Abstract
The VlsE lipoprotein of Borrelia burgdorferi elicits a strong immune response during the course of Lyme disease. The present study was aimed at characterization of the epitopes of VlsE targeted by the antibody response in patients with post-Lyme disease syndrome, a condition characterized by persisting symptoms of pain, fatigue, and/or neurocognitive impairment despite antibiotic treatment of B. burgdorferi infection. Epitope mapping was carried out using microarrays that contained synthesized overlapping peptides covering the full sequence of VlsE from B. burgdorferi B31. In addition to the previously characterized IR6 region in the variable domain, specific sequences in the N- and C-terminal invariable domains of VlsE were found to be major B cell epitopes in affected patients. The crystal structure of VlsE indicated that the newly described epitopes form a contiguous region in the surface-exposed membrane-proximal part of the monomeric form of the protein.
Keywords: Lyme disease, post-Lyme disease syndrome, chronic Lyme disease, VlsE, epitope mapping, antibody
1. INTRODUCTION
Lyme disease is caused by spirochetes of the Borrelia burgdorferi species complex and is the most common vector-borne infection in the United States and Europe [1–3]. It is a multisystem disease that is typically associated with a characteristic skin lesion(s) (erythema migrans (EM)) in the early phase and with extracutaneous manifestations affecting joints, heart, and the nervous system in later stages [2, 4, 5]. Lyme disease is usually successfully treated with antibiotics, although some patients complain of persistent symptoms despite what is currently considered to be adequate antibiotic therapy and in the absence of clear evidence for ongoing infection [6–8]. These symptoms include mild to severe musculoskeletal pain, fatigue, and/or difficulties with concentration and memory [6, 7]. The condition, known as post-Lyme disease syndrome (PLDS or PLS) and sometimes referred to as chronic Lyme disease, can be associated with considerable impairment in the health-related quality of life in some patients [9]. However, despite several years of debate and a number of treatment trials [9–11], few clues to the causes of the symptoms have emerged. Lack of biomarkers to aid in the identification and follow up of PLDS patients or those at risk of becoming affected has been a major barrier to gaining a better understanding of the condition.
The human body's immune response to infection with B. burgdorferi includes production of antibodies to many antigens of the organism. These antibodies are utilized extensively in aiding the clinical diagnosis of Lyme disease [1]. Recently, a specific protein of B. burgdorferi, known as VlsE (variable major protein (Vmp)-like sequence expressed), has emerged as a particularly useful antigen in serologic assays for Lyme disease. VlsE is a surface lipoprotein of B. burgdorferi that undergoes antigenic variation during the course of infection. It consists of two invariable domains located at the N- and C-termini of the protein, as well as six variable regions (VR1-VR6) and six invariable regions (IR1-IR6) within its central variable domain [12]. VlsE elicits a strong antibody response that can be detected throughout the course of the disease (from early to late phase) and which persists for months to years following treatment [13–15]. The major immunodominant epitope of VlsE has been found to be located within the IR6 region [16, 17]. C6, a peptide that reproduces the IR6 epitope, is now utilized in a commercially-available diagnostic test.
While the antibody response to VlsE has been, in general, well-studied, it has not been explored in detail in PLDS patients. Liang et al. found 8 of 13 (62%) CDC criteria-seropositive PLDS patients to be positive for C6 antibodies [15]. A study by Fleming et al., which examined serum specimens from the same clinical trial as used in our study, reported C6 antibody positivity in 53 of 76 (70%) WB-positive and 8 of 51 (16%) WB-negative samples [14]. This study also reported a lack of correlation between longitudinal change in C6 antibody titer and clinical outcome upon additional antibiotic therapy in PLDS patients. In another study it was shown that the C-terminal variable domain of VlsE contains an immunodominant region(s) that is targeted by antibodies in PLDS, as well as in early and late phases of Lyme disease, although the associated epitope(s) was not identified [18]. In the present study, we describe the existence of specific epitopes of VlsE in addition to the IR6 region that are prominently targeted in the anti-VlsE immune response of PLDS patients. Located in the N- and C-terminal invariable domains of VlsE, these target sequences form a contiguous region in the protein's membrane-proximal zone. The newly described epitopes may be associated with later stages and more intractable forms of Lyme disease, or reflect differences in host response, that could lead to persistence of symptoms.
2. MATERIALS AND METHODS
2.1. Study participants
Serum samples were from 54 individuals with PLDS who were seropositive by enzyme-linked immunosorbent assay (ELISA) for IgG antibodies to B. burgdorferi (25 female, 29 male; mean age 56.3 ± 12.8 y (SD); mean elapsed time since the original diagnosis of Lyme disease 4.7 ± 2.8 y (SD)). The source of samples and selection criteria have been previously described in detail [9, 19]. Patients had at least one of the following: a history of EM skin lesion, early neurologic or cardiac symptoms attributed to Lyme disease, radiculoneuropathy, or Lyme arthritis. Documentation by a physician of previous treatment of acute Lyme disease with a recommended antibiotic regimen was also required. Patients had one or more of the following symptoms at the time of enrollment: widespread musculoskeletal pain, cognitive impairment, radicular pain, paresthesias, or dysesthesias. Fatigue often accompanied one or more of these symptoms. The chronic symptoms had to have begun within 6 months after the infection with B. burgdorferi.
The study also included control serum specimens from 14 borrelial IgG ELISA-seropositive individuals who had been treated for early localized or disseminated Lyme disease associated with single or multiple EM with no post-Lyme symptoms after at least 2 years of follow-up (4 female, 10 male; mean age 51.4 ± 18.0 y (SD); mean elapsed time since the original diagnosis of Lyme disease 4.6 ± 3.5 y (SD)). The original diagnosis of acute Lyme disease in these currently healthy subjects was confirmed by recovery of B. burgdorferi in cultures of skin and/or blood. The source of samples and selection criteria were previously described [19].
Serum samples from 20 healthy subjects without history or serologic evidence of past or present Lyme disease were also included in the study (12 female, 8 male; mean age 49.7 ± 15.5 y (SD). This study was approved by the Institutional Review Board of the Weill Cornell Medical College at Cornell University.
2.2. Anti-borrelia antibody seropositivity
2.2.1. B. burgdorferi whole-cell ELISA
IgG anti-borrelia antibody levels were determined by ELISA, as previously described [19].
2.2.2. B. burgdorferi Western blot assay (WB)
IgG antibody response to B. burgdorferi B31 was further characterized by WB, using commercial blots and the Euroblot automated WB instrument, according to the manufacturer's protocols (Euroimmun, Boonton, New Jersey) as previously described [20]. Briefly, nitrocellulose strips containing electrophoresis-separated B. burgdorferi B31 proteins were blocked and then incubated with 1.5 mL of diluted serum sample (1:50) for 30 min. Membrane strips were washed and incubated with AP-conjugated anti-human IgG antibody for 30 min. Bound antibodies were detected using the NBT/BCIP system. Quantitative analysis of bands on each blot was carried out using the EuroLinescan software (Euroimmun). Accurate background correction and determination of cutoff values for positivity were carried out by the software for the p18, p25 (OspC), p28, p30, p39 (BmpA), p41 (FlaB), p45, p58, p66, and p93 borrelial protein bands. Determination of IgG positive serology for Lyme disease was based on the CDC criteria [21, 22].
2.3. Antibody response to VlsE protein of B. burgdorferi
2.3.1. Detection of antibodies to recombinant VlsE
Presence of antibodies to the whole VlsE molecule was determined by immunoblotting, using nitrocellulose strips containing recombinant VlsE protein (Euroimmun) and the Euroblot automated WB instrument, based on the above WB procedure and as previously described [20].
2.3.2. C6 ELISA
IgG antibodies to C6, a peptide that reproduces the IR6 invariable region of the VlsE lipoprotein of B. burgdorferi, was determined using an ELISA kit, according to the manufacturer's instructions (Immunetics, Boston, Massachusetts). Serum specimen dilution was at 1:20. Test results were expressed as an index, calculated by dividing the optical density value for a given sample by that of a positive control included on each plate. The sample was considered positive if the index value was greater than or equal to 1.10, negative if it was less than or equal to 0.90, and equivocal when it was between 0.91 and 1.09.
2.3.3. VlsE peptide microarray and epitope mapping
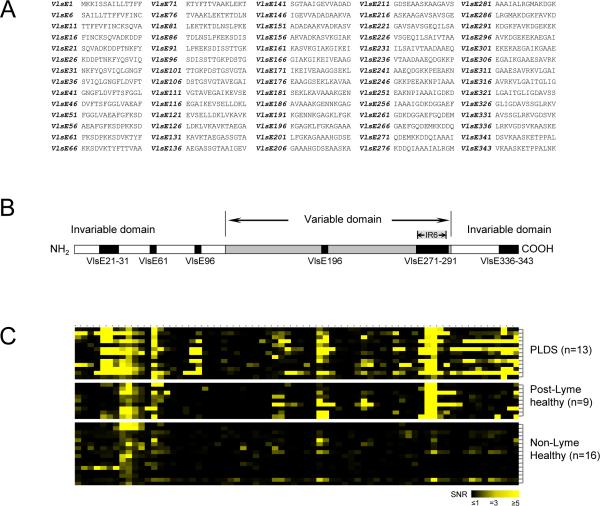
Preliminary mapping of epitopes of the VlsE protein targeted in the anti-borrelia immune response in patients and controls was done using randomly selected PLDS (n=13) and post-Lyme healthy (n=9) individuals who were positive for antibodies to the recombinant VlsE band in the immunoblotting experiment, as well as specimens from non-Lyme healthy subjects (n=16). The designed peptide array consisted of seventy 14mers, with an overlap of 9 amino acids each (except the final peptide, which had an overlap of 12 amino acids with the peptide preceding it), based on the amino acid sequence of the B. burgdorferi B31 VlsE protein (NCBI accession number AAC45733) (Figure 1A). Peptide notation was based on the amino acid number (in the published protein sequence) of the first residue of each peptide (VlsE1 through VlsE343). Amino-oxy-acetylated peptides were synthesized on cellulose membranes using SPOT synthesis technology (JPT, Berlin, Germany), as previously described [23]. Following side chain deprotection, the solid phase bound peptides were transferred into 96 well filtration plates (Millipore, Billerica, Massachusetts) and treated with 200 μL of aqueous triethylamine (0.5 % by vol) in order to cleave the peptides from the cellulose. Peptide-containing triethylamine solution was filtered off and solvent was removed by evaporation under reduced pressure. Resulting peptide derivatives (50 nmol) were re-dissolved in 25 μL of printing solution (70% DMSO, 25% 0.2 M sodium acetate at pH 4.5, 5 % glycerol) and transferred into 384-well microtiter plates. Peptide derivatives were deposited in triplicate onto epoxy-functionalized glass slides (Corning, Corning, New York) using a contact printer. Each array also contained a human IgG feature, which was used as a control. Printed peptide microarrays were kept at room temperature for 5 h and treated for 1 h with 1 % BSA at 42 °C. Slides were washed extensively with water, followed by ethanol, and dried using a microarray centrifuge. Resulting peptide microarrays were stored at 4 °C. Prepared array chips were hydrated and incubated with 1:200 dilutions of serum samples in Tris-buffered saline containing 0.05% Tween-20 (TBST) for 2 h. They were washed with TBST and incubated with Cy5-labeled anti-human IgG in TBST (0.8 μg/mL) (Jackson ImmunoResearch, West Grove, Pennsylvania) for 1 h. Arrays were washed with TBST and de-ionized water, and dried under a stream of nitrogen. The arrays were read using a GenePix 4000B Axon instrument (Molecular Devices, Sunnyvale, California) with excitation at 635 nm and emission filter at ~650–690 nm. The data were analyzed using the GenePix Pro 6.0 software (Molecular Devices). Signals for all features were normalized based on the IgG spot signal on each array. A signal value was considered positive if it was greater than or equal to 3 times its respective background signal (signal to noise ratio (SNR) ≥3) and greater than or equal to 2 times its standard deviation.
2.3.4. ELISA for antibodies to differentially targeted VlsE epitopes
Results of the epitope mapping analysis for VlsE were used to develop an ELISA to measure levels of antibodies against specific epitopes in all of the available specimens. Biotin-labeled peptides representing sequences of 3 epitopes found to be differentially targeted by antibodies from PLDS patients in the peptide microarray epitope mapping analysis were synthesized by utilizing Fmoc chemistry (Sigma-Aldrich, St. Louis, Missouri). These included 1) VlsE21–31 (SQVADKDDPTNKFYQSVIQLGNGF), 2) VlsE96 (SDISSTTGKPDSTG), and 3) VlsE336 (LRKVGDSVKAASKE). Stock solutions of each peptide were prepared in 50% acetonitrile (4–5 mg/mL). Preblocked neutravidin-coated polystyrene plates (Pierce, Rockford, Illinois) were washed with phosphate-buffered saline containing 0.05% Tween-20 (PBST) immediately before use. Wells were incubated with 100 μL of 0.2 μg/mL solutions of biotinylated peptides in dilution buffer (1% BSA in PBST) for 2 h. Control wells were incubated only with buffer. Plates were washed with PBST, followed by incubation with patient serum specimens (1:300 in dilution buffer) for 1 h. Two samples found to have high antibody reactivity in preliminary experiments were included as controls on each plate. Wells were washed as before and incubated with HRP-conjugated sheep anti-human IgG (GE Healthcare, Piscataway, New Jersey) (1:2000 in dilution buffer) for 50 min. Incubation with developing solution, comprising 27 mM citric acid, 50 mM Na2HPO4, 5.5 mM o-phenylenediamine, and 0.01% H2O2 (pH 5), was for 40 min. Absorbance was measured at 450 nm and corrected for non-specific binding by subtraction of the mean absorbance of corresponding wells not coated with the peptide for each specimen. Absorbance values were normalized based on the mean corrected value for the positive samples from the array analysis on each plate. Cutoff for positivity was assigned as three standard deviations above the mean for the non-Lyme healthy control group. The published 3-dimensional crystal structure of VlsE was analyzed for the spatial location of reactive epitopes [24].
2.4. Data analysis
Group differences were analyzed by the two-tailed Welch t test or Mann-Whitney U test (continuous data), and the chi-square test or Fisher's exact test (nominal data). Differences with p values of <0.05 were considered to be significant.
3. RESULTS
3.1. Determination of seropositivity
All selected serum samples from PLDS patients and fully recovered post-Lyme healthy individuals were positive by IgG whole-cell ELISA, while none of the sera from the non-Lyme healthy control group was positive. 47 of 54 (87%) ELISA-positive PLDS subjects, 11 of 14 (79%) ELISA-positive post-Lyme healthy subjects, and none of the non-Lyme healthy subjects were found to be IgG seropositive for anti-borrelia antibodies by WB according to the CDC criteria.
3.2. Antibody response to VlsE protein of B. burgdorferi
3.2.1. Detection of antibodies to recombinant VlsE
45 of 54 (83%) whole-cell ELISA-positive PLDS subjects, 9 of 14 (64%) whole-cell ELISA-positive post-Lyme healthy subjects, and none of the non-Lyme healthy subjects were positive for IgG antibodies to recombinant VlsE.
3.2.2. C6 ELISA
Of the 54 whole-cell ELISA-seropositive PLDS serum specimens, 47 (87%) were positive (n=43) or equivocal positive (n=4) for antibody to the C6 peptide of the borrelial VlsE protein. In comparison, 9 of 14 (64%) whole-cell ELISA-seropositive post-Lyme healthy serum specimens were positive (n=9) or equivocal positive (n=0) for C6 antibody. None of the sera from the non-Lyme healthy control group was positive. The mean C6 antibody index value for the PLDS, post-Lyme healthy, and non-Lyme healthy groups were 3.91 ± 0.36 (SEM), 2.99 ± 0.71 (SEM), and 0.18 ± 0.01 (SEM), respectively.
3.2.3. Peptide microarray
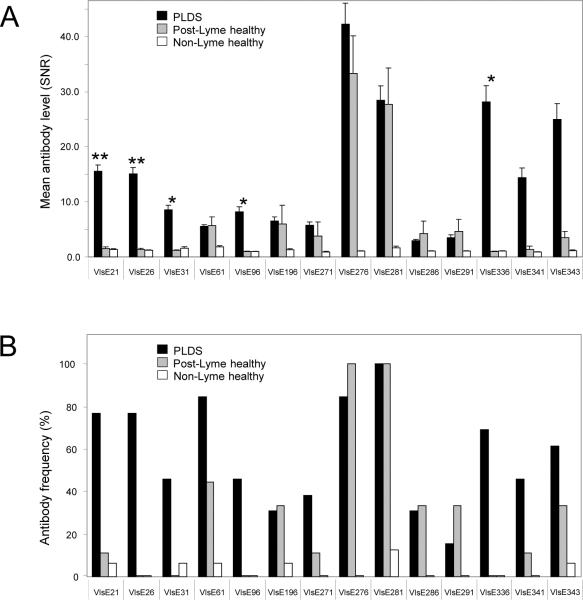
Epitope mapping of the anti-VlsE antibody response in a randomly selected group of PLDS and post-Lyme healthy subjects (all were positive for anti-VlsE antibodies by immunoblotting) identified 14 individual VlsE peptides, comprising 6 unique contiguous amino acid sequences (Fig. 1B). Binding to these peptides (as quantified by the normalized fluorescence signal to noise ratio) for the PLDS and/or post-Lyme healthy serum antibodies was significantly higher than in the non-Lyme healthy control group (p<0.05) (Fig. 1C, Fig. 2). Figure 2 shows the level (Fig. 2A) and frequency of positivity (Fig. 2B) for antibodies to each of the 14 peptides in patient and control groups. Among these peptides were those that form the previously identified IR6 epitope of VlsE (VlsE271–291), which is utilized in the C6 assay. Among the 14 identified peptides, reactivity to 5 peptides covering 3 separate contiguous sequences of amino acids, including VlsE21 through VlsE31 (SQVADKDDPTNKFYQSVIQLGNGF), VlsE96 (SDISSTTGKPDSTG), and VlsE336 (LRKVGDSVKAASKE) was significantly higher in the PLDS group than in the post-Lyme healthy group.
Figure 1. Epitope mapping of VlsE.
A) Synthesized peptides of the VlsE protein of B. burgdorferi B31 used for preparation of microarrays. Peptide notation is according to the amino acid number (in the VlsE protein sequence) of the first residue of each peptide. B) Diagrammatic structure of VlsE showing the 14 peptides (representing 6 contiguous regions) that were found to be the main epitopes of the protein targeted by antibodies in individuals with a history of Lyme disease. These peptides had significantly higher level of IgG antibody reactivity towards them in the post-Lyme groups (n=13 for PLDS, n=9 for post-Lyme healthy) in comparison to the non-Lyme healthy control group (n=16) (p<0.05). C) Heat map of antibody reactivity for tested specimens towards the 70 synthesized VlsE peptides (VlsE1 through VlsE343 from left to right, corresponding to panel B).
Figure 2. Level and frequency of antibody reactivity to differentially targeted peptides of VlsE, as determined by peptide microarray epitope mapping.
A) Mean level of antibody reactivity to each of the 14 peptides in PLDS (n=13), post-Lyme healthy (n=9), and non-Lyme healthy (n=16) groups. Error bars represent the standard error of the mean. Among these 14 peptides, reactivity towards 5 peptides, forming 3 contiguous amino acid sequences, was significantly higher in the PLDS group than in the post-Lyme healthy group. These peptides are shown by asterisks (1 asterisk indicates p<0.05, while 2 asterisks indicates p< 0.01 for the comparison between PLDS and post-Lyme healthy groups. B) Frequency of positivity for IgG antibodies to each of the 14 peptides in the PLDS, post-Lyme healthy, and non-Lyme healthy groups.
3.2.4. ELISA for antibodies to differentially targeted VlsE epitopes
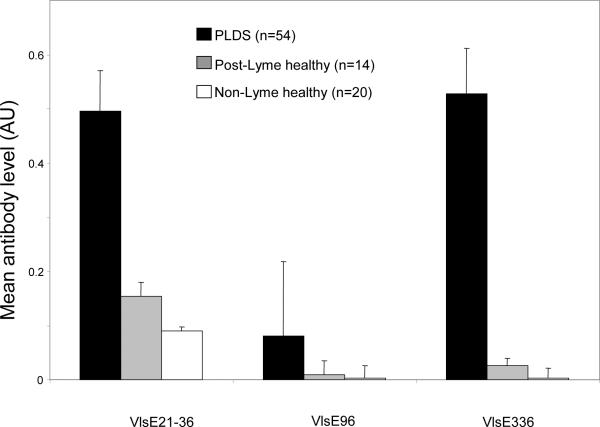
An ELISA protocol was developed to assess the reactivity of antibodies to the above three specific differentially targeted peptides of VlsE in all available specimens. By ELISA, the level of antibody reactivity to VlsE21–31 and VlsE336 (as measured by mean normalized absorbance) was significantly higher in the PLDS group than in the post-Lyme healthy and non-Lyme healthy groups (p<0.001) (Fig. 3). However, the difference in antibody reactivity towards VlsE96 did not reach statistical significance with the ELISA system. Similarly, the frequencies of antibody reactivity towards VlsE21–31 and VlsE336 were significantly greater in the PLDS group (30 of 54, 56%; 21 of 54, 39%, respectively) than the post-Lyme healthy (2 of 14, 14%; 1 of 14, 7%, respectively) (p<0.05 for VlsE21–31 and p<0.01 for VlsE336) and the non-Lyme healthy (0% for both peptides) (p<0.001) groups. When combining the samples that were positive for antibodies to either VlsE21–31 or VlsE336, the frequency of positivity was 65% (35 of 54) for the PLDS group versus 21% (3 of 14) for the post-Lyme healthy group (p<0.05) and 0% in the non-Lyme healthy group (p<0.0001).
Figure 3. Mean levels of antibodies to differentially targeted VlsE epitopes, as measured by ELISA.
Antibody reactivity to VlsE21-31 and VlsE336 remained significantly higher in the PLDS group (n=54) than in the post-Lyme healthy (n=14) and non-Lyme healthy groups (p<0.001 for both peptides). Error bars represent the standard error of the mean.
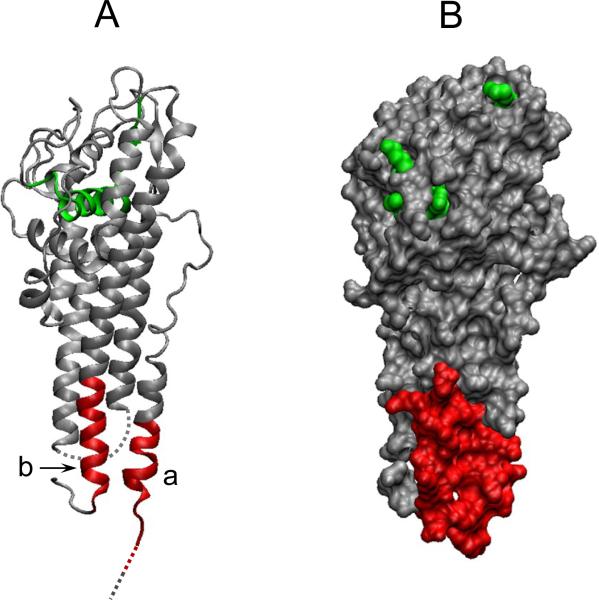
The amino acid sequence and 3-dimensional crystal structure of VlsE indicated that the newly identified epitopes are located in the two invariable domains of VlsE, appearing to form a single contiguous area in the surface-exposed membrane-proximal region of the monomeric form of the protein (Figs. 1B, 4).
Figure 4. Spatial position of epitopes of VlsE for which a differential antibody response is found in the PLDS patient group.
A ribbon diagram (A) and an orthographic molecular surface representation (B) of VlsE monomer are depicted using the VMD molecular graphics program, based on NCBI's 3D-structure database coordinates. Two specific epitopes of the protein believed to be differentially targeted by antibodies in PLDS patients are shown in red: a, VlsE21-31: SQVADKDDPTNKFYQSVIQLGNGF. b, VlsE336: LRKVGDSVKAASKE. Parts of the protein that were missing from the 3D-structure database coordinates are represented as dashed lines in the ribbon diagram. The sequence representing the IR6 epitope of VlsE (used in C6 ELISA) is shown in green. The bottom of the figure represents the membrane proximal region.
4. DISCUSSION
The VlsE protein of B. burgdorferi has emerged as a highly useful diagnostic entity in WB- and ELISA-format assays for active Lyme disease. It is used either as a whole recombinant protein or as a peptide representing its immunodominant epitope located in the IR6 region. While several studies have examined the immune response to VlsE in the course of acute B. burgdorferi infection, no systematic characterization of the targeted epitopes of the protein in the antibody response of PLDS patients has been attempted previously. In order to analyze the anti-VlsE immune response in PLDS, we carried out a detailed epitope mapping of the entire sequence of the VlsE protein of B. burgdorferi B31. Our data show that in addition to the IR6 epitope in the variable domain, PLDS patients have a strong antibody response to specific sequences in the N- and C-terminal invariable domains of VlsE.
We found good concordance between antibody reactivity to the recombinant VlsE molecule (WB) and the IR6 epitope (C6 ELISA). The epitope mapping data demonstrated that the IR6 region is the primary linear epitope in the anti-VlsE antibody response in PLDS patients, as well as in post-Lyme healthy individuals. Interestingly, earlier work had shown a lack of significant antibody reactivity in humans and non-human primates against constituent peptides of the IR6 epitope derived from the amino acid sequence of VlsE from the IP90 strain of B. garinii [25]. In contrast, our data indicate that patients with a history of Lyme disease express antibodies against the VlsE protein of the B. burgdorferi B31 strain that seem to recognize the IR6 region as multiple individual epitopes. The contradiction between our study and the earlier work may be attributed to differences in the amino acid sequences of the synthesized 14mer peptides.
In addition to the IR6 region, two additional sequences, covered by peptides VlsE21 through VlsE31 and by VlsE336 through VlsE343 were found to be major targets in the antibody response of PLDS patients. Specifically, antibodies to sequences covered by VlsE21 through VlsE31 and by VlsE336 were found at significantly lower level and frequency in the post-Lyme healthy group, which was also confirmed by ELISA. These two sequences are located at the N- and C-terminal ends in the invariable domains of VlsE. The 3-dimensional crystal structure of the protein indicates that the two sequences are spatially adjacent to one another, suggesting that they might form a single target region. In addition, they appear to be surface-exposed and located in the membrane-proximal part of the monomeric form of VlsE. Antibodies that bind to the membrane-proximal region of specific proteins in other organisms have been previously described, some of which exert neutralizing or lytic activity [26–28].
VlsE is a membrane protein with a high turnover rate and antigenic variation as a function of time [12]. The B cell memory immune response against specific epitopes in the invariable sections of the protein would be expected to become stronger the longer an infection is left untreated in an individual. Previous work from our group demonstrated increased antibody reactivity in PLDS patients towards borrelial proteins that are associated with later stages of Lyme disease [20]. The fact that PLDS patients in the current study exhibited significantly greater antibody response to VlsE21-31 and VlsE336 epitopes than the fully recovered individuals with a history of early localized or disseminated Lyme disease might indicate that antibodies to the membrane-proximal invariable domains of VlsE become more prominent in later phases of B. burgdorferi infection. Therefore, these antibodies may become useful in patient follow-up and for determination of the stage of active or antecedent infection in Lyme borreliosis and PLDS patients.
A limitation of this study is that it was focused on examining the antibody response to a single sequence variation of the VlsE molecule. It is therefore likely to have missed certain target epitopes in the protein's variable domain. However, in view of the rapid antigenic variation and sequence turnover in these regions, the associated antibody response is not expected to be significant. Nevertheless, follow-up work should consider such sequence variations of the protein, as well as sequence differences among the invariable regions of the various genospecies and strains of borrelia. Another issue to consider is that only seropositive PLDS patients were examined in this study. This was done in order to ensure that all samples had the minimal detectable anti-borrelia antibody response necessary for subsequent analyses. Therefore, our findings do not extend to the seronegative subset of individuals, which formed about 40% of affected PLDS patients in the original treatment study [9]. Future prospective analyses will help to determine whether the newly described antibodies could be useful in predicting the development of post-Lyme disease syndrome or in ascertaining if the treatment of early Lyme disease has been successful. Continuation of these studies, aimed at detailed examination of antigen and epitope specificity of the anti-borrelia immune response in PLDS, may lead to development of specific biomarkers for the condition and provide additional insights into its mode of pathogenesis and potential therapies.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) [grant number AI071180-02 to A. Alaedini] and involved the use of specimens derived from an NIH-supported repository [contract number N01-AI-65308]. It was also supported in part by the Intramural Research Program of the NIH. We are indebted to Dr. Phillip J. Baker at NIH for his invaluable support and guidance throughout this project. We thank Ms. Diane Holmgren, Ms. Donna McKenna, and Ms. Susan Bittker for their assistance with specimen collection and organization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement All authors declare that there are no conflicts of interest.
REFERENCES
- 1.Marques AR. Lyme disease: a review. Curr Allergy Asthma Rep. 2010;10:13–20. doi: 10.1007/s11882-009-0077-3. PMID: 20425509. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Coburn J, Glickstein L. The emergence of Lyme disease. J Clin Invest. 2004;113:1093–1101. doi: 10.1172/JCI21681. PMID: 15085185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanek G, Strle F. Lyme borreliosis. Lancet. 2003;362:1639–1647. doi: 10.1016/S0140-6736(03)14798-8. PMID: 14630446. [DOI] [PubMed] [Google Scholar]
- 4.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. PMID: 17029130. [DOI] [PubMed] [Google Scholar]
- 5.Bratton RL, Whiteside JW, Hovan MJ, Engle RL, Edwards FD. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566–571. doi: 10.4065/83.5.566. PMID: 18452688. [DOI] [PubMed] [Google Scholar]
- 6.Marques A. Chronic Lyme disease: a review. Infect Dis Clin North Am. 2008;22:341–360. doi: 10.1016/j.idc.2007.12.011. PMID: 18452806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feder HM, Jr., Johnson BJ, O'Connell S, et al. A critical appraisal of “chronic Lyme disease”. N Engl J Med. 2007;357:1422–1430. doi: 10.1056/NEJMra072023. PMID: 17914043. [DOI] [PubMed] [Google Scholar]
- 8.Baker PJ. Perspectives on “chronic Lyme disease”. Am J Med. 2008;121:562–564. doi: 10.1016/j.amjmed.2008.02.013. PMID: 18589049. [DOI] [PubMed] [Google Scholar]
- 9.Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85–92. doi: 10.1056/NEJM200107123450202. PMID: 11450676. [DOI] [PubMed] [Google Scholar]
- 10.Krupp LB, Hyman LG, Grimson R, et al. Study and treatment of post Lyme disease (STOP-LD): a randomized double masked clinical trial. Neurology. 2003;60:1923–1930. doi: 10.1212/01.wnl.0000071227.23769.9e. PMID: 12821734. [DOI] [PubMed] [Google Scholar]
- 11.Fallon BA, Keilp JG, Corbera KM, et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology. 2008;70:992–1003. doi: 10.1212/01.WNL.0000284604.61160.2d. PMID: 17928580. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JR, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. PMID: 9108482. [DOI] [PubMed] [Google Scholar]
- 13.Philipp MT, Bowers LC, Fawcett PT, et al. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J Infect Dis. 2001;184:870–878. doi: 10.1086/323392. PMID: 11550127. [DOI] [PubMed] [Google Scholar]
- 14.Fleming RV, Marques AR, Klempner MS, et al. Pre-treatment and post-treatment assessment of the C(6) test in patients with persistent symptoms and a history of Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 2004;23:615–618. doi: 10.1007/s10096-004-1163-z. PMID: 15243815. [DOI] [PubMed] [Google Scholar]
- 15.Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, Philipp MT. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J Clin Microbiol. 1999;37:3990–3996. doi: 10.1128/jcm.37.12.3990-3996.1999. PMID: 10565920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang FT, Alvarez AL, Gu Y, Nowling JM, Ramamoorthy R, Philipp MT. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol. 1999;163:5566–5573. PMID: 10553085. [PubMed] [Google Scholar]
- 17.Embers ME, Jacobs MB, Johnson BJ, Philipp MT. Dominant epitopes of the C6 diagnostic peptide of Borrelia burgdorferi are largely inaccessible to antibody on the parent VlsE molecule. Clin Vaccine Immunol. 2007;14:931–936. doi: 10.1128/CVI.00075-07. PMID: 17567769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang FT, Bowers LC, Philipp MT. C-terminal invariable domain of VlsE is immunodominant but its antigenicity is scarcely conserved among strains of Lyme disease spirochetes. Infect Immun. 2001;69:3224–3231. doi: 10.1128/IAI.69.5.3224-3231.2001. PMID: 11292744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandra A, Wormser GP, Klempner MS, et al. Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav Immun. 2010;6:1018–1024. doi: 10.1016/j.bbi.2010.03.002. PMID: 20227484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra A, Wormser GP, Marques AR, Latov N, Alaedini A. Anti-Borrelia burgdorferi antibody profile in post-Lyme disease syndrome. Clin Vaccine Immunol. 2011;18:767–771. doi: 10.1128/CVI.00002-11. PMID: 21411605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. PMID: 16020686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–591. PMID: 7623762. [PubMed] [Google Scholar]
- 23.Wenschuh H, Volkmer-Engert R, Schmidt M, Schulz M, Schneider-Mergener J, Reineke U. Coherent membrane supports for parallel microsynthesis and screening of bioactive peptides. Biopolymers. 2000;55:188–206. doi: 10.1002/1097-0282(2000)55:3<188::AID-BIP20>3.0.CO;2-T. PMID: 11074414. [DOI] [PubMed] [Google Scholar]
- 24.Eicken C, Sharma V, Klabunde T, et al. Crystal structure of Lyme disease variable surface antigen VlsE of Borrelia burgdorferi. J Biol Chem. 2002;277:21691–21696. doi: 10.1074/jbc.M201547200. PMID: 11923306. [DOI] [PubMed] [Google Scholar]
- 25.Liang FT, Philipp MT. Epitope mapping of the immunodominant invariable region of Borrelia burgdorferi VlsE in three host species. Infect Immun. 2000;68:2349–2352. doi: 10.1128/iai.68.4.2349-2352.2000. PMID: 10722641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Meerten T, Rozemuller H, Hol S, et al. HuMab-7D8, a monoclonal antibody directed against the membrane-proximal small loop epitope of CD20 can effectively eliminate CD20 low expressing tumor cells that resist rituximab-mediated lysis. Haematologica. 2010;95:2063–2071. doi: 10.3324/haematol.2010.025783. PMID: 20851867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zwick MB, Labrijn AF, Wang M, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. PMID: 11602729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanlandschoot P, Beirnaert E, Barrere B, et al. An antibody which binds to the membrane-proximal end of influenza virus haemagglutinin (H3 subtype) inhibits the low-pH-induced conformational change and cell-cell fusion but does not neutralize virus. J Gen Virol. 1998;79(Pt 7):1781–1791. doi: 10.1099/0022-1317-79-7-1781. PMID: 9680143. [DOI] [PubMed] [Google Scholar]