Abstract
Toxigenic C. sordellii strains are increasingly recognized to cause highly lethal infections in humans that are typified by a toxic shock syndrome (TSS). Two glucosylating toxins, lethal toxin (TcsL) and hemorrhagic toxin (TcsH) are believed to be important in the pathogenesis of TSS. While non-toxigenic strains of C. sordellii demonstrate reduced cytotoxicity in vitro and lower virulence in animal models of infection, there are few data regarding their behavior in humans. Here we report a non-TSS C. sordellii infection in the context of a polymicrobial bacterial cholangitis. The C. sordellii strain associated with this infection did not carry either the TcsL-encoding tcsL gene or the tcsH gene for TcsH. In addition, the strain was neither cytotoxic in vitro nor lethal in a murine sepsis model. These results provide additional correlative evidence that TcsL and TcsH increase the risk of mortality during C. sordellii infections.
Introduction
Clostridial species have long been identified as causative agents of human disease, but the importance of these toxigenic bacteria has gained recent attention due to the emergence and spread of pathogens like Clostridium difficile [1] and its close relative C. sordellii [2, 3]. C. sordellii is a sporulating, anaerobic, gram positive bacterium that is often isolated from soil samples and sometimes causes highly lethal human infections [4]. This bacterium is known to cause gynecological infections following childbirth or abortion [5, 6], necrotizing soft tissue infections associated with the injection use of contaminated heroin [7–9], and postoperative infections complicating musculoskeletal transplants performed with contaminated graft material [10]. The incidence of C. sordellii infections is unclear, but an increasing number of cases has been reported over the past 10 years [11–15]. A recent report found that 1 in 200 deaths in women of reproductive age were associated with clostridial toxic shock, due to either C. sordellii or another clostridium, C. perfringens [3].
Perhaps two thirds of C. sordellii infections are associated with a clinically unique toxic shock syndrome (TSS), with mortalities exceeding 70% [4, 16]. However, this percentage may be overestimated, since it is likely that the most dramatic clinical cases (especially those associated with TSS) are reported, while infections that run a more benign course or have a positive outcome remain unpublished.
The pathogenesis of C. sordellii TSS has been a focus of recent studies [16, 17]. Though incompletely understood, the occurrence of TSS depends on the expression of one or both of the large glucosylating cytotoxins (TcsH and TcsL) of C. sordellii, which share structural and functional similarity to the large glucosylating cytotoxins of C. difficile (TcdA and TcdB, respectively). Both TcsH and TcsL intoxicate epithelial and endothelial cells by inactivating small GTPase proteins that are involved in maintaining cytoskeletal integrity [18–20]. Recent data suggest that C. sordellii TcsL is important for the development of TSS [17, 19], though almost nothing is known about the participation of TcsH.
Herein we present a case of invasive C. sordellii infection that was associated with neither TSS nor death. Molecular analyses demonstrated that this clinical strain lacked the tcsH and tcsL genes encoding TcsH and TcsL, respectively. This strain also lacked virulence in a mouse model of peritonitis. These data provide correlative support for the hypothesis that hemorrhagic and lethal toxins are important virulence determinants of the highly lethal and treatment-refractory TSS caused by C. sordellii. Knowledge gained from non-lethal C. sordellii infections such as this one provides new information regarding the pathogenesis of severe infections caused by this organism.
Materials and Methods
Institutional approval
This case report was reviewed and approved by the University of Michigan Institutional Review Board. Animals were treated according to National Institutes of Health guidelines for the use of experimental animals with the approval of the University of Michigan Committee for the Use and Care of Animals.
Animals
Eight-to-ten week old, female C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Bacterial strains
C. sordellii strain HH-310 was isolated from anaerobic culture of a patient’s blood sample (see below). The comparator strain ATCC9714 (tcsL+, tcsH−) was obtained from the American Type Culture Collection (Manassas, VA) and strain JGS6382 (tcsL+, tcsH+) was provided by Dr. J. Glenn Songer (Iowa State University).
PCR
DNA was extracted from C. sordellii strains HH-310, ATCC9714, and JGS6382 using an Easy-DNA™ extraction kit (Invitrogen, Carlsbad, CA). The taxonomic identity of HH-310 was verified using primers specific for a region of the C. sordellii 16S rRNA encoding gene [17]. Subsequent PCR for the tcsL gene encoding lethal toxin (TcsL) was performed using a previously reported primer pair (tcsL primer pair #3) and conditions [17]. New primers were designed to amplify an internal fragment of the tcsH gene encoding hemorrhagic toxin (TcsH) based on the genome sequence of the ATCC9714 strain which was obtained through Roche 454 Titanium genome sequencing (data not shown). Although this strain does not produce an active TcsH, it contains fragments of the tcsH gene at the appropriate genomic location downstream of (5’) the tcsL gene. These remnants were used to design PCR primers. Specifically, these primers were: tcsH_F1 (DLP37): GTAAATAAAACACATTTAAGAGCTTTGG and tcsH_R1 (DLP38): GGAATTTATATATGATAGGCAAAATAGG). Amplification conditions (94° C denaturation for 10 min, then 35 cycles of 94° C for 30 sec, 55° C for 30 sec, and 72° C for 1 min 30 sec, followed by a final extension at 72° C for 5 min) were validated and optimized using DNA extracted from ATCC9714 and JGS6382 prior to assaying HH-310. All PCR amplicons were visualized using electrophoresis (0.8% agarose gels) and amplicon sizes were estimated using a 1 kb DNA ladder (Invitrogen, Carlsbad, CA).
Cytotoxicity assay
To assay for the production of toxins by strains JGS6382 and HH-310, the TechLab C. difficile Toxin/Antitoxin Kit (REF T5000, TechLab®, Blacksburg,VA) was used in conjunction with Vero cell monolayers according to the manufacturer’s instructions (see package insert at http://www.techlab.com/product_details/t5000.shtml). This kit contains a toxin control reagent (positive control) and antitoxins that neutralize C. sordellii hemorrhagic and lethal toxins.. Strains of C. sordellii were grown anaerobically in 10 ml of sterile Reinforced Clostridial Medium (RCM, BD, Franklin Lakes, NJ) for 20 hr at 37° C. Cultures were filter sterilized using 0.2 µm nylon syringe filters (Fisher, Waltham, MA) and the resulting supernatants were assayed for the presence of toxins. Vero cells were cultured as reported elsewhere [21], with the single exception of Dulbecco's Minimal Essential Medium (DMEM) with High Glucose (Invitrogen, Carlsbad, CA) in place of minimal essential media (MEM alpha medium). All assays were performed in triplicate using a final C. sordellii culture supernatant dilution of 1:10. Treated Vero cells were fixed with formalin and stained with Wright-Giemsa Stain Mixture (Ricca Chemical Co.). Cell morphology was observed by microscopy on an Olympus 1X71 inverted microscope (20X magnification). A positive cytotoxic reaction was noted by rounding of the Vero cells compared to wells containing toxin antibodies.
Virulence experiments
Virulence experiments in mice were performed as previously described [17]. Briefly, C. sordellii strains HH-310 or JGS6382 were grown overnight in RCM broth and washed with PBS. Five mice each were then injected intraperitoneally with 100 µl PBS containing approximately 1 × 1010 CFU and 1 × 108 CFU of HH-310 or JGS6382, respectively. Infection was allowed to proceed for 7 d and survival was recorded daily.
Results
Case report
An 81 year-old female presented with the acute onset of stabbing abdominal pain that emanated from her epigastrium and radiated to her right upper abdominal quadrant and back. This was initially intermittent but became continuous and was exacerbated with inspiration. It was associated with fever and chills. The patient had a history of hypertension, diabetes mellitus, coronary artery disease, portal vein thrombosis and a congenital disorder causing non-obstructive dilation of intrahepatic bile ducts (Caroli disease).
As a result of the Caroli disease, the patient suffered repeated episodes of choledocholithiasis and cholangitis requiring multiple endoscopic retrograde cholangio-pancreaticogram (ERCP) procedures and stent placements. Ten months previously the patient was hospitalized with sepsis and cholangitis associated with Klebsiella pneumoniae and Escherichia coli bacteremia. Two recent episodes of right upper quadrant abdominal pain radiating to her back associated with fever were managed successfully as an outpatient with empirical, 10-day courses of levofloxacin.
Physical examination revealed a temperature of 37.9 °C, heart rate 137 beats per min, blood pressure 135/92 mm Hg and transcutaneous oxygen saturation of 92–94%. She had dry mucous membranes, and was tender to palpation in the epigastrium and right upper abdominal quadrant. Initial laboratory investigations showed a white blood cell count of 4.0 thou/µL (normal range 4.0–10.0 thou/µL) with 89.2% neutrophils (normal 36.0–75%), and a platelet count of 112 thou/µL (normal 150–450 thou/µL). Her serum creatinine was 1.0 mg/dL (normal 0.5–1.0 mg/dL), amylase 118 IU/L (normal 30–100 IU/L), lipase 62 U/L (normal 5–50 U/L), aspartate aminotransferase was 40 IU/L (normal 8–30 IU/L), alanine aminotransferase 17 IU/l (normal 7–35 IU/L), alkaline phosphatase 115 IU/L (normal 30–130 IU/L), total bilirubin of 0.8 mg/dL (normal 0.2–1.2 mg/dL) and serum lactate was 4.9 mmol/L (normal 0.5–2.2 mmol/L). The patient’s blood coagulation studies were normal. An ultrasound revealed marked intrahepatic pneumobilia with a complex cystic region in the porta hepatis that contained shadowing echogenic foci, consistent with gas or stones.
Blood cultures were obtained and the patient was treated with intravenous fluids and empirical intravenous levofloxacin and metronidazole. These antibiotics were chosen in light of a history of severe allergic reaction to penicillin and sulfa drugs. Although the patient was initially hemodynamically stable apart from a tachycardia, she soon developed a high fever (temperature 39.6 °C), tachycardia (123 beats per min) and hypotension (blood pressure of 77/41 mmHg). The two sets of aerobic and anaerobic blood cultures drawn on admission grew K. pneumoniae, vancomycin sensitive Enterococcus faecium and C. sordellii. Repeat blood cultures were obtained and parenteral antibiotics were changed to vancomycin, aztreonam, and metronidazole. Her K. pneumoniae strain was sensitive to aztreonam. Management also included intravascular volume resuscitation. The patient’s white blood cell count rose to 18.5 thou/µl and her coagulation parameters became abnormal, with an elevated prothrombin time of 14.3 sec (normal 9.5–11.7 sec) and a partial thromboplastin time of 37.6 sec (normal 21.0–30.0 sec). A repeat pair of aerobic and anaerobic blood cultures again yielded K. pneumoniae, E. faecium, and C. sordellii. An emergent ERCP revealed large amounts of biliary sludge at the hepatic duct bifurcation, and this sludge was removed by balloon extraction but not cultured.
The patient rapidly improved after this procedure and vasopressor agents were never required. Follow up blood cultures obtained three days after presentation were negative. The patient was discharged home and completed two weeks of treatment with parenteral vancomycin combined with oral levofloxacin and metronidazole. Despite ongoing problems with her Caroli disease, the patient remained free of bacteremia eight months after hospital discharge.
Characterization of the C. sordellii HH-310 strain
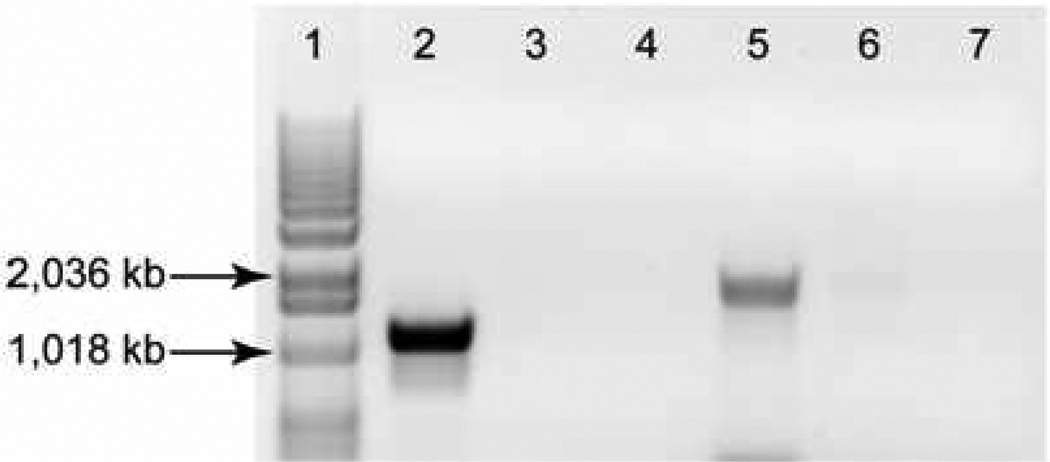
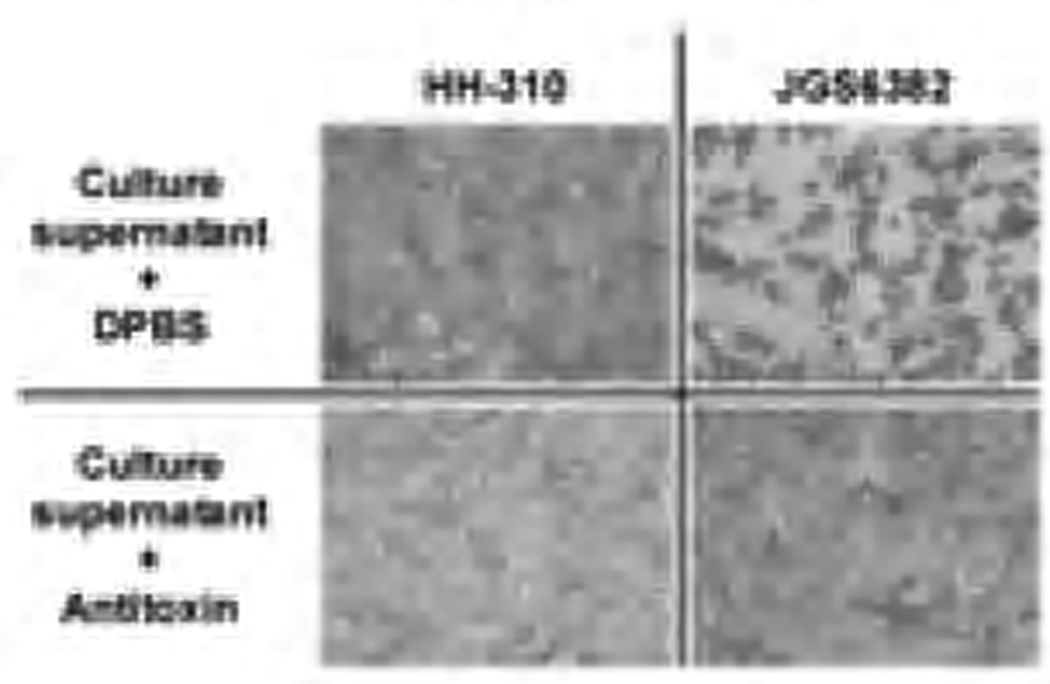
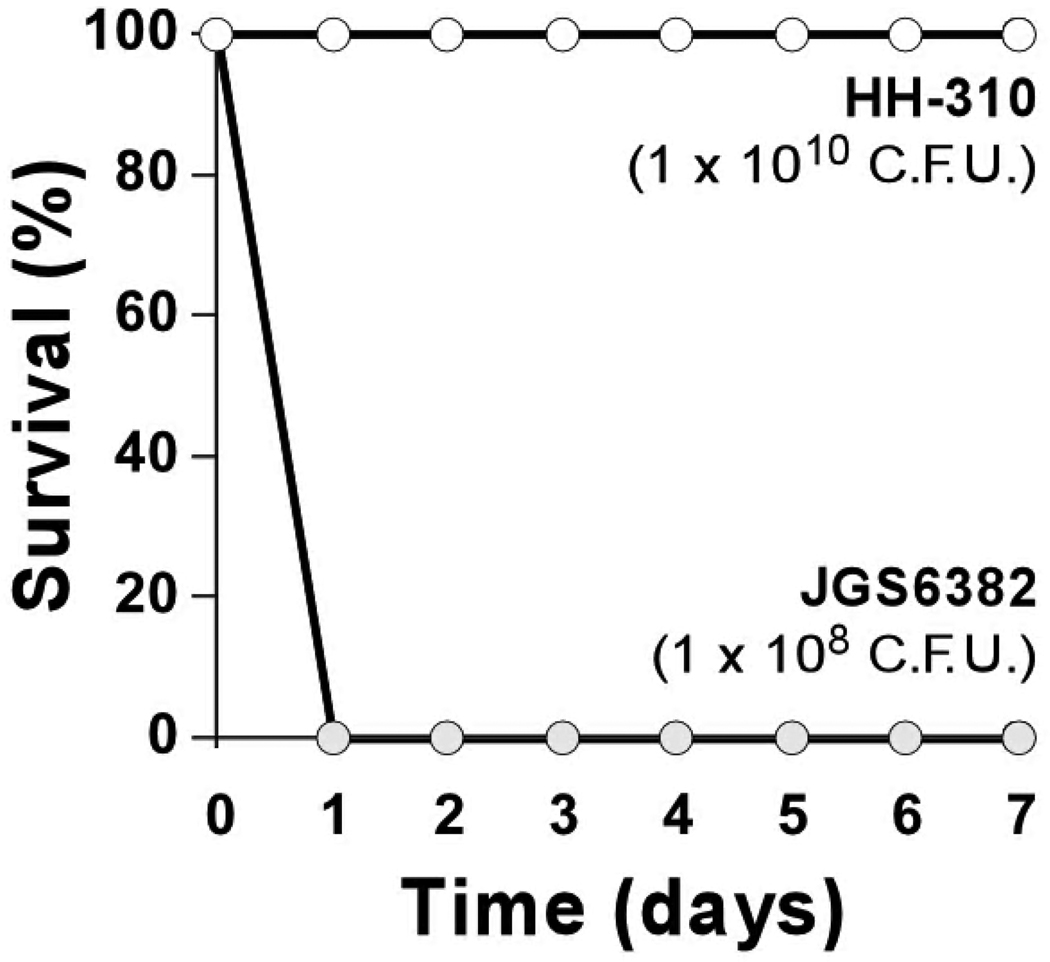
Genomic DNA extracted from the blood borne C. sordellii strain of the patient (named HH-310) was used as template for three separate PCR reactions. The first PCR (C. sordellii-specific 16S rRNA) was used to verify its correct taxonomic identification and resulted in a strong positive amplicon of the correct size (~944 kb, data not shown), verifying that HH-310 is indeed C. sordellii. Two additional PCRs were used to assay for the presence of tcsL and tcsH. Both PCRs successfully amplified toxin genes from the tcsL+ tcsH+ JGS6382 control strain, but failed to do so in HH-310, suggesting that these loci are not present in the genome of this strain (Figure 1). Consistent with PCR results, supernatant from an overnight culture of JGS6382 was highly toxic to Vero cell monolayers, while supernatant from overnight culture of HH-310 was not (Figure 2). Furthermore, C57BL/6J mice inoculated intraperitoneally with a large inoculum (1 × 1010 CFU per mouse) of HH-310 did not exhibit typical sickness behaviors (bradykinesia, huddling, ruffled fur, weight loss; not shown) and survived at least 7 days after infection (Figure 3).
Figure 1. Absence of the tcsH and tcsL genes in the bloodstream C. sordellii strain HH-310.
Two independent PCRs were performed for fragments of the tcsH (lanes 2–4) and tcsL (lanes 5–7) genes using genomic DNA obtained from the C. sordellii strain, HH-310, obtained from the patient discussed in the present report or the comparator strain JGS6382. Lanes 1–7 are: 1kb ladder, JGS6382 tcsH, HH-310 tcsH, water tcsH (tcsH PCR negative control), JGS6382 tcsL, HH-310 tcsL, water (tcsL PCR negative control). Sizes of pertinent ladder fragments are indicated.
Figure 2. No cytoxicity observed with culture supernatant of C. sordellii strain HH-310.
The upper-right panel shows the classic cell rounding phenotype caused by C. sordellii toxins (JGS6382). The bottom-right panel shows that this effect was ameliorated when toxin antibodies were present. HH-310 culture supernatant was non-toxigenic to Vero cells (upper-left panel) and was indistinguishable from the antibody control (bottom-left panel). All panels represent the same (20X) magnification. DPBS, Dulbecco’s phosphate buffered saline (vehicle control for antitoxin).
Figure 3. Failure of C. sordellii strain HH-310 to cause death in a peritonitis model.
Female C57BL/6 mice (n = 5 per group) were inoculated by intraperitoneal injection with C. sordellii strains HH-310 (white circles; 1 × 1010 CFU per mouse) or JGS6382 (gray circles; 1 × 108 CFU per mouse) at time 0 and survival was followed daily for 7 additional days.
Discussion
C. sordellii is an emerging pathogen associated with highly lethal bloodstream and soft tissue infections, particularly in young women following childbirth, abortion, or cervical procedures and also in injection drug users [3, 4]. Herein we describe a non-lethal case of invasive C. sordellii infection in the setting of polymicrobial bacterial cholangitis. This case was not associated with a stereotypical C. sordellii TSS and the patient had a favorable clinical outcome. Her initial sepsis syndrome was potentially due to, or significantly exacerbated by, the presence in her blood of the gram-negative pathogen, K. pneumoniae. Although C. sordellii and E. faecium were also present, K. pneumoniae is a well-known cause of bacteremia and hepatobiliary sepsis and contains the highly inflammatory endotoxin lipopolysaccharide [22]. While the biological basis for the patient’s clinical presentation and hospital course is speculative, it is notable that her strain of C. sordellii lacked the tcsH and tcsL genes encoding hemorrhagic and lethal toxins, respectively.
Although asymptomatic bacteremia can occur with several clostridial species [23], most cases of invasive C. sordellii reported to date have been fatal and associated with refractory hypotension and/or a stereotypical TSS [4]. The TSS includes the sudden onset of weakness, nausea, and vomiting; progressive and refractory hypotension; local and spreading edema; body cavity effusions, severe hemoconcentration; and a marked leukemoid reaction [24, 25]. These features were largely absent from the patient presented here and no lethality was observed when this clinical isolate was tested in a mouse peritonitis model. Non-fatal cases, or infections presenting without shock, are either less common than fulminant, lethal infections or they are underreported. It is therefore important, for both therapeutic and prognostic reasons, to better define the spectrum of clinical presentations and outcomes of C. sordellii infections.
Not all patients infected with C. sordellii die from infection or develop TSS [4, 17, 26]. Also, not all infecting strains express TcsH or TcsL [27] and mounting evidence suggests that these toxins in particular determine why some infected persons rapidly die and others do not [17, 19, 28]. Because of the paucity of reports of non-TSS C. sordellii infections in the literature, case-report data remain scarce to sufficiently support the link between TcsL-TcsH expression and clinical outcome (TSS vs. non-TSS). However, previous studies demonstrated that immunoglobulin therapy directed against TcsL protected against C. sordellii-induced death in rodents [5, 17]. In addition, infection of mice with isogenic tcsL− mutants did not lead to rapid death or cellular toxicity, indicating that TcsL is sufficient to cause disease [21]. The necessary role of TcsH in the development of TSS is less clear. Strains that carry both TcsH and TcsL (JGS6382) and those that carry only TcsL (ATCC9714 [17]) are highly lethal in our experimental mouse model. In addition, no tcsH+, tcsL− strain, to our knowledge, has been isolated from a case of human TSS, indicating that such strains rarely cause this type of disease. Therefore, the pathogenicity of toxigenic C. sordellii and toxigenic C. difficile may be similar, in that TcsL is essential for the development of TSS, much like the orthologous TcdB (TcdB and TcsL are orthologs) which is important for the development of fulminant C. difficile infections [29].
Similar to the case presented here, Hao et al. recently reported a non-TSS case of C. sordellii endometritis [17]. The infecting strain (DA-108) was shown to lack the tcsL gene and was nonlethal in rodents. Screening of this strain with the tcsH primers developed in this study confirmed that DA-108 also lacks tcsH. Taken together, these endometritis and cholangitis cases provide independent support for the importance of C. sordellii glucosylating toxins in the etiology of TSS. However, it remains unclear how often these genes are present (or absent) in the chromosomes of virulent and avirulent strains and if both are essential for TSS. What is more, the possible advantage afforded to certain pathogenic strains by these toxins is equally unclear.
We speculate that patients presenting without toxic shock or severe hypotension are most likely infected with strains not possessing tcsL and/or tcsH. In the future, a rapid molecular assessment of tcsL and tcsH carriage (e.g., by PCR) in clinical isolates might provide therapeutic and diagnostic information at the bedside.
The occurrence of C. sordellii bacteremia is uncommon, with fewer than 20 cases identified in the current literature and a mortality of ~75 % in these patients [14, 30, 31]. Although anaerobes such as Bacteroides and Clostridium have been associated with hepatobiliary infections, C. sordellii is an uncommon pathogen in this setting, which might reflect the low prevalence of gastrointestinal carriage of this bacterium, estimated to be < 10% in culture-based stool assays [32, 33]. We were unable to identify previous cases of C. sordellii associated with cholangitis. However, liver and brain abscesses have been observed with this anaerobe [14, 26, 30]. A rapidly-fatal case of C. sordellii bacteremia and multiple-organ dissemination, including the liver, was reported in the early post-operative period following liver transplantation, in a heavily-immunocompromised patient [34]. Interestingly, experiments conducted with that patient’s C. sordellii strain suggested that it did not produce TcsL because cell-free culture supernatants did not cause death in mice [34].
In summary, this report describes a rare case of C. sordellii bacteremia from a biliary source and correlates the genetic absence of tcsL and tcsH with a good clinical outcome and the absence of TSS. These data support the need for ongoing studies of clostridial pathogenesis to enhance preventive and therapeutic interventions against these emerging pathogens.
Acknowledgments
This work was supported by National Institutes of Health grant HL078727 (D.M.A.) and UL1RR024986 (S.T.W.) from the National Center for Research Resources. The non-animal research was supported by a Doris Duke Charitable Foundation Clinical Scientist Development Award (D.M.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McFee RB. Clostridium difficile: emerging public health threat and other nosocomial or hospital acquired infections. Introduction. Dis Mon. 2009;55:419–421. doi: 10.1016/j.disamonth.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, Garcia P, et al. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 3.Ho CS, Bhatnagar J, Cohen AL, Hacker JK, Zane SB, Reagan S, et al. Undiagnosed cases of fatal Clostridium-associated toxic shock in Californian women of childbearing age. Am J Obstet Gynecol. 2009;201(459):e1–e7. doi: 10.1016/j.ajog.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin Infect Dis. 2006;43:1436–1446. doi: 10.1086/508866. [DOI] [PubMed] [Google Scholar]
- 5.Bitti A, Mastrantonio P, Spigaglia P, Urru G, Spano AI, Moretti G, et al. A fatal postpartum Clostridium sordellii associated toxic shock syndrome. J Clin Pathol. 1997;50:259–260. doi: 10.1136/jcp.50.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer M, Bhatnagar J, Guarner J, Reagan S, Hacker JK, Van Meter SH, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med. 2005;353:2352–2360. doi: 10.1056/NEJMoa051620. [DOI] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Rosen JI, Aragon T, Campbell A, Weir L, Perdreau-Remington F. Clostridial myonecrosis cluster among injection drug users: a molecular epidemiology investigation. Arch Intern Med. 2002;162:517–522. doi: 10.1001/archinte.162.5.517. [DOI] [PubMed] [Google Scholar]
- 8.Dunbar NM, Harruff RC. Necrotizing fasciitis: manifestations, microbiology and connection with black tar heroin. J Forensic Sci. 2007;52:920–923. doi: 10.1111/j.1556-4029.2007.00452.x. [DOI] [PubMed] [Google Scholar]
- 9.Kimura AC, Higa JI, Levin RM, Simpson G, Vargas Y, Vugia DJ. Outbreak of necrotizing fasciitis due to Clostridium sordellii among black-tar heroin users. Clin Infect Dis. 2004;38:e87–e91. doi: 10.1086/383471. [DOI] [PubMed] [Google Scholar]
- 10.Kainer MA, Linden JV, Whaley DN, Holmes HT, Jarvis WR, Jernigan DB, et al. Clostridium infections associated with musculoskeletal-tissue allografts. N Engl J Med. 2004;350:2564–2571. doi: 10.1056/NEJMoa023222. [DOI] [PubMed] [Google Scholar]
- 11.Elsayed S, Zhang K. Positive Clostridium difficile stool assay in a patient with fatal C. sordellii infection. N Engl J Med. 2006;355:1284–1285. doi: 10.1056/NEJMc061165. [DOI] [PubMed] [Google Scholar]
- 12.Aldape MJ, Bryant AE, Ma Y, Stevens DL. The leukemoid reaction in Clostridium sordellii infection: neuraminidase induction of promyelocytic cell proliferation. J Infect Dis. 2007;195:1838–1845. doi: 10.1086/518004. [DOI] [PubMed] [Google Scholar]
- 13.Cohen AL, Bhatnagar J, Reagan S, Zane SB, D'Angeli MA, Fischer M, et al. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstetrics and gynecology. 2007;110:1027–1033. doi: 10.1097/01.AOG.0000287291.19230.ba. [DOI] [PubMed] [Google Scholar]
- 14.Matten J, Buechner V, Schwarz R. A rare case of Clostridium sordellii bacteremia in an immunocompromised patient. Infection. 2009;37:368–369. doi: 10.1007/s15010-008-8192-y. [DOI] [PubMed] [Google Scholar]
- 15.Meites E, Zane S, Gould C. Fatal Clostridium sordellii infections after medical abortions. N Engl J Med. 2010;363:1382–1383. doi: 10.1056/NEJMc1001014. [DOI] [PubMed] [Google Scholar]
- 16.Aronoff DM, Ballard JD. Clostridium sordellii toxic shock syndrome. Lancet Infect Dis. 2009;9:725–726. doi: 10.1016/S1473-3099(09)70303-2. [DOI] [PubMed] [Google Scholar]
- 17.Hao Y, Senn T, Opp JS, Young VB, Thiele T, Srinivas G, et al. Lethal toxin is a critical determinant of rapid mortality in rodent models of Clostridium sordellii endometritis. Anaerobe. 2010;16:155–160. doi: 10.1016/j.anaerobe.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez RD, Wilkins TD. Comparison of Clostridium sordellii toxins HT and LT with toxins A and B of C. difficile. J Med Microbiol. 1992;36:30–36. doi: 10.1099/00222615-36-1-30. [DOI] [PubMed] [Google Scholar]
- 19.Geny B, Khun H, Fitting C, Zarantonelli L, Mazuet C, Cayet N, et al. Clostridium sordellii lethal toxin kills mice by inducing a major increase in lung vascular permeability. Am J Pathol. 2007;170:1003–1017. doi: 10.2353/ajpath.2007.060583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geny B, Popoff MR. Activation of a c-Jun-NH2-terminal kinase pathway by the lethal toxin from Clostridium sordellii, TcsL-82, occurs independently of the toxin intrinsic enzymatic activity and facilitates small GTPase glucosylation. Cell Microbiol. 2009;11:1102–1113. doi: 10.1111/j.1462-5822.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- 21.Carter GP, Awad MM, Hao Y, Thelen T, Bergin I, Howarth PM, et al. TcsL is an essential virulence factor in Clostridium sordellii ATCC9714. Infection and Immunity. 2011;79:1025–1032. doi: 10.1128/IAI.00968-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meatherall BL, Gregson D, Ross T, Pitout JD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122:866–873. doi: 10.1016/j.amjmed.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Haddy RI, Nadkarni DD, Mann BL, Little DR, Domers TD, Clover RD, et al. Clostridial bacteremia in the community hospital. Scand J Infect Dis. 2000;32:27–30. doi: 10.1080/00365540050164173. [DOI] [PubMed] [Google Scholar]
- 24.McGregor JA, Soper DE, Lovell G, Todd JK. Maternal deaths associated with Clostridium sordellii infection. Am J Obstet Gynecol. 1989;161:987–995. doi: 10.1016/0002-9378(89)90768-0. [DOI] [PubMed] [Google Scholar]
- 25.Sosolik RC, Savage BA, Vaccarello L. Clostridium sordellii toxic shock syndrome: a case report and review of the literature. Infect Dis Obstet Gynecol. 1996;4:31–35. doi: 10.1155/S1064744996000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valour F, Boisset S, Lebras L, Martha B, Boibieux A, Perpoint T, et al. Clostridium sordellii brain abscess diagnosed by 16S rRNA gene sequencing. J Clin Microbiol. 2010;48:3443–3444. doi: 10.1128/JCM.00307-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura S, Tanabe N, Yamakawa K, Nishida S. Cytotoxin production by Clostridium sordellii strains. Microbiol Immunol. 1983;27:495–502. doi: 10.1111/j.1348-0421.1983.tb00611.x. [DOI] [PubMed] [Google Scholar]
- 28.Kachman MT, Hurley MC, Thiele T, Srinivas G, Aronoff DM. Comparative analysis of the extracellular proteomes of two Clostridium sordellii strains exhibiting contrasting virulence. Anaerobe. 2010;16:454–460. doi: 10.1016/j.anaerobe.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458:1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronk M, Thomson D, Walpole E. Unusual liver abscess. Intern Med J. 2005;35:134–135. doi: 10.1111/j.1445-5994.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 31.Abdulla A, Yee L. The clinical spectrum of Clostridium sordellii bacteraemia: two case reports and a review of the literature. J Clin Pathol. 2000;53:709–712. doi: 10.1136/jcp.53.9.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan J, Murphy C, Twomey C, Paul Ross R, Rea MC, Macsharry J, et al. Asymptomatic carriage of Clostridium difficile in an Irish continuing care institution for the elderly: prevalence and characteristics. Ir J Med Sci. 2009 doi: 10.1007/s11845-009-0361-1. [DOI] [PubMed] [Google Scholar]
- 33.Moosavian M, Hayati K. A survey of clostridia in the patients with acute diarrhea compared with the control group. Pakistan Journal of Medical Sciences Quarterly. 2008;24:209–212. [Google Scholar]
- 34.Mory F, Lozniewski A, Guirlet MN, Guidat D, Bresler L, Weber M, et al. Severe sepsis caused by Clostridium sordellii following liver biopsy in a liver transplant recipient. Clin Infect Dis. 1995;21:1522–1523. doi: 10.1093/clinids/21.6.1522. [DOI] [PubMed] [Google Scholar]