVIII (by 47.8%), and fIX (by 11.2%), while fX was similar in both groups (Table 1). Antithrombin and PC did not differ between both groups, however free TFPI levels were slightly lower (by 6.6%), but at the level of borderline significance, in COPD patients (Table 1). None of the coagulation variables was associated with age, gender, BMI, current smoking, or hypertension in the COPD group. We found no differences in coagulation factors and inhibitors between patients with COPD stage II vs. stages III and IV, except for protein C activity (104±12 vs. 112±16%, p=0.023). The severity of COPD, fibrinogen or C-reactive protein showed no correlations with coagulation factors or inhibitors (r<0.2, p>0.1).
Thrombin Generation
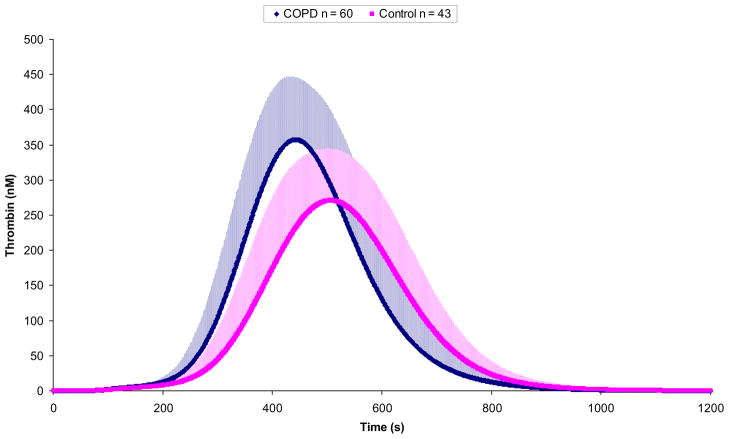
Figure 1 presents the thrombin generation curves for each individual in the COPD group and controls. Exact values of thrombin generation variables for both groups are given in Table 2. Significantly higher maximum thrombin levels (by 28.3%), rates of thrombin formation (by 46.1%), and maximum total thrombin generation (by 14.4%) were observed in the COPD patients when compared to the control group. There were also shorter clot time (time to achieve 10 nM thrombin) and time to maximum levels of thrombin in COPD patients (by 16.3% and 12.7%), respectively.
Figure 1. Thrombin simulations of individuals with COPD.
Computationally derived thrombin generation curves shown as the mean (+SD) for COPD individuals (n=60) and control (n=43) populations.
Table 2.
Thrombin generation in COPD patients and healthy controls.
| COPD patients (n=60) | Healthy controls (n=43) | p | |||
|---|---|---|---|---|---|
| Mean ± SD or median [IQR] | Range | Mean ± SD or median [IQR] | Range | ||
| Maximum level of thrombin, nM | 407 ± 76 | 457–571 | 317 ± 62 | 217–479 | <0.000001 |
| Time to maximum level of thrombin, s | 453 ± 47 | 346–561 | 519 ± 56 | 397–613 | <0.000001 |
| Total thrombin (area under the curve), μM · s | 92.7 ± 23.0 | 54.3–154.3 | 81.0 ± 16.5 | 55.7–129.5 | 0.003 |
| Time to 10 nM of thrombin, s | 197.5 ± 30 | 132–264 | 236 ± 41 | 146–319 | <0.000001 |
| Maximum rate of thrombin, nM · s−1 | 2.82 ± 0.64 | 1.72–5.09 | 1.93 ± 0.51 | 1.22–3.58 | <0.000001 |
| Time to maximum rate of thrombin, s | 363 ± 38 | 279–450 | 413 ± 45 | 317–491 | <0.000001 |
COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SD, standard deviation.
Normally distributed continuous variables were compared using t test for independent variables; otherwise Mann-Whitney U-test was employed.
In COPD patients the major determinant of time to 10 nM thrombin, time to maximum rate and that to maximum level of thrombin formation was free TFPI (r from −0.70 to −0.82, p=0.01 or less). The maximum level of thrombin generation and total production (AUC) are potently determined by prothrombin (both, r=0.69, both p<0.0001) and antithrombin (r=−0.50 and r=−0.57, respectively, both p<0.0001). None of the calculated thrombin generation parameters showed correlations with lung function tests, CRP, IL-6 or TNFα (data not shown).
Thrombin Dependence on Plasma Composition
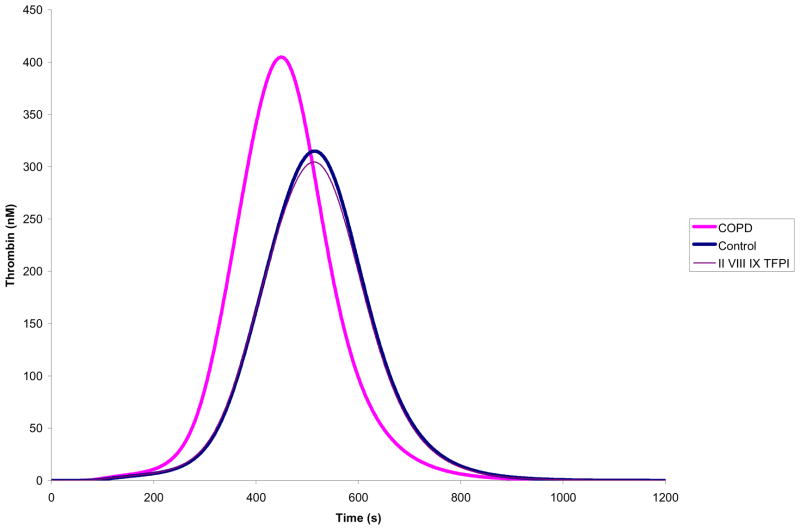
The contribution of each individual protein was evaluated by the relative shift in the thrombin generation curve that occurs after setting that protein to the mean physiologic control. These analyses showed that fII, fVIII, fIX and free TFPI have the greatest impact on thrombin generation. When all the four proteins are set to mean physiologic levels, thrombin generation profiles are very similar (Figure 2).
Figure 2. Plasma factor composition effect on thrombin simulations.
Within the COPD population, fII, fVIII, fIX, and TFPI were able to correct the prothrombotic phenotype when adjusted to concentrations in the controls.
Discussion
The current study shows that there are significant changes in the composition of plasma coagulation factors and inhibitors in the COPD patients that favor prothrombotic tendency. We are the first to show that a marked increase in FVIII, a crucial cofactor of intrinsic tenase leading to more efficient thrombin formation than extrinsic tenase, accompanied by slightly, but significantly, elevated other coagulation factors such as prothrombin, fV, fVII, and fIX, contribute to prothrombotic phenotype observed in COPD patients. In contrast to rheumatoid arthritis patients who also displayed even higher fVIII levels in plasma [14], we did not observe increased levels of coagulation inhibitors, antithrombin and protein C, that could reduce prothrombotic effects of elevated coagulation factors in COPD patients. Of note, coagulation factors were uniformly elevated in COPD patients and showed no associations with the severity of the disease or serum concentrations of inflammatory markers such as CRP, IL-6, or TNFα. This indicates that prothrombotic phenotype related to higher levels of several coagulation factors and lower free TFPI is a common feature of COPD and composition of coagulation factors and/or inhibitors is not driven by IL-6- or TNFα-mediated inflammatory pathways. Since our study was performed on stable COPD patients, it remains to be established to what extent exacerbations of COPD enhance the alterations in plasma coagulation factors thus contributing to increased prothrombotic phenotype.
Simulations of thrombin generation performed as described in previous papers [11–13] demonstrated that in COPD thrombin generation is markedly accelerated and a burst of thrombin formation occurs faster. Importantly, total thrombin formation in our model is also 14% higher in COPD patients from that observed in controls. These findings clearly suggest that relatively small changes in plasma coagulation factor composition determine significant alterations in the kinetics of thrombin formation. Since thrombin generation is predominantly determined by prothrombin, which is higher in COPD, and antithrombin levels, which were similar in both groups, the increase in total thrombin formation in COPD is close to the increase observed in prothrombin levels (14% vs 13%, respectively, Table 1 and 2). The ultimate effect of all the alterations in plasma factor composition facilitates thrombus formation by faster and more massive thrombin formation in COPD.
Altogether, altered thrombin generation profiles associated with slight prothrombotic shifts in coagulation factor levels in COPD indicate that circulating blood thrombogenicity in COPD patients with the background of atherosclerotic vascular disease stimulated by enhanced inflammation might contribute to increased risk for thrombotic cardiovascular events, including myocardial infarction and stroke, in this common disease [4].
A novel and unexpected finding is the presence of slightly reduced free TFPI in plasma of COPD patients compared to healthy controls. Its anticoagulant activity of TFPI, which blocks the TF/FVIIa complex, is associated with the free form of TFPI in plasma and not lipid-bound TFPI [15]. Decreased levels of free TFPI observed in our COPD population could result from impaired TFPI release from dysfunctional endothelial cells [16]. It has been reported that circulating TFPI antigen levels, accompanied by thrombomodulin levels, are significantly higher in patients with COPD compared with controls [17,18]. Lower TFPI concentrations in our COPD patients compared with the controls might be associated with higher FEV1 values in the present study (a mean, 57% vs 44% in the previous study [18]). In other populations increased total TFPI levels associated with lower free TFPI have been observed. In the current study, a small reduction in free TFPI was found and this difference might enhance prothrombotic mechanisms and contribute to the thrombotic potential in COPD patients.
Mechanisms underlying alterations in blood coagulation factors, especially fVIII, in COPD remain to be elucidated. Most likely, COPD is associated with enhanced production of coagulation factors in the liver and endothelial cells, however reduced clearance of these proteins cannot be excluded. Which mediator(s) account for the changes in plasma composition observed in the current study is unknown. It is not certain that prothrombotic phenotype is induced by environmental or disease-specific factors. Since phenotypic blood composition at any time reflects ongoing systemic events (e.g. inflammation) as well as genetic predisposition, we cannot exclude that prothrombotic state observed in COPD is to some extent genetically determined as shown by Vormittag et al. [19] for fVIII activity and a polymorphism in the low-density lipoprotein receptor-related protein 1 gene (663 C>T). Other genetic factors may also be involved in the pathogenesis of prothrombotic phenotype in COPD, for example, prothrombin G20210A mutation is known to be linked with significantly increased prothrombin levels. Recently, it has been reported that hypoxia in COPD patients enhances thrombin formation measured in peripheral blood [20]. Given the fact that the current patients were clinically stable, it is unlikely that this factor contributes to prothrombotic phenotype in COPD.
Our study has several limitations. First, the number of the patients studied is limited. However, it is unlikely that the differences reported here result from significant recruitment bias. Second, we excluded subjects with serious comorbidities frequently encountered in COPD patients such as renal insufficiency or liver injury. However, we can assume that most subjects with comorbidities and COPD may display even more profound prothrombotic alterations in plasma coagulation factor composition with the exception of liver injury which could impair hepatic synthesis of some coagulation factors. The present study did not address the issue of pharmacological modulation of prothrombotic phenotype in COPD. However, oral corticosteroids, which are likely to affect blood coagulation by elevation of fVII and fVIII as well as decreased fibrinogen [21], were not administered in our study population. Inhaled bronchodilators are unlikely to alter coagulation factor levels. Moreover, the effect of statins, known to suppress inflammatory response [22], could not be evaluated in the current study, however they are unlikely to significantly alter inflammatory markers and thrombin formation in COPD patients [6,23]. Finally, clinical implications of the present study remain unknown. A larger study with long-term follow-up is needed to assess the effect of altered kinetics of thrombin generation on clinical endpoints, including myocardial infarction and venous thromboembolism in COPD.
In conclusion, we demonstrated that COPD is associated with altered kinetics of thrombin generation and the changes are largely dependent on plasma coagulation factors composition. The concentration of fVIII was greatest in COPD individuals over controls and when the dynamics of the plasma composition were considered in context of thrombin generation, fII, fVIII, fIX, and TFPI was able to correct the prothrombotic phenotype. These observations yield new insights into the pathogenesis of atherothrombotic events in COPD patients.
Acknowledgments
We would like to thank George Forguites for his technical assistance. This manuscript was funded by grant HL46703 (Project 5) from the National Institute of Health. Portions of this work are submitted to the ISTH July 2011 annual meeting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sin DD, Anthonisen NR, Soriano JB, Agusti AG. Mortality in COPD: role of comorbidities. Eur Respir J. 2006;28:1245–1257. doi: 10.1183/09031936.00133805. [DOI] [PubMed] [Google Scholar]
- 2.Anthonisen NR, Connett JE, Enright PL, Manfreda J. Lung Health Study Research Group. Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med. 2002;166:333–339. doi: 10.1164/rccm.2110093. [DOI] [PubMed] [Google Scholar]
- 3.Huiart L, Ernst P, Suissa S. Cardiovascular morbidity and mortality in COPD. Chest. 2005;128:2640–2646. doi: 10.1378/chest.128.4.2640. [DOI] [PubMed] [Google Scholar]
- 4.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 5.Ashitani J, Mukae H, Arimura Y, Matsukura S. Elevated plasma procoagulant and fibrinolytic markers in patients with chronic obstructive pulmonary disease. Int Medicine. 2002;41:181–185. doi: 10.2169/internalmedicine.41.181. [DOI] [PubMed] [Google Scholar]
- 6.Undas A, Kaczmarek P, Sładek K, Rzeszutko M, Skucha W. Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease. Beneficial effects of simvastatin treatment. Thromb Haemost. 2009;102:1176–1182. doi: 10.1160/TH09-02-0118. [DOI] [PubMed] [Google Scholar]
- 7.Vaidyula VR, Criner GJ, Grabianowski C, Rao AK. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thromb Res. 2009;124:259–261. doi: 10.1016/j.thromres.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowski M, Undas A, Kaczmarek P, Butenas S. Activated factor XI and tissue factor in chronic obstructive pulmonary disease: links with inflammation and thrombin generation. Thromb Res. 2011;127:242–246. doi: 10.1016/j.thromres.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Global Initiative for Chronic Ibstructive Lung Disease. Global strategy for diagnosis, management, and prevention of chronic obstructive pumonary diseaese (updated 2010) www.goldcopd.com.
- 10.Alessandri C, Basili S, Violi F, Ferroni P, Gazzaniga PP, Cordova C. Hypercoagulability state in patients with chronic obstructive pulmonary disease. Chronic Obstructive Bronchitis and Haemostasis Group. Thromb Haemost. 1994;72:343–346. [PubMed] [Google Scholar]
- 11.Brummel-Ziedins KE, Pouliot RL, Mann KG. Thrombin generation: phenotypic quantitation. J Thromb Haemost. 2004;2:281–288. doi: 10.1046/j.1538-7933.2003.00576.x. [DOI] [PubMed] [Google Scholar]
- 12.Brummel-Ziedins KE, Vossen CY, Butenas S, Mann KG, Rosendaal FR. Thrombin generation profiles in deep venous thrombosis. J Thromb Haemost. 2005;3:2497–2505. doi: 10.1111/j.1538-7836.2005.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brummel-Ziedins K, Undas A, Orfeo T, Mann KG. Thrombin generation in acute coronary syndrome and stable coronary artery disease: dependence on plasma factor composition. J Thromb Haemost. 2008;6:104–110. doi: 10.1111/j.1538-7836.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- 14.Undas A, Gissel M, Kwasny-Krochin B, Gluszko P, Brummel-Ziedins K. Thrombin generation in rheumatoid arthritis: dependence on plasma factor composition. Thromb Haemost. 2010;104:224–230. doi: 10.1160/TH10-02-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E, Jr, She D. Cardiovascular disease in patients with in chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16:63–70. doi: 10.1016/j.annepidem.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 17.Cella G, Sbarai A, Mazzaro G, Vanzo B, Romano S, Hoppensteadt T, Fareed J. Plasma markers of endothelial dysfunction in chronic obstructive pulmonary disease. Clin Appl Thromb Haemost. 2001;7:205–208. doi: 10.1177/107602960100700304. [DOI] [PubMed] [Google Scholar]
- 18.Cella G, Saetta M, Baraldo S, Turato G, Papi A, Casoni G, Rigno M. Endothelial cell activity in chronic obstructive pulmonary disease without severe pulmonary hypertension. Clin Appl Thromb Haemost. 2005;11:435–440. doi: 10.1177/107602960501100410. [DOI] [PubMed] [Google Scholar]
- 19.Vormittag R, Bencur P, Ay C, Tengler T, Vukovich T, Quehenberger P, Mannhalter C, Pabinger I. Low-density lipoprotein receptor-related protein 1 polymorphism 663 C>T affects clotting factor VIII activity and increases the risk of venous thromboembolism. J Thromb Haemost. 2007;5:497–502. doi: 10.1111/j.1538-7836.2007.02337.x. [DOI] [PubMed] [Google Scholar]
- 20.Sabit R, Thomas P, Shale DJ, Collins P, Linnane SJ. The effects of hypoxia on markers of coagulation and systemic inflammation in patients with COPD. Chest. 2010;138:47–51. doi: 10.1378/chest.09-2764. [DOI] [PubMed] [Google Scholar]
- 21.van Zaane B, Nur E, Squizzato A, Gerdes VE, Büller HR, Dekkers OM, Brandjes DP. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost. 2010;8:2483–2493. doi: 10.1111/j.1538-7836.2010.04034.x. [DOI] [PubMed] [Google Scholar]
- 22.Undas A, Topor-Madry R, Tracz W. Simvastatin increases clot permeability and susceptibility to lysis in patients with LDL cholesterol below 3.4 mmol/l. Pol Arch Med Wewn. 2009;119:354–359. [PubMed] [Google Scholar]
- 23.Kaczmarek P, Sładek K, Skucha W, Rzeszutko M, Iwaniec T, Szczeklik A. The influence of simvastatin on selected inflammatory markers in patients with chronic obstructive pulmonary disease. Pol Arch Med Wewn. 2010;120:11–17. [PubMed] [Google Scholar]