Abstract
BACKGROUND
We sought to determine the change of PSA doubling time (PSADT) and its association with disease progression during intermittent androgen deprivation (IAD) therapy for prostate cancer.
METHODS
Data were retrospectively analyzed in 96 patients with biochemically relapsed prostate cancer (BRPC) treated with IAD since 1995. IAD consisted of LHRH-agonists ± antiandrogen given usually at PSA threshold (ng/ml) of 10–20, for 6–9 months. Cycles were repeated until the development of castration resistance. Mixed effects model was used to study PSADT change over cycles. Multivariate cox regression model was used to identify outcome-associated variables.
RESULTS
Patients received a mean of 2.8 treatment cycles over a mean follow-up time of 71 months. Fifty-seven (59%) remain on treatment and 39 (41%) developed PSA refractoriness (n = 8) or positive scans (n = 31). First off treatment interval PSADT (median 2.3 months) was significantly shorter than the baseline (median 7.34) but remained stable in subsequent cycles. Off treatment interval PSADT adjusted for testosterone recovery (median 3.7) was significantly longer than that based on all PSA determinations (median 2). Factors associated with disease progression were pre-treatment PSADT (≥6 vs. <6), first off treatment interval PSADT (≥3 vs. <3), and PSA nadir during the first treatment interval (<0.1 vs. ≥0.1).
CONCLUSIONS
During IAD for BRPC, PSADT becomes shorter, and is associated with testosterone recovery. PSADT before treatment and during the first off treatment interval is associated with disease progression. If prospectively validated these data may guide treatment with IAD and clinical trial design.
Keywords: intermittent androgen deprivation therapy, non-metastatic biochemically relapsed prostate cancer, PSA doubling time, serum testosterone, disease progression
INTRODUCTION
Prostate cancer is the most common non-cutaneous cancer in American men with over 210,000 new cases diagnosed annually in the US [1]. Most patients present with early disease and undergo definitive local treatment with prostatectomy and/or radiation therapy. However, 30–60% of patients demonstrate evidence of non-metastatic biochemical relapse within 10 years [2,3].
The clinical paradigm of non-metastatic biochemically relapsed prostate cancer (BRPC) is quite variable and patients may remain asymptomatic and clinically disease-free for many years [4–6]. Extensive data on the natural history in this patient group has identified various factors that predict the probability of metastasis-free and prostate cancer-specific survival [4,7–11]. Among these, the most important predictor of outcome is probably the PSA doubling time (PSADT) [4,11]. Patients with a PSADT of <3 months, 3–9 months, and >9 months have a high risk, intermediate risk, and low risk, respectively, for future development of clinical distant metastatic disease and mortality [7–9].
Unlike the presence of clinically evident metastatic disease, where initiation of androgen deprivation therapy is considered standard approach, there is no standard treatment for non-metastatic BRPC [6]. Salvage radiation therapy after radical prostatectomy may provide long-term benefit for some patients. The use of androgen deprivation therapy remains controversial. It is effective in reducing serum PSA to very low and frequently undetectable levels, and delaying the time to the appearance of clinical distant metastatic disease on scans in high risk patients with a PSA DT ≤12 months and/or pathologic Gleason score >7 [12], but its long-term survival benefit remain undefined at the present time [13,14]. Moreover, it is associated with long-term toxicities that could impact on quality of life and may offset any potential survival benefit from early intervention [15–18].
Intermittent androgen deprivation (IAD) refers to the cyclic administration of androgen deprivation therapy, allowing testosterone recovery between treatment periods, in an attempt to minimize androgen deprivation associated toxicity and to improve patient’s quality of life [17,18]. Studies show it may be as effective as continuous hormonal therapy with respect to biochemical and clinical progression free survival, and overall survival [17–21]. The optimal methodology of treatment cycles during IAD is unclear. Some studies employed a pre-defined duration of treatment cycles (12–24 months). Others used a PSA nadir endpoint to discontinue treatment [17]. Follow-up of serum testosterone during the off treatment interval is often not part of the routine clinical practice [22,23]. The change of baseline PSADT during treatment, and the association of PSADT with disease progression are poorly defined in this scenario.
In the present study we sought to determine the change of PSADT and its association with disease progression in patients with non-metastatic BRPC treated with IAD.
MATERIALS AND METHODS
The study group consisted of known patients who were treated with IAD for non-metastatic BRPC by medical oncologists at the Johns Hopkins hospital Kimmel Cancer Center since 1995. Before the initiation of IAD, all patients had evidence of rising serum PSA levels after local treatment (surgery and/or radiation) for histologically proven prostate cancer, and no evidence of local or distant metastatic recurrence on physical examination, bone scan, and CAT scan. Data were collected retrospectively. Outcome data were frozen on September 30, 2010.
Initiation of IAD
With evidence of biochemical PSA relapse after local therapy (i.e., PSA ≥0.2 ng/ml after surgery and ≥nadir + 2 after radiation therapy), patients had a bone scan and a CAT scan (chest–abdomen–pelvis) to rule out clinically recurrent local or distant metastatic disease. Patients were then typically followed every 3 months by clinical evaluation (history and physical examination including digital rectal exam) and serum PSA level. A CAT scan and bone scan were usually repeated once a year. Patients PSADT was calculated based on all consecutive relapsed PSA levels after the completion of local treatment (including post-surgery salvage radiation therapy). Usually, the PSA threshold for initiation of IAD was 10–20 ng/ml. Androgen deprivation therapy consisted of LHRH-agonist injection every 3 months. A non-steroidal antiandrogen (bicalutamide) was added at physician discretion. Treatment was discontinued after 6–9 months when the PSA nadired.
Subsequent Treatment Cycles
During the off treatment period, patients had a clinical and serum PSA follow-up every 3 months, and serum testosterone measurement at physician discretion. Subsequent treatment re-initiation was done at the same PSA threshold. Relapsed PSA during the off treatment interval was defined as an absolute level >0.1 ng/ml and greater than its post-treatment nadir. Serum testosterone recovery was defined as an absolute level ≥150 ng/dl [24,25]. IAD was continued until the development of a castrate resistant state which was defined as on treatment two consecutive rise of PSA with a castrate level of testosterone [6,26]. At this point scans (bone scan and CAT scan) were repeated to evaluate for clinical distant disease progression.
Statistical Analysis
Follow-up time was defined as the time from initiation of IAD to September 30, 2010. A treatment cycle was defined as the time period from the first LHRH-agonist injection to 3 months after the last injection. The time from 3 months after the last injection of the prior treatment cycle to the first injection of the next treatment cycle was defined as the off treatment interval. Time to disease progression was defined as the time from IAD initiation until evidence of biochemical (castrate resistant state) or clinical (local or distant disease recurrence as evident by physical examination or scans) disease progression at castrate levels of serum testosterone. Baseline and off treatment interval PSADT was calculated using all relapsed PSAs, by natural log of 2 (0.693) divided by the slope of the relationship between the log of PSA and time of PSA measurement for each patient [4]. Changes of PSADT from baseline to the off treatment intervals after IAD initiation were assessed by Wilcoxon test. The association between the PSADT and serum testosterone recovery was analyzed by mixed effects model. Univariate and multivariate Cox regression model was used to analyze clinical predictive factors for disease progression. A survival tree analysis was used to find the best cut-off values for continuous variables as the PSADT.
Regulatory Considerations
The research was carried out in accordance with the approval by the IRB committee of our institution.
RESULTS
Study Group
Ninety-six patients (mean age 66 years) with non-metastatic BRPC after local therapy, who were followed and treated with IAD therapy by medical oncologists at the Johns Hopkins hospital Kimmel Cancer Center between the years 1995 and 2010, were included in the present study. Baseline patient characteristics are listed in Table I.
TABLE I.
Baseline Patient Characteristics
| Parameter | Value |
|---|---|
| Age (years) at initiation of IAD: mean ± SD (range); median | 66.2 ± 8 (48–85); 66 |
| Stage at the initial diagnosis of prostate cancer | |
| T1–2 | 45% (n = 43) |
| T3 | 47% (n = 45) |
| N1 | 8% (n = 8) |
| Combined Gleason grade | |
| Low risk (≤6) | 23% (n = 22) |
| Intermediate risk (7) | 41% (n = 39) |
| High risk (8–10) | 34% (n = 33) |
| Unknown | 2% (n = 2) |
| PSA (ng/ml) at the initial diagnosis of prostate cancer: mean ± SD (range); median | 29 ± 110.95 (2.5–947); 9.25 |
| Primary treatment | |
| Radical prostatectomy | 80% (n = 77) |
| Radiation therapy | 19% (n = 18) |
| Primary androgen deprivation therapy | 1% (n = 1) |
| Time (months) from primary treatment to biochemical relapse: mean ± SD (range); median | 26.8 ± 23.3 (1–108); 18.5 |
| Baseline PSADT (months): mean ± SD (range); median | 9.7±8.6 (1.31–53.9); 7.34 |
| Low risk (PSADT ≥9) | 34% (n = 33) |
| Intermediate risk (PSADT 3–9) | 47% (n = 45) |
| High risk (PSADT<3) | 19% (n = 18) |
| PSA level (ng/ml) before the initiation of intermittent androgen deprivation (IAD): mean ± SD (range); median | 37.66 ± 101 (0.4–947); 18.8 |
| Serum testosterone (ng/dl) before the initiation of IAD: mean ± SD (range); median | 416.6 ± 190.8 (183–1,040); 395.5 |
Treatment Outcomes
With a follow-up of 71.1 ± 33.2 months (mean ± SD, range 22–183, median 65), patients received 2.85 ± 1.65 (mean ± SD, range 1–9, median 2.5) cycles of IAD. A non-steroidal antiandrogen (bicalutamide) was added in 45 patients (47%). Fifty-seven patients (59%) remain on treatment (69.8 ± 34.3 months, mean ± SD, range 26–183, median 65). Thirty-nine patients (41%) have demonstrated evidence of PSA refractoriness (n = 8) or radiological progression (n = 31) after 42.23 ± 23.26 months (mean ± SD, range 14–105, median 37) of follow-up (Table II).
TABLE II.
Treatment Outcomes
| Parameter | Value |
|---|---|
| Follow-up time (months): mean ± SD (range); median | 71.1 ± 33.2 (22–183); 65 |
| Patients for which an antiandrogen was added | 45 (47%) |
| Number of cycles completed | |
| Whole group: mean ± SD (range); median | 2.85 ± 1.65, (1–9); 2.5 |
| Number of patients who got 1 treatment cycle | 19% (n = 18) |
| Number of patients who got 2 treatment cycles | 31% (n = 30) |
| Number of patients who got 3 treatment cycles | 22% (n = 21) |
| Number of patients who got 4 treatment cycles | 17% (n = 16) |
| Number of patients who got ≥5 treatment cycles | 11% (n = 11) |
| On treatment (at 3 months) testosterone nadir (ng/ml): mean ± SD (range); median | 24.8 ± 8.2 (4–50); 20 |
| Time (months) to testosterone recovery ≥150 ng/dl: mean ± SD (range); median | |
| After treatment cycle 1 | 6.8 ± 6 (0–41); 6 |
| After treatment cycle 2 | 6.75 ± 3.9 (0–20); 6 |
| After treatment cycle 3 | 5.7 ± 2.6 (2–12); 6 |
| Off treatment fully recovered testosterone (≥150 ng/dl): mean ± SD (range); median | |
| After treatment cycle 1 | 362.7 ± 173.6 (130–1,012); 350 |
| After treatment cycle 2 | 324.8 ±138.6 (64–665); 301 |
| After treatment cycle 3 | 299.8 ±124.8 (126–576); 284 |
| PSA (ng/ml) response to first treatment cycle: mean ±SD (range); median | |
| At 3 months | 1.47 ± 5.26 (0.01–46); 0.2 |
| Nadir | 0.26 ± 0.68 (0.01–5.7); 0.08 |
| Time to nadir (months) | 6 ±3.2 (3–15); 6 |
| Off treatment PSA doubling time (months): mean ± SD (range); median | |
| After treatment cycle 1 | 3.1 ± 3.38 (0.59–30.5); 2.3 |
| After treatment cycle 2 | 2.18 ± 1.53 (0.49–8.22); 1.65 |
| After treatment cycle 3 | 2.35 ± 1.76 (0.57–8.34); 2.01 |
| After treatment cycle 4 | 3.57 ± 4.75 (0.62–24.06); 2.9 |
Factors Associated With Disease Progression
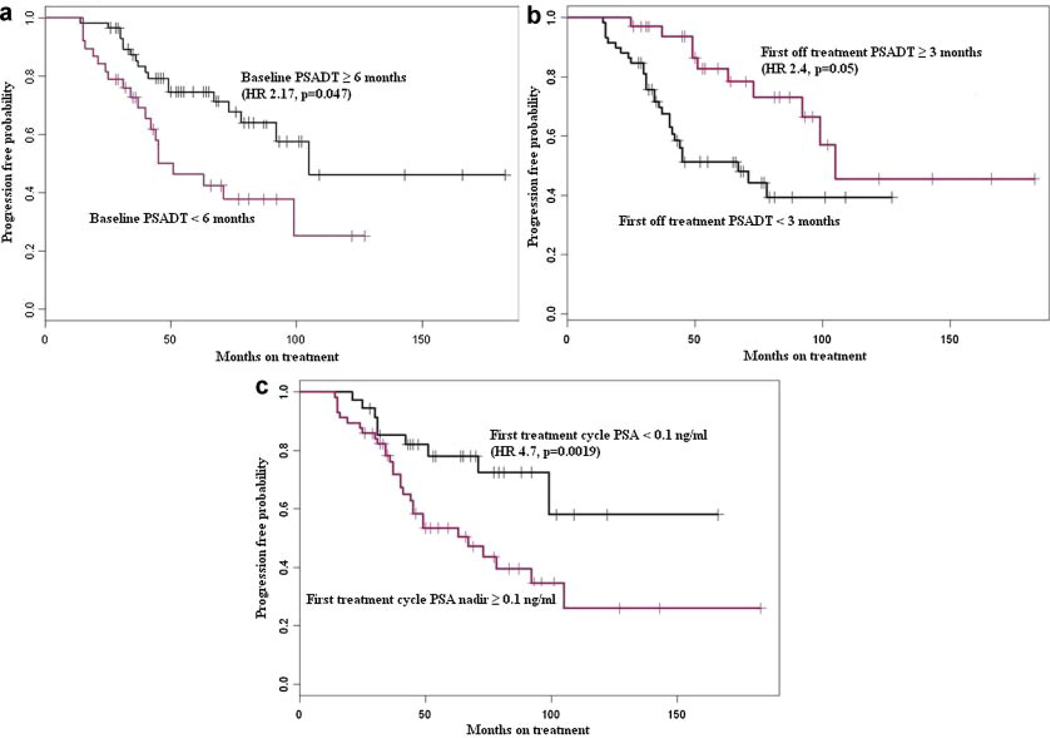
We identified three significant independent factors associated with biochemical (PSA) or clinical (scans) disease progression in patients treated with IAD. These include the baseline pre-treatment PSADT (≥6 vs. <6 months, HR 2.17, P = 0.047; Fig. 1a), first off treatment interval PSADT (≥3 vs. <3 months, HR 2.4, P = 0.05; Fig. 1b), and PSA nadir during the first treatment cycle (<0.1 vs. ≥0.1 ng/ml, HR 4.7, P = 0.0019; Fig. 1c). Factor that were not found to be associated with disease progression include age, pathologic Gleason score of the primary tumor (along its continuous spectrum 6–10 as well as when comparing high Gleason score 8–10 versus low Gleason score 6–7), primary treatment modality (surgery or radiation), post-primary treatment biochemical free survival time, baseline PSA level before the initiation of IAD, and off treatment interval duration during IAD. In view of the spread of some of the curves, and the number of relatively early follow up cases, we repeated the analysis using censoring date September 2009 instead of September 2010. However, we did not find a significant difference in term of the clinico-pathologic factors associated or not associated with disease progression.
Fig. 1.
Kaplan–Meier curves showing progression-free survival (using biochemical and/or clinical criteria), stratified by (a) baseline PSA doubling time (PSADT), (b) first off-treatment PSADT, and (c)PSA nadir <0.1 ng/ml.
PSADT Change and Association With Testosterone Recovery
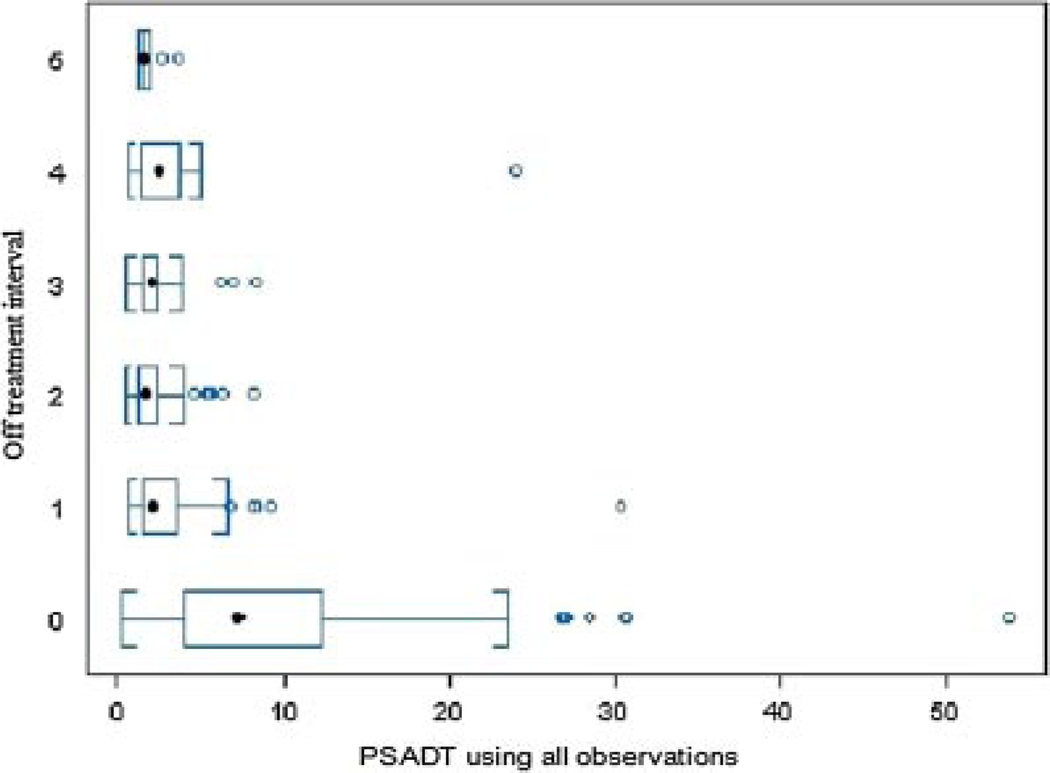
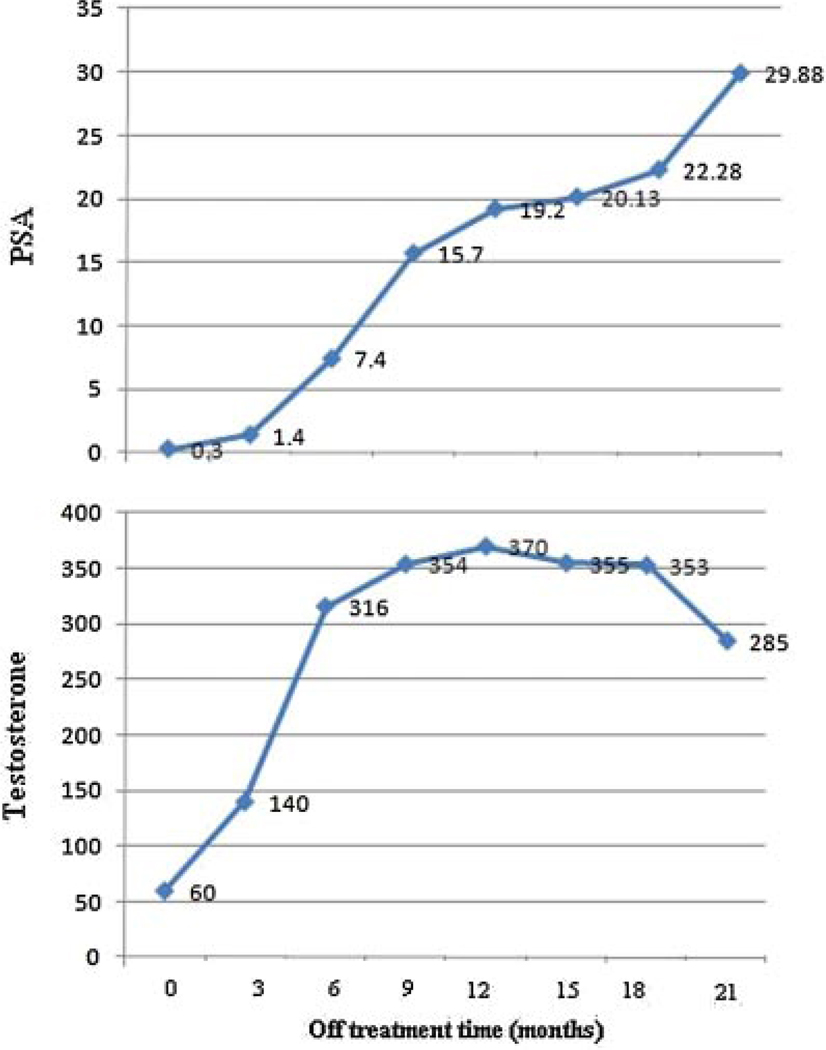
Baseline pre-treatment serum PSA level (ng/ml) level, PSADT and serum testosterone are shown in Table I. PSA response to treatment (decrease at 3 months and nadir), post-treatment serum testosterone nadir level/time to recovery/recovery level, and PSADT during off treatment intervals are shown in Table II. Median PSADT using all relapsed PSAs from time of discontinuation (PSA nadir) to re-initiation of androgen deprivation therapy, during the first off treatment interval, was 2.3 months (mean 3.1), which is significantly (P < 0.0001) shorter than the baseline pre-treatment PSADT (mean 9.7, median 7.34), but stable (P = 0.29) versus PSADT on subsequent off treatment intervals (Fig. 2, Table II). A combined analysis of all off treatment intervals (Table III) revealed that baseline pre-treatment PSADT (PSADT-base) was significantly (P < 0.001) longer than off treatment PSADT based on all relapsed PSAs (PSADT-a), PSADT based only on relapsed PSA values observed after serum testosterone recovery to ≥150 ng/dl (PSADT-b), and the PSADT based only on relapsed PSA values observed ≥3 months after testosterone recovery (PSADT-c). However, PSADT-a was significantly (P < 0.001) shorter than PSADT-b and PSADT-c. Finally, PSADT-c was significantly (P = 0.005) longer than PSADT-b. Figure 3 illustrates the association between PSA and testosterone recovery in one of our patients. If all relapsed PSA levels would be considered regardless of testosterone recovery, then the corresponding PSADT is 1.53 months. In contrast, when considering only PSA values obtained ≥3 months after testosterone recovery to ≥150 ng/dl, the PSADT is 14.44 months.
Fig. 2.
Change of PSA doubling time over several off treatment intervals of intermittent androgen deprivation therapy, using all relapsed PSAs.
TABLE III.
Description of the Different PSADTs
| PSADT (months) | PSADT-base | PSADT-a | PSADT-b | PSADT-c |
|---|---|---|---|---|
| Mean ± SD | 9.67 ± 8.57 | 2.69 ± 2.86 | 5.1 ± 11.65 | 5.45 ± 5.14 |
| (range); median | (0.27–53.9); 7.34 | (0.49–30.5); 2 | (0.84–112.96); 2.54 | (1.31–30.5); 3.7 |
PSADT-base, baseline pre-treatment PSADT; PSADT-a, off treatment PSADT based on all relapsed PSAs; PSADT-b, PSADT based only on relapsed PSA values observed after serum testosterone recovery to ≥150 ng/ml; PSADT-c, PSADT based only on relapsed PSA values observed ≥3 months after testosterone recovery.
Fig. 3.
An illustrative example of the association between PSA and serum test osterone recovery in one of our patients.
DISCUSSION
IAD therapy is frequently employed in patients with isolated evidence of PSA recurrence. To the best of our knowledge, the change of PSADT from baseline to the off treatment intervals, as well as the association between disease progression and PSADT before and after IAD initiation, have not been previously described. Furthermore, follow-up of serum testosterone during the off treatment interval is often not part of the routine clinical practice [22,23]. The present study summarizes our 15 years experience with IAD in patients with non-metastatic BRPC. It indicates that disease progression is driven mainly by PSA kinetics as reflected by the baseline pre-treatment PSADT, first off treatment interval PSADT, and the first treatment cycle PSA nadir that were found to be independent factors associated with biochemical and clinical disease progression.
Our analysis is supported by existing clinical data suggesting that the PSADT is an indicator of disease aggressiveness in patient with isolated PSA relapse [4]. The association between PSA response after the first treatment cycle and IAD outcome observed in the present study confirms prior observations that achieving a low PSA level on ADT might reflect a predominantly androgen-dependent cancer cell population sensitive to hormonal manipulation, and serve as a predictive and prognostic factor [24,27]. These data may help distinguish early on during the course of treatment, the patients who are more likely to benefit from IAD therapy from those who may be candidates for other more aggressive approaches to be tested in clinical trials.
Our data indicate that testosterone recovery influences the calculation of PSADT during off treatment periods. As previously reported [28], off treatment PSA recovery slope has two phases. The initial phase is partially driven by testosterone recovery and is characterized by a sharper PSA slope, which is followed by a slower PSA rise after testosterone recovery. This phenomenon reflects the double-nature of PSA, a marker of androgen receptor activity in androgen changing conditions and a marker of disease progression in stable androgen conditions. PSADT after treatment was significantly shorter using all PSA values >0.1 ng/ml than the same including only PSA values observed after testosterone recovery. These findings which are illustrated on Figure 3, should be taken in consideration if PSADT is used in the decision on PSA threshold for initiating repeated androgen deprivation cycles either in clinical practice or in clinical trials.
Our study has some limitations. This is a retrospective study which limits our ability to exclude the possibility that unequal distribution of unidentified clinical-pathologic parameters in this patient cohort may have biased the observed results. Furthermore, our patient cohort is heterogenous and relatively small. Other clinicopathologic factors that were not found to be significantly associated with disease progression in the present study might have been important in a larger patient cohort, for example, the Gleason score and the off-treatment interval duration [26]. Finally, the median follow-up time is relatively short and therefore we could not examine clinical factors that are associated with overall survival. Despite these limitations that prevent conclusive evidence, the preliminary strong association of various factors with outcomes and between serum testosterone and PSADT calculation observed herein are hypothesis generating that if validated could potentially contribute to treatment decisions, patient selection, and clinical trials design.
CONCLUSIONS
During IAD for BRPC, PSADT becomes shorter, and is associated with testosterone recovery. PSADT before and after IAD initiation is associated with the probability of disease progression. If prospectively validated these data may guide treatment with IAD and clinical trial design.
Footnotes
Previous presentations: The data will be presented at the GU Cancers Symposium—ASCO, Orlando, FL, CA, USA, February 2011.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC. Era specific biochemical recurrence-free survival following radical prostatectomy for clinically localized prostate cancer. J Urol. 2001;166:416–419. [PubMed] [Google Scholar]
- 3.Zietman AL, Coen JJ, Dallow KC, Shipley WU. The treatment of prostate cancer by conventional radiation therapy: An analysis of long-term outcome. Int J Radiat Oncol Biol Phys. 1995;32:287–292. doi: 10.1016/0360-3016(95)00123-G. [DOI] [PubMed] [Google Scholar]
- 4.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 5.Zelefsky MJ, Ben-Porat L, Scher HI, Chan HM, Fearn PA, Fuks ZY, Leibel SA, Venkatraman ES. Outcome predictors for the increasing PSA state after definitive external-beam radiotherapy for prostate cancer. J Clin Oncol. 2005;23:826–831. doi: 10.1200/JCO.2005.02.111. [DOI] [PubMed] [Google Scholar]
- 6.Prapotnich D, Cathelineau X, Rozet F, Barret E, Mombet A, Cathala N, Sanchez-Salas RE, Vallancien G. A 16-year clinical experience with intermittent androgen deprivation for prostate cancer: Oncological results. World J Urol. 2009;27:627–635. doi: 10.1007/s00345-009-0393-1. [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, Partin AW. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 8.Antonarakis ES, Trock BJ, Feng Z, Humphreys EB, Carducci MA, Partin AW, Walsh PC, Eisenberger MA. The natural history of metastatic progression in men with PSA-recurrent prostate cancer after radical prostatectomy: 25-year follow-up. J Clin Oncol. 2009;27(15s) Suppl doi: 10.1111/j.1464-410X.2011.10422.x. abstract 5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95:1376–1383. doi: 10.1093/jnci/djg043. [DOI] [PubMed] [Google Scholar]
- 10.Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D’Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005;23:6992–6998. doi: 10.1200/JCO.2005.01.2906. [DOI] [PubMed] [Google Scholar]
- 11.Partin AW, Eisenberger MA, Sinibaldi VJ, Humphreys E, Mangold LA, Walsh PC. Prostate specific antigen doubling time (PSADT) predicts for distant failure and prostate cancer specific survival (PCSS) in men with biochemical relapse after radical prostatectomy. J Clin Oncol. 2004;22(14s) Suppl abstract 4555. [Google Scholar]
- 12.Moul JW, Wu H, Sun L, McLeod DG, Amling C, Donahue T, Kusuda L, Sexton W, O’Reilly K, Hernandez J, Chung A, Soderdahl D. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol. 2004;171:1141–1147. doi: 10.1097/01.ju.0000113794.34810.d0. [DOI] [PubMed] [Google Scholar]
- 13.Walsh PC, DeWeese TL, Eisenberger MA. A structured debate: Immediate versus deferred androgen suppression in prostate cancer-evidence for deferred treatment. J Urol. 2001;166:508–515. doi: 10.1016/s0022-5347(05)65972-1. discussion 515–516. [DOI] [PubMed] [Google Scholar]
- 14.Tunn UW, Eckart O, Kienle EF, Hillger H. Can intermittent androgen deprivation be an alternative to continuous androgen withdrawal in patients with PSA relapse? First results of the randomized prospective phase III clinical trial EC-507. J Urol. 2003;169(4 Suppl):396a. Abstract. [Google Scholar]
- 15.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 16.Moul JW, Bañez LL, Freedland SJ. Rising PSA in nonmetastatic prostate cancer. Oncology (Williston Park) 2007;21:1436–1445. Review. Discussion 1449, 1452–1454. [PubMed] [Google Scholar]
- 17.Abrahamsson PA. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: A systematic review of the literature. Eur Urol. 2010;57:49–59. doi: 10.1016/j.eururo.2009.07.049. Review. [DOI] [PubMed] [Google Scholar]
- 18.Keizman D, Carducci MA. Intermittent androgen deprivation-questions remain. Nat Rev Urol. 2009;6:412–414. doi: 10.1038/nrurol.2009.145. Review. [DOI] [PubMed] [Google Scholar]
- 19.Miller K, Steiner U, Lingnau A, Keilholz U, Witzsch U, Haider A, Wachter U, Rüssel C, Altwein J. Randomised prospective study of intermittent versus continuous androgen suppression in advanced prostate cancer. J Clin Oncol. 2007;25(18s) Suppl Abstract 5015. [Google Scholar]
- 20.Calais da Silva FE, Bono AV, Whelan P, Brausi M, Marques Queimadelos A, Martin JA, Kirkali Z, Calais da Silva FM, Robertson C. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: Results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–1277. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Tunn UW, Kurek R, Kienle E, Maubach L. Intermittent is as effective as continuous androgen deprivation in patients with PSA-relapse after radical prostatectomy. J Urol. 2004;171:384. (abstract 1458) [Google Scholar]
- 22.Schulman CC, Irani J, Morote J, Schalken JA, Montorsi F, Chlosta PL, Heidenreich A. Testosterone measurement in patients with prostate cancer. Eur Urol. 2010;58:65–74. doi: 10.1016/j.eururo.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Shore ND, Crawford ED. Intermittent androgen deprivation therapy: Redefining the standard of care? Rev Urol. 2010;12:1–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Strum SB, Scholz MC, McDermed JE. Intermittent androgen deprivation in prostate cancer patients: Factors predictive of prolonged time off therapy. Oncologist. 2000;5:45–52. doi: 10.1634/theoncologist.5-1-45. [DOI] [PubMed] [Google Scholar]
- 25.Rathkopf D, Carducci MA, Morris MJ, Slovin SF, Eisenberger MA, Pili R, Denmeade SR, Kelsen M, Curley T, Halter M, Collins C, Fleisher M, Heller G, Baker SD, Scher HI. Phase II trial of docetaxel with rapid androgen cycling for progressive noncastrate prostate cancer. J Clin Oncol. 2008;26:2959–2965. doi: 10.1200/JCO.2007.15.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu E, Gulati R, Telesca D, Jiang P, Tam S, Russell KJ, Nelson PS, Etzioni RD, Higano CS. Duration of first off-treatment interval is prognostic for time to castration resistance and death in men with biochemical relapse of prostate cancer treated on a prospective trial of intermittent androgen deprivation. J Clin Oncol. 2010;28:2668–2673. doi: 10.1200/JCO.2009.25.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, Wilding G, Akdas A, Small EJ, Donnelly B, MacVicar G, Raghavan D Southwest Oncology Group Trial 9346 (INT-0162) Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: Data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 28.Bates AT, Pickles T, Paltiel C. PSA doubling time kinetics during prostate cancer biochemical relapse after external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2005;62:148–153. doi: 10.1016/j.ijrobp.2004.09.048. [DOI] [PubMed] [Google Scholar]