Abstract
Obtaining enough membrane protein in native or native-like status is still a challenge in membrane protein structure biology. Maltose binding protein (MBP) has been widely used as a fusion partner in improving membrane protein production. In the present work, a systematic assessment on the application of mature MBP (mMBP) for membrane protein overexpression and purification was performed on 42 membrane proteins, most of which showed no or poor expression level in membrane fraction fused with an N terminal Histag. It was found that most of the small membrane proteins were overexpressed in the native membrane of E. coli when using MBP. In addition, the proteolysis of the fusions were performed on the membrane without solublilization with detergents, leading to the development of an efficient protocol to directly purify the target membrane proteins from the membrane fraction through a one-step affinity chromatography. Our results indicated that mMBP is an excellent fusion partner for overexpression, membrane targeting and purification of small membrane proteins. The present expression and purification method may be a good solution for the large scale preparation of small membrane proteins in structural and functional studies.
Keywords: membrane protein, maltose binding protein, overexpression, purification, membrane targeting
Introduction
Obtaining enough membrane protein in its native state is still one of the bottlenecks in membrane protein structure biology [1, 2]. Heterologous expression of membrane proteins often results in poor expression or overexpression of misfolded or only partially folded protein, e.g. those in inclusion bodies. The toxic effects of overexpression of membrane proteins and the lack of adequate processing and folding machinery for the overexpressed membrane protein may be responsible for these problems [3, 4].
Even so, in the last decade much has been learned about the biogenesis of membrane proteins, and therefore rational optimization for membrane protein overexpression is possible [3, 4]. Apart from screening different E.coli strains, different expression vectors and optimization of the expression conditions; expression of the target membrane protein as a fusion protein is a proven way to increase the expression level. Different highly soluble proteins, like glutathione Stransferase, maltose binding protein (MBP), thioredoxin, and green fluorescence protein, have been used as carrier proteins through either N terminal or C terminal fusions [5–9]. Among these, MBP is probably the most frequently used fusion protein for membrane proteins. Many proteins have been successfully overexpressed through fusion with MBP for both structural and functional studies, such as the adenosine A2a receptor, a cannabinoid receptor, a neurotensin receptor, an opioid receptor, a pentameric ligand-gated ion channel and so on [10–14]. Remarkably, the precursor MBP (i.e. MBP with its signal peptide, pMBP) was used in these studies to target the N-terminus of the membrane protein to be expressed to the periplasmic side. (Therefore, the N-terminus of the target protein is assumed to be in the periplasmic side). However, there have only been sporadic reports applying the mature MBP (i.e. MBP without its signal peptide, mMBP) as the carrier protein for membrane protein production [6, 15–22], as well as production of small transmembrane peptides/domains [23]. Recently, a comprehensive study of the application of mMBP as the carrier protein for the production of 22 small membrane proteins was reported [7].
In the present work, we further expanded the application of mMBP in membrane protein expression and purification. In total 42 membrane proteins were studied. For these selected target proteins, most of them showed no expression or poor expression in the membrane fraction as N-terminal Histag fusion proteins. It was found that, consistent with our previous report [7], mMBP dramatically increased the expression level of most small and some medium sized membrane proteins. Remarkably, significant amounts of these fusion proteins were overexpressed in the native membranes of E.coli. In addition, the fusions can be conveniently proteolyzed in the native membrane by tobacco etch virus protease (TEV), which leads to the development of an efficient protocol for one-step purification of these membrane proteins from the membrane fraction after proteolysis. The present method is expected to be very useful for structural and functional studies of membrane proteins, especially for those small and medium size proteins suitable for Nuclear Magnetic Resonance studies [24–26].
Material and methods
Construction of expression vectors
The DNA coding E.coli mMBP was inserted into pTBSG, which is derived from pMCGS7 [27] (a generous gift from Dr. Mark I. Donnell from University of Wisconsin), resulting in a vector called pTBMalE according to our previous report [6]. Further modifications on pTBMalE, including substitution of the Histidine residues by Arginine in the N-terminal Histag and insertion of a C-terminal Histag, were made by using QuikChange® Kit (Stratagene), resulting in two vectors called pTBMalE-1 and pTBMalE-2, respectively. Scheme 1 shows the details of the vectors used in the present work.
Scheme 1.
The nomenclature and sequences for the expression vectors used in this work.
Gene cloning
Target genes, either from the Mycobacterium tuberculosis (Mtb) genome or from plasmids (containing the genes encoding the M2 proton channel, KcsA, potassium channel and Diacylglycerol kinase (DAGK) are from our lab, Christopher Miller’s lab at Brandeis University and Charles R. Sanders’ lab at Vanderbilt University, respectively) were amplified by PCR with primers for the following steps of Ligation Independent Cloning. Insertion of the target genes into the expression vector was the same as described previously [6]. After DNA sequencing to confirm correct insertion, the plasmid was transformed into BL21(DE3)-RP codon plus (Stratagene) for expression tests.
Protein expression screening
Cells harboring the expression vector were grown on LB agar plates containing 50 µg/mL ampicillin and 34 µg/mL chloramphenicol. A single clone was picked and inoculated into 3 mL LB media for overnight growth. 500 µL of the overnight culture was then inoculated into 10 mL LB media, and the expression was induced with the addition of 0.4 mM IPTG when OD600 reached 0.6. After induction overnight at room temperature, cells were harvested by centrifugation at 4500 g for 15 min at 4°C, resuspended in 1 mL lysis buffer (20 mM Tris-HCl, pH 7.8, 400 mM NaCl) and lysed by sonication (Sonic Dismembrator, Model 100, Fischer Scientific, Inc.) three times (20 sec each). The lysate was fractionated by centrifugation for 20 min at 10,000 g. The supernatant normally contained soluble proteins and fragmented membranes, while the pellet consisted of insoluble proteins (inclusion body fraction). The supernatant was subjected to ultracentrifugation at 220,000 g for 45 min at 8°C to separate the membrane and soluble protein fractions. The soluble, insoluble and membrane fractions were adjusted to the same volume with lysis buffer, and then 15 µL of each was mixed with 5 µL 4× sample buffer (0.25 M Tris-HCl, pH 6.8, 8% SDS, 20% β-Mercaptoethanol, 40% Glycerol and 0.04% Bromophenol Blue) and 10 µL was loaded onto 12% Tricine SDS-PAGE gels followed by Coomassie staining.
To check whether the overexpressed fusion proteins in the membrane fraction were inserted or weakly attached to the membrane, the membrane fraction prepared above was resuspended in a basic denaturation solution containing 100 mM Na2CO3, pH 11.5, 0.5 M NaCl and 5 M Urea by slight sonication. After gentle shaking for 30 min at room temperature, the sample was centrifuged at 220,000 g for 45 min at 8°C. This procedure was repeated once. The final pellet after ultracentrifugation was resuspended in lysis buffer and the SDS-PAGE sample was prepared as above.
Fusion protein proteolysis on the membrane
The membrane fraction from 10 ml LB culture was suspended in 1 ml lysis buffer with slight sonication until the solution became clear. TEV with an N-terminal Histag was purified as reported previously [23] and added to 0.25 mg/ml and the proteolysis was performed with gentle shaking for two days at room temperature. 15 µL of the reaction solution was mixed with 5 µL sample buffer (4×) and 10 µL was loaded on 12% Tricine SDS-PAGE gels followed by Coomassie staining.
Purification of target membrane proteins
Three membrane proteins, Rv0008c, Rv0011c and Rv2128, were purified by using a one-step purification protocol developed in the present work. The fusion proteins were expressed either in pTBMalE-1 (for Rv0008c and Rv2128) or in pTBMalE-2 (for Rv0011c). After collecting the membrane fraction, proteolysis was performed as mentioned above, and then the reaction solution was centrifuged at 220,000 g for 1 h. The supernatant containing TEV and released MBP was discarded, and the pellet containing the released target protein with C-terminal Histag was resuspended in the binding buffer (20 mM Tris-HCl, pH 7.8, 400 mM NaCl, 20 ml per liter culture). Dodecylphosphocholine (DPC, Anatrace Inc.) was added to this membrane fraction suspension to solubilize the target protein (the final concentration of DPC was 1%). Solubilization was performed at 4°C for 2 hours and the solution was centrifuged at 220,000 g for 1 h. The supernatant was mixed with an appropriate amount of Ni2+-NTA resin (Qiagen) which was pre-equilibrated with the wash buffer (20 mM Tris-HCl, pH 7.8, 400 mM NaCl, 0.2% DPC and 5 mM imidazole). After incubation at 4°C for 2 hours with gentle shaking, the resin was extensively washed with the wash buffer and then eluted with the elution buffer (20 mM Tris-HCl, pH 7.8, 400 mM NaCl, 300 mM imidazole and 0.2% DPC).
Results
Overexpression of membrane proteins in native membrane as mMBP fusions
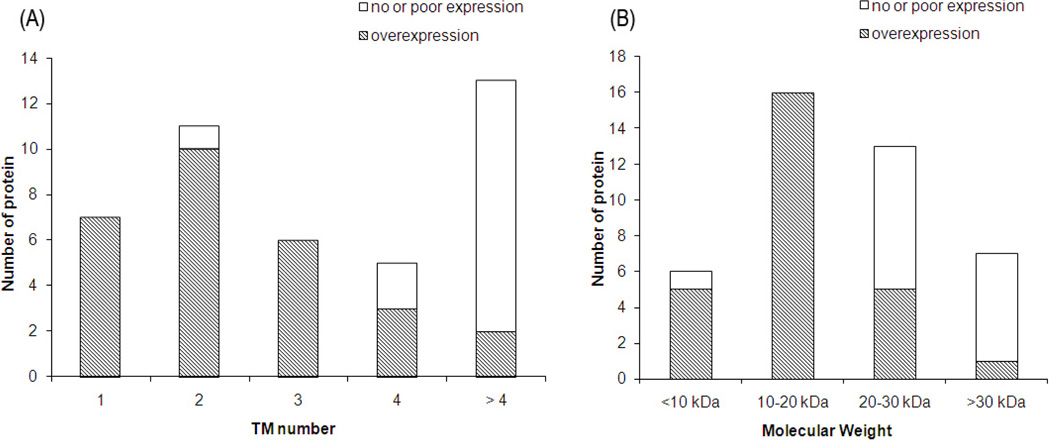
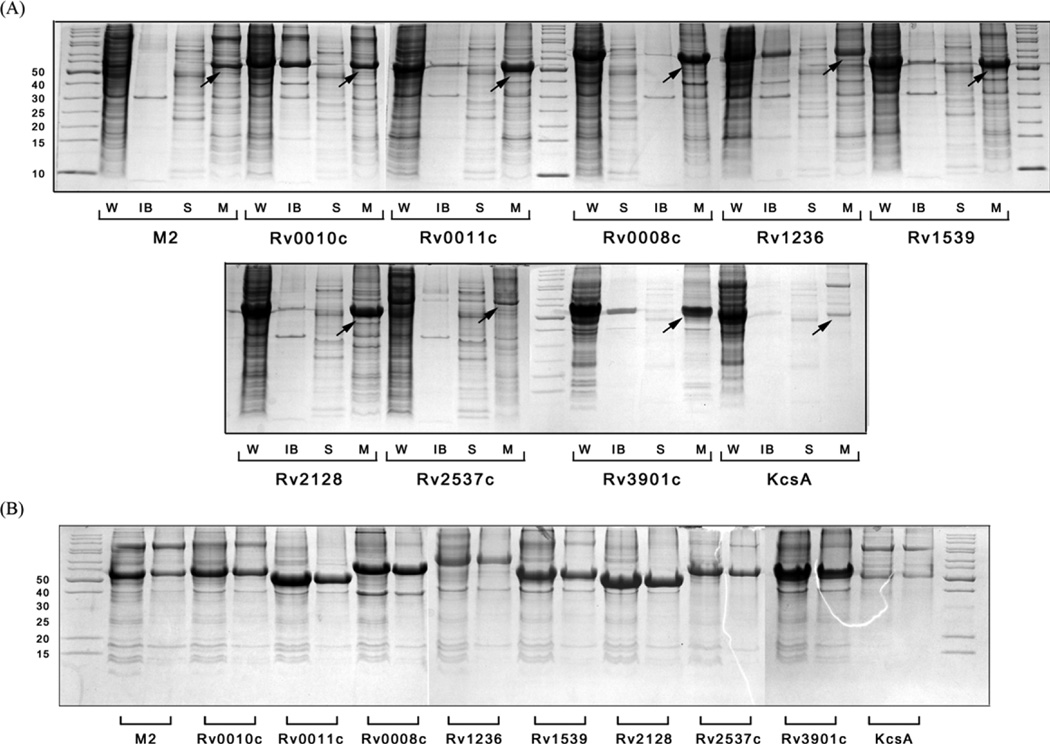
In the present work, 42 membrane proteins (including 39 putative membrane proteins from the Mtb genome and three are well known membrane proteins, M2 protein, KcsA and DAGK) were tested in our expression system. The proteins are listed and ranked by the number of transmembrane helices (for those without experimental data, the transmembrane helix number was predicted by the TMHMM program, http://www.cbs.dtu.dk/services/TMHMM/) in Table 1. Of these proteins, 35 were poorly expressed in the membrane fraction using an N-terminal Histag. As mMBP fusions, 21 of 35 (60%) were overexpressed in the membrane (which means the band corresponding to the fusion protein was visible after Coomassie Blue staining, suggestive of an expression level over 2 mg/L). For the other 7 well expressed membrane proteins, fusion with mMBP did not influence their overexpression in the membrane fraction. As shown in Figure 1, the efficiency of mMBP in promoting overexpression significantly depends on the number of transmembrane helices and the molecular weight of the target protein. For proteins with less than 4 transmembrane helices and a molecular weight less than 20 kDa, there is a very good chance that their expression level in the membrane fraction could be remarkably improved by mMBP. The sharp decrease of the success rate for larger proteins with more transmembrane helices indicates that mMBP fusion strategy may not be a good choice for medium and large membrane proteins. Figure 2A shows the SDS-PAGE of 10 typical membrane proteins studied in the present work, and shows the significant yield of the mMBP fusions expressed in the membrane fraction.
Table 1.
The expression of the 42 membrane proteins as N terminal Histag and mMBP fusions in membrane fraction.
| Protein | # TM | M.W. (kDa) | N Histag | mMBP fusion |
|---|---|---|---|---|
| M2 | 1 | 11.2 | + | + |
| Rv3901c | 1 | 15.4 | ± | + |
| Rv0008c | 1 | 15.7 | + | + |
| Rv2537c | 1 | 15.8 | o | + |
| Rv0875c | 1 | 17.8 | o | + |
| Rv2969c | 1 | 26.8 | o | + |
| Rv0817c | 1 | 28.6 | o | + |
| Rv2128 | 2 | 7.4 | o | + |
| Rv3346c | 2 | 8.9 | o | + |
| Rv0476 | 2 | 9.2 | o | + |
| Rv0544c | 2 | 9.7 | o | o |
| Rv1567c | 2 | 10.3 | + | + |
| Rv0011c | 2 | 10.4 | o | + |
| Rv2876 | 2 | 11.8 | o | + |
| Rv2144c | 2 | 12.0 | o | + |
| Rv1440 | 2 | 12.1 | o | + |
| KcsA | 2 | 13.1 | + | + |
| Rv0010c | 2 | 15.2 | ± | + |
| Rv0460 | 3 | 8.1 | ± | + |
| Rv0882 | 3 | 9.6 | o | + |
| Rv3155 | 3 | 10.9 | o | + |
| Rv3632 | 3 | 13.1 | o | + |
| DAGK | 3 | 13.2 | + | + |
| Rv2612c | 3 | 23.2 | o | + |
| Rv1824 | 4 | 12.8 | o | + |
| Rv1539 | 4 | 21.3 | + | + |
| Rv1811 | 4 | 24.8 | o | o |
| Rv3277 | 4 | 30.0 | o | o |
| Rv3101c | 4 | 32.8 | + | + |
| Rv1304 | 5 | 27.5 | o | o |
| Rv1236 | 5 | 33.0 | o | + |
| Rv1337 | 6 | 25.7 | o | o |
| Rv1457c | 6 | 27.3 | o | o |
| Rv1237 | 6 | 29.1 | o | o |
| Rv2398c | 6 | 29.3 | o | o |
| Rv2938 | 6 | 29.6 | o | o |
| Rv2399c | 6 | 29.7 | o | o |
| Rv2093c | 6 | 33.8 | o | o |
| Rv0929 | 6 | 34.2 | o | o |
| Rv2881c | 7 | 32.0 | o | o |
| Rv1002c | 8 | 55.5 | o | o |
| Rv3877 | 11 | 54.0 | o | o |
Note: “o“ denotes no detectable expression; “±” denotes poor expression which can only be detected by western blot; “+” denotes overexpression which can be visualized by Coomassie blue staining, suggesting the expression level over 2 mg/L culture.
Figure 1.
The relationship between the expression level of the mMBP fusion in membrane fraction and the number of transmembrane helix (A) or the molecular weight (B) of the target membrane protein. The shaded region represents overexpression (≥2 mg/L for the fusion protein), while the unshaded region represents no significant expression.
Figure 2.
(A) The distribution of ten overexpressed mMBP fusions in different cell fractions. “W” denotes whole cell sample, “IB” denotes inclusion body fraction, “S” denotes the soluble fraction and “M” denotes the membrane fraction. The overexpressed fusion proteins in the membrane fraction are indicated by the arrows. (B) Washing of the membrane fraction with basic urea solution. For each protein, left lane: before washing, right lane: after washing.
To exclude the possibility that the mMBP fusion proteins were weakly associated with the membrane, the membrane fractions were washed with basic denaturation solution to remove the weakly associated proteins from the membrane [28]. It is clear that after washing the membrane fraction by basic denaturation solution twice, most of the fusion proteins were still associated with the membrane (Figure 2B), indicating that the fusion proteins were inserted in the native E.coli membrane.
Proteolysis of mMBP fusions on the native membranes and purification of the target membrane proteins
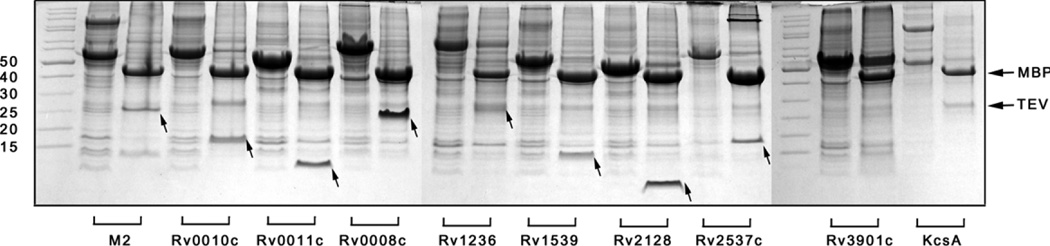
To remove mMBP, proteolysis was performed on the fraction resuspended in the lysis buffer by mild sonication. As shown in Figure 3, all of the ten tested mMBP fusions can be cleaved by TEV. Except for mMBP-Rv3901c, which was approximately 40% cut, the other 9 fusion proteins were completely or nearly completely cleaved. Therefore, for different fusion proteins, the cleavage efficiency by TEV can be different. In order to get better cleavage, the optimizations on reaction time, amount of TEV used, the temperature and even the reaction buffer should be performed for a specific fusion protein. Since mMBP and TEV are highly soluble, they would be left in the supernatant after ultracentrifugation and the target protein would remain in the pellet, the membrane fraction. If the target protein has a C-terminal Histag, it is therefore convenient to purify it from the membrane fraction after solubilization with detergent.
Figure 3.
TEV cleavage of the ten mMBP fusions. The arrows indicate bands corresponding to the released target proteins after proteolysis. Note that Rv 3901 and KcsA are not visible on the gel, probably due to the inadequate amount of the proteins required for Coomassie Blue staining.
In preliminary experiments, it was found that a small fraction of the mMBP was still associated with the membrane fraction after proteolysis, even after washing with a high salt buffer, resulting in a co-purification of N-terminal Histag mMBP and C-terminal Histag target protein by using Ni2+-NTA resin. To resolve this problem, the N-terminal Histag was removed in the initial trial. However, unexpectedly, it was found that most of the proteins in this vector were not well expressed. The failure in expression was not due to the Histag in the C-terminus because the fusion protein with both N-terminal and C-terminal Histag can be well expressed as the original construct (Data not shown). It seems that the N-terminal Histag plays a role in overexpression. Interestingly, when the N-terminal Histag was replaced by an His-His-His-Arg-Arg-Arg tag (H3R3 tag in pTBMalE-1) or an His-Arg-Arg-Arg-Arg-Arg tag (HR5 tag in pTBMalE-2) to keep the positive charges, the fusion proteins were overexpressed well. Since both H3R3 and HR5 tags do not bind to Ni2+-NTA resin, they were used as the expression vectors for the following scale-up expression and purification.
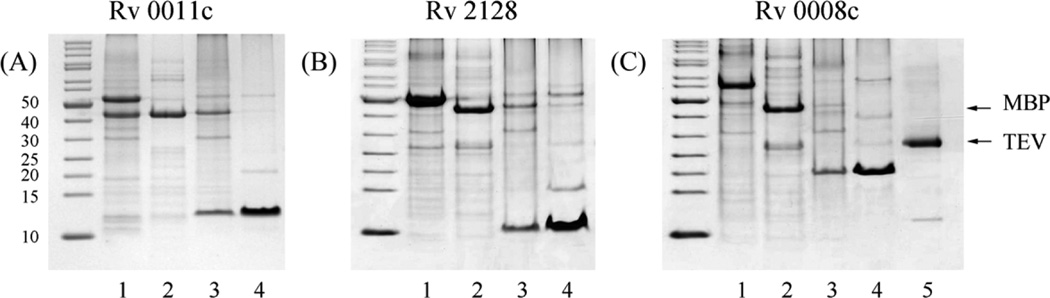
Figure 4 shows the purification of three proteins, Rv0008c, Rv2128 and Rv0011c, by using a one step purification protocol. Rv0008c and Rv2128 were expressed in pTBMalE-1 and Rv0011c was expressed in pTBMalE-2. After TEV cleavage in the membrane at room temperature followed by ultracentrifugation, the membrane fraction was collected and it was shown in Figure 4 that all of the TEV and most of the MBP was in the supernatant, and all of the target protein was in the membrane fraction. After solubilization of the membrane fraction with 1% DPC, the proteins with C Histag were conveniently purified with over 90% purity by just one step of Ni2+-NTA affinity chromatography, and the residual mMBP associated with the membrane with either R3H3 tag or R5H tag were efficiently removed. The final yields of these three proteins were 8.5 mg, 12 mg and 8.0 mg for Rv0008c, Rv2128 and Rv0011c from 1 Liter LB culture, respectively, which are adequate for further structural characterization. Since the uncut fusion protein also has a C-terminal Histag, it can be co-purified with released target protein, therefore forming the main contamination in these three cases. A further gel chromatography step would eliminate such impurities if necessary.
Figure 4.
Purification of three membrane proteins overexpressed as mMBP fusion. (A) Rv 0011c; (B) Rv 2128 and (C) Rv 0008c. For each protein, Lane 1 is the membrane fraction; Lane 2 and Lane 3 are the supernatant and pellet, respectively after TEV cleavage and ultracentrifugation; Lane 4 is the target protein purified from the membrane fraction after TEV cleavage (shown in lane 3). The TEV used in the reaction is shown in Lane 5 in (C).
Discussion
Overexpression in high enough yield and efficient purification of membrane proteins in a native or native-like status for structural studies is always challenging [1, 29]. Up to now, most of the α-helical membrane protein structures in the Protein Data Bank have been isolated from the membrane fraction. In the present work, quite a few membrane proteins that had not been expressed well with an N-terminal Histag were overexpressed in the native membrane of E.coli as mMBP fusion proteins. We also developed a simple protocol to purify the target proteins from the membrane fraction. For the membrane proteins studied here, the transmembrane helix number varies from 1 to 11, and the distribution of the transmembrane helix number is similar to our previous report for all the putative membrane proteins in Mtb genome [30]. In addition, the range in molecular weight (7.4 kDa to 55.5 kDa) is much broader than that in our previous report [7]. Therefore the results shown here may be representative for other bacterial membrane protein genomes.
mMBP facilitates the membrane protein overexpression in the native membrane of E.coli
As shown in Table 1 and Figure 1, mMBP substantially increased the expression level of many small membrane proteins and more interestingly, this fusion enhanced the overexpression in the membrane fraction (Figure 2A). Extensive washing with a basic urea buffer did not remove the fusions from the membrane fraction (Figure 2B), indicating that the fusion proteins were inserted in the membrane. It seems that three factors play important roles in the overexpression of mMBP fusions. Firstly, MBP definitely enhances the overexpression and membrane insertion. Without mMBP fusion, most of the membrane proteins studied here were not expressed with an N-terminal Histag. Secondly, a positive charged tag at the N-terminus of the fusion protein seems to enhance expression. Deletion of the N-terminal positive charged tag seriously reduced the expression level for most of the fusion proteins. The high expression level of the constructs with H3R3 or HR5 tag indicates that it is the positive charge rather than the histidine residues that are crucial for overexpression. However, the C-terminal Histag does not play such a role in expression. Thirdly, the number of the transmembrane helices and the molecular weight of the target membrane protein are also determining factors. mMBP is able to efficiently increase the expression level for small membrane proteins ≤20 kDa with ≤3 transmembrane helices (Figure 1), which is consistent with our previous findings [7].
Since the mMBP fusions studied here have neither signal peptides nor N-terminal hydrophobic transmembrane fragments, it is not likely that their targeting to the membrane is mediated by the signal recognition particle through which most of the integral membrane proteins in bacteria are targeted to the membrane in a co-translational way [31, 32]. It has been well characterized that the newly synthesized MBP can be bound with SecB [33, 34], a chaperone protein that binds the nascent polypeptide in a partially denatured conformation [35]. Then the MBP-SecB complex is transferred to the translocon for membrane crossing through the interaction between SecB and the SecA-translocon. It is conceivable that the mMBP fusions may also be recognized by SecB as MBP and inserted into the membrane with the assistance of the translocon. The recognition by SecB may explain why the mMBP can only increase the membrane expression level of small membrane proteins efficiently. A larger membrane protein with more transmembrane helices may overwhelm the capability of mMBP to keep the fusion protein in a partially denatured state that is necessary for SecB recognition. Such chaperone-assisted membrane targeting may not be limited to the SecB mediated pathway. It has been reported that the expression level of functional CorA, a magnesium transporter in bacteria and archea, can be significantly improved by co-expression of chaperone proteins DnaK and DnaJ [36]. The large soluble domain in the N terminus of CorA suggests that CorA may be targeted to the membrane in a post-translational way, probably with the assistance of chaperones [36]. Considering the similar shape between CorA and mMBP fusions (each of them has a large soluble domain with a relatively small hydrophobic transmembrane domain in the C-terminus.), the involvement of other chaperones in mMBP fusions targeting the membrane is possible.
Purification of the target membrane proteins from the native membrane
To purify the target membrane protein expressed as a fusion typically involves: (1) purification of the fusion protein in detergent by using affinity chromatography; (2) releasing the target protein from the fusion partner by specific proteolysis and (3) removing the fusion partner and protease through an additional chromatography step. This method has been shown to be successful for mMBP fusions [6, 18, 20, 21, 23], though the relatively long process is time consuming. One of the concerns is inefficient proteolysis in detergent solution. It has been reported that the activity of TEV may be significantly reduced in some detergents used to solubilize membrane proteins [37], resulting in incomplete proteolysis. Even worse, as mentioned in our previous report [23], protease cleavage of the peptide chain may not result in separation of the target protein from the mMBP. This is suggested by the detergent solubilization of the hydrophobic cleft on the surface of MBP that may form a binding site for the hydrophobic domain of the target membrane protein potentially resulting in a stable non-covalent complex following peptide cleavage.
To avoid these problems, we developed a protocol to purify the target protein directly from the membrane fraction by one-step chromatography. This method depends on the fact that most of the tested mMBP fusions can be efficiently cleaved by TEV on the membrane in the absence of detergent which may inhibit TEV (Figure 3). Both TEV and mMBP are highly soluble and therefore after proteolysis they can be simply removed by ultracentrifugation, leaving the released target protein in the membrane pellet. In our experiments, a small fraction of mMBP associates with the membrane even after washing, but it can be removed in the following Ni2+-NTA chromatography since the H3R3 tag or HR5 tag cannot bind on the Ni2+ resin (Figure 4). In this way, through only one step of affinity chromatography, the target membrane protein can be conveniently obtained with high purity. Keeping the target protein in the E. coli membrane until the last purification step (Ni2+-NTA affinity chromatography) is a major advantage of this approach, as membrane proteins maybe structurally unstable or lose their functionality when solubilized in detergent for a lengthy period of time. A concern for this method is that the uncut fusion protein may be co-purified with the target protein through the Ni2+-NTA column thereby becoming the main contamination. To avoid this problem, using more TEV and/or performing the reaction for a longer time may resolve this issue. In case the cleavage cannot reach 100%, an additional amylose affinity chromatography as a polishing step may be used to specifically remove uncut mMBP fusions, leaving the purified target membrane protein in the flow through.
Acknowledgment
We thank Dr. Mark I. Donnell from University of Wisconsin for vector pMCSG7. This work was supported in part by NIH grants GM064676 and AI073891 and the National High Magnetic Field Laboratory supported by cooperative agreement DMR-0654118 with the NSF and the State of Florida.
Abbreviations
- MBP
maltose binding protein
- TEV
tobacco etch virus protease
- Mtb
Mycobacterium tuberculosis
- DPC
Dodecylphosphocholine
- DAGK
Diacylglycerol kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lacapere JJ, Pebay-Peyroula E, Neumann JM, Etchebest C. Determining membrane protein structures: still a challenge! Trends Biochem Sci. 2007;32:259–270. doi: 10.1016/j.tibs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Midgett CR, Madden DR. Breaking the bottleneck: eukaryotic membrane protein expression for high-resolution structural studies. J Struct Biol. 2007;160:265–274. doi: 10.1016/j.jsb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Grisshammer R. Understanding recombinant expression of membrane proteins. Curr Opin Biotechnol. 2006;17:337–340. doi: 10.1016/j.copbio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Wagner S, Bader ML, Drew D, de Gier JW. Rationalizing membrane protein overexpression. Trends Biotechnol. 2006;24:364–371. doi: 10.1016/j.tibtech.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Kiefer H, Krieger J, Olszewski JD, Von Heijne G, Prestwich GD, Breer H. Expression of an olfactory receptor in Escherichia coli: purification, reconstitution, and ligand binding. Biochemistry. 1996;35:16077–16084. doi: 10.1021/bi9612069. [DOI] [PubMed] [Google Scholar]
- 6.Qin H, Hu J, Hua Y, Challa SV, Cross TA, Gao FP. Construction of a series of vectors for high throughput cloning and expression screening of membrane proteins from Mycobacterium tuberculosis. BMC Biotechnol. 2008;8:51. doi: 10.1186/1472-6750-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korepanova A, Moore JD, Nguyen HB, Hua Y, Cross TA, Gao F. Expression of membrane proteins from Mycobacterium tuberculosis in Escherichia coli as fusions with maltose binding protein. Protein Expr Purif. 2007;53:24–30. doi: 10.1016/j.pep.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeliseev AA, Wong KK, Soubias O, Gawrisch K. Expression of human peripheral cannabinoid receptor for structural studies. Protein Sci. 2005;14:2638–2653. doi: 10.1110/ps.051550305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drew D, Lerch M, Kunji E, Slotboom DJ, de Gier JW. Optimization of membrane protein overexpression and purification using GFP fusions. Nat Methods. 2006;3:303–313. doi: 10.1038/nmeth0406-303. [DOI] [PubMed] [Google Scholar]
- 10.Weiss HM, Grisshammer R. Purification and characterization of the human adenosine A(2a) receptor functionally expressed in Escherichia coli. Eur J Biochem. 2002;269:82–92. doi: 10.1046/j.0014-2956.2002.02618.x. [DOI] [PubMed] [Google Scholar]
- 11.Yeliseev A, Zoubak L, Gawrisch K. Use of dual affinity tags for expression and purification of functional peripheral cannabinoid receptor. Protein Expr Purif. 2007;53:153–163. doi: 10.1016/j.pep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker J, Grisshammer R. Purification of a rat neurotensin receptor expressed in Escherichia coli. Biochem J. 1996;317(Pt 3):891–899. doi: 10.1042/bj3170891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanasila L, Massotte D, Kieffer BL, Pattus F. Expression of delta, kappa and mu human opioid receptors in Escherichia coli and reconstitution of the high-affinity state for agonist with heterotrimeric G proteins. Eur J Biochem. 1999;260:430–438. doi: 10.1046/j.1432-1327.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- 14.Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 15.Lei X, Ahn K, Zhu L, Ubarretxena-Belandia I, Li YM. Soluble oligomers of the intramembrane serine protease YqgP are catalytically active in the absence of detergents. 2008;47:11920–11929. doi: 10.1021/bi800385r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanamori M, Kamata H, Yagisawa H, Hirata H. Overexpression of the alanine carrier protein gene from thermophilic bacterium PS3 in Escherichia coli. J Biochem. 1999;125:454–459. doi: 10.1093/oxfordjournals.jbchem.a022308. [DOI] [PubMed] [Google Scholar]
- 17.Eliseev R, Alexandrov A, Gunter T. High-yield expression and purification of p18 form of Bax as an MBP-fusion protein. Protein Expr Purif. 2004;35:206–209. doi: 10.1016/j.pep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Sobrado P, Goren MA, James D, Amundson CK, Fox BG. A Protein Structure Initiative approach to expression, purification, and in situ delivery of human cytochrome b5 to membrane vesicles. Protein Expr Purif. 2008;58:229–241. doi: 10.1016/j.pep.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen GQ, Gouaux JE. Overexpression of bacterio-opsin in Escherichia coli as a water-soluble fusion to maltose binding protein: efficient regeneration of the fusion protein and selective cleavage with trypsin. Protein Sci. 1996;5:456–467. doi: 10.1002/pro.5560050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas JL, Trieber CA, Afara M, Young HS. Rapid, high-yield expression and purification of Ca2+ATPase regulatory proteins for high-resolution structural studies. Protein Expr Purif. 2005;40:118–125. doi: 10.1016/j.pep.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Buck B, Zamoon J, Kirby TL, DeSilva TM, Karim C, Thomas D, Veglia G. Overexpression, purification, and characterization of recombinant Ca-ATPase regulators for high-resolution solution and solid-state NMR studies. Protein Expr Purif. 2003;30:253–261. doi: 10.1016/s1046-5928(03)00127-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Gill RL, Jr, Zhu Q, Tian F. Bacterial expression, purification, and model membrane reconstitution of the transmembrane and cytoplasmic domains of the human APP binding protein LR11/SorLA for NMR studies. Protein Expr Purif. 77:224–230. doi: 10.1016/j.pep.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu J, Qin H, Li C, Sharma M, Cross TA, Gao FP. Structural biology of transmembrane domains: Efficient production and characterization of transmembrane peptides by NMR. Protein Sci. 2007;16:2153–2165. doi: 10.1110/ps.072996707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian C, Karra MD, Ellis CD, Jacob J, Oxenoid K, Sonnichsen F, Sanders CR. Membrane protein preparation for TROSY NMR screening. Methods Enzymol. 2005;394:321–334. doi: 10.1016/S0076-6879(05)94012-3. [DOI] [PubMed] [Google Scholar]
- 25.Page RC, Moore JD, Nguyen HB, Sharma M, Chase R, Gao FP, Mobley CK, Sanders CR, Ma L, Sonnichsen FD, Lee S, Howell SC, Opella SJ, Cross TA. Comprehensive evaluation of solution nuclear magnetic resonance spectroscopy sample preparation for helical integral membrane proteins. J Struct Funct Genomics. 2006;7:51–64. doi: 10.1007/s10969-006-9009-9. [DOI] [PubMed] [Google Scholar]
- 26.Gong XM, Franzin CM, Thai K, Yu J, Marassi FM. Nuclear magnetic resonance structural studies of membrane proteins in micelles and bilayers. Methods Mol Biol. 2007;400:515–529. doi: 10.1007/978-1-59745-519-0_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dieckman L, Gu M, Stols L, Donnelly MI, Collart FR. High throughput methods for gene cloning and expression. Protein Expr Purif. 2002;25:1–7. doi: 10.1006/prep.2001.1602. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Y, Chalmers MJ, Gao FP, Cross TA, Marshall AG. Identification of Mycobacterium tuberculosis H37Rv integral membrane proteins by one-dimensional gel electrophoresis and liquid chromatography electrospray ionization tandem mass spectrometry. J Proteome Res. 2005;4:855–861. doi: 10.1021/pr0500049. [DOI] [PubMed] [Google Scholar]
- 29.Sanders CR, Sonnichsen F. Solution NMR of membrane proteins: practice and challenges. Magn Reson Chem. 2006;44(Spec No):S24–S40. doi: 10.1002/mrc.1816. [DOI] [PubMed] [Google Scholar]
- 30.Korepanova A, Gao FP, Hua Y, Qin H, Nakamoto RK, Cross TA. Cloning and expression of multiple integral membrane proteins from Mycobacterium tuberculosis in Escherichia coli. Protein Sci. 2005;14:148–158. doi: 10.1110/ps.041022305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie K, Dalbey RE. Inserting proteins into the bacterial cytoplasmic membrane using the Sec and YidC translocases. Nat Rev Microbiol. 2008;6:234–244. doi: 10.1038/nrmicro3595. [DOI] [PubMed] [Google Scholar]
- 32.Ulbrandt ND, Newitt JA, Bernstein HD. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88:187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Topping TB, Randall LL. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci U S A. 1989;86:9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Randall LL, Hardy SJ. High selectivity with low specificity: how SecB has solved the paradox of chaperone binding. Trends Biochem Sci. 1995;20:65–69. doi: 10.1016/s0968-0004(00)88959-8. [DOI] [PubMed] [Google Scholar]
- 35.Randall LL, Hardy SJ. SecB, one small chaperone in the complex milieu of the cell. Cell Mol Life Sci. 2002;59:1617–1623. doi: 10.1007/PL00012488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Song J, Sui SF, Wang DN. DnaK and DnaJ facilitated the folding process and reduced inclusion body formation of magnesium transporter CorA overexpressed in Escherichia coli. Protein Expr Purif. 2003;32:221–231. doi: 10.1016/S1046-5928(03)00233-X. [DOI] [PubMed] [Google Scholar]
- 37.Mohanty AK, Simmons CR, Wiener MC. Inhibition of tobacco etch virus protease activity by detergents. Protein Expr Purif. 2003;27:109–114. doi: 10.1016/s1046-5928(02)00589-2. [DOI] [PubMed] [Google Scholar]