Abstract
Field studies and laboratory experiments have documented that a key component of resilience is emotional flexibility – the ability to respond flexibly to changing emotional circumstances. In the present study we tested the hypotheses that resilient people exhibit emotional flexibility: a) in response to frequently changing emotional stimuli; and b) across multiple modalities of emotional responding. As participants viewed a series of emotional pictures, we assessed their self-reported affect, facial muscle activity, and startle reflexes. Higher trait resilience predicted more divergent affective and facial responses (corrugator and zygomatic) to positive versus negative pictures. Thus, compared with their low resilient counterparts, resilient people appear to be able to more flexibly match their emotional responses to the frequently changing emotional stimuli. Moreover, whereas high trait resilient participants exhibited divergent startle responses to positive versus negative pictures regardless of the valence of the preceding trial, low trait resilient participants did not exhibit divergent startle responses when the preceding picture was negative. High trait resilient individuals, therefore, appear to be better able than are their low-resilient counterparts to either switch or maintain their emotional responses depending on whether the emotional context changes. The present findings broaden our understanding of the mechanisms underlying resilience by demonstrating that resilient people are able to flexibly change their affective and physiological responses to match the demands of frequently changing environmental circumstances.
Keywords: resilience, emotional flexibility, affect, EMG, startle reflex
In the ebb and flow of daily life, people are often confronted with changing circumstances to which they must adapt. Resilience is the ability to navigate these changes successfully (Block & Kremen, 1996). Resilient people are those who can maintain good mental health while enduring challenges and adversity like economic hardship (Werner & Smith, 1992), terrorist attacks (Fredrickson, Tugade, Waugh, & Larkin, 2003), and daily stressors (Ong, Bergeman, Bisconti, & Wallace, 2006; see Bonanno, 2004).
One key factor in how resilient people adapt to these changes successfully is psychological flexibility. Block described ego-resilience as the flexible deployment of social, cognitive, and emotional resources to meet the fluctuating demands of the environment (Block & Block, 1980; Block & Kremen, 1996); the inability to do so was indicative of psychological rigidity. In a recent review, Kashdan and Rottenberg (2010) summarized evidence to support this theory, showing that good mental health and resilience are characterized by psychological flexibility. For example, people who flexibly deploy different coping strategies to match the demands of the environment are better adjusted and more adept at dealing with stress (Cheng, 2001). Kashdan and Rottenberg also found the inverse to be true - that poor mental health is characterized by psychological rigidity. For example, in a meta-analysis, Bylsma, Morris, & Rottenberg (2008) showed that depressed people exhibit less contextually appropriate emotional reactivity to emotion inductions than do nondepressed individuals.
As highlighted by the above finding of diminished emotional context-sensitivity in depressed people, one important facet of psychological flexibility is emotional flexibility (Bonanno, Papa, Lalande, Westphal, & Coifman, 2004; Waugh, Fredrickson, & Taylor, 2008; Waugh, Wager, Fredrickson, Noll, & Taylor, 2008). Significant changes in the environment often involve shifts from positive to negative life circumstances and vice versa (Sarason, Johnson, & Siegel, 1978). Emotional flexibility is the capacity to produce context-dependent emotional responses to these positive and negative life events (Waugh, Wager, et al., 2008; Westphal, Seivert, & Bonanno, 2010). The term ‘context-dependent response’ is ambiguous and can refer to any number of different contexts and responses. For the purposes of the current study, we operationalize ‘context’ as a discrete emotion-eliciting event and ‘response’ as the initial reactivity to that event. Thus, in this study, context-dependent responding implies that positive events should induce positive emotional reactivity and negative events should induce negative emotional reactivity.
Like the overarching construct of psychological flexibility, emotional flexibility also appears to be a characteristic of resilient people. For example, whereas resilient people respond to negative events with negative affect (Fredrickson, et al., 2003; Waugh, Fredrickson, et al., 2008) and biological stress responses (Tugade & Fredrickson, 2004), they also experience positive emotions during crises (Fredrickson, et al., 2003) and other stressful periods in their lives (Folkman & Moskowitz, 2000). For example, in response to the terrorist attacks on 9/11, those people who reported the fewest depressive symptoms after the attacks reported experiencing both positive emotions, such as gratitude and love, and negative emotions, such as anger and fear, in response to the attacks (Fredrickson, et al., 2003). Beyond naturalistic experience of positive and negative emotions, recent evidence also suggests that more resilient people are better able to strategically enhance both positive and negative emotional experiences than are their less resilient counterparts (Westphal, et al., 2010).
These studies examining emotional flexibility in resilient people are suggestive but are limited in several respects. An important strength of the field studies (e.g., Folkman, Chesney, & Christopher-Richards, 1994) is that they examine people’s emotional responses to real-life circumstances. Because there is typically a practical limit to the number and the timing of emotional assessments in these investigations, however, researchers cannot capture responses to all of the possible emotion-eliciting events. Moreover, the lack of experimental control over the emotion-eliciting events leaves open the possibility that the events experienced by high-resilient individuals are qualitatively different than the events experienced by low-resilient individuals. Although the experimental studies (e.g., Westphal, et al., 2010) address these limitations by testing the ability of resilient people to regulate their emotional responses to specific emotional events, we do not know whether they spontaneously respond flexibly to specific positive and negative events. Finally, a majority of these studies focused on only a single index of emotional responding (e.g., self-reports in the field studies, facial expressions in the experimental studies), thereby examining only small portions of the more elaborate set of responses to emotion-eliciting events.
We address these limitations in the current study by examining the associations between trait resilience and both self-reported and physiological responses to frequently changing positive and negative events in a laboratory setting. One advantage of assessing resilient people’s spontaneous responses to emotional events is that we can begin to examine temporal aspects of emotional flexibility. When emotional events occur in quick succession, maintaining the contextually-dependent emotional responding inherent in emotional flexibility requires the capacity both to switch emotional responses when the valence of the emotional event changes, and to maintain an emotional response when the valence of the emotional event does not change. By assessing spontaneous responses to frequently changing emotional events, we can examine whether resilient people appropriately switch and/or maintain their emotional responses as a function of the valence of the emotional events.
We measured resilience with the ER89 (Block & Kremen, 1996), a scale that was constructed specifically to differentiate those who adapt well from those who do not and that has been demonstrated to have good construct validity (see Method). In the current task, participants viewed positive and negative emotional pictures as multiple indices of emotional responsiveness were assessed. To assess self-reported emotional responsiveness, participants provided continuous ratings of their self-reported affect as they viewed the emotional pictures. To replicate and extend Westphal et al.’s (2008) findings on resilience and expressive flexibility, we assessed facial expressions with facial electromyography (EMG) recorded over two sites. The first site was the corrugator supercilii, the muscle responsible for the brow furrowing (Fridlund & Cacioppo, 1986) typically seen in displays of negative emotion (Tassinary & Cacioppo, 1992). The second site, the zygomatic major, corresponds to the cheek muscle that retracts the corner of the lips (Fridlund & Cacioppo, 1986) in smiles typically seen in displays of positive emotion (Tassinary & Cacioppo, 1992). The third component of emotional responsiveness that we assessed was defensive motivation. Although similar to self-report and facial expressions in differentiating between positive and negative stimuli, defensive motivation also reflects a person’s behavioral readiness to withdrawal from negative situations (Lang, Bradley, & Cuthbert, 1990). To assess defensive motivation, we measured the startle reflex, which refers to the increase in activity recorded from a third facial EMG site, the orbicularis oculi (muscle surrounding the eye), in response to a sudden burst of white noise (Blumenthal, et al., 2005). The startle reflex is potentiated when people are viewing negative pictures and attenuated when people are viewing positive pictures (Bradley, Codispoti, Cuthbert, & Lang, 2001).
These indices form the response profile in which context-dependent responses to positive relative to negative events are reflected by higher self-reported positive affect, lower corrugator activity, higher zygomatic activity, and lower amplitude startle reflex. We hypothesized that high-resilient people would respond with greater emotional flexibility than would their low-resilient counterparts, which would be reflected by two patterns in the data: 1) robust divergence between their responses to positive events and their responses to negative events, reflecting the ability to discriminate between positive and negative events and respond accordingly; and 2) the maintenance of these differential responses to positive and negative events regardless of the valence of the previous event. This maintenance of response divergence reflects the flexibility required to both switch responses when the emotional valence of the events change, and maintain responses when the emotional valence of the events do not change. To examine the possibility that the hypothesized response divergence is due to differential arousal and/or to engagement with the stimuli, we also assessed participants’ skin conductance responses (SCR) and memory for the pictures.
Method
Participants
Participants were recruited through advertisements in local newspapers and classifieds websites (e.g. http://www.craigslist.com). Participation was limited to individuals who did not have any cardiovascular problems, were between the ages of 18 and 55, and were not pregnant. Forty-one individuals (21 females) participated in this study (Mage = 33.7 years, SD = 13.1 years).
Self-report measures
Resilience
We used Block and Kremen’s (1996) ego-resiliency scale (ER89) to assess trait variation in psychological resilience. Participants were asked to indicate the degree to which they agreed with 14 statements (e.g. “I quickly get over and recover from being startled,” “I enjoy dealing with new and unusual situations”) on a scale from 1 (does not apply at all) to 4 (applies very strongly). The ER89 has been shown to have high construct validity. Higher scores on the ER89 have been found to predict the experience of fewer depressive symptoms after the terrorist attacks on 9/11 (Fredrickson, et al., 2003), faster affective and physiological recovery from threat (Tugade & Fredrickson, 2004; Waugh, Fredrickson, et al., 2008), and more successful adaptation to daily stressors (Ong, et al., 2006). The current sample reported similar levels and variability of resilience (M = 3.14, SD = .43) as previous samples (Fredrickson, et al., 2003). The internal reliability was α = .79.
Continuous affective rating
Participants rated their affect continuously throughout the task with a rating dial (Waugh, Fredrickson, et al., 2008), a modification of previously validated continuous affective rating procedures (Levenson & Gottman, 1983; Fredrickson & Kahneman, 1993). The rating dial is a custom-made apparatus (Biopac Systems, Goleta, CA) that features a raindrop-shaped knob that rotates 180 degrees with the pointed end referencing an affective scale labeled 0 – 9 that subtends a 180 degree arc. The position at 0° (‘0’) was labeled ‘negative’; the position at 180° (‘9’) was labeled ‘positive’. Neutral implicitly corresponded to a rating of 4.5. The knob is attached to a voltage potentiometer that translates the position/angle of the knob into a numeric value (0 – 9). We collected continuous affect rating data for the current study because of findings indicating that providing continuous ratings appears to better preserve the underlying affective response (Hutcherson, et al., 2005) than does providing discrete ratings (Taylor, Phan, Decker, & Liberzon, 2003).
Physiological acquisition
Physiological activity was recorded at a sampling rate of 1 kHz with an integrated system and software package (Biopac MP150, AcqKnowledge; Biopac Systems, Goleta, CA). Electromyographic (EMG) activity was recorded with pairs of 4 mm Ag/AgCl electrodes placed over the corrugator supercilii, orbicularis oculi, and zygomatic major muscles following the placement specifications of Fridlund & Cacioppo (1986). Skin conductance was measured with two 6 mm Ag/AgCl electrodes placed on the distal phalanges of the first and second fingers on the non-dominant hand.
Task
Emotional responsiveness
On each trial, participants viewed one of two cues (5s) that indicated whether the following pictures would be positive (Pos; ‘+’ cue) or negative (Neg; ‘−‘ cue). The purpose of the cues was to reduce feelings of uncertainty between sets of stimuli. Participants then viewed three successive pictures from the International Affective Picture Set (IAPS; Lang, Bradley, & Cuthbert, 1997) for 4s each.1 We presented three pictures to increase the potential emotional impact of these images on subsequent trials, although we use the rating and physiological data only from either the first picture (rating, EMG, SCR) or second picture (startle). Finally, participants viewed a blank screen for either 2s or 8s.2
The task consisted of 82 trials (total of 246 pictures) presented in two blocks separated by a 1-minute break. Because we were interested in the impact of the valence of one trial on participants’ responses to the subsequent trial, the trials were sequenced pseudo-randomly to produce 20 trials of each of the 4 temporal pairings of emotional valences (previous → current trial: Pos → Pos, Pos → Neg, Neg → Pos, Neg → Neg). The data from the first picture of each block were not included in the analyses because there was no preceding trial. The pictures were selected by first omitting erotic pictures and then equating all four trial types on normed ratings of emotional intensity (intensity for positive pictures was reverse-coded for comparability: Pos-Pos = 2.09, Pos-Neg = 2.31, Neg-Pos = 1.83, Neg-Neg = 2.30), F(3,236) = 1.1, p > .05, and arousal (Pos-Pos = 4.67, Pos-Neg = 3.93, Neg-Pos = 4.64, Neg-Neg = 3.85), F(3,236) = .45, p > .05.
On a randomly selected 60% of the trials (12 of the 20 trials for each trial type), participants heard a startle probe through the headphones that consisted of instantaneous-rise 50ms bursts of white noise at 95 db (Blumenthal, et al., 2005). The startle probes occurred 1.5s into the second picture of the series (5.5s after onset of first picture) so that the rating and physiological data during the first picture would not be confounded with the occurrence of a startle probe.
Memory
For the incidental recall task, participants viewed a series of 160 IAPS pictures, half of which were positively valenced and half of which were negatively valenced. Within the sets of positively and negatively valenced pictures, half (i.e., 40) were pictures from the emotional responsiveness task (i.e., target pictures), with one picture from each trial of the task being included. The other half of the pictures were foils that were equivalent in normed ratings of emotional intensity and arousal to the target pictures. Further, the 80 positive pictures (i.e., collapsing across positive targets and foils) did not differ significantly from the 80 negative pictures in emotional intensity or arousal. Pictures were randomly ordered and then presented in the same order to each participant. We counterbalanced foil and target picture sets between participants. Participants pressed ‘y’ if they had seen the picture during the previous task or ‘n’ if they had not. Memory performance was calculated as hit rate – percentage of target pictures within each trial type that were correctly identified as having been seen before. Two participants did not complete the memory task.
Procedure
After participants signed the informed consent forms, the experimenter attached the sensors and placed the headphones on the participant. Participants completed some questionnaires during a 10-minute acclimation period. Next, all of the participants’ physiological signals were recorded for a 5-minute baseline period as they rested quietly. The experimenter then explained the emotional responsiveness task to the participants. They were told that they would see a series of cues followed by several pictures. They were informed that seeing a ‘+’ or a ‘−‘ cue meant that they would see a series of either pleasant or unpleasant pictures, respectively. They were instructed to use the rating dial to report how they were currently feeling throughout the entire task and to move the dial as often as they liked to reflect changes in their feelings. The experimenter then introduced the startle probes to the participants and administered five example startle probes. Participants were instructed to ignore the startle probes throughout the task. Skin conductance and facial EMG were measured continuously throughout the task.
After the emotional responsiveness task, participants completed ten minutes of questionnaires and then began the memory task. After they completed the memory task and any remaining questionnaires, participants were debriefed, paid, and thanked for their participation. This procedure was approved by the Stanford University Institutional Review Board.
Data reduction and scoring
The rating and physiology data were reduced and scored with Autonomic Nervous System Laboratory (ANSLab v2.4, Wilhelm & Peyk, 2005) as well as with custom scripts.
Rating Dial
Rating dial values were averaged into 1s bins and extracted from the final 1s bin of the first picture viewed in each series. Analyzing this final bin both allowed enough time for participants’ ratings to stabilize and reduced the potential confound between the current rating and the position of the rating dial from the previous trial. Moreover, examining affect from the first picture avoided any influence of the startle probes during the second picture on concurrent and subsequent ratings.
EMG scoring
EMG signals from the corrugator and zygomatic muscles were high-pass filtered (28hz), rectified, and smoothed with a moving average window of 50ms. The data were then linearly detrended across both the baseline and task periods. To calculate EMG activity during each trial, we first averaged the rectified EMG signal during the first picture on each trial (4s) and standardized it according to each person’s EMG activity during the 5-minute baseline period. Next, to minimize outliers, the resulting EMG responses were winsorized so that responses greater than 3 SD away from the mean (in either direction) were set equal to 3 SD (Tukey, 1977). Three participants were excluded from corrugator analyses due to equipment failure, resulting in 38 participants for the respective analyses. Importantly, resilience was not correlated with the mean or standard deviation of baseline activity in either the corrugator (M: r = .24, p = .15; SD: r = .04, p = .81) or zygomatic (M: r = .19, p = .25; SD: r = −.07, p = .68) muscles.
Startle reflex scoring
After orbicularis activity was low-pass filtered at 100hz and rectified, startle reflexes were scored according to the standards described by Blumenthal et al. (2005). Eye-blinks were counted as reflexive startles if there was a raise in activity within 50 to 150 ms after probe onset that was 5 SD above a proximal baseline, which consisted of the 50 ms pre-stimulus period. To avoid confounding frequency with startle responding, we used the response amplitude metric and only included non-zero eye blinks in our analyses. Each participant’s startle responses were standardized with a T-distribution resulting in a within-participant average of 50 and SD of 10. To minimize outliers, the resulting startle responses were winsorized so that responses greater than 3 SD away from the mean (in either direction) were set equal to 3 SD (Tukey, 1977). Lastly, five participants were excluded from analyses on the startle reflex because they did not have a minimum of 2 startle responses per trial type resulting in data for 36 participants for these analyses. As was the case with zygomatic and corrugator, resilience was not correlated with the mean or standard deviation of baseline activity in the orbicularis muscle (M: r = −.09, p = .61; SD: r = .01, p = .98).
Skin conductance scoring
Skin conductance activity was first low-pass filtered at 1 hz. Skin conductance responses (SCR) were calculated as the difference between the maximum skin conductance change within 1 – 4s post-stimulus (first picture of each series) and the proximal baseline (1s pre-stimulus). Negative changes from the proximal baseline were assumed to reflect a lack of a skin conductance response and were accordingly set to zero. SCRs were then log-transformed to correct for positive skew. Resilience was not correlated with the mean or standard deviation of baseline activity in skin conductance level (M: r = −.09, p = .60; SD: r = .09, p = .58).
Statistical Analyses
The rating dial and physiological variables were each submitted to a 2 × 2 (Previous Picture Set’s Valence [PreVal; positive, negative] × Current Picture Set’s Valence [CurrVal; positive, negative]) repeated measures analysis of variance (ANOVA). Trait resilience was included as a standardized continuous factor that was allowed to interact with the within-subject factors. Significant PreVal and CurrVal main effects and interactions between these within-subjects factors were followed up with paired t-tests. Significant interactions of within-subject factors and trait resilience were followed up with one of two types of simple slopes tests. One type of simple slopes test consisted of a linear regression of trait resilience on the dependent variable of interest within a specific trial type (e.g., previous positive picture sets). The other type of simple slopes test consisted of evaluating the difference between two trial types at both high (+1 SD) and low (−1 SD) levels of trait resilience (Aiken & West, 1991).
Results
Self-report Affect
For self-reported affect, there were significant main effects of CurrVal, F(1,39) = 71.93, p < .001, and PreVal, F(1,39) = 52.87, p < .001. As expected, participants rated their affect during the current positive picture sets as more positive (M = 5.35, SE = .19) than they did during the current negative picture sets (M = 2.90, SE = .19), t(40) = 8.14, p < .001, d = 1.80. Participants also rated their current affect as more positive if the previous picture set was positive (M = 4.56, SE = .13) than they did if the previous set was negative (M = 3.69, SE = .14), t(40) = 7.34, p < .001, d = 1.61, suggesting that there was some carry-over from the previous trial such that the valence of the previous trial was assimilated into the affect reported during the current trial.
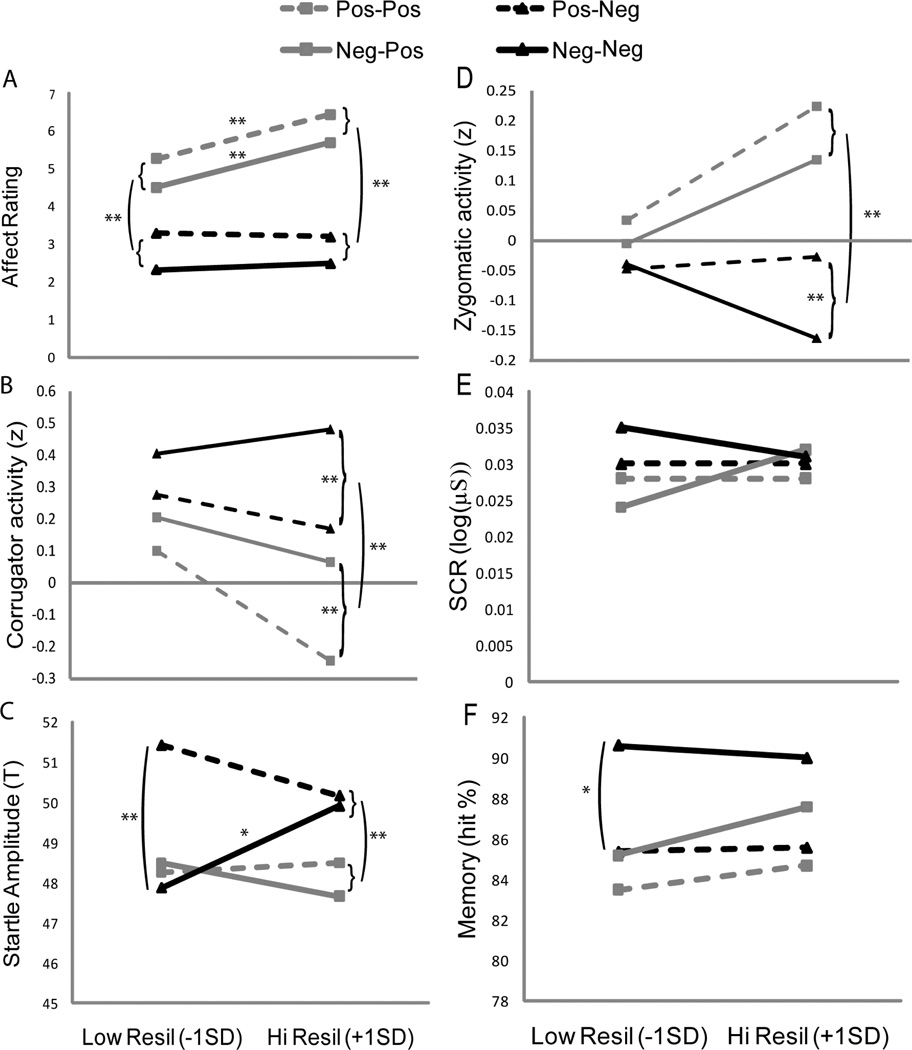
There was also a main effect of trait resilience, F(1,39) = 9.09, p = .005, that was qualified by an interaction of trait resilience and CurrVal, F(1,39) = 4.45, p = .041 (Figure 1a). As predicted, higher trait resilience was associated with a greater difference in self-reported affect between current positive and negative picture sets, β = .32, p = .041 (Table 1). Further, this relation was due to a strong positive association between trait resilience and self-reported affect during positive picture sets, β = .53, p < .001, and no association between trait resilience and self-reported affect during negative picture sets, β = .01, p = .929.
Figure 1.
Responsiveness to emotional picture sets as a function of trait resilience. Responses for positive and negative picture sets that were preceded by either positive (i.e., pos-pos, pos-neg) or negative (i.e., neg-pos, neg-neg) picture sets were estimated at high (+1 SD above the mean) and low (−1 SD below the mean) trait resilience. High trait resilience was associated with greater divergence in A. self-reported affective responses (PA = positive affect, NA = negative affect), B. magnitude of corrugator activity, and D. magnitude of zygomatic activity during positive versus during negative picture sets. Moreover, high trait resilience was associated with greater divergence in C. startle amplitude during positive versus during negative picture sets that followed a negative picture set. There was no significant interaction of trait resilience and divergent E. skin conductance responses or F. memory for the pictures.
*p < .05, **p < .01.
Brackets indicate that these variables were averaged for the illustrated statistical comparison.
Table 1.
Responses to the current trial’s positive and negative picture sets for all participants and by resilience
| All (paired t- test) |
Correlation (r) with resilience: | |||
|---|---|---|---|---|
| Pos – Neg | Pos – Neg | Pos | Neg | |
| Self-reported Affecta | 8.14** | .32* | .50** | .01 |
| Corrugator activityb | −5.29** | −.32* | −.22 | −.01 |
| Zygomatic activitya | 3.71** | .38* | .19 | −.08 |
| Startle Amplitudec | −3.74** | −.13 | −.07 | .11 |
| Skin Conductancea | −.75 | .10 | −.02 | .10 |
| Memoryd | −2.71* | .17 | .10 | −.01 |
Note. Pos = responses on positive trials; Neg = responses on negative trials.
n = 41;
n = 38;
n = 36;
n = 39.
p < .01,
p < .05.
Corrugator Activity
For corrugator activity, there were significant main effects of CurrVal, F(1,36) = 30.33, p < .001, and PreVal, F(1,36) = 15.21, p < .001. As expected, participants exhibited greater corrugator activity during current negative picture sets (M = .33, SE = .09) than they did during current positive picture sets (M = .03, SE = .09), t(37) = 5.29, p < .001, d = 1.22. Participants also exhibited greater current corrugator activity if the previous picture set was negative (M = .29, SE = .08) than they did if the previous picture set was positive (M = .08, SE = .09), t(37) = 3.80, p = .001, d = .85. As was the case with self-reported affect, this finding suggests that the valence of the previous trial was assimilated into corrugator activity during the current trial.
There was no main effect of trait resilience on frequency of corrugator activity F(1,36) = .56, p = .461, but there was an interaction of trait resilience and CurrVal, F(1,36) = 4.19, p = .048 (Figure 1b). As predicted, higher trait resilience predicted a greater difference in corrugator activity during the negative picture sets than during the positive picture sets, β = .32, p = .048 (Table 1). Of note, this relation does not seem to be due to an association between trait resilience and corrugator activity during either positive, β = −.22, or negative, β = −.01, picture sets alone, ps = .184, .937, respectively. Instead, level of trait resilience is a better predictor of the difference in corrugator activity between positive and negative picture sets.
Zygomatic Activity
For zygomatic activity, there was a significant main effect of CurrVal, F(1,39) = 15.71, p < .001, but only a marginal effect of PreVal, F(1,39) = 3.34, p = .074. As expected, participants exhibited greater current zygomatic activity when viewing positive picture sets (M = .097, SE = .066) than when viewing negative picture sets (M = −.069, SE = .058), t(40) = 3.71, p = .001, d = .94.
As predicted, there was a significant interaction of CurrVal and trait resilience, F(1,39) = 6.58, p = .014 (Figure 1d). Follow-up analyses reveal that whereas at high trait resilience there was significant divergence in zygomatic activity when viewing positive versus negative picture sets, β = .28, p < .001, at low trait resilience there was no significant divergence in zygomatic activity, β = .06, p = .342. Like corrugator activity, this relation does not seem to be due to an association between trait resilience and zygomatic activity during either positive, β = .19, or negative, β = −.08, picture sets alone, ps = .250, .656, respectively (Table 1).
Startle Reflex
The analysis on startle amplitude yielded significant main effects of CurrVal, F(1,34) = 13.86, p = .001, and PreVal, F(1,34) = 5.22, p = .029. Consistent with previous literature, participants exhibited higher startle amplitude when viewing negative picture sets (M = 49.85, SE = .32) than when viewing positive picture sets (M = 48.23, SE = .34), t(35) = 3.74, p = .001, d = .88. Participants also exhibited higher startle amplitude when the previous picture set was positive (M = 49.59, SE = .36) than when it was negative (M = 48.49, SE = .33), t(35) = 2.28, p = .029, d = .54. These main effects were qualified by a 3-way interaction of trait resilience, PreVal, and CurrVal, F(1,34) = 5.54, p = .024 (Figure 1c). This interaction was due to a differential effect of the valence of the previous trial on the relation between trait resilience and startle amplitude during the current trial. At high trait resilience there was a difference in startle amplitude between current negative and positive picture sets regardless of whether the previous trial was positive, B = 1.66, SE = .945, p = .088, or negative, B = 2.25, SE = .84, p = .023. On the other hand, at low trait resilience there was a difference in startle amplitude between current negative and positive picture sets only when the previous trial was positive, B = 3.15, SE = .945, p = .002, not when the previous trial was negative, B = −.607, SE = .842, p = .476. Further, this association between trait resilience and the difference in startle amplitude between current negative and positive picture sets that followed a negative trial was due to higher trait resilience predicting higher startle amplitude during the negative picture sets (following negative trials), β = .34, p = .04, and no association between resilience and startle amplitude during the positive picture sets (following negative trials), β = −.17, p = .334.
Skin conductance
There were no significant main effects of CurrVal, F(1,39) = .55, p = .464, or PreVal, F(1,39) = .31, p = .583, and no significant interaction of these factors, F(1,39) = .06, p = .805, on skin conductance. Similarly, there were no main effects or interactions of CurrVal or PreVal with trait resilience. This suggests that the effects of trait resilience on the above variables were not due to differences in general arousal. Moreover, this finding supports our selection of positive and negative pictures as being equivalent in eliciting arousal.
Memory
There were significant main effects of CurrVal, F(1,37) = 7.34, p = .01, and PreVal, F(1,37) = 10.82, p = .002, on memory. Consistent with previous research, participants remembered negative pictures (M = .88, SE = .01) better than they did positive pictures (M = .85, SE = .01), t(38) = 2.71, p = .01, d = .61. Similar to the findings obtained with self-reported affect and corrugator activity, there was a significant effect of the valence of the previous trial on memory for the current picture set: participants had better memory for pictures that were preceded by a negative picture set (M = .88, SE = .01) than they did for pictures that were preceded by a positive picture set (M = .85, SE = .02), t(38) = 3.33, p = .002, d = .78. Importantly, there were no main effects or interactions with trait resilience, suggesting that the previous results for self-reported affect, corrugator activity, zygomatic activity, and startle amplitude were not due to differential engagement or depth of processing.3
Independence of self-report and physiological variables
We next examined whether trait resilience was independently associated with the three emotional response modalities: self-reported affect, facial expressiveness (corrugator [current negative - current positive] and zygomatic [current positive – current negative] activity were highly correlated, r = .61, p < .001, so were averaged together to form a facial expressiveness index), and defensive motivation (startle amplitude).4 These three response modalities were not significantly correlated with each other (rs between .01 and .32, all ps > .05). In a linear regression analysis predicting trait resilience, we entered: (a) the difference in self-reported affect between current positive and negative picture sets; (b) the difference in facial EMG activity (both corrugator and zygomatic) between current negative and positive picture sets; and (c) the difference in startle amplitude between current negative and positive picture sets that followed negative picture sets. All three variables remained at least marginally significant when controlling for each other, βs = .270, .312, .345, ps = .087, .052, .025, for self-report affect, facial EMG activity, and startle amplitude, respectively. These findings suggest that these emotional response modalities were independently associated with trait resilience.
Discussion
This study provides new evidence that when confronted with frequently changing emotional events, higher trait resilience is associated with greater flexibility in multiple emotional response modalities. The first response modality we examined was self-reported affect. As hypothesized, higher trait resilience was associated with more divergent affective responses to positive versus negative events, which in turn was due to a positive association between trait resilience and positive affective responses to positive pictures. This association between resilience and self-reported positive affect is well-supported by previous findings (Fredrickson, et al., 2003; Ong, et al., 2006; Tugade & Fredrickson, 2004); indeed, this association is the basis for theories of emotional responding in resilience (Folkman & Moskowitz, 2000) and for interventions designed to increase resilience (Fredrickson, Cohn, Coffey, Pek, & Finkel, 2008).
Our finding that resilience was not associated with negative affective responses when viewing negative emotional pictures also replicates these previous findings (Fredrickson, et al., 2003; Ong, et al., 2006; Tugade & Fredrickson, 2004), but casts them in a new light by showing that in the midst of both positive and negative experiences, resilient people are not characterized by unconditional positive emotions, but by their ability to flexibly switch their emotional responses to match the demands of these experiences. This conclusion, however, is slightly tempered by our use of a bipolar scale, which was not able to assess the presence of both positive and negative affect to the same stimulus. Future investigations that use stimuli that can induce ambivalent feelings and assess both positive and negative affect simultaneously are needed to examine the circumstances under which these flexibly divergent affective responses may converge.
Our assertion that resilience is more accurately characterized by emotional flexibility than it is by any single emotional response is further supported by the current findings concerning facial EMG activity. Higher trait resilience was associated with more divergent corrugator and zygomatic responses to positive versus negative events; moreover, these divergent responses could not be explained by associations between resilience and facial activity to either positive or negative events alone. Given that facial expressions have evolved, in part, to communicate our feelings and intentions to others (Owren & Bachorowski, 2001), the current finding suggests that resilient people are better at discriminately communicating their emotional experiences through their facial expressions. Being ‘easy to read’ may be one mechanism by which resilient people maintain good social relationships that in turn provide good social support when coping with challenging circumstances (Southwick, Vythilingham, & Charney, 2005).
The startle reflex was the only emotional index in which resilience moderated the influence of the emotional valence of the previous trial on the emotional valence of the current trial. As with self-reported affect, corrugator, and zygomatic activity, high trait resilient participants exhibited robust divergence in startle amplitude to positive versus negative picture sets regardless of the valence of the previous picture set. In contrast, low trait resilient participants did not exhibit this divergence in startle amplitude to positive versus negative picture sets when the previous picture set was negative. Instead, they exhibited an attenuated startle response to negative picture sets that followed negative picture sets. This finding suggests that whereas high resilient people can both switch their defensive motivational state when the valence changes from one emotional event to the next and maintain their defensive motivational state when the valence does not change, low resilient people can switch, but fail to maintain, defensive motivational states. This response attenuation parallels the finding that people diagnosed with major depression exhibit blunted emotional and physiological reactivity (see Bylsma, Morris, & Rottenberg, 2008, for a review). This tendency to exhibit blunted startle reactivity to negative events following other negative events may therefore be one mechanism that confers greater risk for depression in people scoring low on this trait resilience scale (Fredrickson, et al., 2003).
Resilience did not predict skin conductance responses or memory for the pictures, confirming that the demonstrated association between resilience and emotional flexibility was not due to differences in arousal or stimulus engagement. This finding provides discriminant validity for the construct of emotional flexibility by showing that resilience is associated with flexibility in valence-specific response modalities, such as self-reported affect, facial expressions, and defensive motivation, but not in valence-general response modalities, such as arousal and engagement. Moreover, our results demonstrated that these valence-specific response modalities are not redundant with each other, but rather, are independent components of a more general response profile. For example, the startle reflex showed a different pattern of responding than did facial activity and self-reported affect. This discrepancy may be due simply to differences in the timing of these assessments; the startle reflex was assessed later in the emotional response (second picture) than were both facial EMG and self-reported affect (first picture). Alternatively, there may be different mechanisms governing when and how defensive motivation is carried over from one emotional event to another. These possibilities should be examined in future investigations. The present findings advance our understanding of the elements that do and do not comprise emotional flexibility in resilience, which in turn can help guide future studies examining these constructs.
One of the strengths of the current study was that we operationalized resilience as responses to a questionnaire (ER89) that has shown to predict lowered risk for future depressive symptoms (Fredrickson, et al., 2003). In this way, we showed how emotional flexibility may contribute to prospective resilience – how high and low resilient people may differ in their functioning before experiencing psychopathology or not. An important next step in this research is to conduct a longitudinal study in which people at low and high risk for psychopathology are followed over time to examine whether the emotional flexibility demonstrated by resilient people protects them from experiencing psychopathology when they are exposed to life stressors. It will also be important to use other potential measures of resilience besides questionnaires, such as functional genotypes (Caspi, et al., 2003) or neurobiological phenotypes (Charney, 2004).
In some life circumstances, emotional events can occur in rapid succession. For example, the anxious anticipation of a loved one’s impending surgery can quickly give way to relief if the surgery is successful. We have shown in this study that resilient people can fully engage in these quickly changing emotional experiences. In this case, emotional variability is adaptive because the type and intensity of the emotional responses correspond appropriately to the demand required by these real and significant emotional events. It is possible, however, for emotional variability to be maladaptive if the type and/or intensity of individuals’ emotional responses no longer correspond appropriately to the demands of the environment. Indeed, a high level of emotional variability is a hallmark symptom of some mental health difficulties, such as borderline personality disorder (Trull, et al., 2008). It will be important, therefore, for future investigations examining the adaptiveness of emotional variability to assess precisely how people’s emotional responses map onto environmental demands.
In conclusion, we presented evidence that higher trait resilience is associated with increased emotional flexibility. These data extend the findings from field studies (Ong, et al., 2006) and laboratory experiments (Westphal, et al., 2010) by demonstrating that the emotional flexibility of highly resilient people is: a) spontaneous; b) evident in frequently changing situations; c) evident across valence-specific response modalities such as self-reported affect, facial expressions, and defensive motivation; and d) not explained by just heightened positive affectivity, but by divergent positive and negative affective responses. These findings suggest that emotional flexibility is one mechanism by which resilient people adapt successfully to life’s ever-changing circumstances. Future investigations should examine whether this spontaneous emotional flexibility protects resilient people from succumbing to psychopathology in the face of adverse circumstances.
Acknowledgments
This research was supported by a Distinguished Scientist Award from NARSAD and Grant MH74849 from the National Institute of Mental Health to Ian H. Gotlib and a NIMH supplement MH74849 to Renee J. Thompson. The authors thank Maria Lemus, Kalpa Bhattacharjee, and Juliana Gonzales for their help in recruiting and running the participants in this study as well as preprocessing the data.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/emo
The authors report no biomedical financial interests or potential conflicts of interest.
IAPS pictures used in this study: 1050, 1052, 1090, 1111, 1201, 1205, 1280, 1301, 1340, 1463, 1525, 1590, 1710, 1720, 1811, 1999, 2040, 2053, 2055.1, 2058, 2071, 2080, 2120, 2150, 2160, 2208, 2224, 2276, 2278, 2303, 2312, 2344, 2345, 2346, 2351, 2352, 2352.1, 2389, 2399, 2455, 2490, 2550, 2590, 2616, 2682, 2683, 2688, 2691, 2692, 2694, 2700, 2710, 2715, 2722, 2730, 2750, 2751, 2753, 2900, 2900.1, 2981, 3005.2, 30223051, 3061, 3160, 3181, 3190, 3220, 3300, 3400, 3550, 3550.1, 4150, 4220, 4250, 4255, 4503, 4533, 4535, 4538, 4572, 4598, 4599, 4601, 4603, 4606, 4608, 4609, 4610, 4614, 4617, 4621, 4623, 4624, 4625, 4626, 4640, 4641, 4653, 4660, 4689, 5260, 5270, 5450, 5460, 5470, 5480, 5600, 5621, 5622, 5623, 5626, 5628, 5629, 5660, 5700, 5830, 5849, 5910, 5920, 5950, 5971, 6020, 6190, 6200, 6200, 6210, 6211, 6213, 6230, 6241, 6242, 6243, 6244, 6250, 6250.1, 6250.2, 6260, 6300, 6311, 6312, 6315, 6370, 6410, 6510, 6530, 6550, 6555, 6561, 6570.1, 6571, 6610, 6821, 6830, 6831, 6834, 6836, 6838, 6840, 6910, 6940, 7200, 7220, 7230, 7250, 7260, 7270, 7282, 7289, 7291, 7330, 7350, 7359, 7360, 7361, 7380, 7400, 7402, 7430, 7450, 7460, 7470, 7481, 7496, 7501, 7502, 7570, 7600, 7620, 8021, 8030, 8031, 8033, 8034, 8040, 8041, 8060, 8080, 8090, 8116, 8117, 8120, 8130, 8160, 8161, 8162, 8170, 8178, 8179, 8180, 8185, 8186, 8190, 8191, 8192, 8193, 8200, 8210, 8211, 8220, 8230, 8232, 8250, 8251, 8260, 8280, 8300, 8340, 8341, 8350, 8370, 8380, 8400, 8420, 8461, 8470, 8480, 8485, 8490, 8496, 8500, 8501, 8502, 8503, 8510, 8531, 8540, 9000, 9001, 9005, 9006, 9007, 9008, 9041, 9042, 9046, 9050, 9090, 9090, 9101, 9102, 9120, 9156, 9160, 9180, 9182, 9250, 9265, 9280, 9290, 9320, 9330, 9331, 9340, 9341, 9342, 9373, 9390, 9400, 9404, 9409, 9415, 9417, 9420, 9430, 9432, 9440, 9452, 9470, 9471, 9480, 9490, 9495, 9500, 9520, 9530, 9561, 9584, 9592, 9600, 9611, 9620, 9621, 9622, 9630, 9830, 9911, 9912, 9920.
Startle reactivity data collected during this inter-trial ‘recovery’ period are not presented in this paper.
We analyzed hit rate instead of an index that incorporates false alarm rate (e.g. d’) because the novel pictures from which false alarm rate is calculated could be categorized only as positive or negative, not as a function of the valence of the previous picture. Moreover, trait resilience did not correlate with false alarm rate to either the positive, r = .13, p = .417, or negative, r = −.08, p = .64, picture sets.
These analyses were conducted with the 34 participants who had data from all three variables.
Contributor Information
Christian E. Waugh, Department of Psychology, Stanford University
Renee J. Thompson, Department of Psychology, Stanford University
Ian H. Gotlib, Department of Psychology, Stanford University
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- Block J, Block JH. The role of ego-control and ego-resiliency in the origination of behavior. In: Collings WA, editor. The Minnesota Symposia on Child Psychology. Vol. 13. Hillsdale, NJ: Erlbaum; 1980. pp. 39–101. [Google Scholar]
- Block J, Kremen AM. IQ and ego-resiliency: Conceptual and empirical connections and separateness. Journal of Personality and Social Psychology. 1996;70(2):349–361. doi: 10.1037//0022-3514.70.2.349. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Boxtel AV. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bonanno GA. Loss, Trauma, and Human Resilience: Have We Underestimated the Human Capacity to Thrive After Extremely Aversive Events? American Psychologist. 2004;59(1):20–28. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bonanno GA, Papa A, Lalande K, Westphal M, Coifman K. The importance of being flexible: The ability to both enhance and suppress emotional expression predicts long-term adjustment. Psychological Science. 2004;15(7):482–487. doi: 10.1111/j.0956-7976.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1(3):276–298. [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. American Journal of Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Cheng C. Assessing coping flexibility in real-life and laboratory settings: A multimethod approach. Journal of Personality and Social Psychology. 2001;80(5):814–833. doi: 10.1037//0022-3514.80.5.814. [DOI] [PubMed] [Google Scholar]
- Folkman S, Chesney MA, Christopher-Richards A. Stress and coping in caregiving partners of men with AIDS. Psychiatric Clinics of North America. 1994;17(1):35–53. [PubMed] [Google Scholar]
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist. 2000;55(6):647–654. doi: 10.1037//0003-066x.55.6.647. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Cohn MA, Coffey KA, Pek J, Finkel SM. Open hearts build lives: Positive emotions, induced through living-kindness meditation, build consequential personal resources. Journal of Personality and Social Psychology. 2008;95(5):1045–1062. doi: 10.1037/a0013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Tugade MM, Waugh CE, Larkin GR. What good are positive emotions in crisis? A prospective study of resilience and emotions following the terrorist attacks on the United States on September 11th, 2001. Journal of Personality and Social Psychology. 2003;84(2):365–376. doi: 10.1037//0022-3514.84.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JDE, Barrett LF, Gross JJ. Attention and emotion: Does rating emotion alter neural responses to amusing and sad films? Neuroimage. 2005;27:656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psycholological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention, University of Florida; 1997. [Google Scholar]
- Ong AD, Bergeman CS, Bisconti TL, Wallace KA. Psychological resilience, positive emotions, and successful adaptation to stress in later life. Journal of Personality and Social Psychology. 2006;91(4):730–749. doi: 10.1037/0022-3514.91.4.730. [DOI] [PubMed] [Google Scholar]
- Owren MJ, Bachorowski J-A. The evolution of emotional expression: A "Selfish-Gene" account of smiling and laughter in early hominids and humans. In: Mayne TJ, Bonanno GA, editors. Emotions: Current issues and future directions. New York: Guilford Press; 2001. [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the life experiences survey. Journal of Consulting and Clinical Psychology. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Vythilingham M, Charney DS. The psychobiology of depression and resilience to stress: Implications for prevention and treatment. Annual Review of Clinical Psychology. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Tassinary LG, Cacioppo JT. Unobservable facial actions and emotion. Psychological Science. 1992;3(1):28–33. [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. NeuroImage. 2003;18(3):650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Solhan MB, Tragesser SL, Jahng S, Wood PK, Piasecki TM, et al. Affective instability: Measuring a core feature of Borderline Personality Disorder with ecological momentary assessment. Journal of Abnormal Psychology. 2008;117(3):647–661. doi: 10.1037/a0012532. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology. 2004;86(2):320–333. doi: 10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey J. Exploratory data analysis. Reading: Addison-Wesley; 1977. [Google Scholar]
- Waugh CE, Fredrickson BL, Taylor SF. Adapting to life’s slings and arrows: Individual differences in resilience when recovering from an anticipated threat. Journal of Research in Personality. 2008;42:1031–1046. doi: 10.1016/j.jrp.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh CE, Wager TD, Fredrickson BL, Noll DC, Taylor SF. The neural correlates of trait resilience when anticipating and recovering from threat. Social Cognitive and Affective Neuroscience. 2008;3:322–332. doi: 10.1093/scan/nsn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner EE, Smith RS. Overcoming the odds: High risk children from birth to adulthood. Cornell University Press; 1992. [Google Scholar]
- Westphal M, Seivert NH, Bonanno GA. Expressive flexibility. Emotion. 2010;10(1):92–100. doi: 10.1037/a0018420. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Peyk P. ANSLAB: Autonomic Nervous System Laboratory (Version 2.4) 2005 Retrieved from the SPR Software Repository: http://www.sprweb.org. [Google Scholar]