Abstract
Hyperpolarization greatly enhances opportunities to observe in vivo metabolic processes in real time. Accessible timescales are, however, limited by nuclear spin relaxation times, and sensitivity is limited by magnetogyric ratios of observed nuclei. The majority of applications to date have involved direct 13C observation of metabolites with non-protonated carbons at sites of interest (13C enriched carbonyls, for example), a choice that extends relaxation times and yields moderate sensitivity. Interest in 15N containing metabolites is equally high but non-protonated sites are rare and direct 15N observation insensitive. Here an approach is demonstrated that extends applications to protonated 15N sites with high sensitivity. The normally short relaxation times are lengthened by initially replacing protons (H) with deuterons (D) and low sensitivity detection of 15N is avoided by indirect detection through protons reintroduced by H/D exchange. A pulse sequence is presented that periodically samples 15N polarization at newly protonated sites by INEPT transfer to protons while returning 15N magnetization of deuterated sites to the +Z axis to preserve polarization for subsequent samplings. Applications to 15ND2-amido-glutamine are chosen for illustration. Glutamine is an important regulator and a direct donor of nitrogen in cellular metabolism. Potential application to in vivo observation is discussed.
Keywords: Dynamic Nuclear Polarization, glutamine, indirect detection, nitrogen metabolism
Introduction
Hyperpolarization of magnetic nuclei, using a variety of methods [1; 2; 3; 4; 5], has had a tremendous impact on our ability to monitor rapid inter-conversions from substrate to product both in vitro and in vivo [6]. It is now clear that monitoring metabolic processes in vivo will lead to new diagnostic methods for major diseases [7; 8]. Most applications have been based on polarization and observation of 13C at non-protonated sites. There are good reasons for this. Polarization of non-protonated 13C sites relaxes very slowly, allowing monitoring of metabolic conversions over periods that reach many tens of seconds. 13C observation also provides moderate sensitivity with reasonable background suppression using simple direct detection methods and enrichment beyond its 1% natural abundance. However, the pathways that can be followed by hyperpolarizing carbons represent just a part of a total metabolic system. Of equal interest are the pathways that can be followed by hyperpolarizing nitrogen, yet, nitrogen hyperpolarization is seldom exploited [9; 10]. Again there are good reasons. Nitrogens in many substrates of interest are protonated, making their spin-lattice relaxation times relatively short, and direct detection of 15N is very unproductive, being a factor of ~6 less sensitive than that of 13C for equal enrichments and equal levels of polarization. Here we present a scheme for detection of 15N-labeled metabolites that exploits the much longer spin-lattice relaxation times of deuterated nitrogens and enhances sensitivity over direct detection by utilizing indirect detection through protons.
Nitrogen is a major elemental component of proteins and nucleic acids. It is also a component of amino sugars found in the extracellular matrix and on glycoproteins and glycolipids. The side chain nitrogen of glutamine is the direct donor of nitrogen for both nucleic acids and amino sugars. Glutamine is also the most abundant free amino acid in the human body [11]. In the brain glutamine provides a means of neurotransmitter generation through the glutamine-glutamate cycle [12]. It is important in protection of cells from oxidant injury, and metabolism of glutamine is known to be elevated in cultured tumor cells, where cells consume as much as ten times more glutamine than any other amino acid [13]. It is clear that improved methods of monitoring the passage of 15N through various metabolites could be very important.
Hyperpolarization and indirect detection of 15N has been reported previously but primarily in cases where non-protonated nitrogens are involved and indirect detection is done through remote protons [10; 14; 15]. This is somewhat limited because the small couplings to protons two or more bonds removed require long polarization transfer periods. Moreover, the requirement for a non-protonated nitrogen and an adjacent site carrying an easily detectable proton restricts the number of metabolites that might be observed. Detecting hyperpolarized 15N magnetization through a directly bonded proton has also been reported, though this method is sensitive to the decay of 15N polarization due to a dipolar interaction with the proton during dissolution, transfer and storage and is, therefore, less suitable for observing kinetic processes in real time [16]. Here we take advantage of the lengthened spin relaxation time of a deuterated nitrogen and the fact that deuterons directly bonded to nitrogens exchange for protons in aqueous media at rates that can be comparable to the life times of hyperpolarization. While an amide nitrogen with a single directly bonded proton in a molecule of moderate molecular weight (150 Da) at a 11.7T field and 25°C has a predicted spin-lattice relaxation time of 4s, the same molecule with a deuterium attached has a predicted spin-lattice relaxation time of 22s. Deuterons at amide sites in neutral amino acids can be replaced by chemical exchange at a rate of 0.3–1s−1 at 25°C and pH 6.0 [17]. However, this rate can be manipulated through its exponential dependence on hydrogen ion concentration [17] and by the solvent deuterium content. Other compounds may also have inherently slower exchange rates. With exchange of a deuteron for a proton, indirect detection can, in principle, enhance sensitivity substantially. Assuming one starts with the same level of polarization, indirect detection through a proton can enhance sensitivity by a factor of 100 over direct nitrogen detection, one factor of 10 coming from the higher magnetic moment of protons and one factor of 10 coming from the higher rate of precession. The same arguments would lead to enhancement by a factor of 16 over direct carbon detection. Here we present a pulse sequence that allows periodic retrieval of 15N polarization as protons are exchanged into an amide site, and an application to detection of signals from the side-chain nitrogen of glutamine.
Materials and Methods
Materials
All materials, unless otherwise noted, were purchased from Sigma-Aldrich (St. Louis, MO). For each hyperpolarization experiment, a solution of 200 μg – 10 mg of 15N-amido-glutamine was dissolved with gentle heating to a final concentration of 60 mg/ml in 50/50 (v:v) D2O/glycerol containing 15 mM trityl radical (GE-Healthcare, UK). The glycerol had been exchanged repeatedly with D2O to replace all labile protons (H) with deuterons (D). This mixture was added to a polyether ether ketone (PEEK) plastic cup and lowered into an Oxford Hypersense 3.35T DNP polarizer (Oxfordshire, UK). The sample was cooled to 1.4–1.5°K then irradiated for 1–2 hr at 94.007 GHz and 100 mW. The hyperpolarized material was then quickly melted and dissolved in a pre-heated and pressurized buffered aqueous solution containing 0–100% H2O and 100–0% D2O. The buffering agent used for pH 4.5–6.5 was 25 mM citric acid with 25 mM dibasic sodium phosphate; for pH 7.0 it was 25 mM HEPES; for pH 8.0 it was 25 mM TRIS. The dissolved material was flushed into a waiting 8mm NMR tube in a Varian 11.7T Inova spectrometer (Santa Clara, CA) equipped with an XH probe operating at a 31°C sample temperature (~5s transfer time).
NMR spectra
Small tip angle, direct detection, spin lattice relaxation (R1) experiments were collected using a simple pulse of X phase and 14° flip angle (considerably less than the 90° pulse width (pw90) of 32 μs) immediately followed by acquisition of 15N signals. The sweep width was typically 8000 Hz and 1600 complex points were collected. The repetition interval was varied to accommodate different relaxation profiles of deuterated and protonated material. For the observation of protonated (deuterated) glutamine, hyperpolarized material was dissolved with a buffer containing 100% H2O (100% D2O).
The equation describing loss of longitudinal magnetization from the resulting combination of short pulses (T), relaxation (P) can be written as follows:
| Eq. [1] |
where,
IZ (n) is the magnetization that can be measured at cycle n, and I0 is the intensity at the beginning of the experiment. The time of each measurement relative to the beginning is the product of n and τ Considering partial sampling at each pulse, an expression for the measured intensity, I(n), results. This is similar to an equation previously reported for a related hyperpolarization application [18]:
| Eq. [2] |
Equation 2 was fit to the accumulated data to extract R1. The errors reported represent the error associated with fitting the R1 parameter and does not include potential errors in pw90 estimation.
Indirect 15N detection experiments were collected with the experiment to be described below using a sweep width of 6000 Hz and 2000 complex points. For initial experiments the inter-experiment delay was 1s resulting in a repetition interval time τ = 1.35s measured from the start of one cycle to the start of the next. All 90° or 180° pulses were used throughout with 90° pulses being 8.75 μs and 32 μs for H and 15N respectively. The decay of magnetization in an experiment using hyperpolarized, deuterated, 15N-amido-glutamine in a solvent can be described in terms of longitudinal relaxation (P), losses due to sampling (S), and experimental imperfections (E):
| Eq. [3] |
where, I0and P are as described before and S = e−nRsamp and E = e−nRimp.
Rsamp is the rate of signal loss due to sampling, which reflects the H/D exchange that occurred since the previous sampling event. Rimp describes signal loss due to experimental imperfections (errors in 90° pulse width estimation, transverse relaxation during the pulse sequence, diffusion during the time between the gradient pulses, sub-optimal deuterium decoupling, etc). Values of Rsamp and Rimp are described with units of n−1. This equation may be simplified and rearranged to:
| Eq. [4] |
Rsamp may be further deconvoluted considering the effects of H/D exchange during the τ period:
| Eq. [5] |
The fraction of ND2 that has exchanged to NDH during the period τ is described by fEX and ln denotes the natural logarithm. The parameter fEX is also affected by the fraction of H2O in a H2O/D2O dissolution liquid, fH2O, leading to the following expression:
| Eq. [6] |
Thus, Rsamp can, in principle, provide information about the D/H exchange rate (REX).
Results
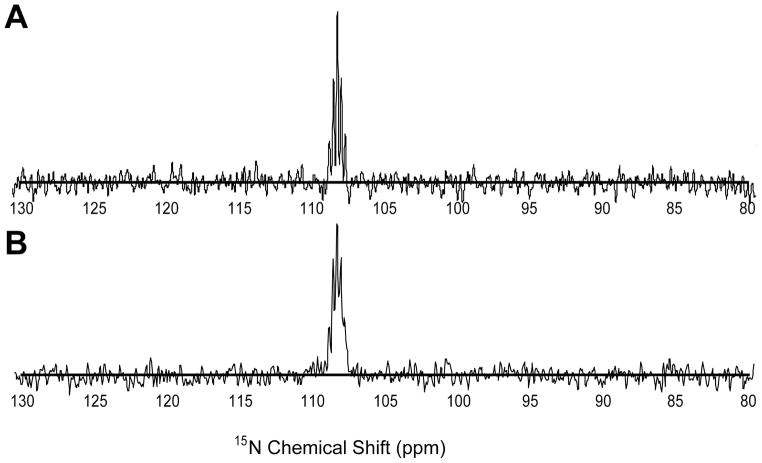
Figure 1 illustrates hyperpolarization efficiency achieved with the particular samples and protocol applied here. Figure 1a shows a signal averaged spectrum of a sample containing 50 mg amide deuterated glutamine (in 4mL of D2O) 15N enriched (>98%) at the sidechain amide nitrogen acquired without hyperpolarization and direct 15N detection. A 90° tip angle was used with a repetition time of 60s over 48 acquisitions. Data were processed with a 5 Hz Gaussian multiplier weighting function. Figure 1b shows the result of 15N direct detection using a single 14° pulse on a sample of 200 μg (in 4 mL of D2O) of the same glutamine 5s after hyperpolarization for 2.5 hrs. The acquisition was processed in a similar way. Correcting for differences in the amount of sample, signal averaging effects, and differences in signal to noise ratios the enhancement is approximately a factor of 8000 The signals show a partially resolved pentet as expected for a nitrogen coupled to two deuterons. The enhancement is short of expectations based on polarization of 13C in pyruvic acid, but parameters known to affect polarization efficiency, including polarization time, transfer time, irradiation frequency, free radical concentration, and solvent conditions have not been optimized. The spectra nevertheless provide a benchmark for comparison to indirectly detected spectra.
Figure 1. Comparison of thermal equilibrium polarized (a) and hyperpolarized (b) 15N-Gln signals at 11.7T.
The top panel is a summation of 48 scans using 50 mg 15N-Gln in D2O with a recycling time of 60s and a 90° tip angle. The bottom panel is 200 μg hyperpolarized 15N-Gln collected with a single 14° tip angle pulse.
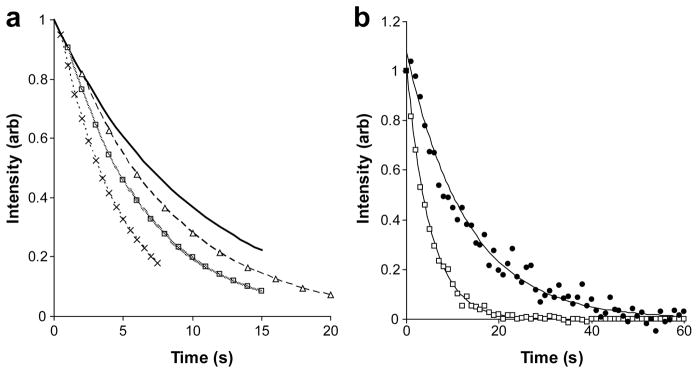
We also used direct 15N direct detection of a hyperpolarized sample using a series of small tip angle pulses to extract fundamental spin relaxation and proton-deuteron exchange parameters. Pulse sequence parameters, such as the repetition rate, and flip angle influence the rate of signal decay independent of spin relaxation and exchange. They must be chosen to allow retention of adequate signal over the expected time course of spin relaxation and exchange decay. Figure 2a shows the effect of the pulse sequence repetition rate with a fixed flip angle of 14° on an observed signal. We anticipate spin relaxation of a deuterated site to be on the order of 0.1s−1 and have chosen a flip angle of 14° and a repetition rate of 1s−1 for our initial experiments.
Figure 2. Longitudinal relaxation of hyperpolarized 15N using repeated small tip experiments.
(a) Theoretical decay of signals observed with a pulse width of 4.9 μs (pw90=21 μs) and sampling rates of 2.0 s−1 (×), 1.0 s−1 (□) and 0.5 s−1 (△). These points are connected for ease of viewing. The heavy black line indicates the sampling independent relaxation. (b) Signal intensity of hyperpolarized deuterated (200 μg, ●) and protonated (10 mg, △) 15N-amido glutamine at 50.5 MHz (pw=5 μs; pw90=32 μs; sampling rate = 1 s−1). These data sets are fitted with Eq. [1] resulting in values of 0.047 ± 0.002s−1 and 0.171 ± 0.002 s−1, respectively.
Figure 2b shows the difference in the decay of 15N signal intensity from 15NH2-glutamine and 15ND2-glutamine. The data in these experiments were fit with Eq. [2]. R1 values of 0.047 ± 0.002 s−1 (T1 of 21s) for the deuterated glutamine and 0.171 ± 0.002 s−1 (T1 of 5.8s) for the protonated glutamine are found. This analysis does not include potential contributions from diffusion or mixing of sample that would introduce unsampled hyperpolarized nuclei into the observation window [19]. These effects, if present, would not dramatically alter these values (at the complete mixing limit, a 2.6 fold decrease in R1 vs. the 3.6 fold decrease observed). Given the small deviations in molecular weight (146), solvent conditions and temperature, the observations are in accord with expectations described in the introduction. Clearly deuteration can enhance spin lifetimes in the spectrometer three-fold or more and would allow an extended period of observation of metabolic conversions. Deuteration also decreases signal loss when transferring the sample from the external hyperpolarization magnet, through a low field condition, to a sample tube in the NMR spectrometer.
Coupling 15N hyperpolarization with H detection offers a substantial sensitivity enhancement over 15N-detected experiments alone. To achieve this combination we developed a method that uses the labile nature of the amido glutamine deuterons to allow replacement with protons from water and detection via these newly added protons. The pH dependent exchange process [17] is depicted in Figure 3a, and the pulse sequence, along with parameters used are depicted in Figure 3b. The pulse sequence is based on a refocused INEPT (insensitive nuclei enhanced by polarization transfer) sequence [20], and parameters are chosen to detect only signals emanating from 15NHD-glutamine (Figure 3c). At the end of the sequence 15N magnetization from NH2 and ND2 glutamine is returned to the +Z axis by the final 90-degree N−x pulse and effectively recycled for later detection. Due to the unique properties of hyperpolarized samples, phase cycling to remove solvent artifacts is not possible (15N magnetization irreversibly decays once the sample is removed from the hyperpolarization apparatus). Artifacts are instead suppressed by the application of symmetric pulsed field gradients during the τa/b – 180 – τ a/b periods and a 3–9–19 WATERGATE sequence during the anti-phase to in-phase H refocusing step. Decoupling of 15N from H spins during acquisition is likewise impossible due to the destruction of the pool of recycled 15N magnetization that would result from the decoupling sequence. However, continuous wave deuterium decoupling is included during periods where 15ND2 magnetization is transverse to minimize loss of magnetization due to exchange among multiplet components on deuterium spin lattice relaxation. Unlike the R1 measurement experiment discussed earlier, 90° detection pulses, rather than small tip angle pulses are used as the object is to detect all magnetization associated with a newly formed 15NHD species.
Figure 3. A refocused INEPT-based experiment detects only signals from the NHD isotopologue of 15N-amido glutamine.
(a) Schematic of sidechain D-H exchange. (b) Pulse sequence for the indirect detection experiment. Narrow (wide) bars denote 90° (180°) pulses. Pulse phases are labeled on the diagram, the phase of the receiver is set to X. Z gradient pulses are 1 ms in duration at 9 G/cm. A 3–9–19 WATERGATE element is used for solvent suppression [25]. Deuterium decoupling is achieved by applying an explicitly gated continuous wave (cw) pulse during the transfer periods with a power of 45 dB. (c) Product operator analysis of the isotopologues analyzed with τa = τb = 1/4J 15N-H. With these delays only the NHD state transfers hyperpolarized 15N magnetization to H for detection. Notice the signals from ND2 and NH2 states are refocused and returned to +Z at the end of the experiment. Note: D = 2H.
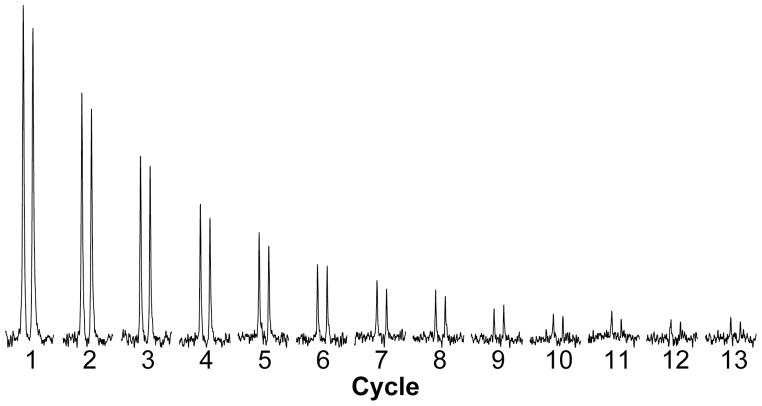
An example of signals from the hyperpolarized 15N of the glutamine sidechain, detected through H, is shown in Figure 4. The sample contains 300 μg of 15N-amido-glutamine in 3.5 ml (25% D2O) at pH 5.0 and 31 °C. The repetition time (τ) is 1.35s. The H chemical shifts of the two amido protons are distinct, and each appears as a doublet due to the evolution of 1JNH coupling during acquisition. For convenience only one of the doublets is shown in Figure 4. Given that the first point is ~7s after dissolution, it is clear that even in this non-optimized trial useable signal exists beyond 20s. The integrated intensity of all resulting signals gives one indication of the sensitivity of the experiment in comparison to direct observation of a similarly polarized sample (Fig 1). Correcting for differences in amount of sample an additional enhancement of a factor of ~20-fold is observed. This is again somewhat short of expectation based on the γ-dependent sensitivity of 15N vs 1H, but suffers from the effects of loss due to multiple pulses, exchange, and relaxation as discussed below.
Figure 4. The signal from 300 μg of hyperpolarized Gln.
Spectra of glutamine amido protons observed using the pulse sequence in Fig 2b with a pH of 5.0, τ of 1.35s, and H2O = 25%.
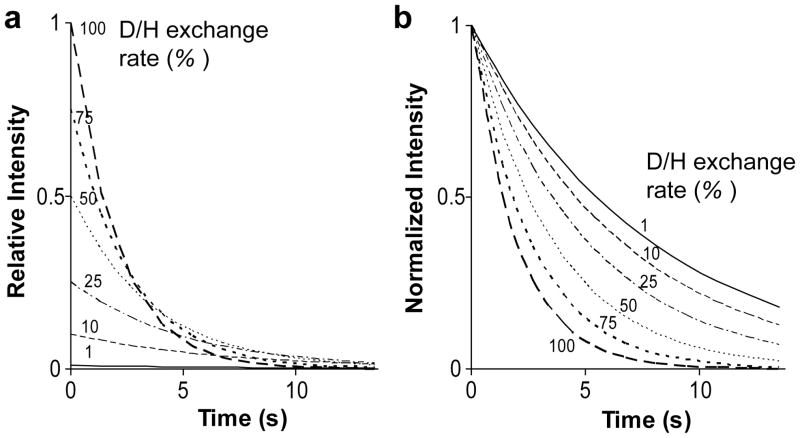
The effect of H/D exchange is of particular interest. Simulations of signal decay for different exchange rates are shown in Figure 5a which depicts relative intensities and Figure 5b which has been normalized to make comparison of decay rates easier. The simulations use the same 1.35s repetition time and the same H2O/D2O composition used for the experiment in Fig. 4. At high exchange rates initial intensities are large (Fig 5a) because a large proportion of protons are introduced at each cycle. Intensities tend to converge to a constant high value (Fig 5a) and decay rates to a constant value (Fig 5b) at the rapid exchange limit where the fraction of polarization sampled by each pulse is simply given by the fraction of protons in the dissolution buffer. However, decay in this limit is rapid with a rate dictated by how frequently polarization is sampled. In principle, one can decrease the decay rate to some extent by using longer spacing between cycles, but the decay will ultimately reflect the average R1 spin relaxation properties of protonated and deuterated species and in most cases will be dominated by the protonated species. This sacrifices the advantage of the long relaxation times of the initially deuterated 15N sites.
Figure 5. Signal longevity is sensitive to D/H exchange.
Simulation demonstrating the effect of the extent of D/H exchange rate on absolute signal intensity as calculated using Eq. [4]. (a) These traces show the expected decay of signals with a repetition time (τ) = 1.35s, R1 = 0.05 s−1 and Rimp = 0.1 in 50% H2O (max fEX = 0.5). Rsamp for each trace was calculated as in Eq. [5]. (b) The same data as in (a) but replotted to show intensity of each trace relative to the first point highlighting the differences in the signal decay.
As exchange rates become slow compared to recycle rates the amount of signal retrieved at any step decreases, and the decay rates eventually are governed by the R1 relaxation rates of the fully deuterated 15N sites. In between the decay rates are quite sensitive to the actual H/D exchange rates and measurement could be used to indirectly probe environmental conditions such as pH through their proportionality to the hydroxide ion concentration (above pH 4). For applications dependent on high sensitivity over long periods of time, exchange rates need to be optimized to extend the lifetime of magnetization. Where possible, this can be accomplished by reducing the pH or reducing the proton content of the sample solution.
An additional influence on apparent decay rate, as described by Eq. [4], comes from imperfections in pulses that return magnetization of fully deuterated sites to the +Z axis. Also relaxation (R2) of magnetization during times spent in the transverse plane contributes to decay by preventing a full return to the +Z axis. Minimizing time spent in the transverse plane and the use of precise or compensated 180° and 90° pulses can help, but there will always be some loss with each repetition cycle (in addition to spin-lattice relaxation loss). Minimization of this loss can be achieved by limiting the number of steps repeated over a desired observation period. The effect of reducing the recycling rate is shown in Fig. 6a. Maintaining the pH at 5 and reducing the H2O content to 10% while increasing τ to 10s resulted in an observed decay rate of 0.073 ± 0.001 s−1, nearly one-third the rate shown in Fig 4 and nearly at the theoretical limit for ND2 longitudinal relaxation (0.05 s−1) as shown in Figure 2. With these parameters clear signal was detected past 60s, a significant improvement in the functional window of the method.
Figure 6. The experiment may be tuned to extend the signal lifetime.
(a) Spectra of glutamine amido protons with a pH of 5.0, τ of 10.35s, and H2O = 10%. (b) The effect of sampling time (τ) on longevity of the signal in a buffer containing 25% H2O, pH 5.0. The decay rates (Robs) are (◆) 1.35s = 0.213 ± 0.005 s−1, (△) 3.35s = 0.145 ± 0.001 s−1, (□) 5.35s = 0.121 ± 0.001 s−1. (c) The signal duration was further enhanced by increasing the time between sampling and reducing the H2O concentration to 10% and resulted in decay rates of (△) 5.35s = 0.103 ± 0.002 s−1, (◆) 10.35s = 0.073 ± 0.001 s−1.
Discussion
The data presented above successfully demonstrate an ability to enhance detection of an important nitrogen donor in mammalian metabolism. Comparison of directly observed 15N spectra collected with the same spectrometer on a normal room temperature sample using signal averaging conditions and on a DNP hyperpolarized sample with a single pulse, show an enhancement in direct 15N observation of a factor of 8000. The enhancement in this comparison would come totally from DNP effects. Several mechanisms can contribute to DNP enhancements [21], however for low γ nuclei using trityl radicals and an apparatus similar to that used here, thermal mixing has been suggested as the dominant mechanism [22]. Under strong microwave saturation the nuclear and electron pools would come to the same spin temperature, one not dependent on the size of nuclear moments. Polarization would vary from one at the low temperature limit to smaller values with an inverse dependence on spin temperature and linear dependence on the nuclear magnetogyric ratio in the high temperature limit. Given that enhancements as high as 44,000 and 23,000 for 13C and 15N respectively have been achieved using DNP and a trityl radical [23], the enhancement of 8000 that we observe for 15N without a great deal of attention to optimizing DNP conditions is reasonable. Some further benefit can clearly be achieved by optimizing conditions.
Our primary objective was, however, to explore additional enhancement that can come from indirect detection of 15N through directly bonded protons. The intensities of the 15N coupled glutamine spectrum, summed over the entire sampling time, provide a reasonable comparison to direct 15N detection of a similarly polarized sample. Making this comparison we find an additional enhancement of a factor of 20. This is short of expectations. In principle a single pulse after INEPT transfer on a fully protonated glutamine sample should yield an additional enhancement of 100, one factor of 10 coming from the higher proton magnetic moment and another factor of 10 coming from the higher precession frequency and associated higher induced voltage in the probe detection coil. The deviations can come from a number of sources. First, we have not included the first sampling in our summation, because this point includes an enhanced level of exchange occurring during dissolution, and transfer to the spectrometer (about 5 s). Second, we are losing signal due to spin-spin and spin-lattice relaxation over the entire sampling time. And third, pulses in the sequence are imperfect and yield additional losses due to an incomplete return of unsampled 15N magnetization to the +Z axis. However, the enhancements coming from indirect detection through protons are significant. When combined with the factor of 8000 coming from DNP enhanced 15N polarization we have achieved a 160,000 fold enhancement over single pulse direct 15N observation on a sample having the polarization of a normal room temperature Boltzmann distribution.
Aside from enhanced sensitivity, the use of a deuterated precursor adds significantly to the time period over which hyperpolarization can be used to monitor kinetic processes. Our spin relaxation experiments show the relaxation time for the fully deuterated sidechain nitrogen of glutamine to be a factor of 3.6 longer than that of a fully protonated sidechain nitrogen (Figure 2). To take full advantage of the longer spin relaxation times of fully deuterated amide groups requires amide deuteron for amide proton exchange times to be comparable to the spin relaxation time scale. The glutamine sidechain amide protons are not optimum in this respect. At pH 6 and 25°C glutamine sidechain amide deuterons exchange rates are on the order of 1 s−1 [17]. As illustrated, we can slow the effective exchange rate. We can likewise tune the exchange rate by altering the pH (data not shown), with rates expected to drop an order of magnitude for every pH unit [17]. It is also possible to slow exchange by partial deuteration of the buffer. Only 10% of the exchanges would be effective in a 90% deuterated buffer. These are not likely options for in vivo studies, but studies of rapid enzymatic reactions in vitro may well tolerate a range of pH and buffer compositions. This method dissolves the hyperpolarized material with a deuterated solvent to maintain the slow relaxation properties of the deuterated and hyperpolarized nucleus during transfer, and the dissolution volume limits the percentage of protonated solvent in the high resolution spectrometer (data not shown). Reducing the dissolution volume or using a different method for D/H exchange, perhaps through enzyme catalysis, would reduce deuterium content in the final mixture to approach a condition closer to 100% H2O.
A relaxation rate on the order of 0.05s−1 and enhancements near 160,000 open wide possibilities for metabolic investigations of nitrogen utilization in enzyme catalyzed reactions. The factor of 160,000 over direct nitrogen detection of normally polarized samples represents a factor of 160 enhancement over direct detection of normally polarized protons. If we assume that the inherent sensitivity of a modern spectrometer allows detection of proton signals from a 50 μM sample of a metabolite of interest with a single pulse, and that one samples polarization spread over 8 repetitions and 40s, a factor of 160 enhancement suggests that one should be able to follow a process that produces a change in substrate or product concentration of approximately 0.5 μMs-1, and do so with the selectivity inherent in an isotope filtered experiment.
For in-cell systems there are a number of interesting processes that lead to concentration changes on the order of our demonstrated sensitivity and over appropriate timescales. For example, transport of glutamine into mitochondrial particles from Ehrlich tumor cells concentrated to a level of 10mg/ml protein would result in a change of 40 μM in external glutamine concentration over the first 5s [24].
The future use of the methodology described for in vivo applications may lie more with compounds for which inherent exchange rates are lower. The choices are broad with nitrogens carrying slowly exchanging protons found in compounds ranging from peptides to nucleic acids to N-acetylated sugars. The methodology itself is also not restricted to 15N. There are a number of compounds with proton carrying 13C sites that exhibit exchange of deuterons for protons in the course of enzyme catalyzed reactions. Similar extensions of relaxation times and enhancements through indirect detection should impact on studies of these systems.
Highlights.
Deuteration of 15N-glutamine reduces R1 ~3-fold
Solvent catalyzed D/H exchange permits indirect detection of 15N polarization.
Hyperpolarized 15N-glutamine is visible for more than 60s.
Acknowledgments
This research was funded by a grant from the NIH, National Center for Research Resources, P41RR005351 awarded to J.H.P., and a Kirschstein NRSA fellowship F32AR058084 awarded to A.W.B. The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Center for Research Resources or the NIH.
Footnotes
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Franken PA, Colegrove FD. Alignment of Metastable Helium Atoms by Unpolarized Resonance Radiation. Physical Review Letters. 1958;1:316–318. [Google Scholar]
- 2.Chekmenev EY, Norton VA, Weitekamp DP, Bhattacharya P. Hyperpolarized (1)H NMR employing low gamma nucleus for spin polarization storage. J Am Chem Soc. 2009;131:3164–5. doi: 10.1021/ja809634u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchiat MA, Carver TR, Varnum CM. Nuclear Polarization in He-3 Gas Induced by Optical Pumping and Dipolar Exchange. Physical Review Letters. 1960;5:373–375. [Google Scholar]
- 4.Abragam A, Goldman M. Principles of Dynamic Nuclear-Polarization. Reports on Progress in Physics. 1978;41:395–467. [Google Scholar]
- 5.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–63. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hilty C, Bowen S. Applications of dynamic nuclear polarization to the study of reactions and reagents in organic and biomolecular chemistry. Org Biomol Chem. 2010;8:3361–5. doi: 10.1039/c004420m. [DOI] [PubMed] [Google Scholar]
- 7.Nomura DK, Dix MM, Cravatt BF. Activity-based protein profiling for biochemical pathway discovery in cancer. Nature Reviews Cancer. 2010;10:630–638. doi: 10.1038/nrc2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cudalbu C, Comment A, Kurdzesau F, van Heeswijk RB, Uffmann K, Jannin S, Denisov V, Kirik D, Gruetter R. Feasibility of in vivo N-15 MRS detection of hyperpolarized N-15 labeled choline in rats. Physical Chemistry Chemical Physics. 2010;12:5818–5823. doi: 10.1039/c002309b. [DOI] [PubMed] [Google Scholar]
- 10.Pfeilsticker JA, Ollerenshaw JE, Norton VA, Weitekamp DP. A selective N-15-to-H-1 polarization transfer sequence for more sensitive detection of N-15-choline. Journal of Magnetic Resonance. 2010;205:125–129. doi: 10.1016/j.jmr.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Godel H, Graser T, Foldi P, Pfaender P, Furst P. Measurement of free amino acids in human biological fluids by high-performance liquid chromatography. J Chromatogr. 1984;297:49–61. doi: 10.1016/s0021-9673(01)89028-2. [DOI] [PubMed] [Google Scholar]
- 12.Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Frontiers in Bioscience. 2007;12:332–343. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- 13.Mates JM, Segura JA, Campos-Sandoval JA, Lobo C, Alonso L, Alonso FJ, Marquez J. Glutamine homeostasis and mitochondrial dynamics. International Journal of Biochemistry & Cell Biology. 2009;41:2051–2061. doi: 10.1016/j.biocel.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Harris T, Giraudeau P, Frydman L. Kinetics from indirectly detected hyperpolarized NMR spectroscopy by using spatially selective coherence transfers. Chemistry. 2011;17:697–703. doi: 10.1002/chem.201002151. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar R, Comment A, Vasos PR, Jannin S, Gruetter R, Bodenhausen G, Hall H, Kirik D, Denisov VP. Proton NMR of (15)N-choline metabolites enhanced by dynamic nuclear polarization. J Am Chem Soc. 2009;131:16014–5. doi: 10.1021/ja9021304. [DOI] [PubMed] [Google Scholar]
- 16.Mishkovsky M, Frydman L. Progress in hyperpolarized ultrafast 2D NMR spectroscopy. Chemphyschem. 2008;9:2340–8. doi: 10.1002/cphc.200800461. [DOI] [PubMed] [Google Scholar]
- 17.Krishna NR, Sarathy KP, Huang DH, Stephens RL, Glickson JD, Smith CW, Walter R. Primary Amide Hydrogen-Exchange in Model Amino-Acids - Asparagine, Glutamine, and Glycine Amides. Journal of the American Chemical Society. 1982;104:5051–5053. [Google Scholar]
- 18.Patyal BR, Gao JH, Williams RF, Roby J, Saam B, Rockwell BA, Thomas RJ, Stolarski DJ, Fox PT. Longitudinal relaxation and diffusion measurements using magnetic resonance signals from laser-hyperpolarized Xe-129 nuclei. Journal of Magnetic Resonance. 1997;126:58–65. doi: 10.1006/jmre.1997.1159. [DOI] [PubMed] [Google Scholar]
- 19.Merritt ME, Harrison C, Kovacs Z, Kshirsagar P, Malloy CR, Sherry AD. Hyperpolarized Y-89 offers the potential of direct imaging of metal ions in biological systems by magnetic resonance. Journal of the American Chemical Society. 2007;129:12942. doi: 10.1021/ja075768z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris GA, Freeman R. Enhancement of Nuclear Magnetic-Resonance Signals by Polarization Transfer. Journal of the American Chemical Society. 1979;101:760–762. [Google Scholar]
- 21.Maly T, Debelouchina GT, Bajaj VS, Hu KN, Joo CG, Mak-Jurkauskas ML, Sirigiri JR, van der Wel PCA, Herzfeld J, Temkin RJ, Griffin RG. Dynamic nuclear polarization at high magnetic fields. Journal of Chemical Physics. 2008;128 doi: 10.1063/1.2833582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ardenkjaer-Larsen JH, Macholl S, Johannesson H. Dynamic nuclear polarization with trityls at 1.2 K. Applied Magnetic Resonance. 2008;34:509–522. [Google Scholar]
- 23.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molina M, Segura JA, Aledo JC, Medina MA, Decastro IN, Marquez J. Glutamine Transport by Vesicles Isolated from Tumor-Cell Mitochondrial Inner Membrane. Biochemical Journal. 1995;308:629–633. doi: 10.1042/bj3080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piotto M, Saudek V, Sklenar V. Gradient-Tailored Excitation for Single-Quantum Nmr-Spectroscopy of Aqueous-Solutions. Journal of Biomolecular Nmr. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]