Abstract
Background & Aims
Saturated free fatty acid (SFA)-stimulated c-Jun NH2-terminal kinase (JNK) activation is associated with the pathogenesis of non-alcoholic fatty liver disease (NAFLD). However, the mechanisms responsible for the effects of SFA are incompletely understood. The goal of this study was to determine the molecular mechanisms by which SFA induce JNK activation in hepatocytes.
Methods
We used siRNA-mediated knockdown in Hepa1c1c7 and AML12 cell lines, as well as primary mouse hepatocytes for these studies.
Results
The current model for JNK activation by SFA involves endoplasmic reticulum (ER) stress, which induces JNK activation through an inositol requiring enzyme 1 (IRE1α)/Apoptosis Regulating Kinase 1 (ASK1)–dependent mechanism. Here, we find that SFA-induced JNK activation is not inhibited in the absence of IRE1α and ASK1. Instead we show that activation of the small GTP-binding proteins Cdc42 and Rac1 is required for SFA-stimulated MLK3-dependent activation of JNK in hepatocytes. In addition, we demonstrate that SFA-induced cell death in hepatocytes is independent of IRE1α, but dependent on Cdc42, Rac1 and MLK3.
Conclusions
Our results demonstrate that Cdc42 and Rac1, rather than ER stress, are important components of a SFA-stimulated signaling pathway that regulates MLK3-dependent activation of JNK in hepatocytes.
Keywords: palmitate, small GTPases, MAP3K, JNK, NAFLD
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a major cause of liver dysfunction in the non-alcoholic, viral hepatitis-negative, population in the USA and Europe [1, 11, 40]. NAFLD is characterized by hepatic steatosis and varying degrees of inflammation and fibrosis. Increasing evidence suggests that saturated free fatty acids (SFA), through induction of signaling pathways, such as the JNK pathway, are a causative link between obesity and NAFLD [26]. Indeed, gene deletion and pharmacological inhibition have established an important role for the JNK signaling pathway in the pathogenesis of NAFLD [13, 19, 30, 33, 36, 39].
Several MAP kinase kinase kinases (MAP3K) are implicated in the regulation of JNK, including MEKK 1–4, MLK 1–4, TAK1, ASK1 and TPL2 [47]. MAP3K provide selectivity for activation of JNK by upstream stimuli. For example, ASK1 mediates ER and oxidative stress-induced JNK activation [27, 42], and we have recently identified MLK3 as an important mediator of SFA-induced JNK activation in mouse embryonic fibroblasts (MEF) [18]. Importantly, JNK activation by different MAP3Ks plays a role in a variety of pathologies. JNK activated by SFA promotes lipoapoptosis and NAFLD [13, 19, 21, 30, 33, 36, 39], while JNK activated by other stimuli may function as a tumor suppressor [6, 34]. Accordingly, while drugs that target JNK directly may be beneficial for the treatment of NAFLD, this approach might be limited by potential side effects. It is likely that inhibition of the specific MAP3K that mediates SFA signaling may provide greater specificity for therapeutic intervention than inhibition of JNK directly, as only SFA activation of JNK would be affected. Therefore, a detailed understanding of the molecular mechanisms by which SFA induce JNK activation is critical to provide specificity for therapeutic intervention.
Molecular mechanisms responsible for the effects of SFA are incompletely understood, but may include ER stress. Cells cope with ER stress by induction of an adaptive protective response to restore homeostasis, known as the unfolded protein response (UPR) [32]. The UPR functions via three signaling pathways mediated by the following stress-sensing ER-resident proteins: PKR like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol requiring enzyme 1 (IRE1) [32]. The goal of this study was to determine the molecular mechanism by which SFA induce JNK activation. Previous studies have linked the UPR to ER stress-induced JNK activation by a mechanism that involves IRE1α and ASK1 [43]. We demonstrate that the small GTPases Cdc42 and Rac1, rather than endoplasmic reticulum (ER) stress, are major contributors to the SFA-stimulated JNK pathway. Together these data establish a new molecular mechanism that mediates the effects of SFA on JNK activation in hepatocytes.
Materials and Methods
Cell lines and cell culture
Hepa1c1c7 and AML12 cells were purchased from ATCC and maintained in αMEM supplemented with 10 % NuSerum (BD) and DMEM:F12 supplemented with 10 % NuSerum (BD), respectively. Primary hepatocytes were isolated from C57BL/6 and MLK3 KO mice as previously described [38]. The animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Cincinnati. IRE1+/+ and IRE1−/− MEF have been described previously [43].
Antibodies and reagents
The antibodies were purchased as follows: MLK3, IRE1, CHOP and ATF4 antibodies from Santa Cruz, Xbp1 antibody from Novus Biologicals, ASK1 antibody from Abcam, tubulin antibody from Sigma, the antibody against the myc-epitope (9B11), phospho-JNK, phospho-p38 MAPK and p38 MAPK from Cell Signaling, Cdc42 and Rac1 antibodies from Upstate Biotechnology and the JNK antibody from Pharmingen. Thapsigargin was from Sigma. Fatty acid free BSA was from Roche. Sodium salts of fatty acids were from Sigma. Preparation of BSA-SFA complexes has been described previously [18].
Immunoprecipitation
Cell extracts were prepared using Triton lysis buffer as described [18]. Cdc42V12 was immunoprecipitated using an antibody against the myc-epitope tag and protein G sepharose. Binding of MLK3 was determined by immunoblot analysis using an antibody against MLK3.
Quantitative PCR
RNA was prepared using the RNeasy Kit from Qiagen according to the manufacturer’s instructions. RNA was reverse transcribed using the iScript cDNA synthesis kit from Biorad and GRP78 and Gadd34 gene expression was determined by quantitative PCR analysis using the Bio-Rad iCycler iQ real-time PCR Detection System and was normalized to the expression of actin using Taqman assays (Mm00517689_g1, Mm00435119_m1, Mm00607939_s1) (Applied Biosystems).
siRNA and transfection
Plasmids were transfected with Lipofectamine 2000 according to the manufacturer’s instructions. siRNAs were transfected using the calcium phosphate method [7]. siRNAs were obtained from Qiagen. The target sequences used for siRNA are: Mm siMLK3 5′–CCCAGACGTCTTGAAGATTCA-3′; Mm siASK1 5′–CTGGATCGAATGAGTATCTTA-3′; Mm siIRE1α 5′–CAGGATGTAAGTGACCGAATA - 3′; Mm Cdc42 5′–TTAAATCAAACTAAAGATTA A-3′; Mm Rac1 5′–AAGCATTTCCTGGAGAGTACA-3′. The Allstars Negative Control siRNA was used as non-targeting control for all siRNA experiments.
GTPase activation assay
CDC42 and Rac1 activation have been analyzed using a Cdc42/Rac1 activation assay kit from Upstate Biotechnology according to the manufacturer’s instructions.
Subcellular fractionation
For subcellular fractionation, cells were homogenized in sample buffer (20 mM Tris, pH 7.4, 300 mM sucrose, 10 mM EGTA, 5 mM EDTA, 0.3 mM PMSF, 5 mM DTT) and centrifuged at 100,000 × g for 1h at 4ºC. The supernatant was collected as cytosolic fraction and the resulting pellet was resuspended in sample buffer containing 1% Triton-X 100 for 30 min, centrifuged at 100,000 × g for 1h and the resulting supernatant was collected as membrane fraction.
Plasmids
The plasmid expression vector for MLK3 was described previously [4]. The point-mutations in the CRIB-domain (I492A/S493A) where introduced using the QuikChange Site-Directed Mutagenesis Kit (Stratagene) with the primer 5′-GACGGCGGCGAGCGTGCCGCCATGCCAC TCGACTTC-3′ and its reverse complement and confirmed by sequencing.
Xbp1 splicing assay
The assay for Xbp1 splicing was described previously [5]. Briefly, RNA was isolated, reverse transcript, amplified and the PCR product was digested with PstI.
Cell death analysis
Apoptosis was evaluated using the Cell Death detection ELISA kit (Roche) and the Caspase-3 cellular assay kit (Enzo) according to the manufacturer’s instructions. Data are presented as mean, with n representing 3 independent experiments. Comparison of two groups was performed using unpaired Student’s t test. A p-value of < 0.05 was regarded as statistically significant.
Results
Role of MLK3 in SFA-induced JNK activation in hepatocytes
We have previously shown that MLK3 deficient mice exhibit reduced levels of steatosis and decreased JNK activation in liver when placed on a high fat diet [18]. However, it is unclear if decreased JNK activation in the liver is due to the loss of MLK3 in hepatocytes or an indirect consequence of failure to develop steatosis. Since we have established that MLK3 is an important mediator of SFA-induced JNK activation in MEF [18], we hypothesized that MLK3 may be important for SFA-induced JNK activation in hepatocytes. To test this hypothesis, we used siRNA to decrease expression of MLK3 in Hepa1c1c7 cells. Decreased expression of MLK3 significantly attenuated, but did not completely abolish SFA-stimulated JNK activation (Supplemental Fig. 1A). To test whether the residual JNK activity is due to incomplete knockdown of MLK3, we prepared primary hepatocytes from wild-type and Mlk3−/− mice. Treatment of wild-type hepatocytes with palmitate caused increased JNK activation, while Mlk3−/− hepatocytes exhibited lower basal JNK activity and markedly reduced JNK activation (Supplemental Fig. 1B), while p38 MAPK phosphorylation was unaffected (Supplemental Fig. 1C). Together, these data suggest that MLK3 serves a major role in SFA-stimulated JNK activation in hepatocytes, although, unlike in MEF, other MAP3K isoforms may also contribute to SFA-induced JNK activation in hepatocytes.
Role of ASK1 in SFA-induced JNK activation
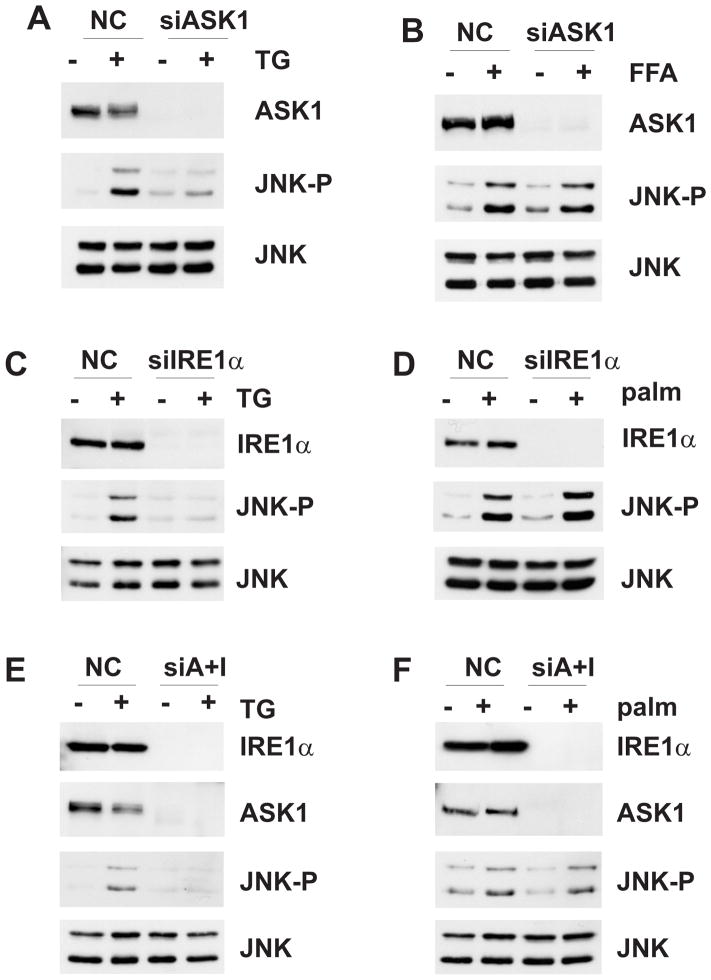
ASK1 is one of the MAP3K isoforms that is likely to contribute to the effects of SFA on JNK activity. Increased levels of SFA have been associated with elevated ER stress [9, 22, 51] and ASK1 is required for ER stress-induced JNK activation [27]. In addition, it has been shown that Ask1−/− mice are protected against diet-induced steatosis [49]. Consistent with earlier studies, we found that treatment of primary wild-type mouse hepatocytes with palmitate induced ER stress as indicated by increased mRNA levels of GRP78 and Gadd34 and increased expression of CHOP, ATF4 and spliced Xbp1 (Supplemental Fig. 2A-C). Similarly, palmitate induced ER stress in MLK3 deficient primary hepatocytes (Supplemental Fig. 2A-C), indicating that MLK3-mediated, SFA-induced JNK activation is either downstream or in parallel with UPR induction. Interestingly, loss of MLK3 attenuated JNK activation by thapsigargin, a compound commonly used to induce ER stress, but did not affect Xbp1 splicing in primary hepatocytes, supporting a role for MLK3 in ER stress-induced JNK activation downstream of the UPR (Supplemental Fig. 2D,E). As expected, depletion of ASK1 inhibited JNK activation by thapsigargin (Fig. 1A). However, loss of ASK1 did not affect palmitate-induced JNK activation (Fig. 1B). Together, these data suggest that ASK1 has a major role in JNK activation by chemically-induced ER stress, but has either no role or a redundant role in SFA-induced JNK activation in hepatocytes.
Fig. 1. Effects of IRE1α and ASK1 depletion on JNK activation.
Hepa1c1c7 cells were transfected with non-silencing control or siRNA specific for ASK1 and incubated with 1 μM thapsigargin for 1 h (A) or with 0.5 mM palmitic acid for 6 h (B). The efficacy of ASK1 depletion and the expression and phosphorylation of JNK was examined by immunoblot analysis. Hepa1c1c7 cells were transfected with non-silencing control or siRNA specific for IRE1α and incubated with 1 μM thapsigargin for 1 h (C) or with 0.5 mM palmitate for 6 h (D). The expression of IRE1α and the expression and phosphorylation of JNK was examined by immunoblot analysis. Hepa1c1c7 cells were transfected with non-silencing control or siRNA specific for IRE1α and ASK1 and incubated with 1 μM thapsigargin for 1 h (E) or with 0.5 mM palmitate for 6 h (F). The expression of IRE1α and ASK1 and the expression and phosphorylation of JNK was examined by immunoblot analysis.
Role of IRE1α in SFA-induced JNK activation
Since the threshold for JNK activation may vary with different types of stresses, and because our siRNA did not completely abolish ASK1 protein, we also depleted IRE1α the ER stress-activated upstream regulator of ASK1 Cells depleted of IRE1α demonstrated inhibition of thapsigargin-induced Xbp1 splicing (Supplemental Fig. 2A) and attenuated thapsigargin-induced JNK activation (Fig. 1C). However, IRE1α depletion had no effect on palmitate-induced JNK activation (Fig. 1D), despite complete inhibition of palmitate-induced Xbp1 splicing (Supplemental Fig. 2B). We next combined depletion of ASK1 and IRE1α by siRNA to further decrease the possibility of activating any residual ASK1 protein. Depletion of IRE1α and ASK1 together resulted in inhibition of thapsigargin-induced JNK activation (Fig. 1E), but did not affect palmitate-induced JNK activation (Fig. 1F). Together, these data suggest that the IRE1α/ASK1 axis has a major role in JNK activation by chemically-induced ER stress, but has either no role or a redundant role in SFA-induced JNK activation.
Activation of Cdc42 and Rac1 by SFA
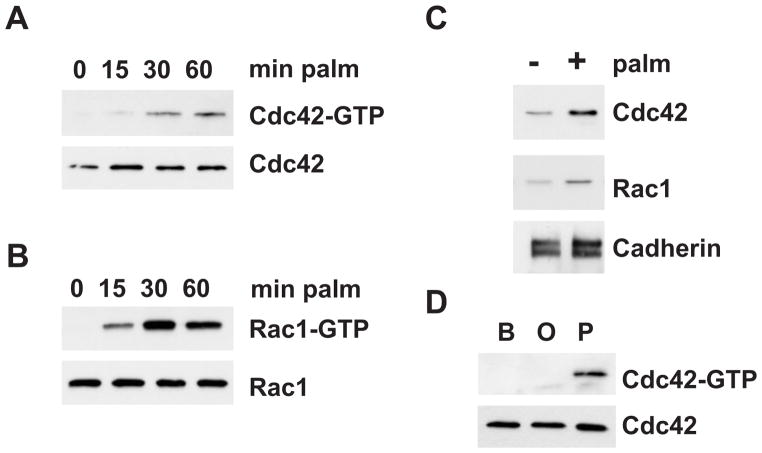
Cdc42 and Rac1 have been established as critical regulators of the JNK pathway [12], and it has been shown that Cdc42 regulates MLK3-dependent JNK activation [2]. Therefore, we examined the role of Cdc42 and Rac1 in SFA-induced JNK activation. Using a GST-PBD pulldown assay that specifically recognizes the active, GTP-bound GTPase, we found that palmitate led to activation of Cdc42 and Rac1 in a time-dependent manner with activation being apparent at 15 min and lasting up to 60 min (Fig. 2A,B). It has been well documented that activation of Cdc42 and Rac1 is associated with their translocation to the membrane [14]. Therefore we performed subcellular fractionations to monitor the distribution of Cdc42 and Rac1 by immunoblot analysis. Indeed, we observed increased Cdc42 and Rac1 translocation to the membrane in response to palmitate treatment (Fig. 2C). To test the requirement of different SFA for Cdc42 activation cells were incubated with oleic or palmitic acid. Palmitate, but not oleate resulted in Cdc42 activation, as measured by GST-PBD pulldown assay (Fig. 2D). Together, these data demonstrate that the small GTPases Cdc42 and Rac1 are activated by saturated SFA.
Fig. 2. Activation of Cdc42 and Rac1 SFA.
AML12 cells were incubated with 0.5 mM palmitate for the indicated time points. Cdc42 (A) and Rac1 (B) activation was determined by binding to GST-PAK PBD and subsequent immunoblot analysis. (C) AML12 cells were incubated with palmitate for 30 min. Cells were fractionated and the membrane fraction was analyzed for translocation of Cdc42 and Rac1 by immunoblot analysis. (D) AML12 cells were incubated with BSA (B), oleate (O) or palmitate (P). Cdc42 activation was determined by binding to GST-PAK PBD and subsequent immunoblot analysis.
Role of Cdc42 and Rac1 in SFA-induced JNK activation
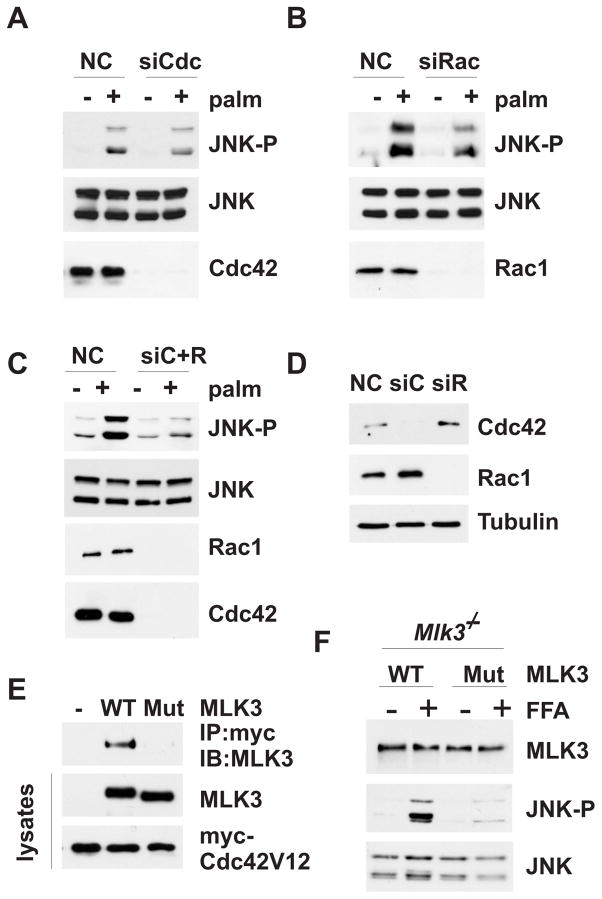
Based on previous studies demonstrating a role for Cdc42 and Rac1 in JNK activation [12] and our observation that Cdc42 and Rac1 are activated by SFA, we hypothesized that Cdc42 and Rac1 may be required for SFA-induced activation of JNK in hepatocytes. Surprisingly, decreased expression of Cdc42 by siRNA treatment had little effect on SFA-stimulated JNK activation (Fig. 3A). Since the Cdc42-related GTPase Rac1 can also activate JNK, and we observed compensatory upregulation of Rac1 upon loss of Cdc42 (Fig. 3D), we also tested the effect of decreased Rac1 expression on SFA-stimulated JNK activation. Depletion of Rac1 only partially decreased SFA-induced JNK activation (Fig. 3B); however, combined depletion of Cdc42 and Rac1 strongly inhibited SFA-stimulated JNK activation (Fig. 3C), indicating that the small GTPases are required for, but function redundantly in SFA-induced JNK activation.
Fig. 3. Requirement of Cdc42 and Rac1 in SFA-induced JNK activation.
(A, B and C) Hepa1c1c7 cells were transfected with non-silencing control or siRNA specific for Cdc42 or Rac1. Cells were incubated with 0.5 mM palmitate. Expression of Cdc42, Rac1 and JNK and JNK phosphorylation was examined by immunoblot analysis. (D) Hepa1c1c7 cells were transfected as in (A-C). The expression of Cdc42 and Rac1 were examined by immunoblot analysis. Tubulin expression was used as loading control. (E) Hepa1c1c7 cells we co-transfected with myc-tagged Cdc42V12 and WT or mutant MLK3. Cdc42V12 was immuno-precipitated using an antibody against the myc-epitope tag. Binding of MLK3 was determined by immunoblot analysis using an antibody against MLK3. Transfection efficiency of Cdc42V12 and MLK3 was determined by immunoblot analysis of total lysates. (F) Primary MLK3 KO hepatocytes were transfected with WT or mutant MLK3 and treated with palmitate for 6 h. The expression of MLK3 and the expression and phosphorylation of JNK was examined by immunoblot analysis.
Previous studies established a role for Cdc42 in MLK3 activation (2). Thus, an activated mutant of Cdc42 (Cdc42V12) will bind to the MLK3 Cdc42/Rac1 binding (CRIB) motif which is thought to promote release of autoinhibition, leucine-zipper-mediated dimerization and autophosphorylation of MLK3. Therefore, we decided to test whether the role of Cdc42 in SFA-stimulated JNK activation is MLK3 dependent. For this experiment, we introduced a point mutation in the MLK3 CRIB motif that has been shown to disrupt binding of MLK3 to Cdc42 [2]. We co-expressed myc-tagged constitutively active Cdc42 (myc-Cdc42V12) and either MLK3 WT or MLK3 Cdc42-binding mutant in Hepa1c1c7 cells to verify the binding characteristics of the overexpressed Cdc42 and MLK3 proteins. Co-immunoprecipitation studies confirmed that wild-type, but not mutant MLK3 binds to activated Cdc42 (Fig. 3E). We then re-expressed either MLK3 wild-type or MLK3 Cdc42-binding mutant in mouse Mlk3−/− primary hepatocytes, examined SFA-induced JNK activation and found that wild-type, but not Cdc42-binding mutant MLK3 rescued SFA-induced JNK activation (Fig. 3F), suggesting that direct interaction between Cdc42 and MLK3 is required for SFA-induced JNK activation. This observation suggests that the activation mechanism of MLK3 is conserved for different stimuli, and that Cdc42 functions in a SFA-stimulated signaling pathway that regulates MLK3-dependent activation of JNK in hepatocytes.
Role of Cdc42, Rac1 and MLK3 in lipoapoptosis
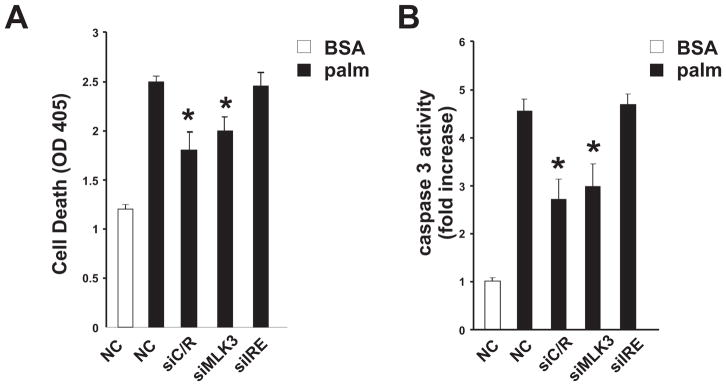
It has been demonstrated that prolonged exposure to elevated levels of SFA leads to cell death that is dependent on JNK activation [21]. Thus, we hypothesized that loss of Cdc42 and Rac1 or MLK3, but not loss of IRE1α would protect from SFA-induced cell death. To test this hypothesis, Hepa1c1c7 cells were transfected with a non-silencing control siRNA or siRNA against Cdc42/Rac1, MLK3 or IRE1α. Transfected cells were then treated with palmitate for 16h and cell death was evaluated by measuring DNA fragmentation and caspase-3 activation. Cells depleted of Cdc42/Rac1 or MLK3 were partially protected from SFA-induced cell death (Fig. 4A,B). In contrast, cells depleted of IRE1α were not protected, supporting the hypothesis that Cdc42/ Rac1 and MLK3, but not IRE1α mediate cell death induced by SFA. These studies suggest that Cdc42/Rac1 and MLK3 are part of a SFA-stimulated signaling pathway that regulates JNK-dependent death in hepatocytes and further support our finding that SFA-induced JNK activation is dependent on Cdc42/Rac1 and MLK3 rather than IRE1α and ASK1.
Fig. 4. Role of Cdc42, Rac1 and MLK3 in lipoapoptosis.
Hepa1c1c7 cells were transfected with non-silencing control or siRNA specific for Cdc42/Rac1, MLK3 or IRE1α and treated with BSA or palmitate for 16 h. Cell death was examined by DNA fragmentation (A) and caspase-3 activation (B) (mean±SD, n=3, *p<0.05).
Discussion
Our studies demonstrate an important role for the small GTPases Cdc42 and Rac1 in SFA-induced JNK activation in hepatocytes. Cdc42 and Rac1 have been established as critical regulators of the JNK pathway. Thus, loss of Cdc42 or expression of dominant-negative mutants of Cdc42 or Rac1 inhibits JNK activation in response to oncogenic exchange factors, growth factors and inflammatory cytokines [12]. Our data using siRNA-mediated depletion of Cdc42 and Rac1 demonstrate that the small GTP-binding proteins are also critically involved in SFA-induced JNK activation (Fig. 3).
Previously it has been demonstrated that activated mutants of Cdc42 induce JNK activation in a manner that is dependent on MLK3 [2]. Here, we show that direct interaction of Cdc42 and MLK3 is required for SFA-induced JNK activation (Fig. 3), indicating that the activation mechanism of MLK3 is conserved for different stimuli. Together, these observations demonstrate that the small GTPases Cdc42 and Rac1 are major components of a SFA-stimulated signaling pathway that regulates MLK3-dependent activation of JNK in hepatocytes.
A major unresolved question is the mechanism by which SFA activate Cdc42 and Rac1. SFA lead to intracellular increases in diacylglycerol (DAG) and ceramide levels [24, 37]. Possible targets for DAG include the PKC family of protein kinases, consistent with previous studies that placed MLK3 downstream of PKC [18]. Interestingly, it has been shown that phorbol ester-treatment leads to PKC-dependent activation of Cdc42 and Rac1 [8]. This mechanism could contribute to the activation of Cdc42 and Rac1 by SFA. Alternatively, SFA could regulate Cdc42 and Rac1 in a ceramide-dependent manner. Indeed, it has been shown that in β-cells, palmitate-induced Rac1 activation requires de novo synthesis of ceramide [41]. A third possibility includes involvement of a specific GEF, GAP or RhoGDI in SFA-induced Cdc42 and Rac1 activation. An important goal for future studies will be to define the molecular mechanisms by which SFA regulate Cdc42 and Rac1.
Previous studies have established that IRE1α in complex with ASK1 is required for ER stress-stimulated JNK activation [43] and that increased levels of SFA result in ER stress [3, 46]. Therefore it has been suggested that IRE1α and ASK1 may play a critical role in SFA-induced JNK activation. Indeed, our studies confirm a major role for IRE1α and ASK1 for JNK activation in response to chemically induced ER stress (Fig. 1). Furthermore, in agreement with previous studies, we observe induction of the UPR in response to elevated SFA levels (Supplemental Fig. 2). However, our data indicate that IRE1α and ASK1 are either not required or are functionally redundant in SFA-stimulated JNK activation (Fig. 1). It has been demonstrated that ER stress-induced JNK activation requires TNFR1 expression and that ER stress increases expression of endogenous TNFα [15, 50] placing TNF signaling downstream of ER stress. Therefore the decrease of thapsigargin-induced JNK activation in MLK3 deficient cells (Supplemental Fig. 2D) may either reflect a direct requirement for MLK3 in ER stress mediated JNK activation or may be an indirect effect, due to the requirement of MLK3 for TNFα induced JNK activation [4]. Treatment of obese mice with chemical chaperones, such as the histone deacetylase inhibitor phenylbutyric acid (PBA) or the bile acid tauroursodeoxycholate (TUDCA) has been shown to reduce ER stress and lower JNK activation [28]. It is possible that chemical chaperones lower obesity-induced JNK activation through other functions, independently of their effects on ER stress. Alternatively, it is possible that other ER stress pathways play a role in SFA-induced JNK activation. For example, recent studies have suggested a role for double-stranded RNA-dependent protein kinase (PKR) in mediating JNK activation in response to ER stress, as well as nutrient stress [25]. Therefore, an important goal for future studies will be to address the role of PKR in SFA-induced JNK activation in hepatocytes.
It has been established that JNK activation promotes lipoapoptosis in hepatocytes [21, 29]. Here we demonstrate that loss of IRE1α does not affect SFA-stimulated JNK activation and lipoapoptosis (Fig. 6). This observation is consistent with the suggested survival function of IRE1α [20] and contrasts with the proapoptotic function of JNK in SFA-induced cell death. Instead, we show that decreased JNK activation in Cdc42/Rac1 and MLK3 deficient cells correlates with attenuation of palmitate-induced cell death, suggesting a role for MLK3 in SFA-induced death in hepatocytes. Indeed, MLK3 has been shown to mediate JNK activation in some forms of stress-induced apoptosis [10, 16, 31, 48]. Interestingly, it was recently demonstrated that GSK-3 promotes JNK-dependent lipoapotosis [17]. Several studies have suggested that GSK-3 cooperates with MLK3 in JNK activation [23, 45]. The mechanism by which GSK-3 regulates MLK3 is unclear. It has been suggested that GSK-3 may directly phosphorylate MLK3 at residues S793 and S789 [23]. Alternatively, since many GSK-3 substrates require prior phosphorylation at a Ser/Thr residue located four residues carboxy-terminal to the site of GSK-3 phosphorylation, it is also possible that S793 is first phosphorylated by a proline-directed “priming kinase” before GSK-3 phosphorylates S789 on MLK3 [23]. Although residue S793 has been reported as in vivo phosphorylation site, the kinase responsible has not been identified [44]. Interestingly, it has been suggested that MLK3 is regulated by JNK-mediated positive feedback phosphorylation [35]. This raises the possibility that the proline-directed kinase JNK may phosphorylate S793, thereby “priming” MLK3 for GSK-3 phosphorylation. This positive feedback loop may lead to sustained JNK activation resulting in lipoapoptosis. Therefore an important goal for future studies will be to address the role of GSK-3 in MLK3-dependent JNK activation.
In summary, we have identified Cdc42 and Rac1 as major contributors to a SFA-stimulated signaling pathway that regulates MLK3-dependent activation of JNK in hepatocytes. These studies reveal a novel mechanism by which SFA activate JNK and have important implications for the use of the JNK pathway as drug target for the treatment of NAFLD.
Supplementary Material
Acknowledgments
We thank Dr. Roger Davis for providing Mlk3−/− mice and plasmid expression vectors for MLK3 and Cdc42. We thank Drs. Patrick B. Dennis and Carol Mercer for critical reading of the manuscript.
Financial Support: These studies were supported in part by NIH grant DK082583 to AJ.
Abbreviations
- SFA
saturated free fatty acids
- JNK
c-Jun NH2-terminal kinase
- Cdc42
cell division cycle protein 42
- Rac1
ras-related C3 botulinum toxin substrate 1
- NAFLD
non-alcoholic fatty liver disease
- IRE1
inositol requiring enzyme 1
- ASK1
Apoptosis Regulating Kinase 1
- MLK3
mixed lineage kinase 3
- MAP3K
mitogen-activated protein kinase kinase k kinase
- MEF
mouse embryonic fibroblasts
Footnotes
Conflict of Interest: The authors who have taken part in this study declare that they have nothing to disclose with respect to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002;17 (Suppl):S186–190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 2.Bock BC, Vacratsis PO, Qamirani E, Gallo KA. Cdc42-induced activation of the mixed-lineage kinase SPRK in vivo. Requirement of the Cdc42/Rac interactive binding motif and changes in phosphorylation. J Biol Chem. 2000;275(19):14231–14241. doi: 10.1074/jbc.275.19.14231. [DOI] [PubMed] [Google Scholar]
- 3.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47(12):2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Brancho D, Ventura JJ, Jaeschke A, Doran B, Flavell RA, Davis RJ. Role of MLK3 in the regulation of mitogen-activated protein kinase signaling cascades. Mol Cell Biol. 2005;25(9):3670–3681. doi: 10.1128/MCB.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 6.Cellurale C, Weston CR, Reilly J, Garlick DS, Jerry DJ, Sluss HK, et al. Role of JNK in a Trp53-dependent mouse model of breast cancer. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CA, Okayama H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques. 1988;6(7):632–638. [PubMed] [Google Scholar]
- 8.Choi WH, Kim J, Lee YR, Lee CK, Kim YS, Choi YJ, et al. Cdc42 contributes to phorbol ester-induced Ca2+-independent contraction of pulmonary artery smooth muscle. J Vet Med Sci. 2005;67(8):787–793. doi: 10.1292/jvms.67.787. [DOI] [PubMed] [Google Scholar]
- 9.Cnop M, Igoillo-Esteve M, Cunha DA, Ladriere L, Eizirik DL. An update on lipotoxic endoplasmic reticulum stress in pancreatic beta-cells. Biochem Soc Trans. 2008;36:909–915. doi: 10.1042/BST0360909. [DOI] [PubMed] [Google Scholar]
- 10.Cole ET, Zhan Y, Abi Saab WF, Korchnak AC, Ashburner BP, Chadee DN. Mixed lineage kinase 3 negatively regulates IKK activity and enhances etoposide-induced cell death. Biochim Biophys Acta. 2009;1793(12):1811–1818. doi: 10.1016/j.bbamcr.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44(1):197–208. doi: 10.1016/j.jhep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, et al. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81(7):1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 13.Czaja MJ. JNK regulation of hepatic manifestations of the metabolic syndrome. Trends Endocrinol Metab. 2010;21 (12):707–713. doi: 10.1016/j.tem.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etienne-Manneville S. Cdc42--the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 15.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26(8):3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Humphrey RK, Newcomb CJ, Yu SM, Hao E, Yu D, Krajewski S, et al. Mixed lineage kinase-3 stabilizes and functionally cooperates with TRIBBLES-3 to compromise mitochondrial integrity in cytokine-induced death of pancreatic beta cells. J Biol Chem. 285(29):22426–22436. doi: 10.1074/jbc.M110.123786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim SH, Akazawa Y, Cazanave SC, Bronk SF, Elmi NA, Werneburg NW, et al. Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol. 2010 doi: 10.1016/j/jhep2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell. 2007;27(3):498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodama Y, Kisseleva T, Iwaisako K, Miura K, Taura K, De Minicis S, et al. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137(4):1467–1477. doi: 10.1053/j.gastro.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318(5852):944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 22.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28(4):360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra R, Barthwal MK, Sondarva G, Rana B, Wong L, Chatterjee M, et al. Glycogen synthase kinase-3beta induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem. 2007;282(42):30393–30405. doi: 10.1074/jbc.M705895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montell E, Turini M, Marotta M, Roberts M, Noe V, Ciudad CJ, et al. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab. 2001;280(2):E229–237. doi: 10.1152/ajpendo.2001.280.2.E229. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, et al. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140(3):338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nehra V, Angulo P, Buchman AL, Lindor KD. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46(11):2347–2352. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- 27.Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, et al. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16(11):1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 298(5):E1027–1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 31.Rangasamy V, Mishra R, Mehrotra S, Sondarva G, Ray RS, Rao A, et al. Estrogen suppresses MLK3-mediated apoptosis sensitivity in ER+ breast cancer cells. Cancer Res. 70(4):1731–1740. doi: 10.1158/0008-5472.CAN-09-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 33.Sabio G, Cavanagh-Kyros J, Ko HJ, Jung DY, Gray S, Jun JY, et al. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10(6):491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saxena NK, Fu PP, Nagalingam A, Wang J, Handy J, Cohen C, et al. Adiponectin Modulates C-Jun N-Terminal Kinase and Mammalian Target of Rapamycin and Inhibits Hepatocellular Carcinoma. Gastroenterology. 2010;139:1762–1773. doi: 10.1053/j.gastro.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schachter KA, Du Y, Lin A, Gallo KA. Dynamic positive feedback phosphorylation of mixed lineage kinase 3 by JNK reversibly regulates its distribution to Triton-soluble domains. J Biol Chem. 2006;281(28):19134–19144. doi: 10.1074/jbc.M603324200. [DOI] [PubMed] [Google Scholar]
- 36.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43(1):163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem. 1999;274(34):24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 38.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 39.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49(1):87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001;35(2):195–199. doi: 10.1016/s0168-8278(01)00094-0. [DOI] [PubMed] [Google Scholar]
- 41.Syed I, Jayaram B, Subasinghe W, Kowluru A. Tiam1/Rac1 signaling pathway mediates palmitate-induced, ceramide-sensitive generation of superoxides and lipid peroxides and the loss of mitochondrial membrane potential in pancreatic beta-cells. Biochem Pharmacol. 2010;80(6):874–883. doi: 10.1016/j.bcp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, et al. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2(3):222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 44.Vacratsis PO, Phinney BS, Gage DA, Gallo KA. Identification of in vivo phosphorylation sites of MLK3 by mass spectrometry and phosphopeptide mapping. Biochemistry. 2002;41(17):5613–5624. doi: 10.1021/bi016075c. [DOI] [PubMed] [Google Scholar]
- 45.Wang MJ, Huang HY, Chen WF, Chang HF, Kuo JS. Glycogen synthase kinase-3beta inactivation inhibits tumor necrosis factor-alpha production in microglia by modulating nuclear factor kappaB and MLK3/JNK signaling cascades. J Neuroinflammation. 2010;7:99. doi: 10.1186/1742-2094-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291(2):E275–281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 47.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Xu Z, Maroney AC, Dobrzanski P, Kukekov NV, Greene LA. The MLK family mediates c-Jun N-terminal kinase activation in neuronal apoptosis. Mol Cell Biol. 2001;21(14):4713–4724. doi: 10.1128/MCB.21.14.4713-4724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto E, Dong YF, Kataoka K, Yamashita T, Tokutomi Y, Matsuba S, et al. Olmesartan prevents cardiovascular injury and hepatic steatosis in obesity and diabetes, accompanied by apoptosis signal regulating kinase-1 inhibition. Hypertension. 2008;52(3):573–580. doi: 10.1161/HYPERTENSIONAHA.108.112292. [DOI] [PubMed] [Google Scholar]
- 50.Yang Q, Kim YS, Lin Y, Lewis J, Neckers L, Liu ZG. Tumour necrosis factor receptor 1 mediates endoplasmic reticulum stress-induced activation of the MAP kinase JNK. EMBO Rep. 2006;(6):622–627. doi: 10.1038/sj.embor.7400687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye J. Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle. Endocr Metab Immune Disord Drug Targets. 2007;7(1):65–74. doi: 10.2174/187153007780059423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.