Abstract
Phrenic motoneurons are located in the cervical spinal cord and innervate the diaphragm muscle, the main inspiratory muscle in mammals. Similar to other skeletal muscles, phrenic motoneurons and diaphragm muscle fibers form motor units which are the final element of neuromotor control. In addition to their role in sustaining ventilation, phrenic motor units are active in other non-ventilatory behaviors important for airway clearance such as coughing or sneezing. Diaphragm muscle fibers comprise all fiber types and are commonly classified based on expression of contractile proteins including myosin heavy chain isoforms. Although there are differences in contractile and fatigue properties across motor units, there is a matching of properties for the motor neuron and muscle fibers within a motor unit. Motor units are generally recruited in order such that fatigue-resistant motor units are recruited earlier and more often than more fatigable motor units. Thus, in sustaining ventilation, fatigue-resistant motor units are likely required. Based on a series of studies in cats, hamsters and rats, an orderly model of motor unit recruitment was proposed that takes into consideration the maximum forces generated by single type-identified diaphragm muscle fibers as well as the proportion of the different motor unit types. Using this model, eupnea can be accomplished by activation of only slow-twitch diaphragm motor units and only a subset of fast-twitch, fatigue-resistant units. Activation of fast-twitch fatigable motor units only becomes necessary when accomplishing tasks that require greater force generation by the diaphragm muscle, e.g., sneezing and coughing.
1. Introduction
Motor output of skeletal muscles is determined by the function of motoneurons and the muscle fibers they innervate which together form a motor unit. Phrenic motoneurons innervate the diaphragm muscle, are located within the ventral horn (lamina IX) of the cervical spinal cord and receive rhythmic excitatory drive from medullary premotor neurons (Ellenberger and Feldman, 1988; Feldman et al., 1985). In mammals, phrenic motoneurons show remarkably similar anatomical location: C3-C5 segments in rats (Mantilla et al., 2009; Prakash et al., 2000; Song et al., 2000), C3-C6 in mice (Qiu et al., 2010), C4-C6 in cats (Webber et al., 1979), C5-C7 in ferrets (Yates et al., 1999), and C3-C5 in humans (Keswani and Hollinshead, 1955). Phrenic motor units are the final element of respiratory neuromotor control.
Respiratory muscles such as the diaphragm muscle are distinct from other skeletal muscles in that they must function from birth onwards in sustaining ventilation and display high levels of activation. In fact, active phrenic motor units in the rat display a daily duty cycle (ratio of active to inactive time) ~ 35% (Kong and Berger, 1986). In the rat hind limb, duty cycles are ~2% for the extensor digitorum longus and ~14% for the soleus muscle (Hensbergen and Kernell, 1997). In addition, phrenic motor units contribute to non-ventilatory behaviors, including swallowing, vocalization, and expulsive behaviors important for airway clearance (Mantilla et al., 2010; Sieck and Fournier, 1989).
Properties of the motor unit population critically determine the function of a skeletal muscle in accomplishing specific motor tasks. Neuromotor control is exerted by the recruitment of motor units with different functional properties (Fournier and Sieck, 1988; Sieck, 1988) and frequency coding of neural activation (Iscoe et al., 1976). Indeed, motor units display considerable diversity in their contractile and fatigue properties (Burke et al., 1973; Fournier and Sieck, 1988). Contractile properties generally match the histochemical and biochemical properties of a motor unit (Butler et al., 1999; Sieck, 1991b, 1994; Su et al., 1997). Importantly, within a single motor unit, these properties are matched (Enad et al., 1989; Sieck et al., 1989a).
The range of muscle force generated during different motor behaviors is determined by the combined contractile and fatigue properties of recruited motor units (Clamann, 1993). Motor unit recruitment also determines the muscle response to the varying mechanical demands that are imposed and thus provides functional limits on the motor tasks that can be accomplished. In this review, we will focus on the role of motor unit recruitment in determining the range of forces generated by the diaphragm muscle in accomplishing both ventilatory (resting – eupneic – ventilation and the response to hypoxic or hypercarbic conditions) and non-ventilatory behaviors (associated with airway occlusion and clearance, e.g., sneezing).
2. Classification of Motor Unit Types
Motor units are commonly classified into different types according to the properties of their muscle fibers (Burke, 1981; Fournier and Sieck, 1988; Kernell, 2006; Sieck et al., 1989a). Importantly, the muscle fibers within a motor unit display homogeneous type composition (Fournier and Sieck, 1988; Hamm et al., 1988; Nemeth et al., 1986). Indeed, all muscle fibers within a phrenic motor unit are of the same fiber type in adult rats (Johnson et al., 1994) and cats (Enad et al., 1989; Sieck et al., 1989a; Sieck et al., 1996).
Four types of motor units are generally considered. Type S motor units exhibit slower contraction times than other motor unit types, but are more resistant to fatigue. Muscle fibers in type S motor units express myosin heavy chain isoform slow (MyHCSlow) and have high mitochondrial volume density and oxidative capacity (Enad et al., 1989; Sieck et al., 1996). Motor units that display faster contraction times comprise 3 types with varying resistance to fatigue. Muscle fibers in fatigue-resistant type FR motor units express MyHC2A and have higher mitochondrial volume density and oxidative capacity compared to highly fatigable type FF motor units, which comprise muscle fibers expressing both MyHC2B and MyHC2X. Fast motor units that are fatigue-intermediate (type FInt) comprise muscle fibers that express only the MyHC2X isoform and have intermediate mitochondrial volume density and oxidative capacity (Sieck et al., 1996).
Muscle fibers also exhibit varying mechanical properties across motor unit types including maximum specific force (force per unit cross-sectional area), force-Ca2+ relationships underlying submaximal activation (Geiger et al., 2000; Geiger et al., 1999) and cross bridge cycling kinetics (Sieck and Prakash, 1997). Diaphragm muscle fibers at type FInt and FF units display greater cross-sectional area (Lewis and Sieck, 1990; Miyata et al., 1995; Prakash et al., 2000; Sieck et al., 1989b; Zhan et al., 1997) and greater maximum specific force (Geiger et al., 2002; Geiger et al., 2001; Geiger et al., 2000; Geiger et al., 1999) than fibers at type S and FR units. In addition, the number of muscle fibers innervated by a phrenic motoneuron (i.e., innervation ratio) varies across motor unit types with greater innervation ratios at type FInt and FF motor units than at type S or FR units (Sieck, 1988). Taken together, the larger fiber size, greater specific force and greater innervation ratio result in substantially greater forces being generated by type FInt and FF motor units compared to type S and FR units (Fig. 1) (Mantilla et al., 2010).
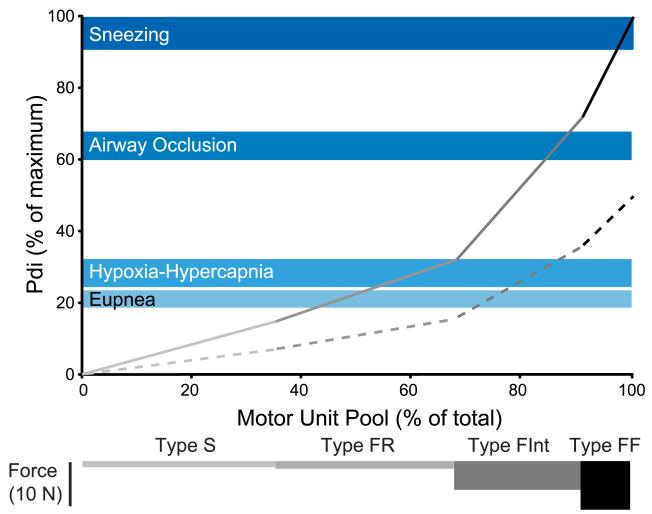
Figure 1.
Model of motor unit recruitment for the rat diaphragm muscle (modified from (Mantilla et al., 2010). Similar models of motor unit recruitment have also been developed in cats and hamsters (Sieck, 1991b, 1994; Sieck and Fournier, 1989). An orderly recruitment of motor units was assumed (type S → type FR → type FInt → type FF) and complete activation of all motor units of each type was assumed before the next type would be recruited. Motor unit properties were based on previous reports of motor unit innervation ratios and measurements of maximum specific force (force per cross-sectional area) in single type-identified fibers, cross-sectional area and proportion of different fiber types in the diaphragm muscle (Geiger et al., 2002; Geiger et al., 2001; Geiger et al., 2000; Geiger et al., 1999; Lewis and Sieck, 1990; Miyata et al., 1995; Prakash et al., 2000; Sieck, 1988; Sieck et al., 1989b; Zhan et al., 1997). Force elicited by maximal motor unit activation for each unit type is shown in bars at the bottom of the figure (motor unit types are identified in shades of gray). Forces generated during ventilatory behaviors (eupnea and hypoxia-hypercapnia) and non-ventilatory behaviors (airway occlusion and sneezing) were obtained from transdiaphragmatic pressure (Pdi) measurements and expressed as percent of maximum Pdi (shown in shades of blue. Shaded area represents the mean ± SE). Maximum Pdi was obtained by bilateral phrenic nerve stimulation. The increasing slope of force development represents additional motor units being recruited at maximal discharge frequency (solid line) and at 50% maximal frequency (dashed line). Forces generated during ventilatory behaviors requires recruitment of type S and FR motor units in the rat diaphragm assuming maximal discharge rates and additional recruitment of type FInt units at submaximal rates. Diaphragm muscle forces generated in response to airway occlusion require recruitment of nearly all type S, FR and FInt units. Sneezing requires recruitment of type FF units.
There is considerable morphological heterogeneity in motoneurons, even within a single pool such as the phrenic motor pool (Burke et al., 1992; Cameron et al., 1985; Cameron and Fang, 1989; Issa et al., 2010; Mantilla et al., 2009; Prakash et al., 2000; Qiu et al., 2010; Torikai et al., 1996). Adult rat phrenic motoneurons show a bimodal distribution with a greater than three-fold variation in motoneuron surface area (Prakash et al., 2000). This heterogeneity is established postnatally at a time when motor unit diversity becomes evident, and in fact, motoneuron soma growth matches the growth of diaphragm muscle fibers (Mantilla and Sieck, 2008; Prakash et al., 2000). Motoneuron morphology, in particular dendritic arborization and somal dimensions, contribute to differences in intrinsic electrophysiological properties across motoneurons (Cushing et al., 2005; Su et al., 1997; van Lunteren and Dick, 1992). Intrinsic properties of motoneurons may depend on motor unit type. For instance, motoneurons at type S motor units are smaller (exhibiting the highest input resistance), more excitable (i.e., lowest rheobase) and display the slower axonal conduction velocities than motoneurons at type FF motor units (Burke, 1981; Zengel et al., 1985). The orderly recruitment of motor units of different types may thus determine the gradation of force development across motor behaviors.
3. Orderly Recruitment of Motor Units
Motor units are recruited in an orderly fashion. Based on the intrinsic electrophysiological properties of motoneurons, for a given synaptic input, smaller more excitable motoneurons with smaller axons and slower conduction velocities would be recruited before larger motoneurons. There is substantial evidence for this recruitment pattern (Butler et al., 1999; Kernell, 2006; Sieck and Fournier, 1989), consistent with the “size principle” (Gordon et al., 2004; Henneman et al., 1965). Indeed, recruitment of motor units generally matches their mechanical and fatigue properties: type S and FR motor units are recruited first, followed by type FInt and FF units. In support of the “size principle”, multiple studies have shown that phrenic motoneurons with slower axonal conduction velocities were recruited first during inspiratory efforts (Dick et al., 1987; Jodkowski et al., 1987, 1988). Also in agreement with Henneman’s “size principle”, motor unit type also is an important determinant of motor unit recruitment order (Burke et al., 1973; Mendell, 2005; Sypert and Munson, 1981). In fact, the force developed by a motor unit is a strong indicator of its recruitment order (Zajac and Faden, 1985).
3.1. Diaphragm Muscle Force – Transdiaphragmatic Pressure
In previous studies in cats (Fournier and Sieck, 1988; Sieck and Fournier, 1989), hamsters (Sieck, 1991b, 1994) and more recently in rats (Mantilla et al., 2010), transdiaphragmatic pressure (Pdi) measurements were used as an estimate of diaphragm muscle force generated during different ventilatory and non-ventilatory behaviors. Bilateral supramaximal phrenic nerve stimulation was used to obtain the maximum Pdi (Pdimax) in anesthetized animals. In cats, Pdi generated during quiet breathing (eupnea) was ~12% of Pdimax, whereas in hamsters and rats, eupneic Pdi was ~27% and ~21% of Pdimax (Fig. 1), respectively. In humans, estimates of the Pdi generated by the diaphragm muscle during eupnea are approximately 10% of Pdimax (Sieck, 1994). These observations suggest that Pdi scales with animal size.
When ventilation was stimulated by exposing animals to gas mixtures that were hypoxic (10% O2) and hypercapnic (5% CO2), diaphragm muscle forces increased to ~28% Pdimax in cats and rats (Mantilla et al., 2010; Sieck and Fournier, 1989). Exposure to hypoxia-hypercapnia represents a robust ventilatory stimulus but it clearly does not approximate maximal force generation by the diaphragm muscle. Indeed, diaphragm muscle forces generated during sustained airway occlusion were ~49% of Pdimax in cats, ~43% of Pdimax in hamsters and ~63% of Pdimax in rats (Mantilla et al., 2010; Sieck, 1991b, 1994; Sieck and Fournier, 1989). Importantly, these responses to airway occlusion were generated within 45 s of forced airway closure, and thus differ from the “asphyxic” response elicited in several other species during late stages of prolonged tracheal occlusion usually requiring more than 90 s (Bucher et al., 1972). The asphyxic response likely is a type of autoresuscitative behavior preceded by a large expiratory component (Bucher et al., 1972) and unrelated to ventilatory behaviors or even expulsive behaviors such as coughing or sneezing (Bolser, 1991).
Only during non-ventilatory behaviors (e.g., coughing, sneezing) were maximal diaphragm muscle forces generated in both cats and rats. In rats (Mantilla et al., 2010), airway irritation induced by intranasal injection of capsaicin results in a sneezing behavior that is associated with generation of near maximal Pdi (94±5% of Pdimax elicited by bilateral phrenic nerve stimulation). In cats, gagging induced by mechanical stimulation of the oropharynx generated forces that sometimes exceeded those generated by bilateral phrenic nerve stimulation (Sieck and Fournier, 1989). Occasional sneezing behaviors in cats were of similar magnitude. Sneezing and gagging behaviors constitute physiological patterns of motor activation that can be elicited in anesthetized animals (Mantilla et al., 2010; Sieck and Fournier, 1989), and thus do not depend on voluntary control of respiratory muscle activity. Whether similarly maximal activation can be achieved in humans is not clear. Regardless, having multiple measures of respiratory muscle activity seems useful in assessing muscle weakness or diminished functional reserve (Steier et al., 2007).
The interspecies differences in normalized Pdi and thus the diaphragm muscle’s reserve capacity for force generation likely reflect the varying ventilatory demands that are imposed on the diaphragm muscle in accomplishing its motor tasks. In this sense, larger species may require more diverse behaviors, with a greater range in motor output reflected in the larger reserve capacity beyond resting breathing (Stahl, 1967). In addition, differences across species may relate to the mechanical properties of the respiratory system itself, e.g., lung or chest wall compliance and airway resistance. In agreement, we found that spontaneous sighs in rats generate forces similar to those involved in the response to airway occlusion (~60% of Pdimax), whereas in the cat sighs generated forces ~30% of Pdimax (Mantilla et al., 2010; Sieck and Fournier, 1989). Regardless, considerable force reserve exists for the diaphragm muscle to generate the forces necessary in sustaining ventilation. This force reserve is likely the result of differences in the population of motor units within these muscles and adaptations in respiratory muscles following injury or disease may impact this force reserve placing patients at increased risk of respiratory failure (Sieck and Mantilla, 2008). These relationships at present are not clear and deserve further study.
3.2. Modeling of Phrenic Motor Unit Recruitment
A simple model was proposed for the recruitment of different motor unit types in the diaphragm muscle when accomplishing a range of ventilatory and non-ventilatory behaviors (Fig. 1). This model of motor unit was based on previous studies in the cat, hamster and rat. First, the force generated per motor unit of each type (Fig. 1) was estimated based on measurements of the specific force (force per cross-sectional area) in single diaphragm muscle fibers (Geiger et al., 2002; Geiger et al., 2001; Geiger et al., 2000; Geiger et al., 1999; Sieck, 1988) and measurements of cross-sectional areas of type-identified fibers (Lewis and Sieck, 1990; Miyata et al., 1995; Prakash et al., 2000; Sieck et al., 1989b; Zhan et al., 1997). Second, the number of motor units of each type was estimated using measurements of the proportion of different fiber types in the diaphragm muscle and assuming that the innervation ratio (i.e., the number of fibers innervated by each motoneuron) was ~15% lower at type S or FR motor units than at type FInt and FF units (Enad et al., 1989; Fournier and Sieck, 1988; Sieck, 1988; Sieck et al., 1989a; Sieck et al., 1996). Third, recruitment of motor units in strict order: type S followed by type FR, FInt and FF units was assumed, with full recruitment of all units of each type being achieved before recruiting the next type. This model is shown in Fig.1 (solid line) for the rat diaphragm muscle (Mantilla et al., 2010), but previous models were generated for the cat and hamster diaphragm (Fournier and Sieck, 1988; Sieck, 1991b, 1994; Sieck and Fournier, 1989).
In these models, diaphragm muscle force generated during ventilatory behaviors (i.e., eupnea and hypoxia-hypercapnia) could be accomplished by recruitment of type S and FR motor units in all species (Mantilla et al., 2010; Sieck, 1991b, 1994; Sieck and Fournier, 1989). Of note and in agreement with these findings, the number of phrenic motoneurons recruited during inspiration in cats (~23% of the total pool) (Jodkowski et al., 1987) is the approximate proportion of type S and FR motor units in the diaphragm muscle (Fournier and Sieck, 1988; Sieck et al., 1989a). During sustained airway occlusion, additional recruitment of type FInt motor units would be required. Importantly, Pdi generated during sneezing was near maximal generated by bilateral phrenic nerve stimulation; thus, only during short-duration expulsive behaviors such as sneezing would recruitment of all motor unit types be required. Similarly, in the cat medial gastrocnemius, a large portion of the motor unit pool would only be recruited during high-intensity, short-duration behaviors such as jumping (Sieck, 1991a; Walmsley et al., 1978).
The gradation of force developed by diaphragm motor units (type FF > FInt > FR > S) results in varying slopes of force development as motor units of each type are sequentially recruited (Fig. 1). The proportionally larger force generated by type FF motor units, for instance, reflects their greater specific force, cross-sectional area and innervation ratio, and the contribution of type FF motor units to the maximal force generated by the diaphragm is disproportionate to the number of motor units of this type.
There are differences in the relative Pdi generated during different motor behaviors (as a fraction of Pdimax) across the three species studied (see section 3.1). However, these differences are reflected in the relative proportion of fatigue-resistant (type S and FR units) and more fatigable motor units (type FInt and FF units) across species. For instance, type S and FR motor units comprise ~65% and ~54% in the rat and hamster, respectively, compared to ~34% in the cat (Sieck, 1991b; Sieck and Fournier, 1989). Thus, the distribution of fatigue-resistant and more fatigable motor units across these species is in agreement with the differences in reserve capacity. In the rat, relatively infrequent (~0.2 min−1), large spontaneous “sigh” breaths were detected during both eupnea and hypoxia-hypercapnia (Mantilla et al., 2010). During these sighs, the diaphragm muscle generated forces ~60% of Pdimax, similar to forces generated during airway occlusion. Thus, it is possible that recruitment of some type FInt motor units would occur during spontaneous ventilatory behaviors.
Similar models can also be generated assuming submaximal activation of motor units. In Fig. 1, activation of motor units at 50% of their maximal discharge rate is shown by the dashed line. However, motor unit discharge frequencies may change during the inspiratory burst (Butler et al., 1999; Kong and Berger, 1986). The discharge frequency of individual motor units or their onset and offset times within an inspiratory burst may also vary (Iscoe et al., 1976), particularly across behaviors of increasing demand (Milano et al., 1992). Unfortunately there is limited information on motor unit discharge rates across behaviors including those associated with highly forceful contractions as such studies are technically exceedingly difficult (Lee and Fuller, 2011).
Clearly, this model does not confirm the “size principle” of motor unit recruitment. Indeed, to the best of our knowledge, no study to date has experimentally confirmed such orderly recruitment in the phrenic motor unit pool. This model represents a first approximation to motor unit recruitment and should be useful in the evaluation of motor unit plasticity following injury or disease.
3.3. Assessment of Diaphragm Muscle Force and EMG
In our recent study in rats (Mantilla et al., 2010), there was a robust correlation between the mechanical forces generated by the diaphragm muscle as measured by the Pdi and its electrical activation as determined by peak root-mean-square (RMS) amplitude of the EMG signal. The RMS EMG amplitude increased progressively from eupnea to hypoxia-hypercapnia, airway occlusion and sneezing, consistent with changes in Pdi (expressed as a percent of Pdimax). Indeed, we suggested that diaphragm muscle EMG activity could be a surrogate for Pdi measurements, particularly when these are impractical (e.g., in chronic measurements or in awake animals).
We recently examined the stability and reliability of longitudinal measurements of diaphragm EMG activity using chronically implanted electrodes during ventilatory and non-ventilatory motor behaviors (Mantilla et al., 2011). Quantitative techniques for recording diaphragm EMG activity over time could allow assessment of muscle function during functional recovery from spinal cord injury or with progression of diseases impairing normal ventilation (Mantilla and Sieck, 2003, 2008, 2009; Sieck and Mantilla, 2009). Unfortunately, simple measures of diaphragm EMG activity (e.g., average rectified integrated (Dow et al., 2006; Dow et al., 2009) or peak RMS amplitude (Mantilla et al., 2010; Sieck and Fournier, 1990; Trelease et al., 1982) are highly variable across animals. Indeed, in the course of 6-weeks (Mantilla et al., 2011), diaphragm EMG amplitude displayed substantial intra-animal variability (coefficient of variation: 29-42% for different behaviors). However, normalization of diaphragm EMG activity to near maximal behaviors such as spontaneous sighs reduced intra-animal variability allowing for quantitative, longitudinal assessment of the diaphragm muscle’s ventilatory activity.
It is important to note that the activity and fiber type composition in the costal and crural regions of the diaphragm muscle is similar (Oyer et al., 1989; Reid et al., 1992; Sieck, 1988), and thus, it may be possible to implant EMG electrodes in either region and obtain representative recordings of phrenic motor unit activation. In humans, esophageal or laparoscopic intramuscular electrode placement are possible (Beck et al., 2001; Beck et al., 1996, 1998; Hemmerling et al., 2001). Previously reported techniques for chronic recordings of diaphragm EMG activity across several species (Chang and Harper, 1989; Cooke et al., 1990; Schoolman and Fink, 1963; Shafford et al., 2006; Trelease et al., 1982; Weinstein et al., 1967) had not examined whether quantitative measurements could be reliably obtained in a longitudinal fashion. These studies primarily evaluated either the presence or absence of activity and not quantitative changes in diaphragm activity over time.
Although changes in EMG activity reflect differences in diaphragm force (Eldridge, 1975; Mantilla et al., 2010; Sieck and Fournier, 1989), several factors may cause variability in EMG amplitude over time and thus limit quantitative assessments of force generation. For instance, problems with the electrode (e.g., movement, dislodgment or failure), tissue scarring and fibrosis around the electrode may reduce the quality of the EMG signal. Muscle fiber growth may also complicate longitudinal assessments of ventilatory activity using EMG recordings by changing the muscle fiber sampling and frequency content. Importantly, by measuring diaphragm EMG activity across multiple behaviors, the progression in peak RMS activity across behaviors of increasing demand was maintained over time, with one exception (Mantilla et al., 2011). Sneezing induced by intranasal application of capsaicin did not generate maximal electrical activation at all time points, in some animals. Indeed, sneezing generated lower electrical activation than airway occlusion or spontaneous sighs, consistent with a change in capsaicin sensitivity following repeated exposures (Bolcskei et al., 2010).
Measuring multiple behaviors may enhance longitudinal studies of diaphragm muscle activity. Following spinal cord injury at C2 in rats, phrenic nerve activity is inconsistently reported during eupnea but can be elicited by increasing inspiratory drive (e.g., with hypoxia or hypercapnia) (Fuller et al., 2009; Golder et al., 2001; Nantwi et al., 1999; Teng et al., 1999). It is worth noting that exposure to hypoxic and hypercapnic conditions only results in ~40% of Pdimax in rats (Mantilla et al., 2011; Mantilla et al., 2010), and thus other behaviors such as spontaneous sighs may inform about the diaphragm muscle’s ability to generate higher levels of force (~65% of Pdimax). Whether the relationship between diaphragm muscle force and EMG activity is preserved following injury or with disease progression will need to be determined experimentally. However, longitudinal measurements of diaphragm EMG activity may prove clinically useful as an initial assessment of the plasticity of phrenic motor units.
3.4. Frequency Coding of Motor Unit Recruitment
Neuromotor control of diaphragm muscle force can also be exerted via coding of motoneuron discharge frequencies (Iscoe et al., 1976; Kong and Berger, 1986; Lee and Fuller, 2011; Lee et al., 2009). In this regard, it may be useful to evaluate changes in motoneuron discharge frequencies using quantitative analyses of the diaphragm EMG signal. Indeed, frequency-domain analyses of EMG signals have been employed under a variety of conditions. Changes in the power spectral density (PSD) of EMG signals with a shift toward higher frequencies have been reported as force generation increases (Arendt-Nielsen and Mills, 1985; Solomonow et al., 1990). Based on models of the EMG power spectrum that consider the varying size and number of activated muscle fibers (Lindstrom and Magnusson, 1977), EMG PSD is primarily dependent on the action potential conduction velocity of activated muscle fibers, and thus on fiber diameter. In agreement, the distribution of sizes for activated muscle fibers and the proportion of motor unit types recruited across different motor behaviors influence EMG PSD (Gerdle et al., 1991; Gerdle et al., 2000; Kupa et al., 1995). Essentially, when muscle fibers with larger diameters and faster conduction velocities become recruited, there will be a shift toward higher frequencies in the EMG PSD (Arendt-Nielsen and Mills, 1985; Lindstrom and Magnusson, 1977). For instance, in the rat diaphragm muscle, muscle fibers of type S and FR motor units are ~1/3 of the cross sectional area of fibers at type FInt and FF units (Aravamudan et al., 2006; Geiger et al., 2000; Lewis et al., 1986; Mantilla et al., 2008; Prakash et al., 1993; Sieck and Fournier, 1989; Verheul et al., 2004). We observed a shift in the diaphragm muscle EMG PSD to higher frequencies during sneezing (reflected by an increase in centroid frequency), consistent with the activation of the larger muscle fibers of the more fatigable fast-twitch motor units (Seven et al., 2011). In agreement, inspiratory resistive loading of the diaphragm muscle increased the centroid frequency of the EMG PSD in both pigs (Hussain et al., 1991) and rabbits (Cairns and Road, 1998). Whether airway occlusion (possibly an extreme case of inspiratory resistive loading) results in a similar shift in EMG PSD in rats has not been reported.
One important aspect that is frequently ignored when studying EMG PSD is the stationarity of the signal in the measurement window. In power spectral analyses, the EMG signal should be at least wide-sense or weakly stationary (Bilodeau et al., 1997; Papoulis, 1984). A common criterion for stationarity is based on the mean square value of the EMG signal for the specified window length (Duchene and Goubel, 1993) and the reverse arrangement test, a non-parametric test for stochastic variables (Bendat and Piersol, 2010; Bilodeau et al., 1997). Thus, diaphragm EMG PSD should be evaluated towards the end of the inspiratory phase when the EMG signal has approached a plateau. Sampling during the onset of inspiration will not satisfy stationarity criteria. Indeed, determining the window length during which the diaphragm EMG signal is wide-sense stationary during bursts associated with different motor behaviors will provide information regarding motor unit recruitment. For instance, the relative timing within an EMG burst when the maximum number of motor units becomes recruited (i.e., when the EMG signal becomes stationary) may provide global information about recruitment of motoneurons within the pool and, possibly changes in their discharge frequency across ventilatory and non-ventilatory behaviors.
4. Conclusions and Future Directions
As the major inspiratory muscle in mammals, recruitment of phrenic motoneurons is of particularly important in the neuromotor control of respiration. In several species, measurements of diaphragm muscle force show that ventilatory behaviors demand only 12-27% of maximal force (Pdimax, elicited by bilateral phrenic nerve stimulation) during eupnea and ~28% during exposure to hypoxic and hypercapnic conditions. Estimates of motor unit recruitment during these motor behaviors indicate that across species, ventilatory behaviors can be accomplished by the recruitment of only fatigue-resistant (type S and FR) motor units. Indeed, only during short-duration expulsive behaviors such as sneezing would recruitment of all motor unit types be required. In rats, spontaneous large breaths (“sighs”) would require recruitment of some type FInt motor units as forces generated by the diaphragm muscle are ~60% of Pdimax, and thus are similar to forces generated during airway occlusion. This large reserve capacity in the diaphragm may confound evaluation of the progression of diseases impairing ventilation unless multiple motor behaviors are examined. Importantly, measurements of diaphragm EMG activity may serve as a useful surrogate for diaphragm muscle force as there is a high degree of correlation between relative RMS EMG and Pdi. This information may assist in evaluating the extent of motor recovery in conditions of injury or disease.
Acknowledgments
Supported by NIH grants HL096750, AR051173 and the Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aravamudan B, Mantilla CB, Zhan WZ, Sieck GC. Denervation effects on myonuclear domain size of rat diaphragm fibers. J. Appl. Physiol. 2006;100:1617–1622. doi: 10.1152/japplphysiol.01277.2005. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Mills KR. The relationship between mean power frequency of the EMG spectrum and muscle fibre conduction velocity. Electroencephalogr. Clin. Neurophysiol. 1985;60:130–134. doi: 10.1016/0013-4694(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Beck J, Gottfried SB, Navalesi P, Skrobik Y, Comtois N, Rossini M, Sinderby C. Electrical activity of the diaphragm during pressure support ventilation in acute respiratory failure. Am. J. Respir. Crit. Care Med. 2001;164:419–424. doi: 10.1164/ajrccm.164.3.2009018. [DOI] [PubMed] [Google Scholar]
- Beck J, Sinderby C, Lindstrom L, Grassino A. Influence of bipolar esophageal electrode positioning on measurements of human crural diaphragm electromyogram. J. Appl. Physiol. 1996;81:1434–1449. doi: 10.1152/jappl.1996.81.3.1434. [DOI] [PubMed] [Google Scholar]
- Beck J, Sinderby C, Lindstrom L, Grassino A. Crural diaphragm activation during dynamic contractions at various inspiratory flow rates. J. Appl. Physiol. 1998;85:451–458. doi: 10.1152/jappl.1998.85.2.451. [DOI] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Random Data: Analysis and Measurement Procedures. 4th edition New York; John Wiley & Sons: 2010. [Google Scholar]
- Bilodeau M, Cincera M, Arsenault AB, Gravel D. Normality and stationarity of EMG signals of elbow flexor muscles during ramp and step isometric contractions. J. Electromyogr. Kinesiol. 1997;7:87–96. doi: 10.1016/s1050-6411(96)00024-7. [DOI] [PubMed] [Google Scholar]
- Bolcskei K, Tekus V, Dezsi L, Szolcsanyi J, Petho G. Antinociceptive desensitizing actions of TRPV1 receptor agonists capsaicin, resiniferatoxin and N-oleoyldopamine as measured by determination of the noxious heat and cold thresholds in the rat. Eur. J. Pain. 2010;14:480–486. doi: 10.1016/j.ejpain.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Bolser DC. Fictive cough in the cat. J. Appl. Physiol. 1991;71:2325–2331. doi: 10.1152/jappl.1991.71.6.2325. [DOI] [PubMed] [Google Scholar]
- Bucher K, Huber B, Baettig P. Expiratory versus inspiratory efforts in suffocating pigeons. Agents Actions. 1972;2:189–192. doi: 10.1007/BF01965859. [DOI] [PubMed] [Google Scholar]
- Burke RE. Motor units: anatomy, physiology and functional organization. In: Peachey LD, editor. Handbook of Physiology. The Nervous System. Motor Control. Am Physiol Soc; Bethesda, MD: 1981. pp. 345–422. [Google Scholar]
- Burke RE, Levine DN, Tsairis P, Zajac FE., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J. Physiol. (Lond.) 1973;234:723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Marks WB, Ulfhake B. A parsimonious description of motoneuron dendritic morphology using computer simulation. J. Neurosci. 1992;12:2403–2416. doi: 10.1523/JNEUROSCI.12-06-02403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, McKenzie DK, Gandevia SC. Discharge properties and recruitment of human diaphragmatic motor units during voluntary inspiratory tasks. J. Physiol. 1999;518(Pt 3):907–920. doi: 10.1111/j.1469-7793.1999.0907p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns AM, Road JD. High-frequency oscillation and centroid frequency of diaphragm EMG during inspiratory loading. Respir. Physiol. 1998;112:305–313. doi: 10.1016/s0034-5687(98)00032-2. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Averill DB, Berger AJ. Quantitative analysis of the dendrites of cat phrenic motoneurons stained intracellularly with horseradish peroxidase. J. Comp. Neurol. 1985;230:91–101. doi: 10.1002/cne.902310108. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Fang H. Morphology of developing motoneurons innervating the medial gastrocnemius of the cat. Dev. Brain Res. 1989;49:253–263. doi: 10.1016/0165-3806(89)90026-6. [DOI] [PubMed] [Google Scholar]
- Chang FC, Harper RM. A procedure for chronic recording of diaphragmatic electromyographic activity. Brain Res. Bull. 1989;22:561–563. doi: 10.1016/0361-9230(89)90112-3. [DOI] [PubMed] [Google Scholar]
- Clamann HP. Motor unit recruitment and the gradation of muscle force. Phys. Ther. 1993;73:830–843. doi: 10.1093/ptj/73.12.830. [DOI] [PubMed] [Google Scholar]
- Cooke IR, Brodecky V, Berger PJ. Easily-implantable electrodes for chronic recording of electromyogram activity in small fetuses. J. Neurosci. Methods. 1990;33:51–54. doi: 10.1016/0165-0270(90)90081-p. [DOI] [PubMed] [Google Scholar]
- Cushing S, Bui T, Rose PK. Effect of nonlinear summation of synaptic currents on the input-output properties of spinal motoneurons. J. Neurophysiol. 2005;94:3465–3478. doi: 10.1152/jn.00439.2005. [DOI] [PubMed] [Google Scholar]
- Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. J. Neurophysiol. 1987;57:245–259. doi: 10.1152/jn.1987.57.1.245. [DOI] [PubMed] [Google Scholar]
- Dow DE, Mantilla CB, Zhan WZ, Sieck GC. EMG-based detection of inspiration in the rat diaphragm muscle. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006;1:1204–1207. doi: 10.1109/IEMBS.2006.260688. [DOI] [PubMed] [Google Scholar]
- Dow DE, Zhan WZ, Sieck GC, Mantilla CB. Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009;1:404–407. doi: 10.1109/IEMBS.2009.5334892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchene J, Goubel F. Surface electromyogram during voluntary contraction: processing tools and relation to physiological events. Crit. Rev. Biomed. Eng. 1993;21:313–397. [PubMed] [Google Scholar]
- Eldridge FL. Relationship between respiratory nerve and muscle activity and muscle force output. J. Appl. Physiol. 1975;39:567–574. doi: 10.1152/jappl.1975.39.4.567. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J. Comp. Neurol. 1988;269:47–57. doi: 10.1002/cne.902690104. [DOI] [PubMed] [Google Scholar]
- Enad JG, Fournier M, Sieck GC. Oxidative capacity and capillary density of diaphragm motor units. J. Appl. Physiol. 1989;67:620–627. doi: 10.1152/jappl.1989.67.2.620. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J. Neurosci. 1985;5:1993–2000. doi: 10.1523/JNEUROSCI.05-08-01993.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J. Neurophysiol. 1988;59:1055–1066. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir. Physiol. Neurobiol. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J. Appl. Physiol. 2002;92:1506–1514. doi: 10.1152/japplphysiol.00095.2001. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Mechanisms underlying increased force generation by rat diaphragm muscle fibers during development. J. Appl. Physiol. 2001;90:380–388. doi: 10.1152/jappl.2001.90.1.380. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J. Appl. Physiol. 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Cody MJ, Sieck GC. Force-calcium relationship depends on myosin heavy chain and troponin isoforms in rat diaphragm muscle fibers. J. Appl. Physiol. 1999;87:1894–1900. doi: 10.1152/jappl.1999.87.5.1894. [DOI] [PubMed] [Google Scholar]
- Gerdle B, Henriksson-Larsen K, Lorentzon R, Wretling ML. Dependence of the mean power frequency of the electromyogram on muscle force and fibre type. Acta Physiol. Scand. 1991;142:457–465. doi: 10.1111/j.1748-1716.1991.tb09180.x. [DOI] [PubMed] [Google Scholar]
- Gerdle B, Karlsson S, Crenshaw AG, Elert J, Friden J. The influences of muscle fibre proportions and areas upon EMG during maximal dynamic knee extensions. Eur. J. Appl. Physiol. 2000;81:2–10. doi: 10.1007/PL00013792. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J. Neurosci. 2001;21:8680–8689. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T, Thomas CK, Munson JB, Stein RB. The resilience of the size principle in the organization of motor unit properties in normal and reinnervated adult skeletal muscles. Can. J. Physiol. Pharmacol. 2004;82:645–661. doi: 10.1139/y04-081. [DOI] [PubMed] [Google Scholar]
- Hamm TM, Nemeth PM, Solanki L, Gordon DA, Reinking RM, Stuart DG. Association between biochemical and physiological properties in single motor units. Muscle Nerve. 1988;11:245–254. doi: 10.1002/mus.880110309. [DOI] [PubMed] [Google Scholar]
- Hemmerling TM, Schmidt J, Wolf T, Hanusa C, Siebzehnruebl E, Schmitt H. Intramuscular versus surface electromyography of the diaphragm for determining neuromuscular blockade. Anesth. Analg. 2001;92:106–111. doi: 10.1097/00000539-200101000-00021. [DOI] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J. Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hensbergen E, Kernell D. Daily durations of spontaneous activity in cat’s ankle muscles. Exp. Brain Res. 1997;115:325–332. doi: 10.1007/pl00005701. [DOI] [PubMed] [Google Scholar]
- Hussain SN, Clement MG, Vanelli G, Albertini M, Aguggini G. The effect of level of contraction on the electromyographic power spectrum of the diaphragm in pigs. Exp. Physiol. 1991;76:765–775. doi: 10.1113/expphysiol.1991.sp003542. [DOI] [PubMed] [Google Scholar]
- Iscoe S, Dankoff J, Migicovsky R, Polosa C. Recruitment and discharge frequency of phrenic motoneurones during inspiration. Respir. Physiol. 1976;26:113–128. doi: 10.1016/0034-5687(76)90056-6. [DOI] [PubMed] [Google Scholar]
- Issa AN, Zhan WZ, Sieck G, Mantilla CB. Neuregulin-1 at synapses on phrenic motoneurons. J. Comp. Neurol. 2010;518:4213–4225. doi: 10.1002/cne.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J. Neurophysiol. 1987;58:105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- Jodkowski JS, Viana F, Dick TE, Berger AJ. Repetitive firing properties of phrenic motoneurons in the cat. J. Neurophysiol. 1988;60:687–702. doi: 10.1152/jn.1988.60.2.687. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Wilson LE, Zhan WZ, Watchko JF, Daood MJ, Sieck GC. Contractile properties of the developing diaphragm correlate with myosin heavy chain phenotype. J. Appl. Physiol. 1994;77:481–487. doi: 10.1152/jappl.1994.77.1.481. [DOI] [PubMed] [Google Scholar]
- Kernell D. The motoneurone and its muscle fibres. Oxford University Press Inc.; New York: 2006. [Google Scholar]
- Keswani NH, Hollinshead WH. The phrenic nucleus. III. Organization of the phrenic nucleus in the spinal cord of the cat and man. Proc. Staff Meet. Mayo Clin. 1955;30:566–577. [PubMed] [Google Scholar]
- Kong FJ, Berger AJ. Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J. Appl. Physiol. 1986;61:1999–2004. doi: 10.1152/jappl.1986.61.6.1999. [DOI] [PubMed] [Google Scholar]
- Kupa EJ, Roy SH, Kandarian SC, De Luca CJ. Effects of muscle fiber type and size on EMG median frequency and conduction velocity. J. Appl. Physiol. 1995;79:23–32. doi: 10.1152/jappl.1995.79.1.23. [DOI] [PubMed] [Google Scholar]
- Lee KZ, Fuller DD. Neural control of phrenic motoneuron discharge. Respir. Physiol. Neurobiol. 2011 doi: 10.1016/j.resp.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns during hypoxia-induced short-term potentiation in rats. J. Neurophysiol. 2009;102:2184–2193. doi: 10.1152/jn.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MI, Sieck GC. Effect of acute nutritional deprivation on diaphragm structure and function. J. Appl. Physiol. 1990;68:1938–1944. doi: 10.1152/jappl.1990.68.5.1938. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Sieck GC, Fournier M, Belman MJ. Effect of nutritional deprivation on diaphragm contractility and muscle fiber size. J. Appl. Physiol. 1986;60:596–603. doi: 10.1152/jappl.1986.60.2.596. [DOI] [PubMed] [Google Scholar]
- Lindstrom L, Magnusson R. Interpretation of Myoelectric Power Spectra: A Model and Its Applications. Proc. IEEE. 1977;65:653–662. [Google Scholar]
- Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic Assessment of Diaphragm Muscle EMG Activity across Motor Behaviors. Respir. Physiol. Neurobiol. 2011;177:176–182. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir. Physiol. Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Mechanisms underlying motor unit plasticity in the respiratory system. J. Appl. Physiol. 2003;94:1230–1241. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J. Appl. Physiol. 2008;104:1818–1827. doi: 10.1152/japplphysiol.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir. Physiol. Neurobiol. 2009;169:133–140. doi: 10.1016/j.resp.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Sill RV, Aravamudan B, Zhan WZ, Sieck GC. Developmental effects on myonuclear domain size of rat diaphragm fibers. J. Appl. Physiol. 2008;104:787–794. doi: 10.1152/japplphysiol.00347.2007. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Retrograde labeling of phrenic motoneurons by intrapleural injection. J. Neurosci. Methods. 2009;182:244–249. doi: 10.1016/j.jneumeth.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM. The size principle: a rule describing the recruitment of motoneurons. J. Neurophysiol. 2005;93:3024–3026. doi: 10.1152/classicessays.00025.2005. [DOI] [PubMed] [Google Scholar]
- Milano S, Grelot L, Bianchi AL, Iscoe S. Discharge patterns of phrenic motoneurons during fictive coughing and vomiting in decerebrate cats. J. Appl. Physiol. 1992;73:1626–1636. doi: 10.1152/jappl.1992.73.4.1626. [DOI] [PubMed] [Google Scholar]
- Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J. Appl. Physiol. 1995;79:1640–1649. doi: 10.1152/jappl.1995.79.5.1640. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab. Neural Repair. 1999;13:225–234. [Google Scholar]
- Nemeth PM, Solanki L, Gordon DA, Hamm TM, Reinking RM, Stuart DG. Uniformity of metabolic enzymes within individual motor units. J. Neurosci. 1986;6:892–898. doi: 10.1523/JNEUROSCI.06-03-00892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyer LM, Knuth SL, Ward DK, Bartlett DJ. Patterns of neural and muscular electrical activity in costal and crural portions of the diaphragm. J. Appl. Physiol. 1989;66:2092–2100. doi: 10.1152/jappl.1989.66.5.2092. [DOI] [PubMed] [Google Scholar]
- Papoulis A. Probability, Random Variables, and Stochastic Processes. McGraw-Hill; New York: 1984. [Google Scholar]
- Prakash YS, Fournier M, Sieck GC. Effects of prenatal undernutrition on developing rat diaphragm. J. Appl. Physiol. 1993;75:1044–1052. doi: 10.1152/jappl.1993.75.3.1044. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J. Appl. Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Qiu K, Lane MA, Lee KZ, Reier PJ, Fuller DD. The phrenic motor nucleus in the adult mouse. Exp. Neurol. 2010;226:254–258. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid WD, Wiggs BR, Pare PD, Pardy RL. Fiber type and regional differences in oxidative capacity and glycogen content in the hamster diaphragm. Am. Rev. Respir. Dis. 1992;146:1266–1271. doi: 10.1164/ajrccm/146.5_Pt_1.1266. [DOI] [PubMed] [Google Scholar]
- Schoolman A, Fink BR. Permanently implanted electrode for electromyography of the diaphragm in the waking cat. Electroencephalogr. Clin. Neurophysiol. 1963;15:127–128. doi: 10.1016/0013-4694(63)90048-8. [DOI] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Frequency-domain analysis of diaphragm muscle EMG activity across ventilatory and non-ventilatory motor behaviors. FASEB J. 2011;25:1111.24. [Google Scholar]
- Shafford HL, Strittmatter RR, Schadt JC. A novel electrode design for chronic recording of electromyographic activity. J. Neurosci. Methods. 2006;156:228–230. doi: 10.1016/j.jneumeth.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Diaphragm muscle: structural and functional organization. Clin. Chest Med. 1988;9:195–210. [PubMed] [Google Scholar]
- Sieck GC. Diaphragm motor units and their response to altered use. Sem. Respir. Med. 1991a;12:258–269. [Google Scholar]
- Sieck GC. Neural control of the inspiratory pump. NIPS. 1991b;6:260–264. [Google Scholar]
- Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin. Chest Med. 1994;15:641–659. [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J. Appl. Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Changes in diaphragm motor unit EMG during fatigue. J. Appl. Physiol. 1990;68:1917–1926. doi: 10.1152/jappl.1990.68.5.1917. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci. Lett. 1989a;97:29–34. doi: 10.1016/0304-3940(89)90134-1. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Prakash YS, Blanco CE. Myosin phenotype and SDH enzyme variability among motor unit fibers. J. Appl. Physiol. 1996;80:2179–2189. doi: 10.1152/jappl.1996.80.6.2179. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Lewis MI, Blanco CE. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J. Appl. Physiol. 1989b;66:2196–2205. doi: 10.1152/jappl.1989.66.5.2196. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB. Effect of mechanical ventilation on the diaphragm. N. Engl. J. Med. 2008;358:1392–1394. doi: 10.1056/NEJMe0801226. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir. Physiol. Neurobiol. 2009;169:218–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Prakash YS. Cross bridge kinetics in respiratory muscles. Eur. Respir. J. 1997;10:2147–2158. doi: 10.1183/09031936.97.10092147. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Baten C, Smit J, Baratta R, Hermens H, D’Ambrosia R, Shoji H. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J. Appl. Physiol. 1990;68:1177–1185. doi: 10.1152/jappl.1990.68.3.1177. [DOI] [PubMed] [Google Scholar]
- Song A, Ashwell KW, Tracey DJ. Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat. Embryol. (Berl.) 2000;202:159–177. doi: 10.1007/s004290000096. [DOI] [PubMed] [Google Scholar]
- Stahl WR. Scaling of respiratory variables in mammals. J. Appl. Physiol. 1967;22:453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- Steier J, Kaul S, Seymour J, Jolley C, Rafferty G, Man W, Luo YM, Roughton M, Polkey MI, Moxham J. The value of multiple tests of respiratory muscle strength. Thorax. 2007;62:975–980. doi: 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CK, Mellen NM, Feldman JL. Intrinsic and extrinsic factors affecting phrenic motoneuronal excitability in neonatal rats. Brain Res. 1997;774:62–68. doi: 10.1016/s0006-8993(97)81688-5. [DOI] [PubMed] [Google Scholar]
- Sypert GW, Munson JB. Basis of segmental motor control: Motoneuron size or motor unit type? Neurosurg. 1981;8:608–621. doi: 10.1227/00006123-198105000-00020. [DOI] [PubMed] [Google Scholar]
- Teng YD, Mocchetti I, Taveira-DaSilva AM, Gillis RA, Wrathall JR. Basic fibroblast growth factor increases long-term survival of spinal motor neurons and improves respiratory function after experimental spinal cord injury. J. Neurosci. 1999;19:7037–7047. doi: 10.1523/JNEUROSCI.19-16-07037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torikai H, Hayashi F, Tanaka K, Chiba T, Fukuda Y, Moriya H. Recruitment order and dendritic morphology of rat phrenic motoneurons. J. Comp. Neurol. 1996;366:231–243. doi: 10.1002/(SICI)1096-9861(19960304)366:2<231::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Trelease RB, Sieck GC, Harper RM. A new technique for acute and chronic recording of crural diaphragm EMG in cats. Electroencephalogr. Clin. Neurophysiol. 1982;53:459–462. doi: 10.1016/0013-4694(82)90011-6. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Dick TE. Intrinsic properties of pharyngeal and diaphragmatic respiratory motoneurons and muscles. J. Appl. Physiol. 1992;73:787–800. doi: 10.1152/jappl.1992.73.3.787. [DOI] [PubMed] [Google Scholar]
- Verheul AJ, Mantilla CB, Zhan WZ, Bernal M, Dekhuijzen PN, Sieck GC. Influence of corticosteroids on myonuclear domain size in the rat diaphragm muscle. J. Appl. Physiol. 2004;97:1715–1722. doi: 10.1152/japplphysiol.00625.2003. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Hodgson JA, Burke RE. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J. Neurophysiol. 1978;41:1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]
- Webber CL, Wurster RD, Chung JM. Cat phrenic nucleus architecture as revealed by horseradish peroxidase mapping. Exp. Brain Res. 1979;35:395–406. doi: 10.1007/BF00236759. [DOI] [PubMed] [Google Scholar]
- Weinstein SA, Annau Z, Senter G. Chronic recording of ECG and diaphragmatic EMG in rats. J. Appl. Physiol. 1967;23:971–975. doi: 10.1152/jappl.1967.23.6.971. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neurosci. 1999;90:1501–1513. doi: 10.1016/s0306-4522(98)00554-5. [DOI] [PubMed] [Google Scholar]
- Zajac FE, Faden JS. Relationship among recruitment order, axonal conduction velocity, and muscle-unit properties of type-identified motor units in cat plantaris muscle. J. Neurophysiol. 1985;53:1303–1322. doi: 10.1152/jn.1985.53.5.1303. [DOI] [PubMed] [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J. Neurophysiol. 1985;53:1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]
- Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J. Appl. Physiol. 1997;82:1145–1153. doi: 10.1152/jappl.1997.82.4.1145. [DOI] [PubMed] [Google Scholar]