Abstract
Several lines of clinical evidence support the idea that fragile X syndrome (FXS) may involve a dysregulation of hypothalamic-pituitary-adrenal axis function (Wisbeck et al, 2000; Hessl et al, 2002). We had tested this idea in a mouse model of FXS (Fmr1 KO) and found that the hormonal response to acute stress was similar to that of wild type (WT) mice (Qin and Smith, 2008). We report here responses to chronic stress (CS) in Fmr1 KO mice. Following restraint for 120 min/day, 10 consecutive days, we assessed dendrite and spine morphology in basolateral amygdala (BLA). We also monitored behavior in an elevated plus maze (EPM) and the hormonal response to this novel spatial environment. After CS, mice of both genotypes underwent adrenal hypertrophy, but effects were greater in WT mice. Behavior in the EPM indicated that only WT mice had the expected increase in anxiety following CS. Serum corticosterone and ACTH levels were both increased following the spatial novelty of EPM, and there were no differences between genotypes in the hormonal responses. BLA dendritic branching increased proximal to the soma in WT, but in Fmr1 KO mice branching was unaffected close to the soma and slightly decreased at one point distal to the soma. Similarly, spine density on apical and basal dendrites increased in WT but decreased in Fmr1 KO mice. Spine length on apical and basal dendrites increased in WT but was unaffected in Fmr1 KO mice. These differences in behavioral response and effects on neuron morphology in BLA suggest a diminished adaptive response of Fmr1 KO mice.
Keywords: fragile X syndrome, FMRP, chronic stress, anxiety, amygdala, dendritic spines
Introduction
Fragile X syndrome (FXS) is the most common known cause of mental retardation and a leading genetic cause of autism. It is due to the silencing of a single gene, FMR1, resulting in the absence of the gene product, fragile X mental retardation protein (FMRP). Symptoms of FXS include a reduction in intellectual ability (Rousseau et al., 1994) and behavioral dysfunction such as hyperactivity, anxiety, attention problems and autistic-like behavior (Miller et al., 1999). Some clinical studies suggest that boys with FXS have dysregulation of hypothalamic-pituitary-adrenal (HPA) axis function (Wisbeck et al., 2000; Hessl et al., 2002). Boys with FXS exhibited higher salivary cortisol levels when exposed to a social stressor and a prolonged recovery back to baseline (Hessl et al., 2002).
In the mouse model of FXS (Fmr1 knockout (KO)), there is also evidence of a dysregulation of HPA axis function. Glucocorticoid receptor immunoreactivity in stratum radiatum of the hippocampus was lower in Fmr1 KO mice (Miyashiro et al, 2003) suggesting that feedback regulation of CORT levels may be diminished in these animals. Immobilization stress resulted in enhanced changes in c-fos expression in the paraventricular nucleus (PVN) (Lauterborn, 2004) and a more prolonged recovery of serum corticosterone (CORT) levels to baseline (Markham et al., 2006). In our previous study, we found no difference between Fmr1 KO and wild type (WT) mice in CORT and ACTH responses to and recovery from acute (30 or 120 min) restraint stress (Qin and Smith, 2008). We also found no difference between genotypes in CORT and ACTH responses to spatial novelty in the elevated plus maze (EPM) despite the fact that Fmr1 KO mice showed less anxiety-like behavior than WT. We now extend these studies to examine the response to chronic stress (CS). In normal rats, CS increases activation of the amygdala, induces dendritic hypertrophy in BLA, and increased anxiety behavior (Vyas et al., 2003; 2006). The alterations in dendrite and dendritic spine morphology in amygdala and anxiety-like behavior reflect experience-dependent forms of plasticity. The purpose of the present study was to investigate experience-dependent plasticity in Fmr1 KO mice.
EXPERIMENTAL PROCEDURES
Animals
Male WT and Fmr1 KO offspring were generated by FVB/NJ-Fmr1tm1Cgr breeding pairs (heterozygous females and WT males). We studied male WT and Fmr1 KO mice at 96±1 days of age. Mice with no previous exposure to these or any other stressful conditions were singly housed beginning one week before the study. Four groups of mice were studied: WT-unstressed control (WT-US) (n=24), Fmr1 KO-unstressed control (KO-US) (n=24), WT-chronic stress (WT-CS) (n=19), Fmr1 KO-chronic stress (KO-CS) (n=20). All mice were housed in a central facility and maintained under controlled conditions of normal humidity and temperature with standard alternating 12-h periods of light and darkness. Food (NIH-31 rodent chow) and water were provided ad libitum. All procedures were carried out in accordance with the National Institutes of Health Guidelines on the Care and Use of Animals and an animal study protocol approved by the National Institute of Mental Health Animal Care and Use Committee.
Genotyping
At the time of weaning, we analyzed genomic DNA extracted (Puregene, Gentra Systems, Inc, Minneapolis, MN, USA) from a small section of tail to test for the presence or absence of the KO allele as previously described (Qin et al., 2002). Primers to screen for the presence or absence of the mutant allele were 5′-ATCTAGTCATGCTATGGATATCAGC-3′ and 5′-GTGGGCTCTATGGCTTCTGAGG-3′. The PCR product at ≈800 bp indicated the presence of the null allele.
Chronic immobilization stress
Mice were subjected to CS by restraint in a plastic cylinder (Model 500M, Braintree Scientific, Inc, Braintree, MA) 120 min once a day from 8 am to 10 am for 10 days.
Elevated plus maze
On the 11th day, mice were tested in an EPM for 5 min. The EPM test took place in the same room as the chronic restraint and at the same time of day. Mice had had no previous exposure to the EPM. The apparatus consisted of two dark arms (30 × 5 × 15 cm) and two open arms elevated 50 cm from the floor. Initially animals were placed at the center facing an open arm. The times spent in the dark and open arms were recorded.
Body weight and adrenal weight change
The body weight of each mouse was measured each day. At the end of the EPM test, mice were decapitated in a separate procedure room and trunk blood was collected for hormone measures as previously described (Qin and Smith, 2008). Adrenal glands were carefully dissected and weighed.
Hormone assays
Blood was collected into tubes containing EDTA (BD Biosciences, Franklin Lakes, NJ), centrifuged 7000 × g for 5 min at 4°C to separate the plasma, and plasma samples were stored at −70°C until assayed. Concentrations of ACTH and CORT in plasma samples were determined by radioimmunoassay (Corticosterone 125I RIA kit and hACTH 125I RIA kit, MP Biomedicals, LLC, Orangeburg, NY). Samples were counted in a Wallac Wizard Gamma Counter 1480 (PerkinElmer, Waltham, MA). Inter- and intra-assay variability was monitored by the use of a standard. For the CORT assay the coefficient of variation within assays ranged from 4% to 8% and between assays was 11%. For the ACTH assay the coefficient of variation within assays ranged from 1% to 10% and between assays was 15%.
Golgi staining and morphological analysis of dendrites and dendritic spines in the basal amygdala (BLA)
After blood sampling, brains were quickly removed and impregnated with the Rapid GolgiStain™ Kit according to the manufacturer’s protocol (FD NeuroTechnologies, Ellicott City, MD). Coronal sections 100 µm in thickness were prepared with a Leica CM1850 cryostat, (Leica Microsystems Inc, Bannockburn, IL). Spiny pyramidal-like neurons from BLA were selected for analysis on the basis of the following criteria: (i) presence of untruncated dendrites, (ii) consistent and dark impregnation along entire extent of all dendrites, and (iii) relative isolation from neighboring impregnated neurons (Mitra et al., 2005; Vyas et al., 2002). BLA neurons analyzed in the present study were located between bregma −1.46mm and 1.82mm. Several aspects of dendritic morphology were analyzed with NIH ImageJ software. The numbers of dendritic branch intersections were determined by means of a Sholl Analysis with 25 µm concentric spheres and total dendrite lengths were measured in 40 neurons per group (8 mice/group) under light objective (16X, 0.63 numerical aperture, Leitz Wetzlar). Length and number of dendritic spines on 50 µm segments of primary basal dendrites starting 25 µm from the soma and on secondary apical dendrites originating 25 µm from the apical trunk were quantified. For the spine measurements we used an oil objective (100 X, 0.63 numerical aperture, Leitz Wetzlar). A total of 30 segments per experimental group in 6 mice per group were analyzed.
Statistical analysis
Data are expressed as mean ± SEM. Effects of CS on weight of adrenal glands, behavior in the EPM, hormone concentrations, dendritic length, and spine density were assessed by means of two-way analysis of variance (ANOVA) with condition and genotype as factors and Bonferroni post-hoc t-tests to assess differences between conditions for each genotype. Body weight data were analyzed by means of repeated measures (RM) 3-way ANOVA with genotype and stress condition as between subjects factors and day of stress as a within subjects factor. Dendritic intersection data were analyzed with a RM 3-way ANOVA with genotype and stress condition as between subjects factors and distance from the soma as a within subjects factor. Spine length distributions were compared by two-way Kruskal-Wallis tests followed by Kolmogorov-Smirnov tests. The criterion for statistical significance was P ≤ 0.05. We used SPSS and Partek Express programs for statistical analyses.
RESULTS
Effects of CS on body weight
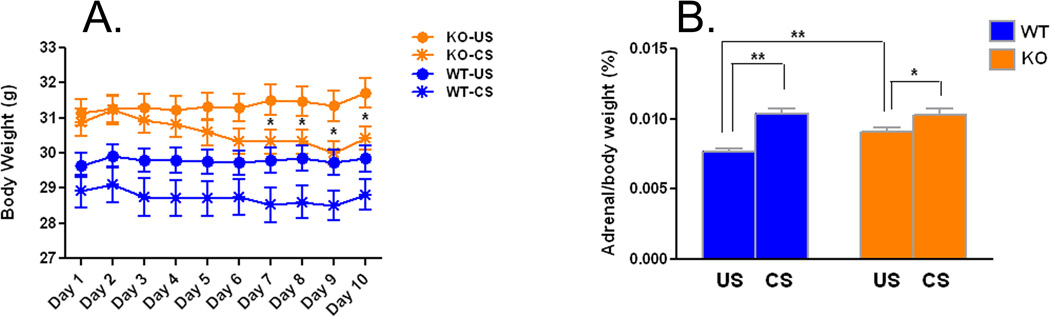
We monitored body weight during the 10 days of CS (Fig.1A). Body weight tended to decrease on Day 3 of CS in both genotypes and continued to decrease in Fmr1 KO mice while leveling off in WT. The genotype × condition × day of stress interaction was statistically significant (F(4.1,316.8) =2.401, P=0.048). At each time point we tested for differences between four pairs of groups by means of post hoc t-tests. Results indicate that in unstressed mice, body weights were higher in Fmr1 KO mice compared with age-matched WT (Day 1, t(41)=2.882, P=0.005; Day 2, t(41)=2.497, P=0.015; Day 3, t(41)=2.765, P=0.007; Day 4, t(41)=2.649, P=0.010; Day 5, t(41)=2.916, P=0.005; Day 6, t(41)=2.929, P=0.004; Day 7, t(41)=3.150, P=0.002; Day 8, t(41)=3.119, P=0.003; Day 9, t(41)=3.095, P=0.003; Day 10, t(41)=3.169, P=0.002). Similarly in mice subjected to chronic stress, body weights were higher in Fmr1 KO mice compared with WT (Day 1, t(36)=2.977, P=0.004; Day 2, t(36)=3.249, P=0.002; Day 3, t(36)=3.335, P=0.001; Day 4, t(36)=3.168, P=0.002; Day 5, t(36)=2.831, P=0.006; Day 6, t(36)=2.320, P=0.023; Day 7, t(36)=2.662, P=0.009; Day 8, t(36)=2.617, P=0.010; Day 9, t(36)=2.256, P=0.027; Day 10, t(36)=2.343, P=0.022). Differences between CS and US weights of Fmr1 KO mice were statistically significant from Day 7–Day 10 (Day 7, t(39)=2.128, P=0.037; Day 8, t(39)=2.174, P=0.033; Day 9, t(39)=2.514, P=0.014; Day 10, t(39)=2.047, P=0.044). In WT mice, body weight was not significantly affected by CS at any of the time points.
Figure 1.
A. Effects of chronic restraint stress on body weight in WT (US, n=22; CS, n=18) and Fmr1 KO (US, n=21; CS, n=20) mice. Each point represents the mean ± SEM for each day of stress. Data were analyzed by means of RM ANOVA with genotype (WT, KO), treatment (US, CS) and day of stress as factors with RM on day of stress. The genotype × treatment × day of stress interaction was statistically significant (F(4.1,316.8) = 2.401, p=0.048). Specific differences between groups at each time point were assessed for statistical significance by means of Bonferroni post-hoc t-tests. Differences between WT-US and KO-US and differences between WT-CS and KO-CS were statistically significant (p<0.05) at all time points. There were no significant differences between WT-US and WT-CS at any time point. Differences between KO-US and KO-CS were statistically significant as indicated on the graph (*, p<0.05).
B. Effects of chronic restraint stress on adrenal gland weight (as a percent of body weight) in WT (US, n=21; CS, n=19) and Fmr1 KO (US, n=20; CS, n=19) mice. Bars are the means ± SEMs. Data were analyzed by means of ANOVA with genotype (WT, KO), treatment (US, CS) as factors. The genotype × treatment interaction was statistically significant (F(1,75) = 5.083, p=0.027). Specific differences between groups were assessed for statistical significance by means of post-hoc Bonferroni t-tests (*, p<0.05; **, p<0.01).
Effects of CS on adrenal weights
Adrenal glands were removed and weighed on Day 11 following the EPM test (Fig. 1B). The genotype × condition interaction was statistically significant (F(1,75) =5.083, P=0.027), so we probed for specific differences between groups by means of Bonferroni t-tests. In unstressed mice, adrenal gland weight was higher (18%) in Fmr1 KO mice compared with age-matched WT (t(39)=3.101, P=0.0027). Adrenal glands showed the expected hypertrophy following CS in both genotypes. In WT mice, adrenal glands increased in weight by 36% after CS (t(38)=5.735, P<0.0001), whereas in Fmr1 KO mice the increase was smaller, c. 13% of KO-US (t(37)=2.498, P=0.015).
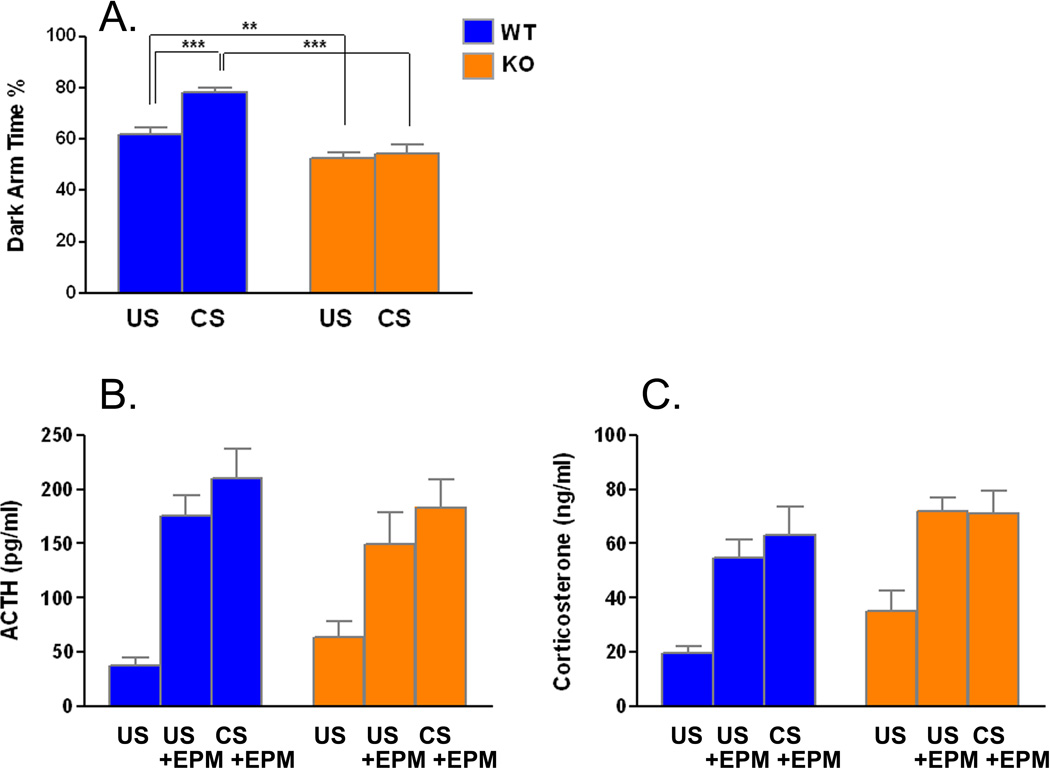
Effects of CS on anxiety and hormonal responses to a novel environment
On Day 11, the day following the last day of restraint stress, we subjected mice to the EPM as a test of anxiety (Fig. 2A). The genotype × condition interaction was statistically significant (F(1,82) =7.753, P=0.007), so we probed for specific differences between groups by means of t-tests. In unstressed mice, we confirmed our previous finding (Qin and Smith, 2008, Liu and Smith, 2009) that Fmr1 KO mice have reduced time in the dark arms suggesting less anxiety than WT (t(46)=2.742, P=0.007). In animals subjected to CS, we found that time spent in the dark arms increased by 28% in WT (t(38)=4.527, P<0.0001), whereas in Fmr1 KO mice there was very little effect. Following EPM we collected trunk blood for measurement of the hormonal response to this novel spatial environment (Fig. 2B & C). We also included a control group of each genotype that was not exposed to either CS or EPM for comparison. Plasma ACTH and CORT concentrations immediately after exposure to the spatial novelty of the EPM were increased in both genotypes regardless of exposure to chronic stress. For both hormones interactions between genotype and condition were not significant. The main effect of genotype regardless of condition was statistically significant for CORT (F(1,51)=5.069, P=0.029) but not for ACTH, indicating that levels of CORT were generally higher in Fmr1 KO mice. Main effects of condition were statistically significant for both ACTH (F(2,51)=23.515, P<0.0001) and CORT (F(2,51)=17.626, P<0.0001). Exposure to the spatial novelty of the EPM increased levels of ACTH 2–5-fold and CORT 2–3-fold in both genotypes.
Figure 2.
A. Effects of chronic restraint stress on percent time spent in closed arms of the elevated plus maze in WT (US, n=24; CS, n=19) and Fmr1 KO (US, n=24; CS, n=19) mice. Bars are the means ± SEMs. Data were analyzed by means of ANOVA with genotype (WT, KO), treatment (US, CS) as factors. The genotype × treatment interaction (F(1, 82) = 7.753, p = 0.007) was statistically significant. Specific differences between groups were assessed for statistical significance by means of post-hoc Bonferroni t-tests (**, p<0.01; ***, p<0.001). Plasma concentrations of ACTH (B.) and corticosterone (C.) in WT (US, n=9; US+EPM, n=9, CS+EPM, n=10) and Fmr1 KO (US, n=10; US+EPM, n=9, CS+EPM, n=10) mice measured after five min in the EPM. Bars are the means ± SEMs. Data were analyzed by means of ANOVA with genotype (WT, KO), treatment (US, US+EPM, CS+EPM) as factors. B. ACTH. The genotype × treatment interaction (F(2, 51) = 0.921, NS) and the main effect of genotype (F(1, 51) = 0.229, NS) were not statistically significant, but the main effect of condition (F(2, 51) = 23.515, P<0.0001) was. C. Corticosterone. The genotype × treatment interaction (F(2, 51) = 0.203, NS) was not statistically significant, but the main effects of both genotype (F(1, 51) = 5.069, P=0.029) and condition (F(2, 51) = 17.626, P<0.0001) were.
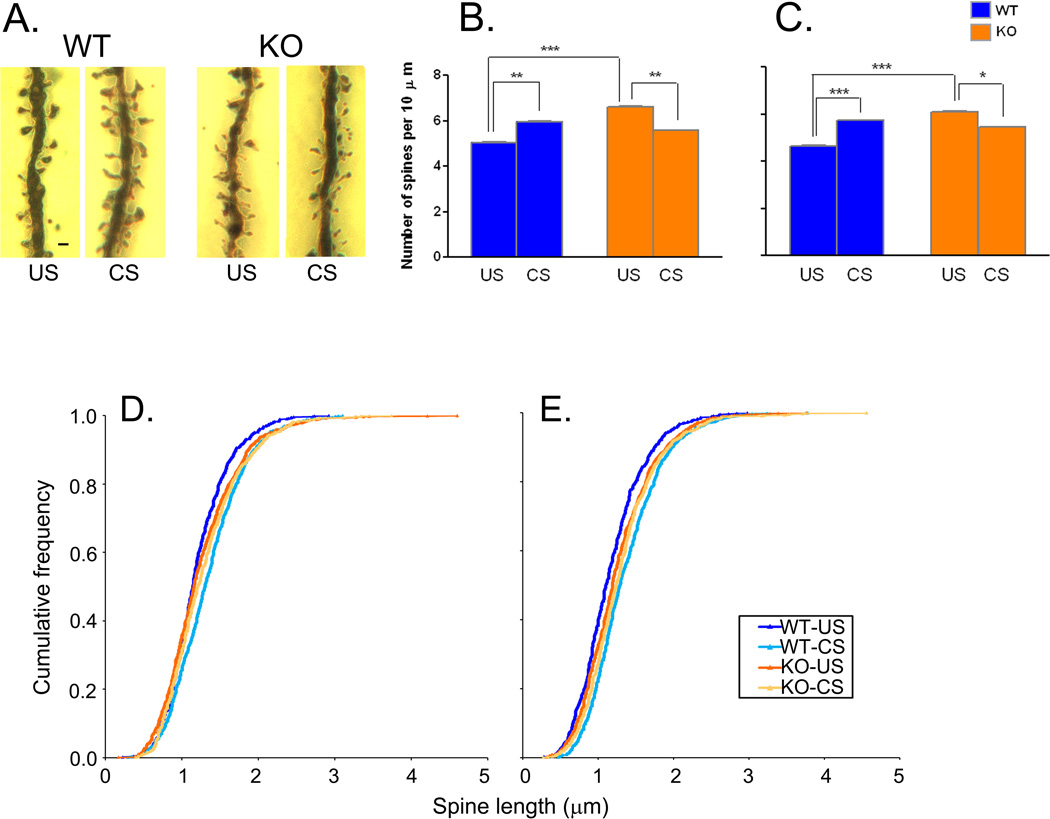
Dendritic analyses in BLA
Tracings of Golgi-impregnated pyramidal-like neurons of BLA from each experimental group are shown in Fig. 3A. Branch intersections on dendrites were analyzed at 25 µm concentric intervals from the soma (Fig. 3B). The curves for the Fmr1 KO mice (both US and CS) are below those for the WT mice indicating reduced complexity of the dendritic arbor in fragile X mice. There appears to be little, if any, effect of CS on dendritic arbors in the Fmr1 KO mice as US-KO and CS-KO curves are nearly superimposable. In contrast, in WT mice there is a clear hypertrophy of the arbor proximal to the soma following CS. The genotype × condition × distance from the soma interaction approached statistical significance (F(4.6,718) =1.803, P=0.116), so we compared the groups at each 25 µm interval with t-tests. In the US groups the number of branch intersections were statistically significantly higher in WT compared with KO between and including 75 and 125 µm (75 µm, t(78)=3.533, p=0.0005; 100 µm, t(78)=3.122, p=0.002; 125 µm, t(78)=2.330, p=0.021) and at 200 µm (t(78)=2.235, p=0.027) from the soma. In the CS groups the number of branch intersections were statistically significantly higher in WT compared with Fmr1 KO between and including 25 and 150 µm (25 µm, t(78)=2.011, p=0.046; 50 µm, t(78)=3.865, p=0.0002; 75 µm, t(78)=3.600, p=0.0004; 100 µm, t(78)=2.379, p=0.019; 125 µm, t(78)=2.451, p=0.015; 150 µm, t(78)=2.595, p=0.010) and at 200 µm (t(78)=2.484, p=0.014) from the soma. In WT mice, the numbers of branch intersections were statistically significantly higher in CS compared with US at 25 (t(78)=1.974, p=0.050) and 50 µm (t(78)=2.803, p=0.006) from the soma. In Fmr1 KO mice, the number of branch intersections was statistically significantly lower in CS compared with US at 175 µm from the soma (t(78)=2.528, p=0.012). We also assessed total dendritic length in these cells (Fig. 3C). This measure also indicates that dendritic arbors in Fmr1 KO mice are less complex (KO-US, 17% lower compared with WT-US; t(78)=3.698, p=0.0003), and that CS results in increased total dendrite length in WT (11%, t(78)=2.320, p=0.022) but has no effect in Fmr1 KO mice.
Figure 3.
- *, p<0.05, **, p<0.01; statistically significant difference between WT-US and KO-US
- §, p<0.05, §§, p<0.01; statistically significant difference between WT-US and KO-US
 , p<0.05,
, p<0.05,  , p<0.01; statistically significant difference between WT-US and WT-CS
, p<0.01; statistically significant difference between WT-US and WT-CS , p<0.05; statistically significant difference between KO-US and KO-CS
, p<0.05; statistically significant difference between KO-US and KO-CS
C. Total dendrite length of pyramidal-like BLA cells in WT (US, n=8; CS, n=8) and Fmr1 KO (US, n=8; CS, n=8) mice. Each point represents the mean ± SEM branches on 40 dendrites (5 per animal) of each genotype. Data were analyzed by means of ANOVA with genotype (WT, KO) and treatment (US, CS) as factors. The genotype × treatment interaction (F(1, 156) = 2.935, p = 0.089) and the main effect of condition (F(1, 156) = 2.458, p = 0.119) approached statistical significance. The main effect of genotype (F(1, 156) = 48.209, p<0.001) was statistically significant. Pairwise comparisons indicate statistically significant differences between genotypes under either US or CS conditions (***, p≤0.001) and between CS and US conditions in WT mice (*, p≤0.05).
Dendritic spine analyses in BLA
Following CS, dendritic spine densities on BLA apical and basal dendrites were affected in both genotypes, but the direction of the effects differed in WT and Fmr1 KO mice. Representative Golgi-impregnated dendrite segments from all four groups are shown in Fig. 4A. In WT mice, spine densities (Fig. 4B & C) increased (18%, apical (t(58)= 2.726, p=0.007); 23%, basal (t(58)=3.692, p=0.0003)) with CS, whereas in Fmr1 KO mice they decreased (−16%, apical (t(58)=3.065, p=0.003); −11%, basal (t(58)=2.302, p=0.023)). Genotype × stress interactions were statistically significant for both apical (F(1,116) =16.77, P<0.0001) and basal (F(1,116) =17.96, P<0.0001) dendrites. Spine densities were higher in US-KO mice compared with US-WT by 31% on both apical (t(58)=4.657, p<0.0001) and basal (t(58)=4.968, p<0.0001) dendrites. Cumulative frequency distributions of spine lengths on both apical and basal dendrites (Fig. 4C) indicate that spines were longer in KO-US mice compared with WT-US (P≤0.05, apical; P≤0.01 basal; Kolmogorov-Smirnov tests), but in mice subjected to CS spines were longer in WT (P≤0.01, apical; P≤0.05 basal; Kolmogorov-Smirnov tests). Chronic stress significantly increased median spine lengths of both apical and basal dendrites 13% and 16%, respectively, in WT mice, but in KO mice CS had no effect.
Figure 4.
Effects of chronic restraint stress on dendritic spines. A. Examples of spine morphology in each experimental group. Scale bar in WT-US segment represents 2 µm. Spine density on secondary apical (B) and primary basal (C) dendrites of pyramidal-like BLA cells in WT (US, n=8; CS, n=9) and Fmr1 KO (US, n=8; CS, n=7) mice. Each point represents the mean ± SEM density of 30 dendrite segments (1–7 per animal) of each genotype. Measurements were made on primary basal dendrites 25 µm from the soma and on secondary apical dendrites 25 µm from the apical trunk. Data were analyzed by means of ANOVA with genotype (WT, KO) and treatment (US, CS) as factors. B. Secondary apical dendrites. The genotype × condition interaction (F(1, 116)=16.769, p<0.0001) was statistically significant. C. Primary basal dendrites. The genotype × condition interaction (F(1, 116)=17.961, p<0.0001) was statistically significant. We probed for specific group differences by means of post-hoc Bonferroni t-tests. Results are shown in the figure as follows: *, p<0.05; **, p<0.01; ***, p<0.001.
Cumulative frequency distributions of spine lengths in WT (US, n=8; CS, n=9) and Fmr1 KO (US, n=8; CS, n=7) mice. (D) Apical dendrites. Lengths were measured in 758, 896, 993, and 836 spines in WT-US, WT-CS, KO-US, KO-CS, respectively. (E) Basal dendrites. Lengths were measured in 699, 861, 917, and 817 spines in WT-US, WT-CS, KO-US, KO-CS, respectively. Statistical analysis of all four cumulative frequency distributions of spine length on both apical and basal dendrites indicate statistically significant differences for both apical and basal dendrites (2- way Kruskal-Wallis test; apical: H = 45.89, p ≤ 0.001; basal: H = 76.03, p ≤ 0.001). Pairs of cumulative frequencies were further probed by means of Kolmogorov-Smirnov Tests; results indicate that for apical dendrites differences between WT-US and WT-CS (p ≤ 0.001), WT-US and KO-US (p ≤ 0.05), and WT-CS and KO-CS (p ≤ 0.01) were statistically significant. For basal dendrites differences between WT-US and WT-CS (p ≤ 0.001), WT-US and KO-US (p ≤ 0.01), and WT-CS and KO-CS, p≤0.05 were statistically significant.
DISCUSSION
The central finding of this study is that stress-induced remodeling of dendritic arbors in murine amygdala is altered in the absence of FMRP. Moreover, Fmr1 KO mice fail to show the increased anxiety induced by chronic stress in rodents. Our findings indicate that these differences in response to CS between WT and Fmr1 KO mice are not the result of a deficiency at the level of circulating stress hormones, because hormone responses are intact in the Fmr1 KO mouse. Results of our study indicate that long term adaptive responses to stress in amygdala are altered in adult fragile X mice.
To our knowledge, this is the first study to demonstrate a deficiency in adaptive response of the amygdala in the mouse model of FXS. The amygdala is involved in storage of memories of fearful and stressful experiences (LeDoux, 2003). It is also thought to be involved in social behavior (reviewed by Kling and Brothers, 1992). Both clinical and animal studies provide evidence for amygdala dysfunction in FXS. Subjects with FXS have reduced amygdala volume (Gothelf et al, 2008; reviewed by Schneider et al, 2009) and aberrant processing of direct gaze stimuli (Watson et al, 2008). Fmr1 KO mice exhibit abnormalities in social behavior (Spencer et al, 2008; McNaughton et al, 2008; Mineur et al, 2006; Liu and Smith, 2009) and decreased freezing responses to both contextual and cued conditioning (Paradee et al, 1999; Zhao et al, 2005). Fear conditioning, like social interaction, is thought to be an amygdala-based function. Consistent with deficits in fear conditioning response is the finding that long-term potentiation (LTP) is reduced in lateral amygdala (LA) in Fmr1 KO mice (Zhao et al, 2005). More recent studies report deficits in mGluR-dependent LTP and surface expression of AMPA receptor subunit GluR1 in LA in Fmr1 KO mice (Suvrathan et al, 2010). In addition, Suvranthan et al (2010) presented evidence that presynaptic transmitter release was also decreased at synapses of thalamic inputs to principal neurons of the LA. Another study found dramatic reductions in measures of GABAergic neurotransmission in BLA in Fmr1 KO mice (Olmos-Serrano et al., 2010). We have shown that in Fmr1 KO mice, rates of energy metabolism and rates of protein synthesis measured in vivo are increased in BLA (Qin et al, 2002; 2005). In the present study, we demonstrate less complex dendritic arbors and increased spine density and spine length on pyramidal-like cells of BLA in US Fmr1 KO mice. We have also reported similar changes in BLA in a study of a mouse model of the fragile X premutation in which FMRP levels are reduced to 10–15% of control (Qin et al., 2011). Taken together these findings indicate that structure and function of the amygdala is clearly affected by reductions in FMRP.
We used restraint for two hours per day for 10 days to induce a state of CS. This approach has been used in many other studies of normal rodents designed to examine the effects of CS on behavior and brain structure (Rao et al., 2009). Rats subjected to this form of CS have increased anxiety-like behavior as demonstrated by their behavior in the elevated plus maze (Vyas et al., 2004). They also have structural changes in amygdala, prefrontal cortex and hippocampus. These changes include increased dendritic arborization on both stellate and pyramidal cells and increased spine density on spiny neurons in BLA (Mitra et al., 2005). CS also results in decreased dendritic arborization in dorsal hippocampus CA3 pyramidal cells (Vyas et al., 2002) and in prefrontal cortex (Liston et al., 2006).
The difference between WT and Fmr1 KO mice in the effects of repeated restraint stress on body weight may reflect a difference between the genotypes in adaptability. We saw an initial tendency (on Day 3) for body weight to decline in both WT and Fmr1 KO mice, but only in the Fmr1 KO mice did the decline in weight continue through Day 9. Repeated daily 3 h of restraint stress in rats produced a pattern of weight loss similar to that observed in our WT mice (Harris et al, 2002). In the rat study, plasma CORT levels were elevated approximately 10-fold on Day 1 of restraint and declined gradually to about 4–5-fold on Day 9 of restraint (Harris et al, 2002). These results are consistent with an adaptation to the stress in the normal animals. It is possible that Fmr1 KO mice do not adapt or take longer to adapt to the stressor.
We tested for anxiety-like behavior with the EPM. The EPM is a standard test of anxiety in rodents. It is based on the conflict between an inclination to explore a novel environment and the aversive attributes of brightly lit and open spaces. In previous studies, we demonstrated reduced anxiety-like behavior in Fmr1 KO mice by their behavior in the open field, EPM, and the elevated zero maze (EZM) (Qin et al, 2002; Liu and Smith, 2009). In the open field, Fmr1 KO mice spent a greater percentage of time in the center of the field and less time close to the walls of the apparatus compared to WT. In the EZM, Fmr1 KO mice spent a greater percentage time in the open quadrants of the maze compared with WT. All of these behaviors indicate reduced generalized anxiety levels in the absence of FMRP. In contrast we found evidence of increased social anxiety in these animals. Behavior of Fmr1 KO mice in a three-chambered test of social interaction indicated reduced social approach and less preference for social novelty suggesting behavior akin to social anxiety (Liu and Smith, 2009). Reduced generalized anxiety and increased social anxiety were all partially normalized by chronic treatment with lithium carbonate (Liu et al, 2010). In the present study CS in WT mice resulted in increased time in the dark enclosed arms of the EPM. Fmr1 KO mice did not have this response. The fact that lithium treatment can normalize behavior on these tests of anxiety in Fmr1 KO mice indicates that differences from WT mice were not due to sensory or physical defects and that Fmr1 KO mice do have the capacity to express generalized anxiety in the EPM.
In our study we measured plasma levels of ACTH and CORT following the 5 min test in the EPM and 24 h after the last administration of restraint stress. Comparison with hormone levels measured after 5 min in the EPM with no prior stress show that both ACTH and CORT levels were similar in both groups. This suggests that hormone levels reflect the response to the spatial novelty of the EPM rather than basal hormone levels at the end of 10 days of CS. Levels of both hormones were much lower than those determined after two hours of acute restraint in which CORT increased 25–30 fold over control (Qin and Smith, 2008). With acute restraint stress we had shown previously that both hormones return to normal after 2 h of recovery (Qin and Smith, 2008). We did not measure hormone levels in groups of mice at various time points during the 10 days of CS, but in a similar study in rats in which animals were subjected to 3 h daily sessions of restraint CORT levels were measured every other day during restraint (Harris et al, 2002). In the rat study CORT levels were highest on Day 1 (c. 20 times control) but progressively decreased over time so that by Day 9 CORT levels were about 4–5 times control. These results indicate some adaptation of the rats to the recurring stress. We don’t know to what extent adaptation occurred in our mice and whether it was similar in both genotypes. These results and comparisons with our previous results on the effects of acute stress suggest that prior chronic restraint had negligible effects on the stress hormonal response to 5 min in the EPM 24 h after the last exposure to restraint.
In the present study, we confirm in WT mice the findings that CS effects structural changes in dendritic arbors and spines in BLA and that these changes are accompanied by increases in anxiety. We also expand on previous studies of the effects of CS in rodents with our finding that spine lengths are significantly increased in BLA. Elongated spines may represent an unstable state of spines during the dynamic plasticity response. Strikingly, none of these effects occurred in Fmr1 KO mice. In Fmr1 KO mice, dendritic arbors and spine length were unaffected by CS. Spine density was decreased in Fmr1 KO mice, an effect in the opposite direction to that found in WT suggesting a maladaptive response in the KO. In keeping with the correlation between morphological changes in BLA and increases in anxiety, Fmr1 KO mice did not show the effects on anxiety-like behavior. Our study does not establish a direct relationship between the morphological and behavioral changes. The evidence for a relationship between the structural remodeling and increased anxiety comes from the finding in rodents that chronic restraint stress, but not chronic unpredictable stress, results in both increased anxiety and dendritic changes in BLA principal neurons (Vyas et al, 2002). Moreover, with recovery from the chronic restraint stress dendritic and the behavioral changes follow similar time courses (Vyas et al, 2004). It is likely that other parts of the amygdala may also participate in the response to CS. In the medial nucleus (MeA), spine loss is induced in spiny stellate neurons by chronic restraint stress, and both spine loss in MeA and the accompanying increased anxiety-like behavior depend on the upregulation of serine protease tissue-plasminogen activator (tPA) (Bennur et al, 2007; Pawlak et al, 2003). Expression of tPA is not seen in BLA (Pawlak et al, 2003).
We propose that the results of our study of CS reveal a deficiency in experience-driven plasticity in amygdala in the absence of FMRP. Disruptions in experience-driven plasticity have been demonstrated in neocortex, hippocampus and cerebellum in the mouse model of FXS. The critical period with respect to thalamus-to-barrel cortex synapses is delayed in perinatal Fmr1 KO mice (Harlow et al, 2010), and the response in visual cortex to chronic monocular deprivation occurs more rapidly in adolescent Fmr1 KO mice compared to WT (Dölen et al, 2007). In adult Fmr1 KO mice, the classical eye blink conditioning response is attenuated (Koekkoek et al, 2005), and this response is dependent on the function of cerebellar Purkinje cells. In our study, Fmr1 KO mice failed to undergo both the behavioral changes and the morphological alterations in BLA seen in WT mice in response to CS.
The differences between the two genotypes in response to stress occurred despite the similar stress hormone response. This suggests that the problem is not at the level of the stress hormones. Rather, our results suggest that there is a defect further down the pathway, e.g., at the level of the glucocorticoid receptor. Glucocorticoid receptor mRNA is one of the cargoes found to associate with FMRP, and in Fmr1 KO mice, immunostaining for glucocorticoid receptor has been shown to be decreased in hippocampal dendrites (stratum radiatum) (Miyashiro et al, 2003). How the glucocorticoid receptor is affected in amygdala is not known, but a reduction in the receptor or an effect on the signaling pathway could be involved in the altered response to stress. Another possibility is that stress could have a differential effect on a pathway modulating the hormonal response. For example, activation of the noradrenergic system in BLA is required for enhanced memory consolidation in response to an emotionally arousing experience (reviewed by Roozendaal et al, 2009). This activation may occur via brain stem-to-BLA circuits stimulated by systemic adrenaline released in response to stress (Clayton and Williams, 2000). Stress has also been shown to result in release of corticotrophin releasing factor (CRF) in the amygdala which interacts with glucocorticoids and β-adrenoreceptors to affect memory consolidation (Roozendaal et al., 2008). Endocannabinoids are also released in BLA (Marsicano et al., 2002) in response to stress and endocannabinoid receptor activation in BLA can enhance memory consolidation (Campolongo et al, 2009). GABA (γ-aminobutyric acid) receptor antagonists infused in BLA also enhance memory consolidation (Brioni et al, 1989) and it has been proposed that endocannobinoids and glucocorticoids may enhance memory consolidation by inhibiting GABAergic activity in BLA (Roozendaal et al, 2009). The deficient response to stress in Fmr1 KO mice could be mediated via an effect on any of these factors.
The fact that GABAergic transmission is already markedly reduced in BLA in Fmr1 KO mice (Olmos-Serrano et al, 2010) suggests to us that there may be insufficient latitude in the system in BLA to allow for its modulation. Further inhibition via endocannaboids, glucocorticoids, or some other mechanism may be inadequate to enhance memory consolidation. This is consistent with the model proposed by Rao et al (2009) in which the disinhibition caused by stress permits glutamatergic synapses to undergo plastic changes. With repeated bouts of stress, inputs are strengthened over time by means of spinogenesis and increased dentritic arborization. We propose that in Fmr1 KO mice in which inhibition is reduced in the unstressed state, the stress response does little to further disinhibit and, consequently, cannot induce the adaptive changes.
Conclusions
Long term adaptive changes are essential for optimal function of the nervous system. They endow the nervous system with the ability to respond to the environment and make adjustments important for survival. In WT mice such changes occur in response to chronic stress and are manifest as increased anxiety-like behavior and structural remodeling in the amygdala. Fmr1 KO mice appear to lack this capacity to respond to CS in BLA at least in the time frame that we have studied. Our findings indicate that this fundamental property is deficient in the BLA in the absence of FMRP and suggests that FMRP is essential for the normal expression of experience-driven plasticity in BLA.
Bullet Points.
Chronic stress (CS) on anxiety and amygdala dendrites in WT and Fmr1 KO mice
Control Fmr1 KO mice had reduced dendritic arbors and elevated spine densities
ACTH and CORT increased in both genotypes in response to spatial novelty after CS
CS increased anxiety and dendritic branching in WT but not in Fmr1 KO mice
Following CS, spine density increased in WT but decreased in Fmr1 KO mice
Acknowledgements
We thank Dr. Zhong-Hua Liu for help with the statistical analyses. The research was supported by the Intramural Research Program of the NIMH, NIH and the Fragile X Research Foundation.
Abbreviations
- FXS
fragile X syndrome
- FMRP
fragile X mental retardation protein
- Fmr1 gene
fragile X mental retardation-1
- WT
wild-type
- KO
knockout
- US
unstressed
- CS
chronic stress
- HPA
hypothalamic-pituitary-adrenal
- CORT
corticosterone
- ACTH
adrenocorticotrophic hormone
- EPM
elevated plus maze
- LTP
long term potentiation
- LA
lateral amygdala
- BLA
basal lateral amygdala
- RM
repeated measure
- ANOVA
analysis of variance
- GABA
γ-aminobutyric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdale is mediated by tissue-plasminogen activator. Neurosci. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Nagahara AH, McGaugh JL. Involvement of the amygdale GABAergic system in the modulation of memory storage. Brain Res. 1989;487:105–112. doi: 10.1016/0006-8993(89)90945-1. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consoldation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci, USA. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EC, Williams CL. Adrenergic activation of the nucleus tractus solitarius potentiates amygdala norepinephrine release and enhances retention performance in emotionally arousing and spatial memory tasks. Behav Brain Res. 2000;112:151–158. doi: 10.1016/s0166-4328(00)00178-9. [DOI] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Shankaranarayana R, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile x syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O’Hara R, Erba HW, Ringel J, Hayahi KM, Patnail S, Golianu B, Kraemer HC, Thompson PM, Piven J, Reiss AL. Neuroanatomy of fragile X syndrome with aberrant behavior and the fragile x mental retardation protein (FMRP) Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of Fmr1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RBS, Mitchell TD, Simpson J, Redmann SM, Youndblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regulatory Integrative Comp Physiol. 2002;282:R77–R89. doi: 10.1152/ajpregu.2002.282.1.R77. [DOI] [PubMed] [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, Blasey C, Hastie T, Gunnar M, Reiss AL. Cortisol and behavior in fragile X syndrome. Psychoneuroendocrin. 2002;27:855–872. doi: 10.1016/s0306-4530(01)00087-7. [DOI] [PubMed] [Google Scholar]
- Kling AS, Brothers LA. The amygdala and social behavior in The Amygdala: Neurobiological Aspects of Emotion. In: Aggleton P, editor. Memory and Mental Dysfunction. New York: Wiley-Liss, Inc; 1992. pp. 353–377. [Google Scholar]
- Koekkoek SKE, Yamaguchi K, Milojkovic BA, Dortland BR, Rulgrok TJH, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, Willemsen R, Ikeda T, Kakizawa S, Onodera K, Nelson DL, Mientjes E, Joosten M, De Schutter E, Oostra BA, Ito M, De Zeeuw CI. Deletion of Fmr1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC. Stress induced changes in cortical and hypothalamic c-fos expression are altered in fragile X mutant mice. Mol Brain Res. 2004;131:101–109. doi: 10.1016/j.molbrainres.2004.08.014. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and amygdala. Cell Mol Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Mill MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortex xortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z-H, Smith CB. Dissociation of social and nonsocial anxiety in a mouse model of fragile X syndrome. Neurosci Lett. 2009;454:62–66. doi: 10.1016/j.neulet.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z-H, Chuang D-M, Smith CB. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int J Neuropsychopharm. 2010 May 25; doi: 10.1017/S1461145710000520. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariscano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Herman H, Tang J, Hofmann C, Zieglgansberger W, DiMarzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Markham J, Beckel-Mitchener AC, Estrada CM, Greenough WT. Corticosterone response to acute stress in a mouse model of fragile X syndrome. Psychoneuroendocrin. 2006;31:781–785. doi: 10.1016/j.psyneuen.2006.02.008. [DOI] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. Am J Med Genet. 1999;83:268–279. [PubMed] [Google Scholar]
- Mineur YS, Huynh LX, Crusio WE. Social behavior deficits in the Fmr1 mutant mouse. Behavioural Brain Research. 2006;168:172–175. doi: 10.1016/j.bbr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the balolateral amygdala. Proc Natl Acad Sci, USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Plauszkiewicz AM, Martin BS, Kaufmann WE, Corbin JG, Hintsman MM. Defective GABAergic neurotransmission and pharmacoloigal rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: Strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neurosci. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdale is critical for stress-induced anxiety-like behavior. Nature Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- Qin M, Entezam A, Usdin K, Huang T, Liu Z-H, Hoffman GE, Smith CB. A mouse model of the fragile X premutation: effects on behavior, dendrite morphology, and regional rates of cerebral protein synthesis. Neurobiology of Disease. 2011 January 8; doi: 10.1016/j.nbd.2011.01.008. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Smith CB. Increased rates of cerebral glucose metabolism in a mouse model of fragile X mental retardation. Proc Natl Acad Sci, USA. 2002;99:15758–15763. doi: 10.1073/pnas.242377399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Smith CB. Unaltered hormonal response to stress in a mouse model of fragile X syndrome. Psychoneuroendocrinology. 2008;33:883–889. doi: 10.1016/j.psyneuen.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RP, Suvrathan A, Mill MM, McEwen BS, Chattarji S. PTSD: From neurons to networks. In: Shiromani PJ, Keane TM, LeDoux JE, editors. Post-Traumatic Stress Disorder. NY: Humana Press; 2009. pp. 151–184. [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the β-adrenoreceptor -cAMP pathway dependence on glucocorticoid receptor activation. J Neurosci. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdale. Nature Reviews Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rousseau FD, Heitz D, Tarleton J, et al. A multicenter study on genotype-phenotype correlations in fragile X syndrome, using direct diagnosis with probe StB12:3: The first 2253 cases. Am J Hum Genet. 1994;55:225–237. [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Hagerman RJ, Hessl D. Fragile X syndrome — From genes to cognition. Dev Disabil Res Rev. 2009;15:333–342. doi: 10.1002/ddrr.80. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Graham DF, Yuva-Paylor LA, Nelson DL, Paylor R. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122:710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- Suvrathan A, Hoeffer CA, Wong H, Klann E, Chattarji S. Characterization and reversal of synaptic defects in the amygdala in a mouse model of fragile X syndrome. Proc Natl Acad Sci, USA. 2010;107:11591–11596. doi: 10.1073/pnas.1002262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Chattarji S. Enhanced anxiety and hypertrophy in basolateral amygdala neurons following chronic stress in rats. Ann NY Acad Sci. 2003;985:554–555. [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neurosci. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana R, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neurosci. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Watson C, Hoeft F, Garrett AS, Hall SS, Reiss AL. Aberrant brain activation during gaze processing in boys with fragile X syndrome. Arch Gen Psychiatry. 2008;65:1315–1323. doi: 10.1001/archpsyc.65.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisbeck JM, Huffman LC, Freund L, Gunnar M, Davis EP, Reiss AL. Cortisol and social stressors in children with fragile X syndrome: A pilot study. Dev Behav Ped. 2000;21:278–282. doi: 10.1097/00004703-200008000-00004. [DOI] [PubMed] [Google Scholar]
- Zhao M-G, Toyoda H, Ko SW, Ding H-K, Wu L-J, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]