Abstract
Background
The need, safety and effectiveness of vitamin D supplementation during pregnancy remain controversial.
Design
In this randomized controlled trial, women with a singleton pregnancy at 12–16 weeks’ gestation received 400, 2000 or 4000 IU vitamin D3/day until delivery. The primary outcome was maternal/neonatal circulating 25(OH)D at delivery, with secondary outcomes 25(OH)D ≥80 nmol/L achieved and 25(OH)D concentration required to achieve maximal 1,25(OH)2D production.
Results
Of the 494 women enrolled, 350 women continued until delivery: Mean 25(OH)D by group at delivery and 1-month before delivery were significantly different (p<0.0001), and percent who achieved sufficiency was significantly different by group, greatest in 4000 IU group (p<0.0001). The relative risk (RR) for achieving ≥80 nmol/L within one month of delivery was significantly different between 2000 vs. 400 IU (RR 1.52 [CI 1.24–1.86]); 4000 vs. 400 (RR 1.60 [CI 1.32–1.95]), but not between 4000 vs. 2000 (RR 1.06 [CI 0.93–1.19]). Circulating 25(OH)D had a direct influence on circulating 1,25(OH)2D concentrations throughout pregnancy (p<0.0001) with maximal production of 1,25(OH)2D in all strata in the 4000 IU group. There were no differences between groups on any safety measure. Not a single adverse event was attributed to vitamin D supplementation or circulating 25(OH)D levels.
Conclusions
Vitamin D supplementation of 4,000 IU/day for pregnant women was safe and most effective in achieving sufficiency in all women and their neonates regardless of race while the current estimated average requirement was comparatively ineffective at achieving adequate circulating 25(OH)D, especially in African Americans.
Introduction
The function of vitamin D during pregnancy for both mother and fetus remains largely undefined. Vitamin D is known to be involved in skeletal homeostasis during pregnancy as evidenced by a recent publication dealing with craniotabes in the newborn, and severe vitamin D deficiency may lead to neonatal seizures in those neonates with profound hypocalcemia (1–5). The function of vitamin D during this sensitive period, however, may also have potential effects on other systems, including immune (6–10), pancreatic (11–13), musculoskeletal (14–17), and cardiovascular function (18–20) as well as neural development (21–24). Recent publications suggest relationships between maternal vitamin D status and adverse pregnancy outcomes such as preeclampsia and cesarean section (25–28).
A Cochrane Review published in 2000 highlighted the relative dearth of data dealing with vitamin D supplementation during human pregnancy (29). This review listed seven studies on the topic (30–36), of which four reported clinical outcomes (30–32,36). From these limited data, the Cochrane Review concluded that there was insufficient evidence to evaluate the effects of vitamin D supplementation during pregnancy (29). Since that time, there have been few studies that have addressed this issue (37–39).
In 2004, we initiated an NICHD-sponsored6-year randomized double-blind placebo-control trial of vitamin D supplementation during pregnancy to assess safety and pregnancy outcomes with an approved Investigational Drug Application from the FDA (#66,346). We hypothesized that 4000 IU/day vitamin D3 would be more efficacious and effective than the standard dosing regimen of 400 IU/day and 2000 IU (the former upper limit for vitamin D)(40)/day dosing regimen in achieving a total circulating 25 (OH)D level of at least 80 nmoL/L (32/ng/mL) in pregnant women regardless of race throughout pregnancy and at the time of delivery without causing any safety concerns. This minimal value of 80 nmol/L was based on years of research with regard to circulating 25 (OH)D levels suppressing secondary hyperthyroidism, optimal intestinal calcium absorption and bone mineral density (41). These results are presented here.
Methods
Study Design
This study was a single center, randomized, controlled, double-blinded study of vitamin D supplementation stratified by race (FDA IND #66,346; ClinicalTrials.gov # NCT00292591). Women less than 16 weeks’ gestation with a singleton pregnancy were eligible for participation in the study.
Study Participants and Setting
This study was approved by MUSC’s Institutional Review Board for Human Research (HR# 10725), and was conducted from January 4, 2004 through July 31, 2009 at the Medical University of South Carolina (MUSC), Charleston, South Carolina. The inclusion criteria for the subjects included the following: (1) maternal age of 16 years or greater at the time of consent; (2) confirmed singleton pregnancy of less than 16 completed weeks of gestation at the time of consent; (3) planned to receive ongoing prenatal care in the Charleston, SC area; and (4) the ability to provide written informed consent at the first visit. If a woman received her obstetrical care at a facility separate from MUSC, then she came to MUSC’s Clinical and Translational Research Center (CTRC) outpatient research facility for each of the study visits. Women were consented at their first prenatal visit, at which time baseline 25(0H)D levels were measured. Irrespective of gestational age at enrollment, subjects began vitamin D supplementation between the start of the 12th and the start of the 16th weeks of gestation (12 0/7th and 16 0/7th weeks)as defined by their last menstrual period.
Exclusion Criteria
Those women with a pregnancy greater than 16 weeks of gestation as calculated by last menstrual period were not eligible to participate. Pregnant women with pre-existing calcium or parathyroid conditions, or who required chronic diuretic or cardiac medication therapy including calcium channel blockers, or chronic hypertension were not eligible for enrollment into the study. Pregnant women with active thyroid disease (e.g., Graves, Hashimoto’s or thyroiditis) also were excluded; however, mothers on thyroid supplement with normal serological parameters could participate in the study if they were without any other endocrine dysfunction
Study Protocol
Gestational Age at Enrollment
Subjects could be consented and enrolled into the study before the initiation of vitamin D supplementation at 12–16 weeks of gestation. Gestational age was based on last menstrual period. If a woman was unsure of her gestational age, the obstetrical estimate at the time of the visit was used. If, at the 20-week fetal ultrasound it was determined by the obstetrician that the gestational age was incorrect, the revised gestational age was used and the discrepancy noted.
Initial Study Visit
Baseline blood and urine samples were obtained following subject consent at the initial visit; however, the earliest time of randomization following measurement of baseline total circulating 25(OH)D was 12 weeks’ gestation with the target upper limit of gestation of 16 weeks’ gestation. Irrespective of enrollment gestational age, vitamin D supplementation did not begin before the twelve week of gestation (12and0/7th weeks).
Subsequent Study Visits
Subjects were followed with monthly study visits, which continued until delivery. The visits coincided with routine obstetrical visits or were performed in conjunction with those visits if the obstetrical care was provided outside of MUSC. The subjects also were seen at the GCRC/CTRC for a study visit at 16 weeks of gestation, and with their infant at 2weeks’ postpartum.
Completion of Questionnaires
Following their written, informed consent, mothers completed questionnaires regarding sociodemographic information, baseline health status and medical history at the first visit. At the second visit, the Block Food Frequency Questionnaire was completed to ascertain generalized eating pattern, with specific calculation of calcium and vitamin D intake (Block, Berkeley, California).(42–47). Each completed FFQ form was sent to the processing center (Berkeley, California) and this data were later reviewed for accuracy by a registered dietician who was blinded to subject treatment group assignment. Total caloric intake, vitamin D and calcium intake were recorded for each subject.
An interim maternal health history questionnaire also was completed at each visit with the assistance of the study coordinator to ascertain adverse events, discussing type and frequency of acute illnesses such as respiratory, gastrointestinal, and other viral and/or bacterial illnesses. A review of medications and doctor’s visits was obtained at that time.
After delivery, the newborn record of each infant was reviewed for mode of delivery and level of neonatal care required (normal newborn nursery, Level 2 or Level 3 intensive care). Birth weight (grams) and gestational age also were recorded.
Blood and Urine Samples
Maternal blood and urine samples were collected at each visit. Cord blood was obtained at delivery. If the cord blood sample could not be obtained, a neonatal blood sample was drawn within two weeks of delivery.
Intervention
Multi-Vitamin and Vitamin D Supplementation
Pregnant women who presented for prenatal care at ≤16 weeks’ gestation were randomized into one of three treatment regimens of vitamin D3 after establishing their baseline serum 25(OH)D level. All patients received a total of two pills daily: a standard prenatal multivitamin vitamin containing 400 IU of vitamin D and an additional vitamin D3 supplement of 0 IU (placebo), 1600 IU, or 3600 IU of vitamin D3 for a total of 400 IU, 2000 IU and 4000 IU of vitamin D supplementation, respectively.
In order to obtain Institutional Review Board approval for the study, the following safety measure was put into place: baseline total circulating 25(OH)D levels were measured and women with levels ≤100 nmol/L (40 ng/mL) were eligible for randomization into one of the three arms (400, 2000 or 4000 IU vitamin D3/day) with further substratification by race within each treatment group. Women with baseline 25(OH)D levels >100 nmol/L to 150 nmol/L (>40–60 ng/mL, levels considered to be in the normal range at the time of study implementation) were randomized into one of two treatment groups (400 or 2000 IU vitamin D3/day), while women with a baseline 25(OH)D level >150 nmol/L (>60 ng/mL) were given 400 IU vitamin D3/day. The doses of vitamin D utilized in our study were selected based on current recommendations (400 IU/day), the upper safe intake level established in 1997 (2000 IU/day)(40) and the amount we calculated to be required to achieve nutritional vitamin D sufficiency (4000 IU/day)(48).
Adherence to Medication Regimen
Adherence to the prescribed vitamin D supplementation regimen of 1 prenatal vitamin and the vitamin D supplement was measured by maternal self-report and pill counts at each follow-up visit (49). The number of vitamin D pills returned was divided by the expected number of pills that would have been taken between study visits to generate a percentage that served as a measure of adherence of medication regimen between study visits. The adherence measures were used to generate an average adherence for each subject (49). If a woman missed one prenatal visit, her next month supply of vitamins was either mailed to her or dropped off at her residence. In such cases, medication adherence was based on the pill count from the date of the last visit to the current prenatal visit over the expected number of pills taken. If a woman had more than two missed visits or if she failed to take at least 50% of the prescribed vitamin D pills, she was exited from the study.
Randomization
Our study utilized stratified blocked randomization to balance by ethnicity and also to balance by enrollment (as a cautionary measure against a potential temporal or seasonal bias). A randomization scheme was separately developed for each of the three ethnic groups (i.e., the stratum). Within each stratum, the treatments were assigned within blocks. Since there were three treatment groups, the block size had to be divisible by three; the data team selected a block size of six, which was unknown to the investigators or the pharmacists. In this way, at the end of each block (i.e., enrollment of six subjects), each ethnic group was balanced in the number randomly assigned to 400, 2000, and 4000 IU treatment groups.
Materials
Source of Vitamin D
Vitamin D tablets were manufactured by Tishcon Corporation, Westbury, NY, a Good-Manufacturing-Practice (GMP) facility. The cholecalciferol contained in the vitamin D tablet was supplied to Tishcon Corporation by Hoffman-La Roche Ltd., Basel, Switzerland. The tablet vitamin D concentration was verified by the company every 6 months and by an independent laboratory chosen by the investigators (Heartland Assays, Ames, IO) using HPLC-UV to ensure the tablets met label claim throughout the study; these results were reported to the Investigational Drugs Department at MUSC. Tablets were maintained in MUSC’s Research Pharmacy until the time that they were dispensed to each enrolled subject.
Source of Prenatal Vitamins
Prenatal vitamins prescribed at the time of each subject’s enrollment were Myadec Multivitamin-Multimineral Supplement (distributed by Pfizer Consumer Healthcare, Morris Plains, NJ) with 400 IU vitamin D3/tablet. Those mothers who were unable to swallow a prenatal vitamin were given Flintstones™ Complete chewable vitamin (Bayer Healthcare, Morristown, NJ), which provided 400 IU vitamin D3 per vitamin.
Measures
Maternal Sociodemographic Measures included maternal age at time of enrollment, her self-defined race, insurance status, educational status, occupation and employment outside of the home.
Pregnancy Health Status, and Labor and Delivery Characteristics and Complications
Characteristics of each mother’s health status and complications during pregnancy, labor, and delivery were recorded and reviewed by an obstetrician (DDJ blinded to treatment). If the mother required hospitalization, a copy of the hospital record was obtained after she had signed a release of medical information form. Any acute illnesses, hospitalizations or development of pregnancy-related conditions that were not preexisting also were recorded. The Data Monitoring and Safety Committee (DSMC) was notified of all such events.
Anthropomorphic Measurements
Pre-pregnancy height and weight of each mother were recorded at the first outpatient visit to determine Body Mass Index or BMI (weight [kg]/height2 [m2]). During subsequent visits, only the subject’s weight was recorded. Birth weight (grams) was recorded for each infant.
Laboratory Measurements
Maternal and Cord Blood/Neonatal Vitamin D and Metabolite Assays
Circulating vitamin D2 and D3 were measured in serum using direct ultraviolet detection preceded by organic extraction and high performance liquid chromatography as previously described (50). This assay has a coefficient of variation of ≤10% and a 5 nmol/L vitamin D detection limit. There is no normal established circulating range of vitamins D2 or D3 in human subjects.
A rapid, direct RIA developed in the Hollis laboratory and manufactured by Diasorin Corporation (Stillwater, MN) was used to measure total circulating 25(OH)D concentration in serum samples (51). This RIA is an FDA-cleared device and, in fact, is the FDA predicate device for the measurement of circulating 25(OH)D in humans.
Based on clinical laboratory classifications (52,53), a priori, deficiency was defined as total circulating 25(OH)D <50 nmol/L (20 ng/mL), insufficiency as ≥50 to <80 nmol/L (≥20 to <32 ng/mL), and sufficiency as ≥80 nmol/L (≥32 ng/mL) (41,53–55). The inter- and intra-assay coefficient of variation is ≤10%.
An RIA manufactured by Diasorin Corporation and developed in the Hollis laboratory was used to measure total circulating 1,25(OH)2D concentration (56). This assay uses an I125-labeled tracer and samples are processed using acetonitrile followed by solid-phase extraction and quantitation. This RIA is an FDA-cleared device. The normal circulating level of 1,25(OH)2D is 48–120 pmol/L (20–50 pg/mL). The inter-and intra-assay coefficient of variation is ≤15%.
Maternal and Cord Blood/Neonatal Circulating intact PTH Concentrations
Intact PTH (iPTH) was measured by immunoradiometric (IRMA) that utilizes 2 different polyclonal antibodies (Diasorin, Stillwater, MN). The first antibody, specific for PTH 39–84, is bound to a solid phase bead. The second antibody is specific for PTH 1–34 and is labeled with 125I. The adult normal range for iPTH in our laboratory is 1.3–5.4 pmol/L. Higher vitamin D levels are associated with lower iPTH; as vitamin D status improves iPTH declines (57).
Maternal Baseline and Follow-up Serum Calcium, Creatinine and Phosphorus Studies
Maternal serum total calcium, creatinine and inorganic phosphorus were measured by MUSC’s Clinical Chemistry Laboratory using standard methodology and laboratory normative data. Results were reported to the PI and downloaded to the research database from the clinical chemistry registry. All results were reviewed by the Clinical PI of the study on a weekly basis for any abnormal values and reported to the DSMC.
Circulating Vitamin D Binding Protein (VDBP)
VDBP was measured using a commercial ELISA purchased from R&D Systems (Minneapolis, MN). Circulating VDBP levels in normal individuals using this ELISA is stated by the manufacturer to be 3.93 ± 1.62 μmol/L.
Maternal Urinary Calcium/Creatinine Ratio
A non-fasting urine sample was obtained from the mother at each obstetrical visit and was sent to the Clinical Chemistry Laboratory at MUSC for urinary calcium (Ca) and creatinine (Cr) measurements1 and derivation of the urinary Ca (mg/dL): Cr (mg/dL) ratio (converted to mmol/L/mmol/L).
Safety Measures throughout the Study
All study subjects were monitored for hypervitaminosis D. The circulating 25(OH)D level of 225 nmol/L (90 ng/mL) was used to define hypervitaminosis D as required by the FDA and our IRB. This conservative maternal level was arbitrarily chosen to ensure the safety of all study patients, particularly those assigned to the 4,000 IU vitamin D3/day regimen (58). Subsequent vitamin D supplementation trials have demonstrated that circulating levels of 25(OH)D exceeding 300 nmol/L (120 ng/mL) do not cause hypercalciuria, the first indicator of hypervitaminosis D (48). Even in women who are vitamin D deficient, urinary calcium excretion increases during pregnancy secondary to increased glomerular filtration rate (59). Given this, urinary calcium: creatinine ratio was used and is the most sensitive, early indicator of hypervitaminosis D. Operationally, we defined a priori caution limits for hypervitaminosis D as a non-fasting urinary calcium/creatinine ratio ≥0.8 mg/mg or 2.27 mmol/mmol.
Whenever any patient exceeded the caution limit or had an abnormal clinical chemistry value, a specific case study by the Data Safety and Monitoring Committee (DSMC) was to be initiated to examine the contribution of confounding factors (e.g., diet, sunlight exposure, etc.). Operationally, vitamin D3 supplementation stopped if the urinary calcium: creatinine ratio exceeded 1.0 (mg/dL/mg/dL) or if the circulating 25(OH)D level exceeded 225 nmol/L (90 ng/mL).
Statistical Methods
Sample Size and Power Considerations
To detect a statistically significant increase in 25(OH)D by 10 ng/mL between any two groups was calculated to require a minimum of 32 patients per group at 90% power, alpha = 0.05, two tailed test for the primary analysis. This calculation assumed that the standard deviation of 25(OH)D measurements at a single time point was approximately 10, that there would be a low correlation (r = 0.25) between the baseline and final measurements, and that a substantial proportion (up to 50%) of participants may be lost to follow-up due to moving, termination of care, or discontinuation of participation. Because the primary outcome—maternal and neonatal vitamin D status at or around the time of delivery, a prerequisite for inclusion in the final analysis was that the mother had to have had a livebirth and had to have been a study participate until the day of delivery. Lastly, as one of the secondary goals of this study was to explore vitamin D differences by ethnicity, the three supplemented groups (400, 2,000 and 4,000 IU/day) were balanced by ethnicity (equal numbers of Caucasian, African American, and Hispanic).
Statistical Analysis
The main variables of interest were: (1) differences in mean maternal and infant total circulating 25(OH)D at the time of delivery between supplement groups (ANOVA); and (2) differences, between supplement groups, in the proportion of women achieving 25(OH)D of ≥80 nmol/L within one month and at the time of delivery (Chi-square). Secondary analyses employed Chi-square for categorical variables, ANOVA or Student’s t-test, as appropriate, for normally distributed variables (with the Bonferonni option for pair-wise analysis in ANOVA), and paired Student’s t-test for within group changes from baseline to delivery. Multiple regression was used to assess the association between final vitamin D concentrations with baseline values, ethnicity, dose group and the dose *race interaction. Stratified analysis was used to more fully explore any evidence of interaction. Variables that were not normally distributed were analyzed with Wilcoxon-Mann-Whitney test. The association between vitamin D, 25(OH)D and 1,25(OH)2D; D3; and urinary calcium: creatinine ratio were explored with a combination of exponential and linear models. Data were analyzed with SAS 9.22(60)(Cary, NC) and SigmaPlot software (Systat Software, Inc., San Jose, CA).
The analysis was conducted as an intention-to-treat (ITT)(61). The ITT approach (effectiveness) compares the outcomes between supplement groups, as assigned, and makes no assumption regarding whether or not subjects were adherent to the dosing regimen.2 The intention-to-treat design was used as a measure of the effectiveness of increasing vitamin D levels via oral dosing. This approach presents a conservative finding of potential benefits that could be shown from a population-or public health-basedintervention.3
Results
Study Population
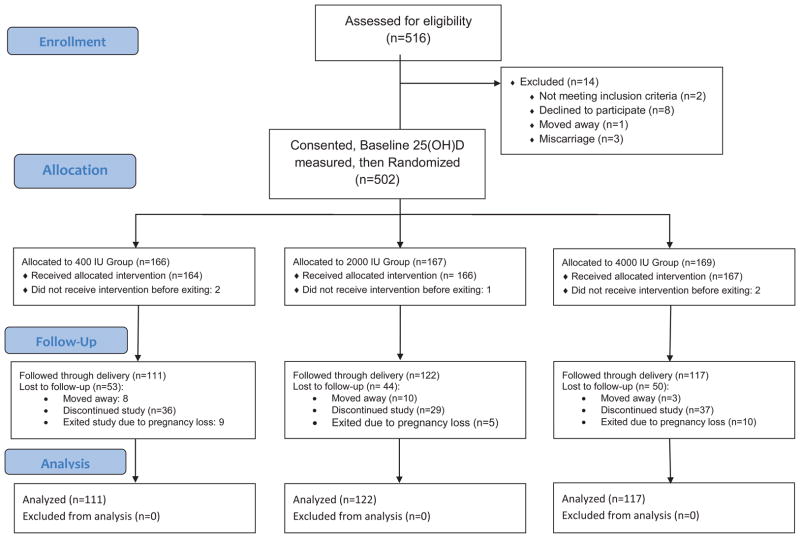
Figure 1 shows the enrollment, allocation, and follow-up of the women who participated in the trial. A total of 516 women were interviewed and 502 consented to participate in this study and were randomly assigned to a treatment group: 166 were assigned to Group 1 (400 IU group); 167 were assigned to Group 2 (2000 IU group); and 169 were assigned to Group 3 (4000 IU group). Of the 502 women consented, there were 23 women with a valid initial 25(OH)D greater than 100 nmol/L (40 ng/mL) who were not eligible for enrollment in the 4000 IU group: 2 African American, 6 Hispanic and 15 Caucasian women. Of those, 12 were enrolled into the 400 IU group, 10 were enrolled into the 2000 IU group, and 1 was enrolled in the 4000 IU group (the latter being a protocol deviation early in the study where one woman with a baseline 25(OH)D of 41 was randomized to the 4000 IU group): 17 continued until delivery: 1 African American, 6 Hispanic and 10 Caucasian women;10 were in the400 IU group, and 6 were in the 2000 IU group, and 1 was in the 4000 IU group. Finally, there was one Caucasian woman whose baseline 25(OH)D level was 172.5 nmoL/L (69 ng/mL) who was placed into the 400 IU group. After allocation into treatment groups, there were no statistically significant differences among the groups with regard to lost to follow-up, drop-outs or pregnancy losses.
Figure 1. Flow Diagram of Pregnancy Study.
1IU denotes International Units
The sociodemographic characteristics of the active cohort are found in Table 1. Baseline characteristics were similar between the groups on the basis of race/ethnicity, maternal age, gestational age at enrollment, educational and employment status, health rating, planned pregnancy, body mass index and season at study entry. There was a trend toward differences between the groups on the basis of maternal gravidity and parity and insurance status. A total of 62 women (12.4%) were taking a prenatal vitamin at the time of randomization. Of the 502 women who were randomized to treatment, 350 women continued in the study until delivery and had outcome data available for analysis: 98 African American, 137 Hispanic and 115 Caucasian women evenly distributed into the 3 treatment groups, with 111 controls, 122 in 2000 IU and 117 in 4000 IU groups. There were no differences in baseline vitamin D status among treatment groups.
Table 1.
Maternal Sociodemographic and Clinical Characteristics at Study Enrollment by Vitamin D Supplementation Group
| Maternal Characteristic | 400 IU Group | 2000 IU Group | 4000 IU Group | p-value |
|---|---|---|---|---|
| N=111 | N=122 | N=117 | ||
| Race/Ethnicity,1 N (%) | 0.9 | |||
| African American | 28 (25.2) | 37 (30.3) | 33 (28.2) | |
| Hispanic | 45 (40.5) | 48 (39.3) | 44 (37.6) | |
| Caucasian | 38 (34.2) | 37 (30.3) | 40 (34.2) | |
|
| ||||
| Maternal Age (years), Mean ± SD | 27.0 ± 5.6 | 27.4 ± 5.7 | 26.6 ± 5.4 | 0.6 |
| Range | 18 – 41 | 17 – 41 | 17 – 44 | |
|
| ||||
| Gestation at Enrollment, weeks | ||||
| Mean ± SD | 12.5 ± 1.9 | 12.6 ± 1.6 | 12.4 ± 2.0 | 0.8 |
| Range | 7.1 – 18.4 | 8.4 – 17.6 | 6.4 – 21.4 | |
|
| ||||
| Maternal Gravidity, Median | 2 | 2 | 2 | 0.08 |
| Range | 1 – 8 | 1 – 7 | 1 –9 | |
|
| ||||
| Maternal Parity, Median | 2 | 2 | 1 | 0.052 |
| Range | 0–5 | 0–7 | 0 – 9 | |
|
| ||||
| Education, N (%) | 0.4 | |||
| < HS Education | 18 (17.3) | 23 (19.7) | 13 (11.6) | |
| HS graduate | 17 (16.4) | 24 (20.5) | 22 (19.6) | |
| College or more | 69 (66.4) | 70 (59.8) | 77 (68.8) | |
|
| ||||
| Employed at Study Entrance, N (%) | 61 (55.0) | 67 (54.9) | 65 (55.6) | 0.9 |
|
| ||||
| Insurance, N (%) | 0.07 | |||
| Medicaid/None | 62 (55.9) | 85 (69.7) | 69 (59.0) | |
| Commercial | 49 (44.1) | 37 (30.3) | 48 (41.0) | |
|
| ||||
| Subjective Health Rating Scale | 0.4 | |||
| Median2 | 9 | 10 | 10 | |
| Range | 5–10 | 5–10 | 1–10 | |
|
| ||||
| Planned Pregnancy, N (%) | 59 (54.6) | 61 (50.4) | 59 (50.4) | 0.8 |
|
| ||||
| BMI, N (%)3 | 0.6 | |||
| ≤30 | 78 (70.3) | 87 (71.3) | 89 (76.1) | |
| > 30 | 33 (29.7) | 35 (28.7) | 28 (23.9) | |
|
| ||||
| Season at study entry, N (%) | 0.9 | |||
| April – September | 54 (48.7) | 60 (49.2) | 56 (47.9) | |
| October – March | 57 (51.4) | 62 (50.8) | 61 (52.1) | |
|
| ||||
| Vitamin D Intake in IU,4 Mean ± SD | 181.6 ± 108.4 | 195.8 ± 135.0 | 204.2 ± 148.2 | 0.6 |
| Range | 21.4 – 470.6 | 8.2 – 693.8 | 5.3 – 737.3 | |
|
| ||||
| Calcium Intake, mg/day, Mean ± SD | 1063.6 ± 539.6 | 993.9 ± 514.0 | 1073.6 ± 491.9 | 0.6 |
| Range | 252.9 – 2888.1 | 285.4 – 2754.1 | 275.6 – 2925.9 | |
|
| ||||
| Kcal Intake, Mean ± SD | 2148.3 ± 778.6 | 2059.4 ± 803 | 2212.9 ± 920.8 | 0.5 |
| Range | 977.3 – 4668.2 | 993.4 – 4793.4 | 929.3 – 5516 | |
Race/Ethnicity as defined by mother.
Self-reported maternal health status rating from 1 (poor) -10 (excellent)
BMI, Prepregnancy body mass index (BMI)
International Units, IU: dietary intake calculated from Block 1998.2 Food Frequency Questionnaire; amount did not include prenatal vitamin intake
A comparison of those women who completed the study and those who electively discontinued the study is found in Table 2. Women who had a pregnancy loss or who moved away were excluded in this analysis. Individuals who exited the study did not differ by treatment group. Those women who electively exited the study were more likely to be African American than Hispanic or Caucasian. Compared to women who continued in the study until delivery, women who exited the study were more likely to be younger (p=0.01), African American (p=0.003), of higher gravidity (p<0.0001), less educated (p=0.01), employed at entrance into the study (p=0.04), with an unplanned pregnancy (p=0.01), and with a BMI <30 (p=0.02). Baseline vitamin D status by ethnicity of those who completed vs. those who exited also did not differ (see Table 2).
Table 2.
Subjects Who Completed the Study Compared to Subjects Exited Before Delivery1
| Maternal Characteristic | Delivered | Exited | p-value |
|---|---|---|---|
| N=350 | N=129 | ||
| Treatment Group, N (%): | 0.5 | ||
| 400 IU | 111 (74.0) | 39 (26.0) | |
| 2000IU | 122 (79.7) | 31 (20.3) | |
| 4000IU | 117 (75.5) | 38 (24.5) | |
|
| |||
| Ethnicity,2 N (%): | 0.003 | ||
| African American | 98 (66.7) | 49 (33.3) | |
| Hispanic | 137 (81.1) | 32 (18.9) | |
| Caucasian | 115 (81.0) | 27 (19.0) | |
|
| |||
| Maternal Age (years), Mean ± SD | 27.0 ± 5.6 | 25.5 ± 5.1 | 0.01 |
| Range | 17–44 | 18–42 | |
|
| |||
| Gestational Age at Enrollment (weeks), | 0.053 | ||
| Mean ± SD | 12.5 ± 1.8 | 12.1 ± 2.1 | |
| Range3 | 6.3–21.4 | 6.1–17.7 | |
|
| |||
| Maternal Gravidity, Median (range) | 2.0 (0–9) | 3.0 (1–10) | <.0001 |
|
| |||
| Education, N (%): | 0.01 | ||
| < High School Education | 54 (73.0) | 20 (27.0) | |
| High School graduate | 63 (71.6) | 25 (28.4) | |
| College or more | 216 (84.4) | 40 (15.6) | |
|
| |||
| Employed at Entrance into Study, N (%) | 0.04 | ||
| Yes | 193 (81.1) | 45 (18.9) | |
| No | 157 (44.9) | 48 (51.6) | |
|
| |||
| Insurance, N (%): | 0.4 | ||
| Medicaid/None | 216 (75.3) | 71 (24.7) | |
| Commercial | 134 (78.4) | 37 (34.3) | |
|
| |||
| Subjective Health Rating Scale,4 Median (range) | 9.0 (1–10) | 9.0 (5–10) | 0.6 |
|
| |||
| Planned Pregnancy, N (%) | 0.01 | ||
| Yes | 179 (51.7) | 34 (37.4) | |
| No | 167 (74.6) | 57 (25.5) | |
|
| |||
| BMI,5 N (%): | 0.02 | ||
| ≤30 | 254 (73.8) | 90 (26.2) | |
| > 30 | 96 (84.2) | 18 (15.8) | |
|
| |||
| Season at study entry, N (%): | 0.6 | ||
| April – September | 170 (75.9) | 54 (24.1) | |
| October – March | 180 (77.9) | 51 (22.1) | |
|
| |||
| Baseline 25(OH)D, nmol/L, Mean ± S.D. (range) | |||
| Total | 59.5 ± 23.8 (6.0–172.5) | 50.5 ± 25.1 (6.5–120.5) | 0.001 |
| African American | 39.4 ± 18.6 (6.0–108.8) | 37.4 ± 17.6 (6.5–87.8) | 0.6 |
| Hispanic | 59.3 ± 20.0 (17.3–103.8) | 54.7 ± 20.6 (23.0–95.3) | 0.3 |
| Caucasian | 74.6 ± 20.2 (29.5–172.5) | 68.8 ± 28.8 (23.3–120.5) | 0.2 |
Exited included those patients who chose not to continue. It does not include those with pregnancy losses, who became medically ineligible or who moved from geographic area.
Race/Ethnicity as defined by mother
Gestational age at enrollment based on last menstrual period; change of gestational age occurred in 11 cases at time of 20 week fetal ultrasound.
Self-reported maternal health status rating from 1 (poor) to 10 (excellent)
BMI, Prepregnancy body mass index (BMI)
With regard to pregnancy losses, there were 8 women in the 400 IU group (baseline mean ± SD: 16.5 ± 7.6 weeks; median 15.5; range 10.0–34.0); 5 in the 2000 IU group (baseline mean ± SD: 17.2 ± 4.6; median 15.0; range 12.0–23.0); and 10 in the 4000 IU group (baseline mean ± SD: 16.4 ± 6.3; median 16.0; range 9–32) who experienced a loss after enrollment into the study. The 25(OH)D level around or at the time of the loss did not differ by treatment groups (p=0.8) There were no statistically significant differences in mean gestational age at loss among the treatment groups (p=0.9) or in the % losses per treatment group (p=0.4). When looking at baseline 25(OH)D of those women who delivered a liveborn infant vs. those who experienced a pregnancy loss, the mean levels were 57.8 ± 24.4 vs. 50.5 ± 23.3 nmol/L; however, this did not reach statistical significance.
Among the 350 women who continued in the study until delivery, the median ratio of the number of study capsules taken to the number that should have been taken between the time of randomization and delivery was similar between the groups. Adherence to protocol was not statistically different between treatment groups: 69% (400 IU group), 68% (2000 IU group), and 69% (4000 IU group)(p=0.9).
Study Outcomes
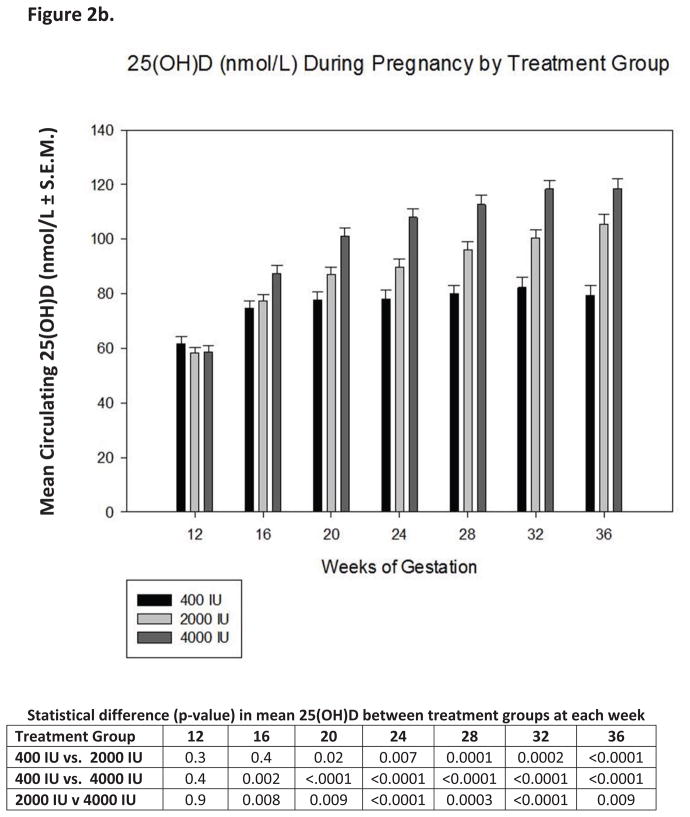
As shown in Table 3, the primary outcome—mean circulating 25(OH)D one month prior to delivery and at delivery was statistically different between treatment groups, with the highest mean level achieved in the 4000 IU group.. Overall, the mean 25(OH)D by dose group one month before delivery and at delivery, and as chronic levels measured the average from 20–36 weeks of gestation were significantly different between control and 2000, control and 4000, and 2000 vs. 4000 (p<0.0001).
Table 3.
Total Circulating 25(OH)D Concentration (nmoL) during Pregnancy by Treatment Group
| Measure | 400 IU Group | 2000 IU Group | 4000 IU Group | p-value |
|---|---|---|---|---|
| 25(OH)D at baseline, Mean ± SD | 61.6 ± 27.1 | 58.3 ± 22.3 | 58.2 ± 21.8 | 0.5 |
| (Range) | (6.0 – 172.5) | (14.0 – 115.3) | (11.8 – 109.3) | |
|
| ||||
| 25(OH)D 1 month before delivery | ||||
| Mean ± SD | 79.4 ± 34.3 | 105.4 ± 35.7 | 118.5 ± 34.9 | <0.0001 |
| (Range) | (16.0 – 193.0) | (17.3 – 176) | (26.3 – 243.5) | |
|
| ||||
| 25(OH)D at delivery, Mean ± SD | 78.9 ± 36.5 | 98.3 ± 34.2 | 111.0 ± 40.4 | <0.0001 |
| (Range) | (12.5 – 159.5) | 18.0 – 177.0 | 25.0 – 251.0 | |
|
| ||||
| 25(OH)D, 20–36 weeks,1 Mean ± SD | 79.1 ± 29.5 | 94.4 ± 26.1 | 110.8 ± 28.3 | <0.0001 |
| (Range) | (17.1 – 162.3) | (16.7 – 149.1) | (26.5 – 212.3) | |
|
| ||||
| Achieved 25(OH)D Level ≥80 nmoL at 1 month prior to delivery, N (%) | 51 (50.0) | 82 (73.9) | 91 (82.0) | <0.0001 |
|
| ||||
| Achieved 25(OH)D Level ≥80 nmoL at delivery, N (%) | 43 (50.0) | 63 (70.8) | 68 (82.0) | <0.0001 |
|
| ||||
| Achieved 25(OH)D Level ≥80 nmoL at 1 month prior to delivery or at delivery, N (%) | 57 (52.3) | 93 (79.5) | 94 (83.9) | <0.0001 |
|
| ||||
| Infant birth 25(OH)D, Mean ± SD | 18.2 ± 10.1 | 22.8 ± 9.8 | 26.5 ± 10.3 | <0.0001 |
| (Range) | (2.4 – 48.4) | (3.6 – 47.9) | (2.4 – 52.0) | |
The secondary outcome measure of attaining a total circulating 25(OH)D of at least 80 nmol/L at the time of delivery was met by 43/86(50%) women in the 400 IU group, 63/80 (70.8%) in the 2000 IU Group, and 68/83(82%) in the 4000 IU Group (see Table 3); however, there were 92 women with missing levels at delivery (p<0.0001). Because of the high correlation (r=0.72, p<0.0001) between one month prior to delivery and delivery 25(OH)D values, one month prior to delivery values were used as a surrogate for delivery values for the women with missing delivery room values. When combined, 57/109 (52.3%) women in the 400 IU Group, 93/117(79.5%) in the 2000 IU Group, and 94/112(83.9%)women in the 4000 IU Group achieved aminimal circulating 25(OH)D level of at least 80 nmol/L around the time of delivery (p<0.0001). Expressed as relative risk ratios, as shown in Table 4, there were significant differences between the 2000 vs. 400 IU groups (RR 1.52; 95% CI 1.24–1.86) and between the 4000 vs. 400 IU groups (RR 1.60; 95% CI 1.32–1.95); however, there was not a significant difference between the 2000 and 4000 IU groups in this regard.
Table 4.
Secondary Outcome: Achieving Total Circulating 25(OH)D ≥ 80 nmoL around Time of Delivery by Treatment Group
| 25(OH)D nmol/L | 2000 IU N (%) | 400 IU N (%) | Risk ratio (95% CI) | Risk difference (95% CI) |
|---|---|---|---|---|
| ≥80 nmol/L | 93 (79.5) | 57 (52.3) | 1.5200 (1.2426–1.8594) | 0.2719 (0.1530–0.3909) |
| 4000 IU N (%) | 400 IU N (%) | Risk ratio (95% CI) | Risk difference (95% CI) | |
| ≥80 nmol/L | 94 (83.9) | 57 (52.3) | 1.6049 (1.3183–1.9540) | 0.3163 (0.2005–0.4322) |
| ≥80 nmol/L | 4000 IU N (%) | 2000 IU N (%) | Risk ratio (95% CI) | Risk difference (95% CI) |
| 94 (83.9) | 93 (79.5) | 1.0559 (0.9340–1.1936) | 0.0444 (−0.0555–0.1443) |
Vitamin D sufficiency defined a priori as a total circulating 25(OH)D concentration of ≥80 nmoL (32 ng/mL). The following comparisons were made: 2000 IU group vs. the 400 IU group; 4000 IU group vs. 400 IU group; and lastly, the 4000 IU group vs. the 2000 IU group. Risk ratios and risk differences were reported for each comparison with 95% confidence intervals (CI).
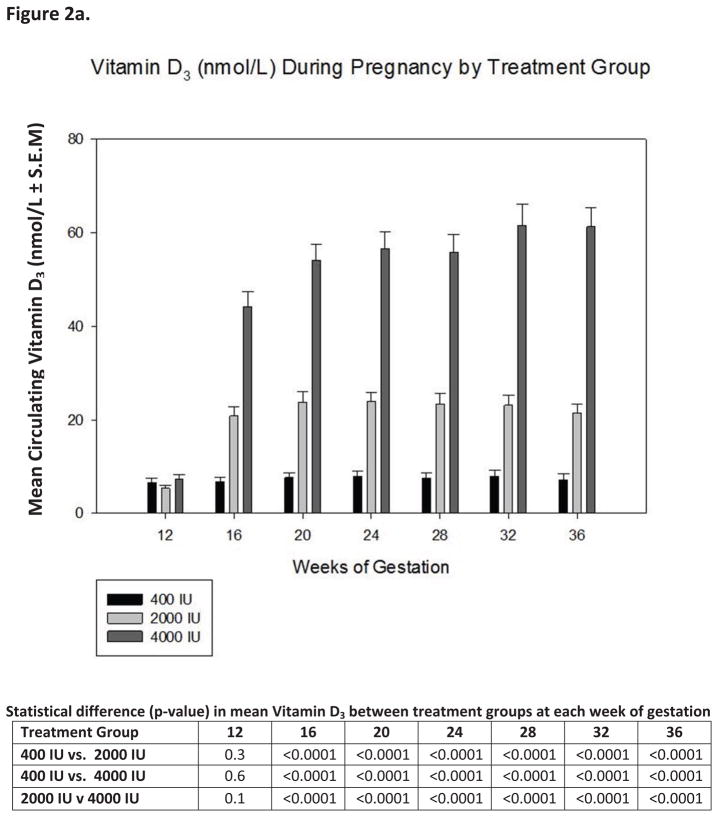
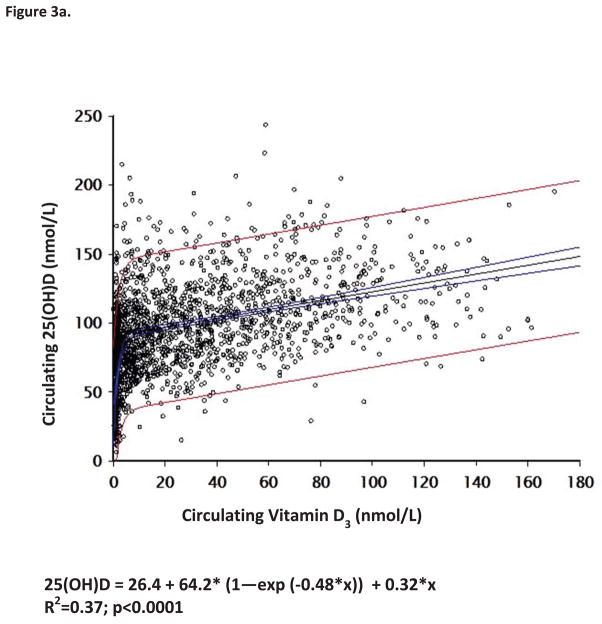
Vitamin D supplementation at various treatment doses given to our pregnant population had a variable effect on circulating levels of vitamin D3 and its metabolites (Figure 2a). Supplementation of vitamin D3 at double the prior 1997 IOM recommendation of 200 IU/d(40) and the current IOM EAR (62) provided essentially no increase in circulating vitamin D3 levels and only a minimal 5ng/mL rise in circulating 25(OH)D levels over the duration of the study (Figures 2a,2b). Conversely, supplementing 2000 or 4000 IU/d vitamin D3 had a profound effect on increasing both circulating levels of vitamin D3 and 25(OH)D (Figures 2a, 2b and Table 5a and b). Figure 3a describes the substrate-product relationship in all patients between vitamin D3 and 25(OH)D. The relationship is biphasic with respect to 25(OH)D production requiring at least 10 ng/mLl circulating vitamin D3 to saturate the vitamin D-25-hydroxylase.
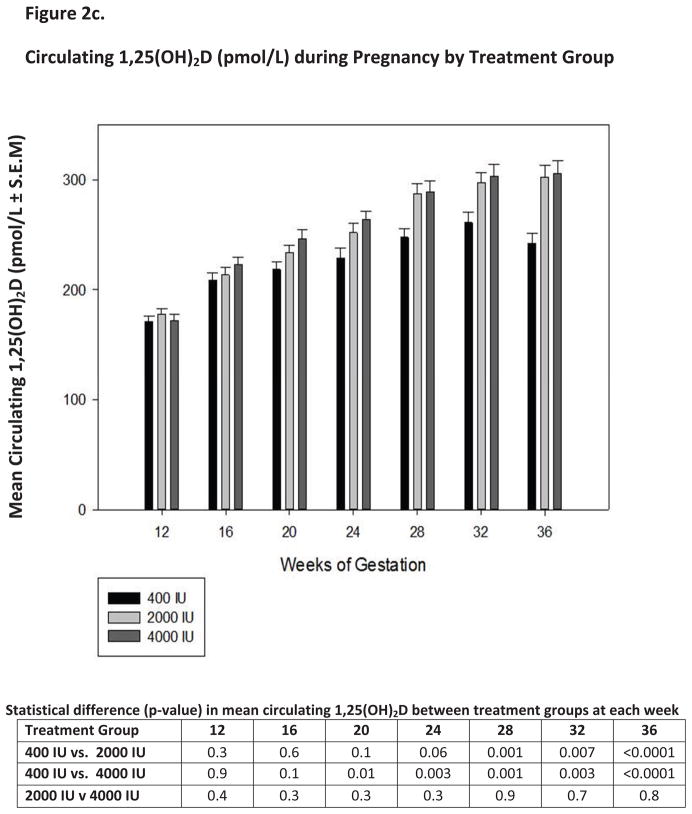
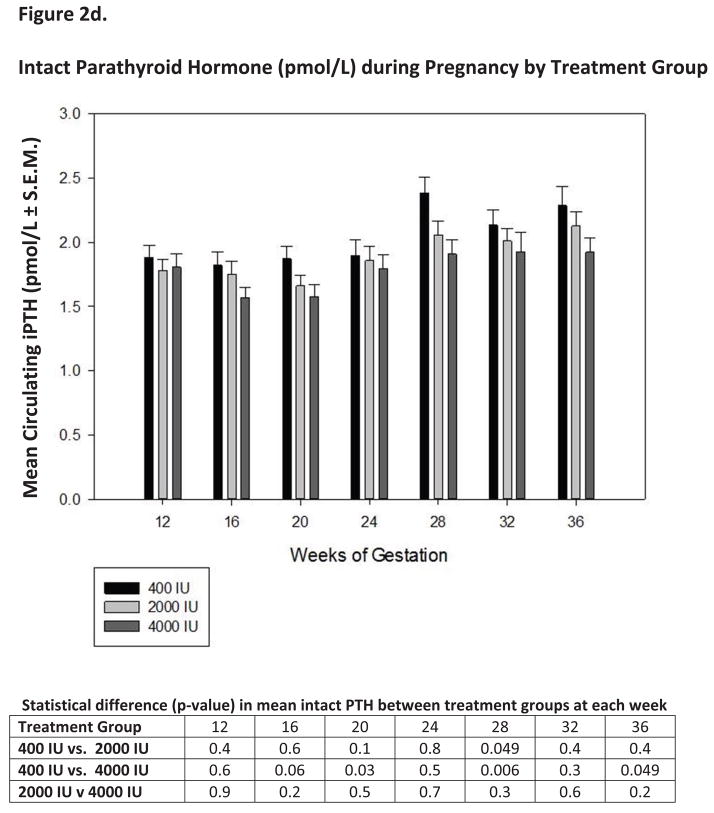
Figure 2. Circulating vitamin D3, its metabolites and intact parathyroid hormone as a function of vitamin D3 dose and time during pregnancy.
Panels A, B, C and D show the mean (± S.E.M.) circulating concentrations of vitamin D3, 25(OH)D, 1,25(OH)2D and intact parathyroid hormone at defined time points during pregnancy.
Table 5.
Pill Adherence, Mode of Delivery, and Neonatal Characteristics by Vitamin D Supplementation Group
| a. Circulating 25(OH)D (nmol/L) by Trimester Stratified by Treatment Group
| |||
|---|---|---|---|
| Treatment Group | Baseline 25(OH)D Mean ± SD | 2nd Trimester1 Mean ± SD | 1 month prior delivery Mean ± SD |
| 400 IU | 61.2 ± 27.1 | 76.1 ± 27.5 | 81.2 ± 35.9 |
| 2000IU | 57.5 ± 22.4 | 84.2 ± 23.0 | 102.6 ± 36.4 |
| 4000IU | 59.8 ± 25.4 | 98.6 ± 27.3 | 114.2 ± 35.5 |
| p-value | 0.5 | <0.0001 | <0.0001 |
| b. Circulating 25(OH)D (nmol/L) By Trimester Stratified by Treatment Group and Race/Ethnicity
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400 IU | 2000 IU | 4000 IU | ||||||||||
| Characteristic | Baseline 25(OH)D Mean ± SD | 2nd Trimester Mean ± SD | 1 month prior to delivery Mean ± SD | Δ baseline to 1 month prior2 (p-value) | Baseline 25(OH)D Mean ± SD | 2nd Trimester Mean ± SD | 1 month prior to delivery Mean ± SD | Δ baseline to 1 month prior (p-value) | Baseline 25(OH)D Mean ± SD | 2nd Trimester Mean ± SD | 1 month prior to delivery Mean ± SD | Δ baseline to 1 month prior (p-value) |
| African American | 37.3 ± 17.1 | 48.8 ± 21.1 | 49.4 ± 28.4 | 12.7(0.009) | 41.0 ± 19.1 | 72.2± 28.4 | 91.2 ± 45.1 | 49.4 (<0.0001) | 40.7 ± 20.1 | 81.0 ± 26.4 | 97.8 ± 42.4 | 57.4 (<0.0001) |
| Hispanic | 59.1 ± 21.6 | 76.9 ± 21.7 | 79.5 ± 30.3 | 20.3 (<0.0001) | 59.2 ± 18.9 | 85.2 ± 16.8 | 102.1 ± 28.7 | 42.1 (<0.0001) | 63.3 ± 27.6 | 101.4 ± 28.2 | 121.1 ± 30.9 | 60.1 (<0.0001) |
| Caucasian | 81.3 ± 23.8 | 95.2 ± 20.6 | 106.9 ± 26.4 | 25.0 (<0.0001) | 71.9 ± 19.0 | 94.9 ± 18.3 | 115.7 ± 31.8 | 44.4 (<0.0001) | 71.3 ± 17.3 | 109.8 ± 19.2 | 120.4 ± 29.7 | 50.4 (<0.0001) |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.02 | <0.0001 | <0.0001 | 0.008 | |||
| c. Intact PTH (pmol/L) by Trimester Stratified by Treatment Group
| |||
|---|---|---|---|
| Treatment Group | Baseline PTH Mean ± SD | 2nd Trimester3 Mean ± SD | 1 month prior delivery Mean ± SD |
| Control | 1.9 ± 1.0 | 1.9 ± 1.0 | 2.2 ± 1.3 |
| 2000IU | 1.8 ± 0.9 | 1.7 ± 0.9 | 2.1 ± 1.1 |
| 4000IU | 1.8 ± 1.1 | 1.6 ± 0.8 | 1.9 ± 1.1 |
| p-value | 0.5 | 0.1 | 0.1 |
| d. PTH (pmol/L) By Trimester Stratified by Treatment Group and Race/Ethnicity
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 400 IU | 2000IU | 4000IU | |||||||
| Characteristic | Baseline PTH Mean ± SD p-value | 2nd Trimester Mean ± SD p-value | 1 month prior Mean ± SD p-value | Baseline PTH Mean ± SD p-value | 2nd Trimester Mean ± SD p-value | 1 month prior Mean ± SD p-value | Baseline PTH Mean ± SD p-value | 2nd Trimester Mean ± SD p-value | 1 month prior Mean ± SD p-value |
| African American | 2.5 ± 1.2 | 2.6 ± 1.2 | 3.1 ± 1.8 | 2.1 ± 1.1 | 2.0 ± 0.9 | 2.3 ± 1.3 | 2.0 ± 0.9 | 1.9 ± 0.9 | 2.3 ± 1.1 |
| Hispanic | 1.8 ± 0.9 | 1.8 ± 0.7 | 2.0 ± 1.0 | 1.7 ± 0.9 | 1.8 ± 1.0 | 2.2 ± 1.1 | 1.8 ± 1.1 | 1.5 ± 0.7 | 1.7 ± 0.9 |
| Caucasian | 1.6 ± 0.9 | 1.4 ± 0.8 | 1.7 ± 1.0 | 1.6 ± 0.7 | 1.5 ± 0.7 | 1.9 ± 1.0 | 1.7 ± 1.2 | 1.6 ± 0.8 | 1.7 ± 1.3 |
| p-value | 0.001 | <0.0001 | 0.0002 | 0.01 | 0.055 | 0.3 | 0.6 | 0.06 | 0.06 |
Mode of delivery was categorized a priori as either a vaginal delivery (defined as spontaneous vaginal delivery or assisted vaginal delivery [which included use of forceps or vacuum extraction]) or cesarean section (further subdivided as cesarean following labor, cesarean without labor, and repeat elective cesarean) Primary cesarean section included women who had undergone a cesarean section with or without labor for either a maternal or fetal indication and did not include women who underwent a repeat, elective cesarean section.
Pill Adherence Measure was calculated as follows: the number of pills taken divided by the number of pills predicted to have been taken based on the number of days between visits. Of note, women who missed a study visit had additional vitamin D study pills delivered to them to ensure a continued supply of the study drug. If a woman missed two consecutive visits, she exited the study.
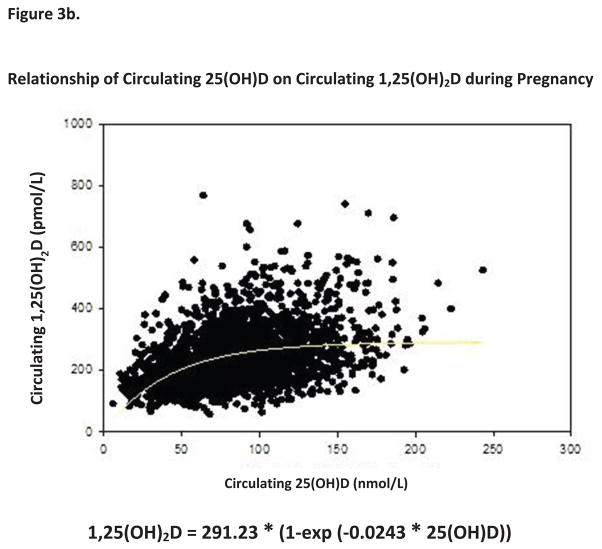
Figure 3. Substrate-Product Relationships of Vitamin D Metabolites during Pregnancy.
Panel A demonstrates the relationship between circulating vitamin D3 to control the production of 25(OH)D during pregnancy. Panel B demonstrates the relationship of circulating 25(OH)D to control the production of 1,25(OH)2D during pregnancy. All data points for all subjects in all groups were included in this analysis.
Table 5a and b provide additional information with respect to circulating 25(OH)D concentrations analyzed by race, vitamin D dose, and stage of gestation. Clearly, race and duration of supplementation have profound effects on the circulating level of 25(OH)D attained. African Americans lag at every time point and dose in relation to circulating 25(OH)D. This is especially noticeable in the 400 IU group. In contrast, a greater proportion of African American women achieved 25(OH)D ≥80 nmol/L by the second trimester in the 4000 IU group when compared to both the 400 and 2000 IU groups, (see Table 5b).
One of the most interesting biochemical findings in our study was the association between circulating 1,25(OH)2D levels and of circulating 25(OH)D (Figures 2c and 3b). In exploring the association between 25(OH)D and 1,25(OH)2D, 25(OH)D was found to have a direct influence on 1,25(OH)2D levels throughout pregnancy (p<0.0001). While the baseline 1,25(OH)2D level in all groups at 12 weeks’ gestation were not significantly different (Figure2c), within a few weeks, however, the circulating 1,25(OH)2D levels became significantly elevated in the 2,000 and 4,000 IU groups as opposed to the 400 IU group.
The relationship between these vitamin D metabolites is examined more closely in Figure 3b. This figure clearly demonstrates a biphasic relationship between circulating 25(OH)D and 1,25(OH)2D with circulating levels of 25(OH)D of at least 100 nmol/L (40 ng/ml) required to support maximum 1,25(OH)2D output in the pregnant women. It is also worthy to note that circulating 1,25(OH)2D levels at 12 weeks’ gestation are approximately triple that of normal, nonpregnant female and normal male subjects as previously reported (Fig. 2c) (56).
Figure 2d and Table 5c and d also display circulating intact PTH levels. The trend of PTH in all subjects was higher as the subjects progressed through pregnancy, but was not significantly different by treatment group. Decreases in circulating PTH was observed if the levels attained were analyzed by race. The African American group clearly had decreased circulating PTH as circulating 25(OH)D levels increased (Table 5c and d).
Circulating levels of VDBP were measured in 80 selected subjects based on their circulating 1,25(OH)2D levels, which ranged from 224.9 to 768.0 pmol/L at various stages of gestation. The average level of VDBP with this ELISA detected in these subjects was 5.45 ± 1.26 μmol/L, which represented a 39% increase as compared to normal subjects. Further, using linear regression, no relationship was observed between circulating VDBP and 1,25(OH)2D.
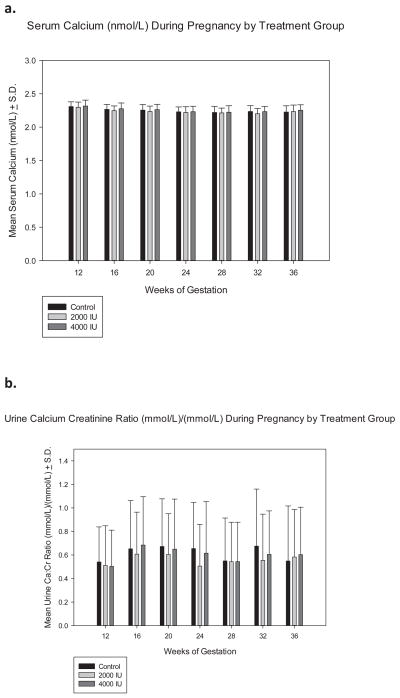
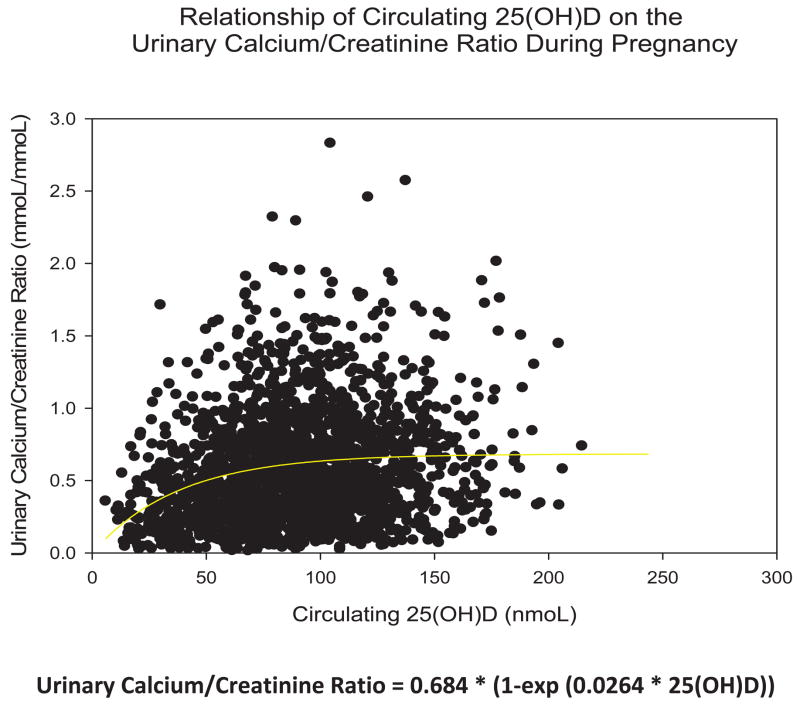
With respect to the effect of circulating 25(OH)D on either blood calcium or urinary calcium, no significant effects were observed with one exception: that being the relationship between low circulating 25(OH)D and urinary calcium levels (Figures 4a, 4b, 5). From Figure 5, it would appear that approximately 75 nmol/L (30 ng/mL) circulating 25(OH)D was required in the pregnant women to normalizeurinary calcium excretion. Above that threshold, 25(OH)D appeared not to influence urinary calcium and subsequent excretion.
Figure 4. Serum Calcium and Urinary Calcium/Creatinine Ratio as a Function of Vitamin D3 Dose and Time during Pregnancy.
Panels A and B show the mean (± S.D.) serum calcium and urinary calcium/creatinine ratio at defined time points during pregnancy.
Figure 5. Relationship of Circulating 25(OH)D on the Urinary Calcium: Creatinine Ratio during Pregnancy.
All data points are included for all study patients. Urinary calcium and urinary creatinine were measured in mmol/L. Ratio was calculated from measurement of urinary calcium (mmol/L) divided by measurement of urinary creatinine (mmol/L).
Throughout the study, there were no statistically significant differences between groups on any safety measure: serum calcium, creatinine, and phosphorus, and urinary calcium: creatinine ratios (p-value not significant [pNS] between groups). Review of adverse events by the DSMC showed that not a single adverse event in this trial was attributed to vitamin D supplementation or circulating 25(OH)D levels. There was one safety measure stop implementation: in the 4000 IU group, one woman with a baseline circulating 25(OH)D level of 29.3 nmol/L (13.3 ng/mL) increased to 233.3 nmol/L (93.3 ng/mL) at visit 2. Her follow-up circulating 25(OH)D level at the return visit prior to stopping supplementation had decreased to 66.6 ng/mL. Although her urinary calcium/creatinine ratio and all serum biochemical indices were within normal limits, the woman ceased supplementation per protocol.
Mode of delivery and neonatal characteristics by maternal treatment group are found in Table 6. There were no differences between the groups in terms of gestational age at delivery or birth weight. In addition, there were no significant differences in level of care (newborn nursery vs. higher level of care [level 2 or neonatal intensive care admission]) or increased adverse outcomes of pregnancy related to maternal vitamin D intake. There were several differences, however, in terms of neonatal vitamin D status by treatment group: Neonatal 25(OH)D was significantly correlated with maternal 25(OH)D overall, 1-month prior, and at delivery (r2=0.6; OR 0.50); and was significantly different by treatment group: 45.5±25.3 nmol/L (18.2±10.1 ng/mL, control), 57.0±24.5 nmol/L (22.8±9.8 ng/mL, 2000 IU) and 66.3±25.8 nmol/L (26.5±10.3 ng/mL, 4000 IU), (p<0.0001). By treatment group, using IOM guidelines for sufficiency (total circulating 25(OH)D ≥50 nmol/L or 20 ng/mL) (62), 31/78(39.7%)neonates in the 400 IU group, 53/91 (58.2%)in the 2000 IU group, and 66/84(78.6%)in the 4000 IU group had a cord blood/neonatal 25(OH)D level in the deficient range (p<0.0001).
Table 6.
Circulating 25(OH)D and PTH Changes during Pregnancy by Treatment Group and Race/Ethnicity
| Characteristic | 400 IU Group | 2000 IU Group | 4000 IU Group | p-value |
|---|---|---|---|---|
| N=111 | N=122 | N=117 | ||
| Maternal age at delivery (years), Mean ± SD | 27.4 ± 5.7 | 28.0 ± 5.7 | 27.1 ± 5.5 | 0.49 |
|
| ||||
| Mode of Delivery1: N (%) | ||||
| Uncomplicated vaginal | 69 (62.2%) | 81 (66.4%) | 81 (69.8%) | |
| Assisted vaginal | 2 (1.8%) | 4 (3.3%) | 9 (7.8%) | |
| C/S after Labor | 23 (20.7%) | 19 (15.6%) | 19 (16.4%) | |
| C/S without Labor | 17 (15.3%) | 18 (14.8%) | 7 (6.0%) | |
| Vaginal, Any type | 71 (74.7%) | 85 (79.4%) | 90 (85.7%) | 0.15 |
| Primary Cesarean Section | 24 (25.3%) | 22 (20.6%) | 15 (14.3%) | |
|
| ||||
| Gestational Age (weeks) at Delivery, Mean ± SD | 38.6 ± 2.2 | 38.8 ± 1.8 | 39.1 ± 1.8 | 0.17 |
|
| ||||
| Birth Weight (grams) at Delivery, Mean ± SD | 3221.8 ± 674.9 | 3360.1 ± 585.0 | 3284.6 ± 597.6 | 0.23 |
|
| ||||
| Admission to Level II or III, N (%) | 12 (10.8%) | 14 (11.5%) | 11 (9.4%) | 0.9 |
2nd trimester mean value was the average 25(OH)D value obtained at visits between 16 and 24 weeks of gestation.
Δ baseline to 1 month prior connotes the change from the baseline 25(OH)D level to the level achieved at one month prior to delivery.
2nd trimester mean value was the average PTH value obtained at visits between 16 and 24 weeks of gestation.
Discussion
In this randomized controlled trial of a diverse group of pregnant women living at latitude 32°N supplemented from 12–16 weeks of gestation until delivery, compared to the 400 (control) and 2000 IU groups, a daily vitamin D dose of 4000 IU was associated with improved vitamin D status at throughout pregnancy, one month prior, and at delivery in both mother and neonate. Irrespective of race and ethnicity, this improvement in vitamin D status was achieved without any evidence of hypervitaminosis D or an increase in adverse events during pregnancy and with optimization of 25(OH)D and 1,25(OH)2D. From a standpoint of enzyme kinetics, this simply means that in the case of vitamin D being converted to 25(OH)D and subsequently to 1,25(OH)2D, enzyme saturation is occurring, i.e., reaction rates are moving from first order to zero order enzyme kinetics. In simple terms, it means that an appropriate amount of substrate is being supplied to produce maximum product, i.e., 25(OH)D and 1,25(OH)2D: as such, no substrate “starvation” is occurring.
At no point in human nutrition is it more critical to ensure adequate nutrient intake than during the state of pregnancy. Folate intake during pregnancy and its role in the development of neural tube defect serves as a stark example (63,64). The limited clinical investigation into meaningful dietary vitamin D supplementation during pregnancy can be traced back to post-World War II Britain. Because of the British experience with idiopathic infantile hypercalcemia attributed to hypervitaminosis D, an inaccurate association occurred that had a profound effect on the potential of vitamin D supplementation, not only during infancy but also during pregnancy. In 1963, Black and Bonham-Carter (65) recognized that elfin facies observed in patients with severe idiopathic infantile hypercalcemia resembled the peculiar facies observed in patients with supravalvular aortic stenosis syndrome. By 1966 vitamin D was viewed by the medical community as the cause of SAS syndrome. (66,67) With the advent of molecular genetics, the children with SAS Syndrome were discovered to have Williams Syndrome, an example of unipaternal disomy, with abnormal vitamin D metabolism. (68–75)
The perception that vitamin D can inflict harm during pregnancy still lives on today as many obstetrical specialists are afraid to undertake vitamin D repletion during this period. Research efforts in this area were further hampered when in 1997, the Institute of Medicine issued guidelines that defined the adequate intake (AI) for vitamin D during pregnancy to be 200 IU/d with intakes greater than 2000 IU/d causing potential harm. (40) Recently, the IOM issued new guidelines with respect to pregnant women that define the estimated average requirement (EAR) and recommended dietary allowance (RDA) to be 400 and 600 IU/day, respectively. They also increased the tolerable upper intake limit (UL) to 4000 IU/day. (62)These new guidelines, with the exception of the UL, are based on old data since limited new data exist. The result of prior and current guidelines is that most prenatal vitamins only contain 400 IU of vitamin D. In our experience, many of today’s practicing obstetricians are unaware of the vitamin D content in prenatal vitamins or have a fear of administering additional vitamin D supplements to the pregnant women.
Our study was based on two previous vitamin D supplementation studies in non-pregnant adults that appeared to be safe. (48,58) Prior to undertaking the NIH-funded study described here, however, we had to obtain an Investigational Drug Number from the FDA which entailed writing a complete investigational drug application. This was required by the FDA since we proposed using a vitamin D3 dose of 4,000 IU/d, 20 times the adequate intake (AI), and twice the safe limit put forth by the IOM in 1997, (40) but currently put forth as the UL. (62) Thus, our study is the first one to test this current UL in pregnant women.
The only known avenue of vitamin D toxicity is manifested through hypercalcemia and hypercalciuria, (76) neither of which was observed in our RCT. In fact, our Data and Safety Monitoring Committee concluded that not a single adverse event in this RCT could be attributed to vitamin D intake. Hypervitaminosis D is largely arbitrarily defined as circulating levels of 25(OH)D that exceed 375 nmol/L (150 ng/mL), a level we never attained with our dosing regimen. As has been observed in other human supplementation studies, the conversion of vitamin D to 25(OH)D appears to be controlled. (77) Further, it has been known for decades that during pregnancy 1,25(OH)2D levels become extremely elevated. (78,79) This increase in circulating 1,25(OH)2D levels has particularly been attributed to an increase in the serum vitamin D-binding protein that would regulate the amount of “free” 1,25(OH)2D available in the circulation. (79) While this rise in VDBP during pregnancy has been shown to be 46–103%, depending on the assay employed, (80) it cannot account, however, for a nearly 3–4-fold increase in circulating 1,25(OH)2D observed in our study. Bikle, et al., (81) clearly demonstrated that free 1,25(OH)2D levels are increased during pregnancy despite the significant increase in VDBP levels. We were unable to measure “free” circulating levels of 1,25(OH)2D in our subjects, however, our data agree with that of Bikle, et al., in that no relationship was observed during pregnancy between circulating VDBP and “total” circulating 1,25(OH)2D(81). New data from our study suggests that a circulating 25(OH)D level of approximately 100 nmol/L (40 ng/mL) is required to optimize production of 1,25(OH)2D during human pregnancy through renal and/or placental production of the hormone (Figure 2c, 3b). It is also of great interest that production of circulating 1,25(OH)2D in the fetus is directly linked to circulating 25(OH)D (10).
Clearly, vitamin D metabolism is greatly altered during pregnancy, and that pregnancy itself is the primary driver for these extraordinary circulating 1,25(OH)2D levels. From our data, it is evident that production of 1,25(OH)2D is really not under the control of the classic regulators of calcium, phosphorus and PTH. The dramatic rise in maternal circulating 1,25(OH)2D following conception is remarkable for many reasons: by 12 weeks of gestation, maternal circulating 1,25(OH)2D levels are already triple that of the nonpregnant female (Figure 2c). From that point in gestation, the 1,25(OH)2D levels rise much higher and are driven by substrate—25(OH)D availability (Figure 3b). This substrate dependence of 1,25(OH)2D production is never observed in normal human physiology driven by classical calcium homeostasis (10,82,83).
Another remarkable factor in pregnant women is how they can attain supra-physiologic levels of 1,25(OH)2D, sometimes exceeding 700 pmol/L in our study, and never exhibit hypercalciuria or hypercalcemia. These tremendous circulating levels of 1,25(OH)2D during pregnancy are possibly of placental origin or from the renal 1-α-hydroxylase that would have to be uncoupled from feedback control and for reasons other than maintaining calcium homeostasis. The second scenario is most likely because women with nonfunctional renal 1-α-hydroxylase and normal placental function fail to increase circulating 1,25(OH)2D during pregnancy (84). The increased levels of 1,25(OH)2D may be due to the methylation of the catabolic CYP24A1 placental gene (85). It is possible that calcitonin may be a contributor to this process in that calcitonin rises during pregnancy (86), is known to stimulate the renal 1-α-hydroxylase gene independent of calcium levels (87,88), and also protects by opposing hypercalcemia (89). Another possible stimulator of the 1-α-hydroxylase during pregnancy is prolactin (90). If prolactin was a major contributor, however, the effect should continue into lactation, which we do not see; and would be accompanied by elevated circulating 1,25(OH)2D, which also is not seen (91). Further, the physiologic function of this altered vitamin D metabolism may be related to increased reliance on innate immune function during pregnancy as well as decreased adaptive immune responses (7,8,10,92), protecting the newborn from respiratory infection and subsequent wheezing (93,94), and possibly epigenetic alterations in invariant NKT cells, which can lead to increased autoimmune disease prevalence (95,96). As supported by this and prior studies, it is important to remember that for cord blood to attain 25(OH)D of 50 nmol/L, the maternal 25(OH)D level would need to be at least 80 nmol/L (97).
Our data also suggest that a circulating level of approximately 75 nmol/L (30 ng/mL) 25(OH)D is required to normalize calcium excretion into the urine. Interestingly, this value is virtually identical to the value obtained by Heaney et al. with respect to the equilibration of intestinal calcium absorption (98). This increased level of circulating 25(OH)D in the pregnant woman also appears to reduce circulating parathyroid hormone, especially in African American subjects. It is also important to compare our study results with respect to two recent reports dealing with vitamin D supplementation during pregnancy (62,99). The IOM report recommends a vitamin D intake of 400–600 IU/day and states that this level can be obtained solely from the diet. Further, this intake level would be sufficient to meet their circulating 25(OH)D target of 20 ng/mL (50 nmol/L) (62). Even using this conservative 25(OH)D level, their recommendation would have left >50% of our total cohort and >80% of African American women in the cohort deficient at study entry. The Endocrine Society’s recommendation of a daily vitamin D intake of 1,500–2,000 IU and target 25(OH)D level of >30 ng/mL (75nmol/L) (99) is more sound advice yet is still conservative when compared to our study results. It must be pointed out that the purpose of the IOM report was to guide food manufacturers and fortifiers and is not intended to guide clinical practice (62). On the other hand, clinical practice guidance is precisely the purpose of The Endocrine Society’s recommendations (99).
This study has certain limitations: this study was conducted at a southernly latitude, and therefore, the vitamin D requirements of women living at more northern latitudes could be greater. While women with preexisting hypertension and diabetes were excluded from the study, these women may be at greater risk of vitamin D deficiency, and therefore, may receive the greatest benefit from vitamin D supplementation of 4000 IU/day. Because of safety concerns, women were not allowed to remain in the study if their total circulating 25(OH)D level rose above 225 nmol/L. There were three women who attained this threshold, none of whom had any associated hypercalciuria or hypercalcemia. Lastly, due to safety concerns that surrounded the use of 4000 IU vitamin D supplementation during pregnancy, the study was designed to begin supplementation starting at the 12th week of gestation, beyond the period of early organogenesis. Hence, we cannot ensure the safety before the 12th week of gestation. With regard to vitamin D intake during pregnancy, it is interesting that our study largely confirms the observations of Obermer in England more than 60 years ago (100). Obermer’s suggestions largely were ignored because of greatly flawed associations between vitamin D and Supravalvular Aortic Stenosis Syndrome (65,66,101). The data in our paper put us back on the path suggested by Obermer with respect to vitamin D intake during pregnancy. Additional studies will be necessary to ascertain safety of 4000 IU/dayvitamin D supplementation before the 12th week of gestation.
Conclusions
In summary, starting at 12–16 weeks of gestation, vitamin D supplementation with 4,000 IU/day was most effective in achieving vitamin D sufficiency throughout pregnancy, one month prior to delivery and at delivery in a diverse group of women and their neonates without increased risk of toxicity. These findings suggest that the current vitamin D EAR and RDA for pregnancy women issued in 2010 by the Institute of Medicine (62) should be raised to 4,000 IU vitamin D per day so that all women regardless of race attain optimal nutritional and hormonal vitamin D status throughout pregnancy.
Acknowledgments
Funded by the National Institute of Children’s Health and Human Development #R01 HD47511, NIH #RR01070 and by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCRR Grant number UL1 RR029882.
FDA IND Number: 66,346; ClinicalTrials.gov Number: NCT00292591
The authors would like to acknowledge the hundreds of women who participated in this study, without whose participation this study would not have been possible; our dedicated study coordinator team: Judy Shary, M.S., Pamela G. Smith, R.N., Martha Murphy, B.S., Betty Bivens, R.A., and Deanna Fanning, R.N., who truly made the study possible; our Data Safety and Monitoring Committee, without whose due diligence this study could not have taken place; and lastly, our data management team Melanie Floyd and Frilatha Bandlamuri, B.S., M.S. for their meticulous care of the data.
Footnotes
Presented in part at the 14th Vitamin D Workshop, Brugge, Belgium, October 2009, and the Pediatric Academic Societies meeting, Vancouver, Canada, May 2010.
Disclosures
Bruce W. Hollis, Ph.D. serves as a consultant for Diasorin Inc., Stillwater, MN.
All other authors (DDJ, TCH, ME, and CLW) state that they have no conflicts of interests.
To convert mg/dL of calcium to mmol/L, multiply the value by 0.25. To convert mg/dL of creatinine to mmol/L, multiply by 0.088.
Data of adherent subjects will be made available to any investigator upon request following publication.
Adherence efficacy and outcome data with detailed pharmacokinetics will be presented in a separate paper.
Authors’ roles
Study design: all authors contributed to study design (BWH, DDJ, TCH, ME, CLW).
Study conduct: all authors contributed to study conduct (BWH, DDJ, TCH, ME, CLW).
Data collection: all authors contributed to data collection (BWH, DDJ, TCH, ME, CLW).
Data analysis: BHW, TCH, ME, CLW
Data interpretation: all authors contributed to data interpretation (BWH, DDJ, TCH, ME, CLW).
Drafting manuscript: BWH, TCH, CLW
Revising manuscript content: all authors contributed to revising the manuscript content (BWH, DDJ, TCH, ME, CLW).
Approving final version of manuscript: All authors approved the final version of the manuscript (BWH, DDJ, TCH, ME, CLW).
TCH and ME take responsibility for the integrity of the data analysis.
Literature Cited
- 1.Hatun S, Ozkan B, Orbak Z, Doneray H, Cizmecioglu F, Toprak D, Calikoglu AS. Vitamin D Deficiency in Early Infancy. J Nutr. 2005;135(2):279–282. doi: 10.1093/jn/135.2.279. [DOI] [PubMed] [Google Scholar]
- 2.Ladhani S, Srinivasan L, Buchanan C, Allgrove J. Presentation of vitamin D deficiency. Arch Dis Child. 2004;89:781–784. doi: 10.1136/adc.2003.031385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner CL, Greer FR. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–1152. doi: 10.1542/peds.2008-1862. [DOI] [PubMed] [Google Scholar]
- 4.Yorifuji J, Yorifuji T, Tachibana K, Nagai S, Kawai M, Momoi T, Nagasaka H, Hatayama H, Nakahata T. Craniotabes in normal newborns: the earliest sign of subclinical vitamin D deficiency. J Clin Endocrinol Metab. 2008;93(5):1784–1788. doi: 10.1210/jc.2007-2254. [DOI] [PubMed] [Google Scholar]
- 5.Anatoliotaki M, Tsilimigaki A, Tsekoura T, Schinaki A, Stefanaki S, Nicolaidou P. Congenital rickets due to maternal vitamin D deficiency in a sunny island of Greece. Acta Paediatr. 2003;92 (3):389–391. doi: 10.1080/08035250310009347. [DOI] [PubMed] [Google Scholar]
- 6.Hewison M. Vitamin D and the Immune System: New Perspectives on an Old Theme. Endocrinology & Metabolism Clinics of North America. 2010;39(2):365–379. doi: 10.1016/j.ecl.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zügel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1β and VDR Activation Pathways in Human TLR2/1-Induced Antimicrobial Responses. PLoS ONE. 2009;4(6):e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 9.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker VP, Zhang X, Rastegar I, Liu PT, Hollis BW, Adams JS, Modlin RL. Cord Blood Vitamin D Status Impacts Innate Immune Responses. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2010-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svoren BM, Volkening LK, Wood JR, Laffel LM. Significant vitamin D deficiency in youth with type 1 diabetes mellitus. J Pediatr. 2009;154(1):132–134. doi: 10.1016/j.jpeds.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boucher BJ, Mannan N, Noonan K, Hales CN, Evans SJ. Glucose intolerance and impairment of insulin secretion in relation to vitamin D deficiency in east London Asians. Diabetologia. 1995;38 (10):1239–1245. doi: 10.1007/BF00422375. [DOI] [PubMed] [Google Scholar]
- 13.Maghbooli Z, Hossein-Nezhad A, Karimi F, Shafaei AR, Larijani B. Correlation between vitamin D(3) deficiency and insulin resistance in pregnancy. Diabetes Metab Res Rev. 2007 Jul 2; doi: 10.1002/dmrr.737. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Fuleihan E, Nabulsi M, Tamim H, Maalouf J, Salamoun M, Khalife H, Choucair M, Arabi A, Vieth R. Effect of vitamin D replacement on musculoskeletal parameters in school children: A randomized controlled trial. J Clin Endocrinal Metab. 2006;91:405–412. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 15.Adams JS. Vitamin D as a defensin. J Musculoskelet Neuronal Interact. 2006;6(4):344–346. [PubMed] [Google Scholar]
- 16.Plotnikoff G, Quigley J. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–1470. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 17.Boyan BD, Sylvia VL, Dean DD, Schwartz Z. Membrane mediated signaling mechanisms are used differentially by metabolites of vitamin D(3) in musculoskeletal cells. Steroids. 2002;67(6):421–427. doi: 10.1016/s0039-128x(01)00178-7. [DOI] [PubMed] [Google Scholar]
- 18.Zittermann A, Schleithoff SS, Koerfer R. Vitamin D insufficiency in congestive heart failure: why and what to do about it? Heart failure reviews. 2006;11(1):25–33. doi: 10.1007/s10741-006-9190-8. [DOI] [PubMed] [Google Scholar]
- 19.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83(4):754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 20.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D Deficiency and Risk of Cardiovascular Disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui X, McGrath JJ, Burne THJ, Mackay-Sim A, Eyles DW. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. International Journal of Developmental Neuroscience. 2007;25 (4):227–232. doi: 10.1016/j.ijdevneu.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Brown J, Bianco J, McGrath J, Eyles D. 1,25-Dihydroxyvitamin D-3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343:139–143. doi: 10.1016/s0304-3940(03)00303-3. [DOI] [PubMed] [Google Scholar]
- 23.Eyles D, Brown J, MacKay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118(3):641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 24.Eyles D, Smith S, Kinobeb R, Hewison M, McGrath J. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. Journal of Chemical Neuroanatomy. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Halhali A, Tovar AR, Torres N, Bourges H, Garabedian M, Larrea F. Preeclampsia is associated with low circulating levels of insulin-like growth factor 1 and 1,25-dihydroxyvitamin D in maternal and umbilical cord compartments. J Clin Endocrinol. 2000;85(5):1828–2833. doi: 10.1210/jcem.85.5.6528. [DOI] [PubMed] [Google Scholar]
- 26.Robinson CJ, Wagner CL, Hollis BW, Baatz JE, Johnson DD. Maternal vitamin D and fetal growth in early-onset severe preeclampsia. Am J Obstet Gynecol. 2011 doi: 10.1016/j.ajog.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal Vitamin D Deficiency Increases the Risk of Preeclampsia. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940–945. doi: 10.1210/jc.2008-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahomed K, Gulmezoglu AM. Vitamin D supplementation in pregnancy (Cochrane Review) Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; Chicester, UK: 1999. [DOI] [PubMed] [Google Scholar]
- 30.Mallet E, Gugi B, Brunelle P, Henocq A, Basuyau J, Lemeur H. Vitamin D supplementation in pregnancy: a controlled trial of two methods. Obstet Gynecol. 1986;68:300 –304. doi: 10.1097/00006250-198609000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Brooke OG, Brown IRF, Bone CDM, Carter ND, Cleeve HJW, Maxwell JD, Robinson VP, Winder SM. Vitamin D supplements in pregnant Asian women: Effects on calcium status and fetal growth. Br Med J. 1980;1:751–754. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell J, Ang L, Brooke O, Brown I. Vitamin D supplements enhance weight gain and nutritional status in pregnant Asians. Br J Obstet Gynaecol. 1981;88:987–991. doi: 10.1111/j.1471-0528.1981.tb01686.x. [DOI] [PubMed] [Google Scholar]
- 33.Ala-Houhala M, Koskinen T, Terho A, Koivula T, Visakorpi J. Maternal compared with infant vitamin D supplementation. Arch Dis Child. 1986;61:1159–1163. doi: 10.1136/adc.61.12.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marya R, Rathee S, Lata V, Mudgil S. Effects of vitamin D supplementation in pregnancy. Gynecol Obstet Invest. 1981;12:155–161. doi: 10.1159/000299597. [DOI] [PubMed] [Google Scholar]
- 35.Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: effect on neonatal calcium homeostasis. J Pediatr. 1986;109(2):328–334. doi: 10.1016/s0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- 36.Brooke OG, Butters F, Wood C. Intrauterine vitamin D nutrition and postnatal growth in Asian infants. Brit Med J. 1981;283:1024. doi: 10.1136/bmj.283.6298.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datta S, Alfaham M, Davies D, Dunstan F, Woodhead S, Evans J, Richards B. Vitamin D deficiency in pregnant women from a non-European ethnic minority population: an interventional study. Br J Obstetr Gynaecol. 2002;109:905–908. doi: 10.1111/j.1471-0528.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- 38.Arora P, Arora RS. Vitamin D supplementation for non-Western pregnant women: the British experience. Am J Clin Nutr. 2007;85(4):1164–1165. doi: 10.1093/ajcn/85.4.1164. [DOI] [PubMed] [Google Scholar]
- 39.Yu C, Sykes L, Sethi M, Teoh T, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clinical Endocrinology. 2009;70(5):685–690. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 40.Food and Nutrition Board. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academy Press; Washington, D.C: 1997. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 41.Hollis B. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 42.Block G, DiSogra C. Validation, self-administered, in low-income white, African-American and Hispanic women. Food and Nutrition Service, U.S. Department of Agriculture; Alexandria, VA: 2001. [Google Scholar]
- 43.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc. 1992;92:686–693. [PubMed] [Google Scholar]
- 44.Mares-Perlman JA, Klein BEK, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle-aged and older men and women similarly to multiple food records. J Nutr. 1993;123:489–501. doi: 10.1093/jn/123.3.489. [DOI] [PubMed] [Google Scholar]
- 45.Coates RJ, Eley JW, Block G, Gunter EW, Sowell AL, Grossman C. An evaluation of a food frequency questionnaire for assessing dietary intake of specific carotenoids and vitamin E among low-income black women. Am J Epidemiol. 1991;134:658–671. doi: 10.1093/oxfordjournals.aje.a116138. [DOI] [PubMed] [Google Scholar]
- 46.Sinha R, Patterson BH, Mangels AR, Levander OA, Gibson T, Taylor PR, Block G. Determinants of plasma vitamin E in health males. Cancer Epidemiology Biomarkers and Prevention. 1993;2:473–479. [PubMed] [Google Scholar]
- 47.Sobell J, Block G, Koslowe P, Tobin J, Andres R. Validation of a retrospective questionnaire assessing diet 10–15 years ago. Am J Epidemiol. 1989;130:173–187. doi: 10.1093/oxfordjournals.aje.a115310. [DOI] [PubMed] [Google Scholar]
- 48.Heaney R, Davies K, Chen T, Holick M, Barger-Lux M. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 49.Appelgren KE, Nietert PJ, Hulsey TC, Hollis BW, Wagner CL. Analyzing adherence to prenatal supplement: does pill count measure up? Int J Endocrinol. 2010;2010:631971. doi: 10.1155/2010/631971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hollis BW. Detection of vitamin D and its major metabolites. In: Feldman D, Glorieux F, Pike J, editors. Vitamin D. Academic Press; New York, N.Y: 2005. pp. 932–950. [Google Scholar]
- 51.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD. Determination of vitamin D status by radioimmunoassay with a 125I-labeled tracer. Clin Chem. 1993;39:529–533. [PubMed] [Google Scholar]
- 52.Laboratories MC. Laboratory Reference Data. Mayo Clinic; Rochester, MN: 2003. [Google Scholar]
- 53.Hollis BW, Wagner CL, Kratz A, Sluss PM, Lewandrowski KB. Normal serum vitamin D levels. Correspondence. N Engl J Med. 2005;352:515–516. doi: 10.1056/NEJM200502033520521. [DOI] [PubMed] [Google Scholar]
- 54.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79:717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 55.Hollis BW, Wagner CL. Vitamin D requirements during lactation: High-dose maternal supplementation as therapy to prevent hypovitaminosis D in both mother and nursing infant. Amer J Clin Nutr. 2004;80S:1752S–1758S. doi: 10.1093/ajcn/80.6.1752S. [DOI] [PubMed] [Google Scholar]
- 56.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with a 125I-labeled tracer. Clin Chem. 1996;42:586–592. [PubMed] [Google Scholar]
- 57.Vieth R, Ladak Y, Walfish P. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinal Metab. 2003;88:185–191. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 58.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73(2):288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 59.Pitkin RM. Endocrine regulation of calcium homeostasis during pregnancy. Clin Perinatol. 1983;10 (3):575–592. [PubMed] [Google Scholar]
- 60.System S. SAS Software. 9.22. SAS Institute, Inc; Cary, NC: 2010. [Google Scholar]
- 61.Gillings D, Koch G. The application of the principle of intention-to-treat in the analysis of clinical trials. Drug Inf J. 1991;25:411–424. [Google Scholar]
- 62.Food and Nutrition Board. Dietary Reference Intakes for Vitamin D and Calcium. National Academy Press; Washington, D.C: 2010. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 63.Pitkin RM. Nutritional influences during pregnancy. Med Clin North Am. 1977;61(1):3–15. doi: 10.1016/s0025-7125(16)31346-3. [DOI] [PubMed] [Google Scholar]
- 64.Georgieff MK. Nutrition and the developing brain: nutrient priorities and measurement. Am J Clin Nutr. 2007;85(2):614S–620. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- 65.Black J, Bonham-Carter J. Association between aortic stenosis and facies of severe infantile hypercalcemia. Lancet. 1963;2:745–749. doi: 10.1016/s0140-6736(63)90553-1. [DOI] [PubMed] [Google Scholar]
- 66.Friedman WF, Mills LS. The production of “elfin facies” and abnormal dentition by vitamin D during pregnancy: relationship to the supravalvular aortic stenosis syndrome. Proc Soc Pediatr Res. 1967;37:80. [Google Scholar]
- 67.Taussig HB. Possible injury to the cardiovascular system from vitamin D. Ann Intern Med. 1966;65:1195–1200. doi: 10.7326/0003-4819-65-6-1195. [DOI] [PubMed] [Google Scholar]
- 68.Morris CA, Mervis CB. William’s syndrome and related disorders. Ann Dev Genomics Human Genet. 2000;1:461–484. doi: 10.1146/annurev.genom.1.1.461. [DOI] [PubMed] [Google Scholar]
- 69.Aravena T, Castillo S, Carrasco X, Mena I, Lopez J, Rojas JP, Rosembert C, Schroter C, Aboitiz F. Williams syndrome: clinical, cytogenetical, neurophysiological and neuroanatomic study. Rev Med Child. 2002;130:631–637. [PubMed] [Google Scholar]
- 70.Taylor A, Stern P, Bell N. Abnormal regulation of circulating 25-hydroxyvitamin D on the Williams Syndrome. N Engl J Med. 1982;306:972–975. doi: 10.1056/NEJM198204223061607. [DOI] [PubMed] [Google Scholar]
- 71.Garabedian M, Jacqz E, Guillozo H, Grimberg R, Guillot M, Gagnadoux MF, Broyer M, Lenorr G, Balsan S. Elevated plasma 1,25-dihydroxyvitamin D concentrations in infants with hypercalcemia and an elfin facies. N Engl J Med. 1985;312:948–952. doi: 10.1056/NEJM198504113121503. [DOI] [PubMed] [Google Scholar]
- 72.Knudtzon J, Aksnes L, Akslen LA, Aarskog D. Elevated 1,25-dihydroxyvitamin D and normocalcaemia in presumed familial Williams Syndrome. Clin Genet. 1987;32:369–374. doi: 10.1111/j.1399-0004.1987.tb03151.x. [DOI] [PubMed] [Google Scholar]
- 73.Wozikowska J, Wojtanowska H, Kozuszko K, Slowik J, Blazewska K, Balo G. A case of idopathic hypercalcemia, hypersensitivity to vitamin D3. Wiad Lek. 1992;45:229–232. [PubMed] [Google Scholar]
- 74.McTaggart SJ, Craig J, MacMillan J, Burke JR. Familial occurrence of idiopathic intantile hypercalcemia. Pediatr Nephrol. 1999;13:668–671. doi: 10.1007/s004670050678. [DOI] [PubMed] [Google Scholar]
- 75.Mathias RS. Rickets in an infant with Williams Syndrome. Pediatr Nephrol. 2000;14:489–492. doi: 10.1007/s004670050800. [DOI] [PubMed] [Google Scholar]
- 76.Vieth R. The mechanisms of vitaminD toxicity. Bone Miner. 1990;11(3):267–272. doi: 10.1016/0169-6009(90)90023-9. [DOI] [PubMed] [Google Scholar]
- 77.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87(6):1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 78.Kumar R, Cohen WR, Silva P, Epstein FH. Elevated 1,25-dihydroxyvitamin D plasma levels in normal human pregnancy and lactation. J Clin Invest. 1979;63(2):342–344. doi: 10.1172/JCI109308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouillon R, Van Assche FA, Van Baelen H, Heyns W, DeMoor P. Influence of the Vitamin D-binding protein on serum concentrations of 1,25(OH)2D. J Clin Invest. 1981;67:589–596. doi: 10.1172/JCI110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haughton MA, Mason RS. Immunonephelometric assay of vitamin D-binding protein. Clin Chem. 1992;38(9):1796–1801. [PubMed] [Google Scholar]
- 81.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest. 1984;74(6):1966–1971. doi: 10.1172/JCI111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sowers MR, Wallace RB, Hollis BW. The relationship of 1,25-dihydroxyvitamin D and radial bone mass. Bone Miner. 1990;10(2):139–148. doi: 10.1016/0169-6009(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 83.Gloth FM, Gundberg CM, Holllis BW, Haddad JG, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 84.Greer FR, Hollis BW, Napoli JL. High concentrations of vitamin D2 in human milk associated with pharmacologic doses of vitamin D2. J Pediatrics. 1984;105:61–64. doi: 10.1016/s0022-3476(84)80361-3. [DOI] [PubMed] [Google Scholar]
- 85.Novakovic B, Sibson M, Ng HK, Manuelpillai U, Rakyan V, Down T, Beck S, Fournier T, Evain-Brion D, Dimitriadis E, Craig JM, Morley R, Saffery R. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J Biol Chem. 2009;284(22):14838–14848. doi: 10.1074/jbc.M809542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stevenson JC, Hillyard CJ, MacIntyre I, Cooper H, Whitehead MI. A physiological role for calcitonin: protection of the maternal skeleton. Lancet. 1979;2(8146):769–770. doi: 10.1016/s0140-6736(79)92117-2. [DOI] [PubMed] [Google Scholar]
- 87.Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1alpha-hydroxylase gene in normocalcemic rats. Proc Natl Acad Sci U S A. 1999;96(14):8253–8258. doi: 10.1073/pnas.96.14.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhong Y, Armbrecht HJ, Christakos S. Calcitonin, a regulator of the 25-hydroxyvitamin D3 1alpha-hydroxylase gene. J Biol Chem. 2009;284(17):11059–11069. doi: 10.1074/jbc.M806561200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zaidi M, Moonga BS, Abe E. Calcitonin and bone formation: a knockout full of surprises. J Clin Invest. 2002;110(12):1769–1771. doi: 10.1172/JCI200217425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ajibade DV, Dhawan P, Fechner AJ, Meyer MB, Pike JW, Christakos S. Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin. Endocrinology. 2010;151(7):2974–2984. doi: 10.1210/en.2010-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carneiro RM, Prebehalla L, Tedesco MB, Sereika SM, Hugo M, Hollis BW, Gundberg CM, Stewart AF, Horwitz MJ. Lactation and bone turnover: a conundrum of marked bone loss in the setting of coupled bone turnover. J Clin Endocrinol Metab. 2010;95(4):1767–1776. doi: 10.1210/jc.2009-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4(2):80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, Town GI, Pattemore PK, Espinola JA, Crane J. Cord-Blood 25-Hydroxyvitamin D Levels and Risk of Respiratory Infection, Wheezing, and Asthma. Pediatrics. 2011;127(1):e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 94.Belderbos ME, Houben ML, Wilbrink B, Lentjes E, Bloemen EM, Kimpen JL, Rovers M, Bont L. Cord Blood Vitamin D Deficiency is Associated With Respiratory Syncytial Virus Bronchiolitis. Pediatrics. 2011 doi: 10.1542/peds.2010-3054. [DOI] [PubMed] [Google Scholar]
- 95.Yu S, Cantorna MT. Epigenetic Reduction in Invariant NKT Cells following In Utero Vitamin D Deficiency in Mice. J Immunol. 2010 doi: 10.4049/jimmunol.1002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.van der Vliet H, von Blomberg B, Nishi N, Reijm M, Voskuyl A, van Bodegraven A, Polman C, Rustemeyer T, Lips P, van den Eertwegh A, Giaccone G, Scheper R, Pinedo H. Circulating V[alpha]24+ V[beta]11+ NKT Cell Numbers Are Decreased in a Wide Variety of Diseases That Are Characterized by Autoreactive Tissue Damage. Clinical Immunology. 2001;100(2):144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 97.Hollis BW, Pittard WB. Evaluation of the total fetomaternal vitamin D relationships at term: Evidence for racial differences. J Clin Endorcrinol Metab. 1984;59:652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 98.Heaney R, Dowell M, Hale C, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Amer College Nutr. 2003;22(2):142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 99.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 100.Obermer E. Vitamin-D requirements in pregnancy. Br Med J. 1947;2(4535):927. doi: 10.1136/bmj.2.4535.927-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Friedman WF. Vitamin D as a cause of the supravalvular aortic stenosis syndrome. Am Heart J. 1967;73:718–720. doi: 10.1016/0002-8703(67)90186-x. [DOI] [PubMed] [Google Scholar]