Abstract
Objective
Examine the effect of conjugated equine estrogens alone (ET), conjugated equine estrogens plus medroxyprogesterone (EPT), calcitriol alone, calcitriol plus EPT/ET or placebo on serum lipid profile and analyze the interaction with estrogen receptor alpha gene single nucleotide polymorphisms (ESR-α SNPs) on response to therapy.
Methods
489 postmenopausal women > 65 years age enrolled in 3-year double blind placebo-controlled clinical trial
Results
In both intent to treat and complier (>80% adherent) analysis, there was significant increase in serum high-density lipoproteins (HDL), significant decrease in serum low-density lipoproteins (LDL) and LDL:HDL ratio in all hormone treatment groups compared to placebo (p<0.05). However, serum triglycerides and very low-density lipoproteins (VLDL) increased in EPT and ET+ calcitriol groups' versus placebo (p <0.05). ESR-α SNPs PvuII and XbaI appeared to have a significant effect on response to treatment. Genotypes containing p allele showed significantly greater decrease in serum cholesterol and VLDL than those having P allele in the ET plus calcitriol group (p<0.05) and those with x allele had significantly greater decrease in serum cholesterol in HT plus calcitriol group at the end of 3 years versus X allele and a greater decrease in serum LDL in alleles x versus X in ET plus calcitriol group (p<0.05).
Conclusions
ET with or without progesterone had a favorable effect on lipid profile in postmenopausal elderly women and this was dependent on estrogen receptor SNP's – PvuII and XbaI. However, this interaction with ESR-α SNPs need to be confirmed in larger studies.
Keywords: hormone therapy, lipids, conjugated equine estrogen, medroxyprogesterone acetate, postmenopausal women, estrogen receptor SNPs
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of mortality in both men and women in the United States and other developed countries. However, the incidence of the disease is delayed in women by about a decade, probably as a consequence of a protective effect of estrogen before menopause (1–3). The risk of CVD in women increases markedly after menopause (4,5). Several lines of evidence indicated that the unfavorable lipid-lipoprotein changes seen after menopause were related to lack of estrogen and had led to the suggestion that hormone therapy (HT) might reduce the risk of CVD.
A number of observational studies reported a lower risk of CVD in women taking estrogen. A meta-analysis of more than 30 observational studies showed a 56% reduction in risk of a major coronary event in healthy current estrogen users compared with women who have never used estrogen (6,7). The cardio-protective effect of ET (Estrogen Therapy) has been attributed to a number of potential mechanisms of which favorable changes in lipid-lipoprotein profile is estimated to contribute to at least one third of cardio-protective effect (8). Observational studies and clinical trials that evaluated the effect of exogenous estrogen on cardiovascular risk have demonstrated favorable changes in serum lipid and lipoprotein levels including a decrease in serum levels of total cholesterol and LDL and increased serum HDL and triglyceride levels (8–13). However other factors including route, type and dose of estrogen administration and the co-administration of a progestin affects the response in terms of serum lipids (11–19). Medroxyprogesterone acetate (MPA) is the most commonly used progestogen in HT regimens in United States, for women with intact uterus to reduce the increased incidence of endometrial cancer that accompanies unopposed estrogen use. MPA was reported to attenuate the beneficial effects of estrogen on cardiovascular system in postmenopausal estrogen progestin intervention trial (11,20) and also by other investigators (21). Debate is continuing on whether there is an impact of the added progestins on the beneficial effects of estradiol on the cardiovascular system (22,23). Also studies in the cynomolgus macaque had previously indicated an attenuation of ET effects' by MPA on the development of atherosclerotic plaque, as well as coronary artery reactivity (24–27). However, observational studies of HT report no differences in risk for clinical cardiovascular events between users of estrogen alone and users of estrogen plus progestins, including women using predominantly MPA (18,19,28). In two large randomized clinical trials, HERS (Heart and Estrogen/progestin Replacement Study) and ERA (Estrogen Replacement and Atherosclerosis Study) which examined the use of HT in postmenopausal women with established CHD, no beneficial effect of HT in reducing the CHD risk was found (29,30). Then came the results from the Women's Health Initiative (WHI), a large, randomized trial that reported that women on estrogen in combination with progestin had increased incidence of coronary heart disease compared to placebo (31).
The biological rationale for the beneficial effect of ET is that estrogen increases the hepatic synthesis of LDL receptor (apo B-100), resulting in an increased LDL uptake and therefore decreased circulating LDL levels (32). It also increases the activity of the enzyme lipoprotein lipase, thus raising HDL levels (33).
Though the positive role of ET treatment on serum lipids is well known, the influence of HT on lipid parameters is still controversial.
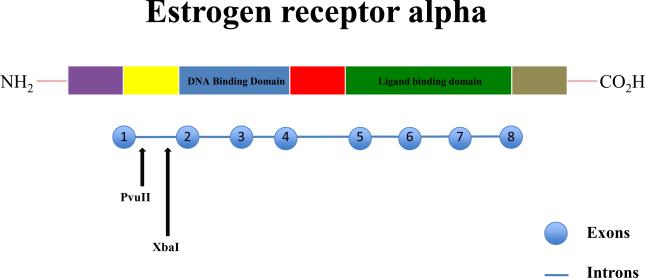
Most of the biological actions of estrogen are exerted through estrogen receptors (ER) that belong to the family of ligand gated nuclear transcription factors (34,35). There are two types of estrogen receptors – alpha (α) and beta (β). ER-α is considered to be the main receptor for estrogen. There are various single nucleotide polymorphisms (SNPs) present in ER-α - PvuII and XbaI. These are present in intron 1 of the ER-α gene (Figure 1). PvuII is present −397 base pairs from exon 2 and produces C to T transition (36). XbaI is present −351 base pairs from exon 2 and produces A to G transition (37). These SNPs have previously been shown to be associated with bone markers (38), bone mineral density and fractures (39,40) and cardiovascular disease including myocardial infarction (41,42) and stroke (43). Few studies have examined the association of change in serum lipids in response to HT with estrogen receptor alpha gene SNPs (37, 44,45).
FIG. 1.
Schematic diagram–estrogen receptor-α gene: it consists of eight exons encompassing six domains. PvuII and XbaI restriction fragment length polymorphisms produce C/T and A/G variations, respectively.
In a randomized, double blinded clinical trial; we examined the effect of conjugated equine estrogen alone (ET, in women who underwent hysterectomy) or in combination with medroxyprogesterone (EPT, in women with intact uterus) on lipid profile in elderly postmenopausal women and further analyzed the impact of estrogen receptor α gene single nucleotide polymorphisms (SNPs) on the response to therapy.
METHODS
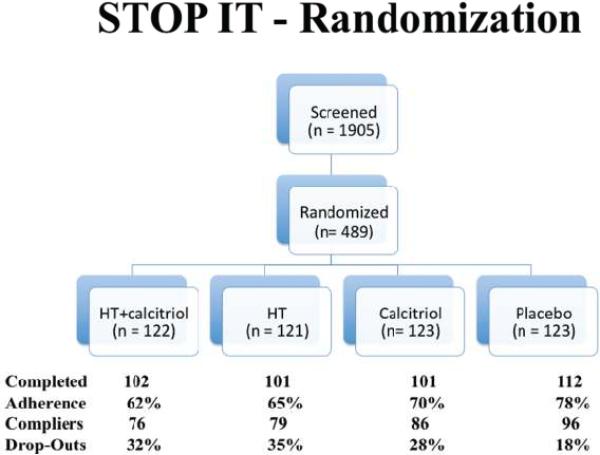
Four hundred and eighty nine elderly women aged 65–77 years were recruited to participate in a 3-year double blind placebo-controlled clinical trial, STOP IT (Sites Testing Osteoporosis Prevention or Intervention Treatment). The study was originally designed to test the effects of estrogen in women without a uterus (ET) plus medroxyprogesterone acetate in women with a uterus (EPT), calcitriol, and the combination of ET/EPT and calcitriol on bone density, biochemical markers of bone remodeling, falls and fractures, compared to placebo. The women were recruited into the study through advertisements in local newspapers or by mass mailing of letters inviting them to participate in a three-year study. Women were excluded if they had severe chronic illness, had primary hyperparathyroidism or active renal stone disease, and were on medications, such as bisphosphonates, anticonvulsants, estrogen, fluoride, or thiazide diuretics in the previous 6 months. Additional inclusion criteria were normal liver and kidney function. All participants were free living, in good health and ambulatory. Of the 489 women, 470 were white, 13 were black, 4 were Hispanic, 1 Asian and one of mixed race. Creighton University Institutional Review Board approved the study and all participants signed an informed consent to participate in the study. .
Women were randomly assigned to either conjugated equine estrogens (Premarin) 0.625 mg daily plus Medroxyprogesterone acetate (Provera) 2.5 mg daily (EPT), calcitriol (Rocaltrol) 0.25 mcg b.i.d., the combination of Premarin plus Provera + calcitriol, or matching placebos; hysterectomized women (n=290) assigned to estrogen were not given the progestin (ET). The detailed study design has been depicted in Fig 2. Details about the safety assessment and study compliance have been published elsewhere (46).
FIG. 2.
STOP IT randomization. STOP IT, Sites Testing Osteoporosis Prevention or Intervention Treatment; HT, hormone therapy.
Dietary intake at baseline and at the end of the study (36 months) was assessed using 7-day food diaries. A dietitian asked the women to complete a 7-day food dairy and nutrient supplement record. Plastic food models (NASCO, Fort Artinson, WI) were used to help participants to better estimate the quantities consumed. The average daily caffeine and calcium intakes were calculated using the FOOD PROCESSOR II PLUS nutrition and diet analysis system (version 5.1; Esha Research, Salem, OR).
On entry into the study, women underwent a physical examination and a detailed medical history was obtained from a questionnaire. At baseline recruitment and at 36 months, participants were also provided with a questionnaire to report their smoking and alcohol history, reproductive history, and present use of medications, vitamins and mineral supplements. Current smokers were considered as smokers while past and women who never smoked were classified as nonsmokers. Alcohol use was stratified into drinkers and nondrinkers.
At baseline and at the end of study, a fasting blood sample was collected from each participant between 0700 h and 0900 h for measurement of serum total cholesterol, triglycerides, HDL cholesterol, and LDL cholesterol levels. Serum total cholesterol, triglycerides and HDL cholesterol were estimated using an auto-analyzer (Vitros 750 and Vitros 950 chemistry analyzer, Ortho-Clinical Diagnostics, NY). The method used for total cholesterol measurement is based on an enzymatic method proposed by Allain et al (47). Triglyceride analysis is based on an enzymatic method described by Spayd et al (48). HDL cholesterol is estimated after pretreatment of the sample to precipitate LDL and VLDL. VLDL cholesterol level is calculated as triglyceride/5. LDL cholesterol is calculated as, [total cholesterol-(HDL+VLDL)].
Estrogen Receptor Genotype Analysis
Genomic DNA was isolated from peripheral white blood cells using the Puregene kit (Gentra systems). The samples were genotyped for PvuII (rs2234693) and XbaI (rs9340799) restriction fragment length polymorphisms (RFLPs) as has been published by us before (38). Briefly, the oligonucleotide primers used included forward, 5'-CTGCCACCCTATCTGTATCTTT-3' and reverse, 5'-ACCCTGGCGTCGATTATCTGA-3'. Conventional polymerase chain reaction (PCR) was performed for 30 cycles under the following conditions: denaturation for 60 seconds at 94°C, annealing at 50°C for 60 seconds and extension at 72°C for 90 seconds. The PCR products (~1.3 kbp long fragments) were digested with restriction endonuclease enzymes – PvuII and XbaI. The resulting fragments were separated by gel electrophoresis on 2% agarose gel stained with ethidium bromide and visualized on a transilluminator under UV light and photographed. These were coded as P/p (PvuII) and X/x (XbaI) with upper case letters signifying the absence and lower case signifying the presence of restriction sites respectively.
Statistical analysis
Data were analyzed using SAS package 8.0. The following procedures were performed for both the intent to treat group and adherent to treatment group. In each case, the baseline characteristics between the six treatment groups were compared using One-Way ANOVA and the averages and standard errors were computed. The percentage of smokers and alcohol drinkers by treatment group, and their standard errors, were also found by Chi-Square test. The unadjusted (crude means) baseline lipid variables and the unadjusted percent change over baseline lipid parameters of the six treatment groups were compared again using One-Way ANOVA. When the treatment effect was significant at 0.05 level in the ANOVA models, multiple comparisons of the least squared means by treatment were performed using Tukey's method. In addition to these “crude” means, in an ANCOVA (analysis of covariance) model, the treatment effects on (a) the baseline values and (b) the percent change from the baseline in variables of interest were assessed. The effects of the covariates, age weight, height, BMI, dietary calorie intake, fiber intake, calcium intake, caffeine intake, alcohol status, smoking status and age at menopause were adjusted for in these analyses. When the percent change was the outcome variable, the corresponding baseline value was also used for the adjustment. The least–squares means (i.e. the adjusted means) were computed by the MIXED procedure of SAS (v. 8.2). When the treatment effects were significant at 0.05 level in the ANCOVA models, multiple comparisons of the least squared means by treatment were performed using Tukey's method. The impact of RFLP on the treatment response in terms of serum lipids was assessed by ANCOVA models after adjusting for the above mentioned confounders plus statin users and the baseline serum lipid for each of the corresponding values, for example in the analysis of final serum cholesterol at 36 months, baseline serum cholesterol was used a co-variate in the analysis in addition to the above mentioned covariates. When the treatment effects were significant at 0.05 level in the ANCOVA models, multiple comparisons of the least squared means by treatment were performed using Bonferroni adjustment.
RESULTS
Of the 489 women enrolled into the study, 416 women came for the final visit (36 month), of which 337 women were adherent to treatment (> 80 % compliant with study medication). The data of the 416 women were included for the intent to treat analysis. Of these, one woman with suspected Paget's disease was excluded from the analyses and 3 women had no lipid profile data available. So the final intent to treat analyses was performed on the data of 412 women. The data of the 337 women who were adherent to the treatment regimen were used for complier analyses (> 80% compliance with study medication). The initial randomization of women into different treatment groups has been depicted in Fig 2.
In both intent to treat and complier analyses, there were no significant differences between the treatment groups with regard to their age, height, weight, BMI, dietary calcium and caffeine intake (Table 1). The distribution of smokers and alcohol drinkers was also not different between the six treatment groups (Table 1).
Table 1.
Baseline Characteristics
| Placebo | Calcitriol | EPT | ET | EPT+Calcitriol | ET+Calcitriol | P value | |
|---|---|---|---|---|---|---|---|
| N | 112 | 101 | 57 | 42 | 62 | 38 | |
| Age (y) | 71.0 ± 0.4 | 71.4 ± 0.3 | 71.4 ± 0.5 | 71.3 ± 0.5 | 71.1 ± 0.4 | 71.9 ± 0.6 | 0.83 |
| Height (cm) | 160.0 ± 0.6 | 159.7 ± 0.6 | 158.9 ± 0.7 | 159.7 ± 1.6 | 159.0 ± 0.8 | 158.2 ± 0.8 | 0.67 |
| Weight (Kg) | 69.3 ± 1.3 | 67.7 ± 1.3 | 67.4 ± 1.4 | 73.3 ± 2.2 | 66.7 ± 1.5 | 69.4 ± 2.1 | 0.14 |
| BMI | 27.2 ± 0.5 | 26.5 ± 0.5 | 26.8 ± 0.6 | 28.7 ± 0.8 | 26.4 ± 0.5 | 27.7 ± 0.8 | 0.14 |
| Calcium intake (mg/d) | 782.2 ± 28.6 | 762.1 ± 25.6 | 806.3 ± 41.1 | 691.2 ± 39.8 | 767.0 ± 38.0 | 654.3 ± 34.5 | 0.07 |
| Caffeine intake (mg/d) | 267.0 ± 22.5 | 218.8 ± 16.1 | 234.5 ± 22.6 | 261.2 ± 22.7 | 252.4 ± 19.3 | 330.5 ± 51.7 | 0.09 |
| Smokers (%)a | 11.6 ± 3.0 | 8.9 ± 2.8 | 17.5 ± 5.1 | 21.4 ± 6.4 | 11.3 ± 4.1 | 13.2 ± 5.6 | 0.35 |
| Alcohol use (%)a | 32.1 ± 4.4 | 40.6 ± 4.9 | 45.6 ± 6.7 | 21.4 ± 6.4 | 27.4 ± 5.7 | 31.6 ± 7.6 | 0.08 |
| Statin Use N (%)a | 8 (7.14) | 5 (5) | 2 (3.5) | 2 (4.8) | 1 (1.6) | 5 (13.2) | 0.243 |
Abbreviation: EPT, estrogen plus medroxyprogesterone therapy; ET, estrogen therapy
The values represented in the table are means ± SE.
Comparisons were done using the One-Way Analysis of Variance or Kruskal-Wallis test, as appropriate.
The distribution was tested using the Chi-square statistic.
Intent to Treat Cross- sectional Analysis
At baseline, there were no significant differences in means of serum cholesterol and HDL cholesterol between any of the treatment groups (Table 2). Unadjusted mean serum triglycerides and serum VLDL were significantly lower in women assigned to EPT+calcitriol treatment regimen compared to women assigned to ET treatment (123.4 ± 7.7 vs 180.7 ± 15.0; p <0.01 and 24.7 ± 1.5 vs 34.8 ± 2.7; p <0.05 respectively -Table 2), however, after adjusting for differences in clinical characteristics and dietary factors, the changes became non significant (adjusted means for serum triglycerides were 124.6 ± 11.5 mg/dl in EPT+calcitriol group vs 174.2 ± 13.3 on ET, p >0.05 and for serum VLDL were 24.4 ± 2.1 vs 33.0 ± 2.5, p >0.05 respectively). Adjusted mean serum LDL cholesterol was significantly higher in women receiving the EPT treatment at baseline compared to women receiving the calcitriol (145.8 ± 4.6 in EPT group vs 128.5 ± 4.0 in calcitriol group; p <0.05). The unadjusted ratio of mean LDL/ HDL cholesterol in the EPT group was significantly greater compared to women in the calcitriol group (3.15 ± 0.15 vs 2.65 ± 0.08; p < 0.05) and was similar in adjusted analysis (3.05 ± 0.13 vs 2.49 ± 0.11; p <0.01 respectively). The unadjusted LDL/HDL cholesterol was also significantly higher on ET compared to women in the calcitriol group (3.20 ± 0.19 vs 2.65 ± 0.08; p <0.05 respectively: see Table 2). Also, the mean LDL/HDL ratio in women on EPT+calcitriol was significantly different from the group on EPT (both adjusted and unadjusted) (Table 2).
Table 2.
Baseline lipid profile in treatment groups of Intent to treat and complier analysesa
| Placebo | Calcitriol | EPT | ET | EPT+Calcitriol | ET+Calcitriol | |
|---|---|---|---|---|---|---|
| INTENT TO TREAT (n) | 112 | 101 | 57 | 42 | 62 | 38 |
| Serum cholesterol | 224.4 ± 2.8 | 218.2 ± 3.6 | 231.6 ± 5.1 | 232.8 ± 6.2 | 218.6 ± 4.7 | 220.0 ± 5.6 |
| Serum triglycerides | 148.2 ± 7.8 | 144.9 ± 8.0 | 155.9 ± 10.4 | 180.7 ± 15.0 | 123.4 ± 7.7f | 154.3 ± 13.8 |
| Serum HDL | 50.5 ± 1.1 | 53.3 ± 1.2 | 50.9 ± 1.7 | 48.9 ± 1.9 | 54.2 ± 1.2 | 51.8 ± 2.4 |
| Serum VLDL | 28.5 ± 1.4 | 28.5 ± 1.5 | 30.2 ± 1.9 | 34.8 ± 2.7 | 24.7± 1.5e | 30.9 ± 2.8 |
| Serum LDL | 143.2 ± 2.7 | 135.1 ± 3.3 | 150.7 ± 4.7 | 146.1 ± 5.6 | 139.7 ± 4.3 | 136.2 ± 5.3 |
| LDL : HDL cholesterol | 2.96 ± 0.08 | 2.65 ± 0.08 | 3.15 ± 0.15b | 3.20 ± 0.19b | 2.67 ± 0.10be | 2.78 ± 0.13 |
| COMPLIERS (n) | 96 | 86 | 41 | 38 | 43 | 33 |
| Serum cholesterol | 223.0 ± 3.0 | 221.1 ± 4.0 | 235.7 ± 6.0 | 231.8 ± 6.6 | 217.1 ± 5.9 | 218.7 ± 5.9 |
| Serum triglycerides | 148.9 ± 8.8 | 145.1 ± 9.1 | 156.5 ± 12.1 | 177.5 ± 16.2 | 130.3 ± 9.8 | 155.4 ± 14.7 |
| Serum HDL | 50.5 ± 1.1 | 53.3 ± 1.3 | 49.9 ± 1.9 | 49.2 ± 2.0 | 53.6 ± 1.4 | 51.3 ± 2.7 |
| Serum VLDL | 28.5 ± 1.6 | 28.4 ± 1.7 | 29.9 ± 2.1 | 34.0 ± 2.9 | 26.1 ± 1.9 | 31.1 ± 2.9 |
| Serum LDL | 143.9 ± 2.9 | 137.9 ± 3.7 | 156.3 ± 5.1b | 145.2 ± 6.0 | 137.4 ± 5.3 | 134.9 ± 5.7 |
| LDL : HDL cholesterol | 2.97 ± 0.09 | 2.70 ± 0.09 | 3.30 ± 0.17c | 3.16 ± 0.20 | 2.66 ± 0.13d | 2.78 ± 0.14 |
Abbreviation: EPT, estrogen plus medroxyprogesterone therapy; ET, estrogen therapy
The values are means ± SE in mg/dl. Comparison between the treatment groups was done using either ANOVA (for crude means) or ANCOVA (for adjusted means; adjusted for age, weight, BMI dietary factors). Post-hoc significance was computed by Tukey's multiple comparison method.
p<0.05 compared to calcitriol
p<0.01 compared to calcitriol
p<0.05 compared to EPT
p<0.05 compared to ET
p<0.01 compared to ET
Compliers analysis
At baseline, there were no significant differences in unadjusted and adjusted means between the six treatment groups with regard to serum cholesterol, triglycerides, HDL cholesterol and VLDL cholesterol (Table 2) but There was a significant difference in baseline adjusted serum LDL cholesterol between the women assigned to EPT and calcitriol treatment regimens (154.9 ± 5.4 vs 132.9 ± 4.3;p<0.01), between the women assigned to EPT+calcitriol and EPT treatment regimens (132.0 ± 5.4 vs 154.9 ± 5.4;p<0.05) and between the women of ET+calcitriol and EPT treatment groups (128.5 ± 6.3 vs 154.9 ± 5.4;p<0.05). The LDL:HDL cholesterol ratio (both unadjusted and adjusted) was significantly higher in women receiving EPT treatment compared to that of women assigned to calcitriol alone (3.30 ± 0.17 vs 2.70 ± 0.09 respectively p<0.01 in unadjusted analysis and 3.22 ± 0.15 vs 2.55 ± 0.12; p <0.01 in adjusted analysis respectively), EPT+calcitriol treatment (2.66 ± 0.13 in EPT+calcitriol vs 3.30 ± 0.17 in EPT;p<0.05 for unadjusted and p<0.01 for adjusted) and ET+calcitriol treatment (2.54 ± 0.18 in ET+calcitriol vs 3.22 ± 0.15 in EPT; adjusted, p<0.05).
Longitudinal Analysis
Intent to Treat
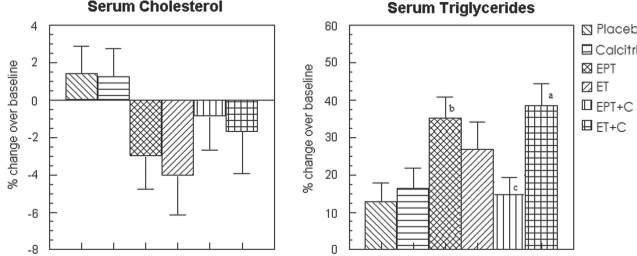
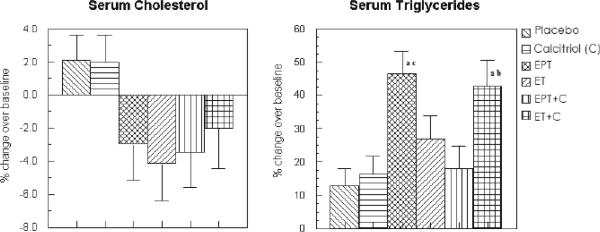
Serum total cholesterol - The results of unadjusted analysis are presented in Table 3. The decrease in total cholesterol in women receiving EPT and ET alone was significantly greater than placebo (−12.1 ± 4.4 in EPT and −14.4 ± 4.8 in ET vs 3.5 ± 2.4 mg/dl in placebo; p <0.05: see Table 3), however, it became non-significant after adjustment for relevant confounders (Fig 3).
Table 3.
Unadjusted change from baseline (mg/dl) of lipid parameters in treatment groups of Intent to treat and complier analyses
| Placebo | Calcitriol | EPT | ET | EPT+Calcitriol | ET+Calcitriol | |
|---|---|---|---|---|---|---|
| INTENT TO TREAT | ||||||
| N | 112 | 101 | 57 | 42 | 62 | 38 |
| Serum cholesterol | 3.5 ± 2.4 | 0.95 ± 2.8 | −12.1 ± 4.4a | −14.4± 4.8a | −2.04 ± 3.5 | −3.5 ± 5.6 |
| Serum triglycerides | 10.5 ± 6.0 | 17.1 ± 5.3 | 48.4 ± 19 | 32.3 ± 9.6 | 16.1 ± 7.3 | 41.6 ± 10.7a |
| Serum HDL | 1.3 ± 0.7 | −1.2 ± 0.8 | 6.4 ± 1.3bd | 7.3 ± 1.5bd | 6.2 ± 1.3ad | 8.4± 1.6bd |
| Serum VLDL | 2.7 ± 1.0 | 3.7 ± 0.9 | 5.8 ± 2.0 | 5.02 ± 1.8 | 3.2 ± 1.4 | 8.3 ± 2.1a |
| Serum LDL | −1.3 ± 2.1 | −0.84 ± 2.3 | −25 ± 4.2bd | −26.3 ± 4.7bd | −11.5 ± 3.5 | −19.9 ± 5.5bd |
| LDL: HDL cholesterol | −0.05 ± 0.06 | 0.05 ± 0.05 | −0.84± 0.12bd | −0.82 ± 0.11bd | −0.41 ± 0.09bd | −0.68± 0.13bd |
| COMPLIERS | ||||||
| N | 96 | 86 | 41 | 38 | 43 | 33 |
| Serum cholesterol | 4.7 ± 2.7 | 1.7 ± 3.0 | −13.4 ± 5.3a | −14.4 ± 5.2a | −6.8 ± 4.4 | −4.2 ± 6.2 |
| Serum triglycerides | 9.3 ± 6.6 | 16.5 ± 6.0 | 69.5 ± 22.3bd | 30.9 ± 10.5 | 18.3 ± 9.9 | 45.8 ± 12.0ad |
| Serum HDL | 1.4 ± 0.8 | −1.4 ± 0.9 | 9.7 ± 1.5bd | 8.6 ± 1.4bd | 8.6 ± 1.4bd | 9.8 ± 1.6bd |
| Serum VLDL | 2.5 ± 1.1 | 3.6 ± 1.0 | 8.9 ± 2.5ac | 4.5 ± 2.0 | 3.7 ± 2.0 | 9.2 ± 2.4ac |
| Serum LDL | −0.03 ± 2.3 | 0.31 ± 2.5 | −32.5 ± 4.6bd | −27.2 ± 5.0bd | −19.04 ± 4.3bd | −22.9 ± 5.8bd |
| LDL : HDL cholesterol | −0.02 ± 0.06 | 0.09 ± 0.06 | −1.1 ± 0.14bd | −0.9 ± 0.1bd | −0.65 ± 0.09bd | −0.82± 0.12bd |
Abbreviation: EPT, estrogen plus medroxyprogesterone therapy; ET, estrogen therapy
The values are means ± SE. Comparison between the treatment groups was done using ANOVA. Post-hoc significance was computed by Tukey's multiple comparison method.
p<0.05 compared to placebo
p<0.01 compared to placebo
p<0.05 compared to calcitriol
p<0.01 compared to calcitriol
FIG. 3.
Percent change in serum cholesterol and serum triglycerides in the various treatment groups after adjustment for confounders (intent-to-treat analysis). EPT, estrogen + medroxyprogesterone therapy; ET, estrogen therapy; C, calcitriol. aP < 0.05 as compared with placebo. bP < 0.01 as compared with placebo. cP < 0.05 as compared with EPT.
Serum triglycerides - The increase in serum triglycerides was significantly higher in women receiving ET+calcitriol compared to placebo (41.6 ± 10.7 vs 10.5 ± 6.0 mg/dl; p<0.05 -Table 3); the adjusted increase was significantly higher in EPT and ET+calcitriol groups compared to placebo and EPT+calcitriol (only EPT group) groups (35.3 ± 5.6 % in EPT vs 12.7 ± 4.6 % in placebo; p<0.01; 37.7 ± 7.1 % in ET+calcitriol vs 12.7 ± 4.6 % in placebo; p<0.05 and 14 ± 5.7 % in EPT+calcitriol vs 35.3 ± 5.6 % in EPT alone; p<0.05 – see Fig 3). No significant differences existed between other treatment groups of intent to treat analyses.
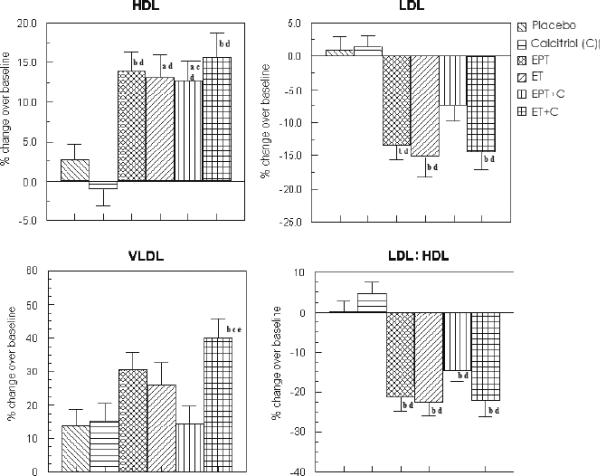
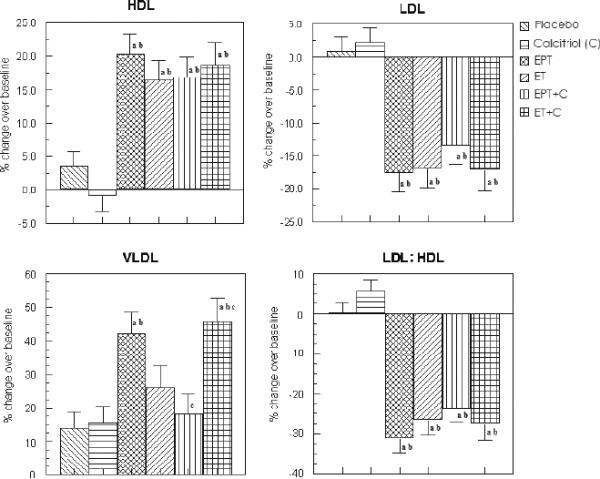
Serum HDL - There was a significantly greater increase in serum HDL in all of the hormone treatment groups as compared to placebo and calcitriol groups; both unadjusted and after adjustment for confounders (Unadjusted change was 6.4 ± 1.3, 7.3 ± 1.5, 6.2 ± 1.3, 8.4 ± 1.6 mg/dl in EPT, ET, EPT+calcitriol and ET+calcitriol groups vs 1.3 ± 0.7 and −1.2 ± 0.8 in placebo and calcitriol respectively, p<0.05 – see Table 3). For adjusted analysis, serum HDL increased by 13.9 ± 2.5 % in EPT, 13.1 ± 2.9 % in ET, 12.7 ± 2.5 % in EPT+calcitriol and 15.6 ± 3.2 % in ET+calcitriol vs 2.66 ± 2.1 % in placebo (p<0.05) and decreased in calcitriol only group (−0.1 ± 2.2 %; p <0.05 – see Figure 4).
FIG. 4.
Percent change in serum HDL, LDL, LDL/HDL ratio, and VLDL in the various treatment groups after adjustment for confounders (intent-to-treat analysis). EPT, estrogen + medroxyprogesterone therapy; ET, estrogen therapy; C, calcitriol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low density lipoprotein. aP < 0.05 as compared with placebo. bP < 0.01 as compared with placebo. cP < 0.05 as compared with C. dP < 0.01 as compared with C. eP < 0.01 as compared with EPT + C.
Serum LDL - There was a significantly greater decrease in serum LDL in all of the hormone treatment groups as compared to placebo and calcitriol groups; both unadjusted and after adjustment for confounders (Unadjusted change was −25 ± 4.2, −26.3 ± 4.7, −11.5 ± 3.5, −19.9 ± 5.5 mg/dl in EPT, ET, EPT+calcitriol and ET+calcitriol groups vs −1.3 ± 2.1 and −0.84 ± 2.3 in placebo and calcitriol respectively, p<0.05 – see Table 3). For adjusted analysis, serum LDL decreased in EPT (−13 ± 2.6 %), ET (−15.5 ± 3 %), EPT+calcitriol (−7.2 ± 2.5 %) and ET+calcitriol (−14.2 ± 3.2 %) vs 0.3 ± 2.1 % increase in placebo (p<0.05) and 1.1 ± 2.2 % increase in calcitriol only group (p <0.05 – see Figure 4). The exception was the adjusted and unadjusted percent change in LDL- C in EPT+calcitriol group, which was not significantly different.
Serum LDL/HDL ratio - The adjusted and unadjusted serum LDL/HDL ratio was also significantly lowered in all the 4 treatment groups receiving the hormones compared to that of placebo and calcitriol groups (Unadjusted change was −0.84 ± 0.12, −0.82 ± 0.11, −0.41 ± 0.09, −0.68 ± 0.13 in EPT, ET, EPT+calcitriol and ET+calcitriol groups vs −0.05 ± 0.06 and 0.05 ± 0.05 in placebo and calcitriol respectively, p<0.05 − see Table 3). For adjusted analysis, the results were similar: −21.1 ± 3.3 % in EPT, −22.3 ± 3.9 % in ET, −14 ± 3.3 % in EPT+calcitriol and −22.5 ± 4.1 % in ET+calcitriol groups vs 0.35 ± 2.7 % and 4.7 ± 2.8 % in placebo and calcitriol respectively, p<0.05 – see Figure 4).
Serum VLDL - The unadjusted serum VLDL of the women receiving ET+calcitriol treatment increased significantly more than placebo (8.3 ± 2.1 mg/dl increase in ET+calcitriol vs 2.7 ± 1.0 in placebo, p <0.05; see Table 3). However, adjusted percent change in VLDL levels was significantly higher in ET+calcitriol group compared to that of women belonging to placebo, calcitriol and EPT+calcitriol treatment groups (40 ± 6.6 % in ET+calcitriol vs 13.7 ± 4.4 %, 15.8 ± 4.6 %, 13.6 ± 5.3 % in placebo, calcitriol and EPT+calcitriol respectively, p< 0.05; see Fig 4).
Complier Analysis
There were no major differences from the intent to treat analysis in terms of serum total cholesterol, serum HDL, serum LDL, LDL/HDL ratio and serum VLDL (Table 3, Figures 5 and 6). Serum triglycerides increased more in EPT and ET+calcitriol groups compared to placebo and calcitriol groups (Unadjusted analysis: Change in serum triglycerides was 69.5 ± 22.3, 45.8 ± 12.0 mg/dl in EPT and ET+calcitriol vs 9.3 ± 6.6 and 16.5 ± 6.0 mg/dl in placebo and calcitriol respectively, p <0.05; see Table 3). For adjusted analysis, change in serum triglycerides was 46.5 ± 6.8 % in EPT, 42.7 ± 7.8 % in ET+calcitriol vs 12.8 ± 5.1 % in placebo and 16.4 ± 5.4 % in calcitriol respectively (p < 0.05, see Figure 5).
FIG. 5.
Percent change in serum cholesterol and serum triglycerides in the various treatment groups after adjustment for confounders (complier analysis). EPT, estrogen + medroxyprogesterone therapy; ET, estrogen therapy; C, calcitriol. aP < 0.01 as compared with placebo. bP < 0.05 as compared with calcitriol. cP < 0.01 as compared with calcitriol.
FIG. 6.
Percent change in serum HDL, LDL, LDL/HDL ratio, and VLDL in the various treatment groups after adjustment for confounders (complier analysis). EPT, estrogen + medroxyprogesterone therapy; ET, estrogen therapy; C, calcitriol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very low density lipoprotein. aP < 0.01 as compared with placebo. bP < 0.01 as compared with C. cP < 0.05 as compared with EPT.
Effect of Estrogen Genotypes
Estrogen receptor alpha gene RFLPs were done in 324 women for both PvuII and XbaI. These were in apparent linkage disequilibrium and the distribution did not differ from that expected under Hardy-Weinberg equilibrium. There were no significant differences in baseline serum lipids according to genotypes.
There was a significant association between PvuII RFLP and the response to treatment in terms of serum cholesterol and VLDL. Genotypes containing the p allele had a greater decrease in serum cholesterol (−19 mg/dl in pp vs 7 mg/dl in Pp, p = 0.06) and increase in serum VLDL (13.3 mg/dl in pp vs −5 mg/dl in PP, p = 0.0006) compared to those having the P allele in the ET plus calcitriol group (Table 4). In terms of serum triglycerides and serum LDL, there was a trend (overall p value < 0.05) for greater decrease in the genotypes containing p allele in the ET plus calcitriol group (−66.8 mg/dl in pp vs −25.8 mg/dl in PP for serum triglycerides and −40.4 mg/dl in pp vs −19.5 mg/dl in PP for serum LDL) (Table 4).
Table 4.
Change in serum lipids from baseline to the end of 3 years according to treatment group and estrogen receptor alpha genotypes
| Treatment | Change in mean serum cholesterol; mg/dl | |||||||
|---|---|---|---|---|---|---|---|---|
| PP | Pp | pp | Overall P-value | XX | Xx | xx | Overall P-value | |
| N | 67 | 164 | 93 | 45 | 150 | 129 | ||
| ET+Calcitriol | −21 | 7ac | −19b | 0.0006 | −5.9 | 3.8 | −19.1 | 0.08 |
| Placebo | −3.8 | 3.4 | 12.1 | 0.13 | −6.4 | 3.8 | 10.2 | 0.06 |
| ET | −8.6 | −16 | −23 | 0.52 | −19.6 | −9.6 | −20 | 0.47 |
| Calcitriol | 1.7 | 1.4 | −5.4 | 0.96 | 17.8 | −5.8 | −2 | 0.14 |
| EPT+Calcitriol | 10 | −11.8 | −6.3 | 0.32 | 36.8 | −8.9d | −8.2e | 0.036 |
| EPT | −17.1 | −13.3 | −8.5 | 0.35 | −22.7 | −8.4 | −17.6 | 0.27 |
| Change in mean serum triglycerides; mg/dl | ||||||||
|---|---|---|---|---|---|---|---|---|
| PP | Pp | pp | Overall P-value | XX | Xx | xx | Overall P-value | |
| ET+Calcitriol | −25.8 | −40.3 | −66.8 | 0.01 | −14.0 | 37.8 | 66.8 | 0.07 |
| Placebo | −11.3 | 8.4 | 19 | 0.43 | 4.3 | 8 | 11.6 | 0.85 |
| ET | 15.4 | 18.9 | 42.3 | 0.99 | 12.4 | 14 | 34.8 | 0.99 |
| Calcitriol | 16.8 | 21.6 | 11.3 | 0.98 | 35.5 | 4.8 | 21 | 0.42 |
| EPT+Calcitriol | 15.6 | 15.8 | 13.6 | 0.93 | 28.7 | 9.1 | 19.9 | 0.78 |
| EPT | 39.9 | 60.9 | 60.2 | 0.94 | 67.2 | 75.9 | 24.8 | 0.09 |
| Change in mean serum VLDL; mg/dl | ||||||||
|---|---|---|---|---|---|---|---|---|
| PP | Pp | pp | Overall P-value | XX | Xx | xx | Overall P-value | |
| ET+Calcitriol | −5fg | 8 | 13.3 | 0.0005 | −2.5 | 7.3 | 13.3 | 0.006 |
| Placebo | 2.9 | 1.7 | 1.7 | 0.34 | 3.8 | 1.7 | 0.6 | 0.34 |
| ET | 3.3 | 1.3 | −1.6 | 0.94 | 2.5 | −1.2 | 1.8 | 0.87 |
| Calcitriol | −1 | 4.2 | 2.2 | 0.96 | 2.5 | 0.3 | 4.1 | 0.93 |
| EPT+Calcitriol | 3 | 3.1 | 2.8 | 0.91 | 5.2 | 1.8 | 4.0 | 0.75 |
| EPT | −0.7 | 5.8 | 12.1 | 0.21 | −2.3 | 8.1 | 5.0 | 0.42 |
| Change in mean serum LDL; mg/dl | ||||||||
|---|---|---|---|---|---|---|---|---|
| PP | Pp | pp | Overall P-value | XX | Xx | xx | Overall P-value | |
| ET+Calcitriol | −19.5 | −9.1 | −40.4 | 0.007 | −19 | −9.2 | −40.4h | 0.01 |
| Placebo | −7.6 | −0.3 | 2.4 | 0.28 | −12.3 | −0.6 | 4.7 | 0.05 |
| ET | −24.3 | −25.3 | −34.5 | 0.65 | −36.1 | −17.9 | −32.4 | 0.63 |
| Calcitriol | 6.3 | 2.3 | −8.2 | 0.69 | 15.1 | 0.6 | −5.2 | 0.36 |
| EPT+Calcitriol | −5.6 | −21.5 | −14.2 | 0.53 | 18.4 | −19.5 | −16.7 | 0.06 |
| EPT | −26.1 | −29.5 | −31.2 | 0.75 | −37.8 | −25.6 | −33.8 | 0.50 |
| Change in mean serum HDL; mg/dl | ||||||||
|---|---|---|---|---|---|---|---|---|
| PP | Pp | pp | Overall P-value | XX | Xx | xx | Overall P-value | |
| ET+Calcitriol | 3.8 | 10.7 | 8 | 0.31 | 15.5 | 8.1 | 8.0 | 0.54 |
| Placebo | 1.4 | 1.5 | 2 | 0.68 | 1.6 | 2.6 | 0.5 | 0.9 |
| ET | 12.3 | 8 | 5.2 | 0.08 | 14 | 7.9 | 6.8 | 0.046 |
| Calcitriol | −3.2 | −2.6 | 0.65 | 0.58 | −0.8 | −2.6 | −0.9 | 0.48 |
| EPT+Calcitriol | 12.7 | 6.6 | 5.2 | 0.13 | 13.2 | 8.9 | 4.5 | 0.13 |
| EPT | 4.6 | 8.7 | 10.6 | 0.54 | 3.7 | 7.5 | 11.2 | 0.28 |
Abbreviation: EPT, estrogen plus medroxyprogesterone therapy; ET, estrogen therapy
p = 0.001 for PP as compared to Pp
p = 0.057 for PP as compared to pp
p = 0.06 for Pp as compared to pp
p = 0.03 for XX as compared to Xx
p = 0.07 for XX as compared to xx
p = 0.01 for PP as compared to Pp
= 0.0006 for PP as compared to pp
p = 0.01 for Xx as compared to xx
XbaI RFLP also showed a significant association in the response to treatment in terms of serum cholesterol and serum LDL. Genotypes including the x allele had a greater decrease in serum cholesterol in the EPT plus calcitriol group at the end of 3 years compared to X allele alone (−8.9 mg/dl in Xx vs 36.8 mg/dl in XX, p = 0.03) and a greater decrease in serum LDL in ET plus calcitriol group (−40.4 mg/dl in xx vs 9.2 mg/dl in Xx, p = 0.01) (Table 4). In terms of serum VLDL, there was a trend (overall p value < 0.05) for greater increase in xx vs XX (13.3 mg/dl vs −2.5 mg/dl respectively). There were no significant differences in other serum lipids in terms of response to treatment according to genotypes.
Discussion
The results of this study show that hormone therapy (estrogen with or without medroxyprogesterone acetate) favorably lowers serum LDL and increases serum HDL but also increases serum triglycerides in healthy elderly post-menopausal women, and further indicate that the effect of treatment (HT and/or calcitriol) is modified by estrogen receptor single nucleotide polymorphisms (SNPs). This is the first study of its kind looking at the interaction between estrogen receptor SNPs, hormone therapy, calcitriol and treatment response in terms of serum lipids in a longitudinal follow up.
The positive effects of estrogen therapy on serum lipids have been reported in previous studies. In a randomized, double blind trial in younger postmenopausal women, treatment with ET resulted in a significant dose-dependent reduction in LDL-cholesterol and increase in serum HDL over the dose range 0.3–0.625 mg/day. At all three doses of ET studied (0.3, 0.45, and 0.65 mg/day), LDL-cholesterol was significantly reduced and HDL was increased relative to both baseline and placebo treatment (49). In addition, there were favorable effects on hemostatic factors including fibrinogen and plasminogen activator inhibitor −1. Similar findings were reported in another randomized double blind cross-over trial (50).
There has been interest in the cardiovascular effect profile of HT in postmenopausal women. At the center of debate are two large studies, one observational – Nurses Health Study (NHS) and the other a randomized controlled trial – Women's Health Initiative (WHI) (31,51).
NHS was a large observational study involving 121,700 female nurses 30 to 55 years of age at the time of enrollment in 1976 (18, 52). In the latest analysis involving 70,533 postmenopausal women with no previous cardiovascular disease followed for up to 20 years, there was a significant reduction in major CHD (nonfatal myocardial infarction and coronary death) after adjustment for age, body mass index, history of diabetes, hypertension, high cholesterol level, age at menopause, smoking status and parental history of premature heart disease (Relative Risk, RR = 0.61, 95% Confidence Limits (CL) = 0.52–0.71 for current users and RR = 0.82, CL = 0.72–0.94 for past users of hormone therapy). There was a small difference in risk reduction based on the type of hormone therapy (RR = 0.55, CL = 0.45–0.68 for women taking oral conjugated estrogen alone and RR = 0.64, CL = 0.49–0.85 for women taking estrogen plus progestin) (52).
However, the initial results of WHI trial showed that estrogen plus progestin therapy increased the risk of coronary heart disease (CHD) (nonfatal myocardial infarction [MI] and CHD death), stroke and venous thromboembolism (31). These results were later modified based on more data and after central adjudication – estrogen plus progestin therapy still significantly increased the risk of CHD (HR = 1.24, nominal 95% confidence interval (nCI) = 1.00–1.54, adjusted confidence interval (aCI) = 0.97–1.60) and stroke (HR = 1.31, nCI = 1.02–1.68, aCI = 0.93–1.84) (53). The absolute risk was still very low – the findings suggest that over one year, one per 1600 users women taking EPT compared with placebo developed 6 more CHD events. There was no increase in the CHD death or overall mortality, congestive heart failure and a composite of CHD, revascularization or angina. Therefore, although the risk for MI increased the risk for angina was not increased. But there were other adverse events- invasive breast cancers, strokes, VTEs, PEs and benefits- less colorectal cancers, hip fractures and total fractures that increased the risk benefit of HT. In the ET arm of the WHI trial, there was no significant effect on CHD although risk of stroke and VTE was elevated however; none of the cardiovascular events were significant after adjustment (51). There was no increase in breast cancer on ET.
In a subgroup analysis to determine specific populations at increased risk of CHD, no significant interaction between age or years since menopause and treatment was observed (P = 0.36 for the age analysis and P = 0.33 for years-since-menopause analysis), although there was a numerical trend towards a higher risk with EPT more years after menopause (53). Although the tests for interaction were non-significant, women not using statins or aspirin (≥80 mg/d) were at increased risk of CHD. In addition, women with baseline serum LDL cholesterol levels (> 155 mg/dl) had increased risk of CHD when started on EPT compared to those with lower LDL-C. Age was also a determinant of CHD risk. The average age of women started on HT was 63 years. BMI was also a possible risk because the average BMI was 28.5 kg/m2 (53). In the Nurses Health study the risk of CHD increased with increasing BMI; the risk was increased 356% in women with a BMI of ≥ 29 kg/m2 compared to women with a BMI of < 21 kg/m2 (54).
In a randomized double blind trial involving 222 post-menopausal women ≥ 45 years old without pre-existing cardiovascular disease and serum LDL ≥ 130 mg/dl, the average rate of progression of subclinical atherosclerosis measured by change in intimal-media thickness of the right distal common carotid artery was slower in women taking ET with 17beta-estradiol than in women taking placebo (55). However, the results of other studies are less convincing (30).
These apparent differences in observational and intervention studies could be partly explained by findings of various animal studies carried out by Clarkson et al and others (56,57). Specifically, these studies demonstrate that the extent of subclinical atherosclerosis at baseline appears to be a significant modulator of the atheroprotective effects of HT and that estrogen inhibits the initiation of fatty streaks in vasculature but does not inhibit the progression of established lesions. This “timing hypothesis” might play an important role in determining the effects of estrogen therapy in various populations. In a meta-analysis of 30 trials of hormone therapy involving 26708 women with mean follow of 4.5 years, hormone replacement reduced mortality in the younger age group < 60 years, (OR 0.61; CI, 0.39 to 0.95), but not in the older age group > 60 years, (OR 1.03; CI, 0.90 to 1.18) (58).
Estrogen receptor SNPs appear to affect the response of serum lipids to HT treatment. The genotypes that included the x and p alleles showing significantly greater response in terms of serum cholesterol, serum LDL and serum VLDL compared to X and P alleles respectively. We have shown previously from the same dataset that PvuII and XbaI SNPs interact with treatment response to hormone therapy in terms of bone loss measured by changes in bone mineral density and bone markers (38).
Only a few studies have looked at the interaction between ERα genotypes and the response in serum lipids to hormone therapy. In fact, the first study done by Herrington et al (37) found a significant association between PvuII SNPs and treatment response to hormone therapy in terms of serum HDL with greater increase in serum HDL in women with PP genotype as compared to Pp or pp. Other small studies have found similar results (44,45).
The mechanism by which ERα-SNPs lead to changes in serum lipids is not clear. Initial reports had suggested an alteration in protein expression secondary to changes in mRNA splicing of PvuII gene (59). As these are present in the non-coding region (activation domain) of gene, the structure of receptor protein should not be altered. Instead there is growing evidence that these SNPs affect the binding of transcription factors like myb to ERα gene leading to decreased expression of ERα receptor. Herrington el al (60) found that the presence of P allele resulted in > 10-fold increase in a downstream reporter activity as compared to only 2.5-fold increase in activity with p allele; both sets of gene stimulated by transcription factor myb. However, whether these SNPs affect the quality or quantity of estrogen receptor alpha gene mRNA transcripts or protein expression remains to be established. Schuit et al. (61) demonstrated that these SNPs (p and x alleles) are associated with lower serum estradiol levels in postmenopausal women. They hypothesized that these SNPs affected the expression of one of the subtypes of 17β-hydroxysteroid dehydrogenase enzyme that catalyzes the transformation of estrone into estradiol. It might also be possible that these SNPs are in linkage disequilibrium with another SNP in ERα gene that affects the expression of ERα.
In a prospective study involving 1739 men and women from Framingham cohort followed for 27 years, the investigators found a significant association between ERα SNPs and MI risk with 3 times greater risk of MI (95% CI = 1.7–5.2) in men with PP genotype as compared to Pp or pp (41). Similar results were found for stroke in 2709 males of the Second Northwick Park Heart study with increased risk in PP genotype independent of established cardiovascular risk factors including diabetes (43). In an autopsy study from Finland, the investigators found that men aged 53 years or over with P/p and P/P genotype had two to five-fold larger complicated lesions and odds ratio of 6.2 and 10.6 respectively for coronary thrombosis than men with the p/p genotype after adjusting for age and body mass index (62). As noted, all these studies found worse cardiovascular outcomes in men associated with P allele. In contrast, the results of Rotterdam study in 3791 postmenopausal women followed for 8–10 years suggested that p and x alleles were associated with increased MI risk (42). These apparent opposing results in men and women can be explained by the fact that there is no cessation of hypothalamic-pituitary axis in men leading to sufficiently high serum estradiol levels leading to protective effects of p allele in men (42). However in women, the low estradiol levels after menopause coupled with the presence of p allele reducing the expression of ERα receptor gene, leads to worse clinical consequences in terms of cardiovascular endpoints.
There is also the potential effect of calcitriol on the expression of estrogen receptor alpha gene expression. Many studies in human cells have shown that calcitriol down regulates the expression of estrogen receptor alpha gene with major impact on gene transcription (63, 64). These studies also demonstrate a decrease in the bio-responses to estrogen with calcitriol. Although these findings were predominantly found in breast cancer models, it could occur in other tissues also. The results of our study support these findings by showing a more favorable response in serum lipids to hormone therapy and calcitriol in women with p allele as compared to P allele. In women with p allele, ERα receptor gene is already under expressed and calcitriol has little effect on its expression and treatment with hormone therapy infact leads to better response in serum lipids as is found in men (42).
There are some limitations in our study. This is a relatively small sample size for comparing the effects of estrogen receptor SNPs on serum lipids although it is still one of the largest studies reporting the effect of interaction between estrogen receptor alpha SNPs and hormone therapy on serum lipids. Moreover, the genotypes were done by restriction endonuclease digestion and not by Taqman probes that are more accurate.
Although these findings are in older postmenopausal women, if applicable to younger postmenopausal women could partially explain the cardio-protective effect of estrogen when started early after menopause. The results of this study especially the genotype analysis are preliminary in nature and further large trials need to be carried out to confirm our findings in both younger and older postmenopausal women and also the overall impact on event rate in terms of MI and other outcomes.
Acknowledgments
Financial Support This study was supported by NIH funds (Grants UO1-AG10373 and AG10358)
JCG has received consulting fees and honoraria from Wyeth-Pfizer and Roche. He has also served on the Advisory Council and as a speaker for Pfizer and Roche.
Footnotes
Disclosures: AJS and XF have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gordon T, Kannel WB, Hjorland MC, McNamara PM. Menopause and coronory heart disease. The Framingham study. Annals of Intern Med. 1978;89:157–161. doi: 10.7326/0003-4819-89-2-157. [DOI] [PubMed] [Google Scholar]
- 2.Witteman JC, Grobbee DE, Kok FS, Hofman A, Valkenburg HA. Increased risk of atherosclerosis in women after the menopause. Br Med J. 1989;298:642–44. doi: 10.1136/bmj.298.6674.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. J Am Med Assoc. 1991;265:1861–1867. [PubMed] [Google Scholar]
- 4.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann.Intern.Med. 1976 Oct;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. Menopause and the risk of coronary heart disease in women. N.Engl.J.Med. 1987 Apr 30;316(18):1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 6.Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev.Med. 1991 Jan;20(1):47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Wenger NK, Grady D, Mosca L, Collins P, Kornitzer M, et al. Hormone and nonhormone therapy for the maintenance of postmenopausal health: the need for randomized controlled trials of estrogen and raloxifene. J.Womens Health. 1998 Sep;7(7):839–847. doi: 10.1089/jwh.1998.7.839. [DOI] [PubMed] [Google Scholar]
- 8.Bush TL, Barrett-Connor E, Cowan LD, Criqui MH, Wallace RB, Suchndran CM, et al. Cardiovascular mortality and noncontraceptive use of estrogen in women: Results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 9.Walsh BW, Schiff I, Rosner B, Greenberg L, Ravnikar V, Sacks FM. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N Eng J Med. 1991;325:1196–204. doi: 10.1056/NEJM199110243251702. [DOI] [PubMed] [Google Scholar]
- 10.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 11.The writing group for the PEPI Trial Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women: The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273:199–208. [PubMed] [Google Scholar]
- 12.Lobol RA, Pickar JH, Wild RA, Walsh B, Hirvonen E, For the Menopause Study Group Metabolic inpact of adding medroxy-progesterone acetate to conjugated estrogen therapy in postmenopausal women. Obstet Gynecol. 1994;84:987–95. [PubMed] [Google Scholar]
- 13.Mendelson ME, Karsas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 14.Luciano AA, Miller BE, Schoenenfeld MJ, Schaser RJ, Ogen/Provera Study Group Effects of estrone sulfate alone or with medroxyprogesterone acetate on serum lipoprotein levels in postmenopausal women. Obstet.Gynecol. 2001 Jan;97(1):101–108. doi: 10.1016/s0029-7844(00)01081-4. [DOI] [PubMed] [Google Scholar]
- 15.Meschia M, Bruschi F, Soma M, Amicarelli F, Paoletti R, Crosignani P. Effects of oral and transdermal hormone replacement therapy on lipoprotein(A) and lipids: a randomized controlled trial. Menopause. 1998 Fall;5(3):157–162. [PubMed] [Google Scholar]
- 16.Sendag F, Karadadas N, Ozsener S, Bilgin O. Effects of sequential combined transdermal and oral hormone replacement therapies on serum lipid and lipoproteins in postmenopausal women. Arch.Gynecol.Obstet. 2002 Jan;266(1):38–43. doi: 10.1007/pl00007497. [DOI] [PubMed] [Google Scholar]
- 17.Zegura B, Guzic-Salobir B, Sebestjen M, Keber I. The effect of various menopausal hormone therapies on markers of inflammation, coagulation, fibrinolysis, lipids, and lipoproteins in healthy postmenopausal women. Menopause. 2006 Jul–Aug;13(4):643–650. doi: 10.1097/01.gme.0000198485.70703.7a. [DOI] [PubMed] [Google Scholar]
- 18.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N.Engl.J.Med. 1996 Aug 15;335(7):453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 19.Psaty BM, HeckbET SR, Atkins D, Lemaitre R, Koepsell TD, Wahl PW, et al. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch.Intern.Med. 1994 Jun 27;154(12):1333–1339. [PubMed] [Google Scholar]
- 20.Barrett-Connor E, Slone S, Greendale G, Kritz-Silverstein D, Espeland M, Johnson SR, et al. The Postmenopausal Estrogen/Progestin Interventions Study: primary outcomes in adherent women. Maturitas. 1997 Jul;27(3):261–274. doi: 10.1016/s0378-5122(97)00041-8. [DOI] [PubMed] [Google Scholar]
- 21.Seeger H, Wallwiener D, Mueck AO. Effect of medroxyprogesterone acetate and norethisterone on serum-stimulated and estradiol-inhibited proliferation of human coronary artery smooth muscle cells. Menopause. 2001 Jan–Feb;8(1):5–9. doi: 10.1097/00042192-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Sarrel PM. How progestins compromise the Cardioprotective Effects of Estrogens. Menopause. 1995;2(4):187–190. [Google Scholar]
- 23.Kuhl H. Effects of progestogens on haemostasis. Maturitas. 1996 May;24(1–2):1–19. doi: 10.1016/0378-5122(96)00994-2. [DOI] [PubMed] [Google Scholar]
- 24.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler.Thromb.Vasc.Biol. 1997 Jan;17(1):217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 25.Williams JK, Honore EK, Washburn SA, Clarkson TB. Effects of hormone replacement therapy on reactivity of atherosclerotic coronary arteries in cynomolgus monkeys. J.Am.Coll.Cardiol. 1994 Dec;24(7):1757–1761. doi: 10.1016/0735-1097(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 26.Williams JK, Anthony MS, Honore EK, Herrington DM, Morgan TM, Register TC, et al. Regression of atherosclerosis in female monkeys. Arterioscler.Thromb.Vasc.Biol. 1995 Jul;15(7):827–836. doi: 10.1161/01.atv.15.7.827. [DOI] [PubMed] [Google Scholar]
- 27.Williams JK, Delansorne R, Paris J. Estrogens, progestins, and coronary artery reactivity in atherosclerotic monkeys. J.Steroid Biochem.Mol.Biol. 1998 Apr;65(1–6):219–224. doi: 10.1016/s0960-0760(98)00020-x. [DOI] [PubMed] [Google Scholar]
- 28.Sidney S, Petitti DB, Quesenberry CP., Jr. Myocardial infarction and the use of estrogen and estrogen-progestogen in postmenopausal women. Ann.Intern.Med. 1997 Oct 1;127(7):501–508. doi: 10.7326/0003-4819-127-7-199710010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group JAMA. 1998 Aug 19;280(7):605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 30.Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N.Engl.J.Med. 2000 Aug 24;343(8):522–529. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 31.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 32.Kovanen PT, Brown MS, Goldstein JC. Increased binding of low density lipoproteins to liver membranes from rats treated with 17 alpha ethinyl estradiol. J Biol Chem. 1979;254:11367–73. [PubMed] [Google Scholar]
- 33.Wild RA, Taylor EL, Knehans A. The gynecologist and the prevention of cardiovascular disease. Am J Obstet Gynecol. 1995;172:1–13. doi: 10.1016/0002-9378(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 34.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995 Dec 15;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acevedo ML, Kraus WL. Transcriptional activation by nuclear receptors. Essays Biochem. 2004;40:73–88. doi: 10.1042/bse0400073. [DOI] [PubMed] [Google Scholar]
- 36.Castagnoli A, Maestri I, Bernardi F, Del Senno L. PvuII RFLP inside the human estrogen receptor gene. Nucleic Acids Res. 1987 Jan 26;15(2):866. doi: 10.1093/nar/15.2.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrington DM, Howard TD, Hawkins GA, Reboussin DM, Xu J, Zheng SL, et al. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N.Engl.J.Med. 2002 Mar 28;346(13):967–974. doi: 10.1056/NEJMoa012952. [DOI] [PubMed] [Google Scholar]
- 38.Rapuri PB, Gallagher JC, Knezetic JA, Haynatzka V. Estrogen receptor alpha gene polymorphisms are associated with changes in bone remodeling markers and treatment response to estrogen. Maturitas. 2006 Mar 20;53(4):371–379. doi: 10.1016/j.maturitas.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Ioannidis JP, Ralston SH, Bennett ST, Brandi ML, Grinberg D, Karassa FB, et al. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. JAMA. 2004 Nov 3;292(17):2105–14. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- 40.van Meurs JB, Schuit SC, Weel AE, van der Klift M, Bergink AP, Arp PP, et al. Association of 5' estrogen receptor alpha gene polymorphisms with bone mineral density, vertebral bone area and fracture risk. Hum Mol Genet. 2003 Jul 15;12(14):1745–54. doi: 10.1093/hmg/ddg176. [DOI] [PubMed] [Google Scholar]
- 41.Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, et al. Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA. 2003 Nov 5;290(17):2263–70. doi: 10.1001/jama.290.17.2263. [DOI] [PubMed] [Google Scholar]
- 42.Schuit SC, Oei HH, Witteman JC, Geurts van Kessel CH, van Meurs JB, Nijhuis RL, et al. Estrogen receptor alpha gene polymorphisms and risk of myocardial infarction. JAMA. 2004 Jun 23;291(24):2969–77. doi: 10.1001/jama.291.24.2969. [DOI] [PubMed] [Google Scholar]
- 43.Shearman AM, Cooper JA, Kotwinski PJ, Humphries SE, Mendelsohn ME, Housman DE, et al. Estrogen receptor alpha gene variation and the risk of stroke. Stroke. 2005 Oct;36(10):2281–2. doi: 10.1161/01.STR.0000181088.76518.ec. [DOI] [PubMed] [Google Scholar]
- 44.Nogueira-de-Souza NC, Guerreiro da Silva ID, Carvalho CV, Pulchinelli A, Haidar MA, Baracat EC, et al. Effect of estrogen receptor-alpha (ESR1) gene polymorphism on high density lipoprotein levels in response to hormone replacement therapy. Braz.J.Med.Biol.Res. 2009 Dec;42(12):1138–1142. doi: 10.1590/s0100-879x2009001200003. [DOI] [PubMed] [Google Scholar]
- 45.Lamon-Fava S, Asztalos BF, Howard TD, Reboussin DM, Horvath KV, Schaefer EJ, et al. Association of polymorphisms in genes involved in lipoprotein metabolism with plasma concentrations of remnant lipoproteins and HDL subpopulations before and after hormone therapy in postmenopausal women. Clin Endocrinol (Oxf) 2010 Feb;72(2):169–75. doi: 10.1111/j.1365-2265.2009.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallagher JC, Fowler SE, Detter JR, Sherman SS. Combination treatment with estrogen and calcitriol in the prevention of age-related bone loss. J.Clin.Endocrinol.Metab. 2001 Aug;86(8):3618–3628. doi: 10.1210/jcem.86.8.7703. [DOI] [PubMed] [Google Scholar]
- 47.Allain CC, et al. Enzymatic determination of Total cholesterol in serum. Clin Chem. 1974;20:470. [PubMed] [Google Scholar]
- 48.Spayd R, et al. Multilayer film elements for clinical analysis. Clin Chem. 1978;24:1348–1350. [PubMed] [Google Scholar]
- 49.Lobo RA, Bush T, Carr BR, Pickar JH. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on plasma lipids and lipoproteins, coagulation factors, and carbohydrate metabolism. FertilETil.Steril. 2001 Jul;76(1):13–24. doi: 10.1016/s0015-0282(01)01829-5. [DOI] [PubMed] [Google Scholar]
- 50.Koh KK, Shin MS, Sakuma I, Ahn JY, Jin DK, Kim HS, et al. Effects of conventional or lower doses of hormone replacement therapy in postmenopausal women. Arterioscler.Thromb.Vasc.Biol. 2004 Aug;24(8):1516–1521. doi: 10.1161/01.ATV.0000133683.65877.bc. [DOI] [PubMed] [Google Scholar]
- 51.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 52.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann.Intern.Med. 2000 Dec 19;133(12):933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 53.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, et al. Estrogen plus progestin and the risk of coronary heart disease. N.Engl.J.Med. 2003 Aug 7;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 54.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, et al. Weight, weight change, and coronary heart disease in women. Risk within the 'normal' weight range. JAMA. 1995 Feb 8;273(6):461–465. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 55.Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, et al. Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann.Intern.Med. 2001 Dec 4;135(11):939–953. doi: 10.7326/0003-4819-135-11-200112040-00005. [DOI] [PubMed] [Google Scholar]
- 56.Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007 May–Jun;14(3 Pt 1):373–384. doi: 10.1097/GME.0b013e31803c764d. [DOI] [PubMed] [Google Scholar]
- 57.Rosenfeld ME, Kauser K, Martin-McNulty B, Polinsky P, Schwartz SM, Rubanyi GM. Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis. 2002 Oct;164(2):251–259. doi: 10.1016/s0021-9150(02)00178-8. [DOI] [PubMed] [Google Scholar]
- 58.Salpeter SR, Walsh JM, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women: a meta-analysis. J.Gen.Intern.Med. 2004 Jul;19(7):791–804. doi: 10.1111/j.1525-1497.2004.30281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill SM, Fuqua SA, Chamness GC, Greene GL, McGuire WL. Estrogen receptor expression in human breast cancer associated with an estrogen receptor gene restriction fragment length polymorphism. Cancer Res. 1989 Jan 1;49(1):145–8. [PubMed] [Google Scholar]
- 60.Herrington DM, Howard TD, Brosnihan KB, McDonnell DP, Li X, Hawkins GA, et al. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E-selectin but not C-reactive protein. Circulation. 2002 Apr 23;105(16):1879–82. doi: 10.1161/01.cir.0000016173.98826.88. [DOI] [PubMed] [Google Scholar]
- 61.Schuit SC, de Jong FH, Stolk L, Koek WN, van Meurs JB, Schoofs MW, et al. Estrogen receptor alpha gene polymorphisms are associated with estradiol levels in postmenopausal women. Eur J Endocrinol. 2005 Aug;153(2):327–34. doi: 10.1530/eje.1.01973. [DOI] [PubMed] [Google Scholar]
- 62.Lehtimaki T, Kunnas TA, Mattila KM, Perola M, Penttila A, Koivula T, et al. Coronary artery wall atherosclerosis in relation to the estrogen receptor 1 gene polymorphism: An autopsy study. J Mol Med. 2002 Mar;80(3):176–80. doi: 10.1007/s00109-001-0311-5. [DOI] [PubMed] [Google Scholar]
- 63.Swami S, Krishnan AV, Feldman D. 1alpha,25-dihydroxyvitamin D3 down-regulates estrogen receptor abundance and suppresses estrogen actions in MCF-7 human breast cancer cells. Clin Cancer Res. 2000 Aug;6(8):3371–9. [PubMed] [Google Scholar]
- 64.Krishnan AV, Swami S, Feldman D. Vitamin D and breast cancer: Inhibition of estrogen synthesis and signaling. J Steroid Biochem Mol Biol. 2010 Jul;121(1–2):343–8. doi: 10.1016/j.jsbmb.2010.02.009. [DOI] [PubMed] [Google Scholar]