Abstract
The goal of this study is to determine whether treatment with methylselenocysteine (MSC) results in differential uptake of irinotecan and its active metabolite (SN-38) between tumors of head and neck squamous cell carcinomas and normal tissue. The in vivo synergy between MSC and irinotecan is influenced by treatment schedule and associated with enhancement of tumor vessel maturation, intra-tumor concentration of SN-38 and apoptotic death of tumor cells. Normal tissue drug concentrations of were not impacted by selenium treatment. The finding is of clinical relevance for enabling the delivery of higher doses of irinotecan to reverse tumor resistance, recurrence and ultimately enhancing cure rates.
Keywords: Methylselenocysteine, selenium, FaDu, A253, irinotecan
1. Introduction
Irinotecan is a pro-drug that is activated by carboxylesterases into the active metabolite, SN-38 (7-ethyl-10-hydroxyl-camptothecin) [1]. Irinotecan treats multiple solid tumors [2] by targeting topoisomerase I and resulting in double-stranded DNA breaks [3]. Methylselenocysteine (MSC) is a selenium containing compound that is activated by β-lyase into the active metabolite methylselenol [4]. MSC has modest partial response of anti-tumor activity when given alone. However, MSC enhances the anti-tumor activity (cure rates) of irinotecan against FaDu and A253 xenografts [5].
FaDu and A253, head and neck squamous cell carcinomas (HNSCC), were previously characterized [6]. FaDu is poorly differentiated and p53 mutant. A253 is well differentiated and null p53. Both untreated tumors have similar level of carboxylesterase-2, irinotecan activating enzyme [6].
In nude mice bearing human FaDu xenografts, sequential combination treatment of MSC (0.2mg/d × 28) and irinotecan (100mg/kg/wk × 4) increased the complete response (CR) rate from 30% after irinotecan alone to 100% after the combination treatment [5]. The CR rate increased from 10% after irinotecan alone to 60% after the combination treatment in mice bearing A253 xenografts. Treatment with MSC alone resulted in 30% partial response (PR) but 0% CR. Optimal therapeutic selectivity is achieved only when MSC is administered at least 7 days prior to irinotecan and continued throughout treatment schedule [5].
In our previously published study [7], we demonstrated that the enhanced CR rates of HNSCC xenografts after combination treatment of MSC and irinotecan were due to multi-factorial alterations. Collectively, the shared changes in both xenografts after the first course treatment of irinotecan alone or in combination translated into increased intra-tumor SN-38 concentration [7]. Based on these findings, we designed this study to address the following questions: (i) Does MSC administered concurrently with irinotecan alter plasma and intra-tumor concentrations of irinotecan and SN-38? (ii) What is the effect of adding MSC on concentrations of irinotecan and SN-38 after the second course of irinotecan treatment? (iii) What is the effect of adding MSC on normal tissue concentrations of irinotecan and SN-38? (iv) What is the effect of adding MSC on tumor microvessel maturation? (v) What is the effect of adding MSC on apoptotic cell death?
The answers to these questions are critical to confirm whether giving MSC sequentially alters SN-38 intra-tumor concentration but does not affect its normal tissue concentration.
In this study using our preclinical xenografts models, we confirmed that the increase in vessel maturation and drug concentration needed at least 15 days to be achieved, which resulted in an increase in apoptotic cell death. Thus, the increase of anti-tumor selectivity and activity by adding MSC might be due in part to the increase of the intra-tumor drug availability, thus SN-38 binds more to its target and produces more cell death.
2. Materials and Methods
2.1. Tumors transplantation
FaDu and A253 cell lines were purchased from American Type Culture Collection (Rockville, MD), and maintained in RPMI 1640 supplemented with 10% fetal bovine serum (GIBCO BRL, Life Technologies, Grand Island, NY). Cell lines were tested every two months for mycoplasma using Mycoplasma T. C. Rapid Detection System (Gen-Probe Inc., San Diego, CA). Human HNSCC xenografts were initially established by implanting 106 cultured cells subcutaneously into female athymic nude mice (nu/nu) from Harlan Sprague-Dawley, Inc. (Indianapolis, IN). Xenografts were passed several generations by transplanting ~ 50mg non-necrotic tumor tissues before treatment. FaDu and A253 tumors were implanted bilaterally to eliminate host variability. Mice were housed 5 per cage under specific pathogen-free conditions following an institutionally-approved protocol.
2.2. Drugs and treatments
Irinotecan was purchased from Pfizer Pharmacia Upjohn Corporation (New York, NY), as a ready-to-use clinical formulation solution. MSC was supplied by Sigma (St. Louis, MO), and dissolved in 0.9% NaCl. Irinotecan (100mg/kg, intravenously) was given alone or in combination with MSC (0.2mg/d/mouse, orally). Two schedules were used, concurrent and sequential. In concurrent combination, MSC and irinotecan were given for 2h (figure 1A). In sequential combination, MSC was given daily for a total of 7 days prior to irinotecan (first course treatment, figure 2A) or daily for a total of 14 days (second course treatment, figure 3A).
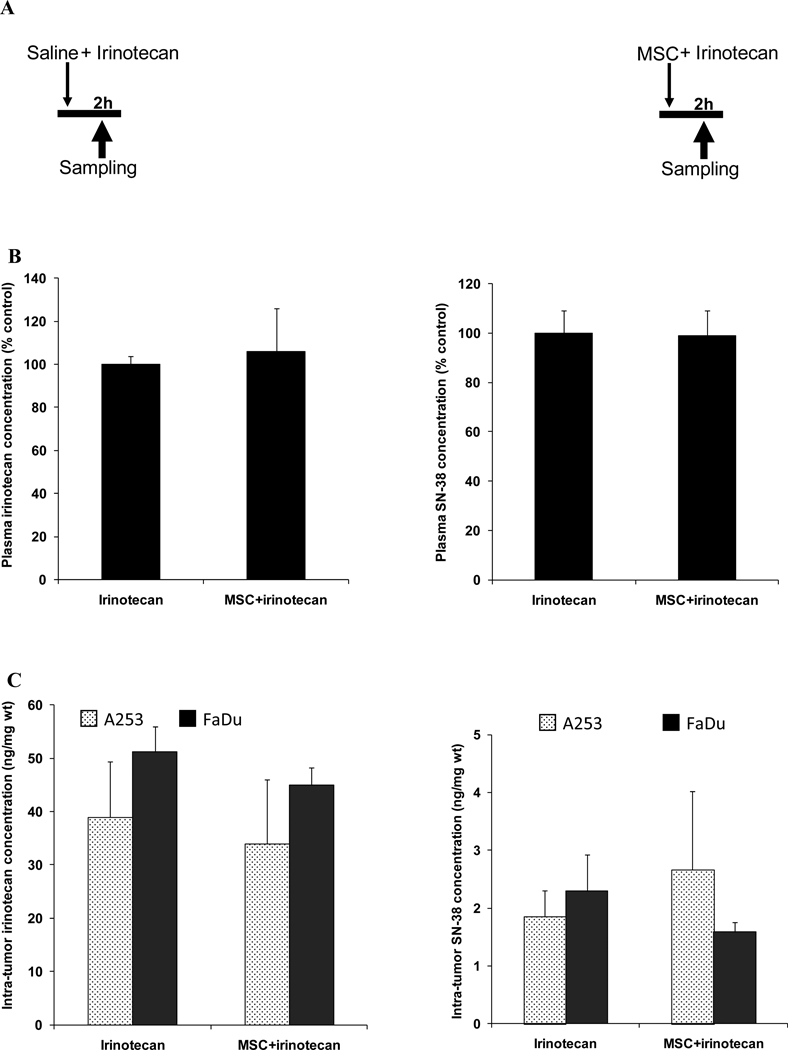
Figure 1. Drug concentrations after the concurrent treatment schedule.
Panel A is the treatment schedule with irinotecan alone or in concurrent with MSC.
Panel B is plasma irinotecan or SN-38 concentrations 2h after treatment with 100mg/kg irinotecan alone or after the concurrent combination treatment with MSC (0.2 mg/d) and irinotecan.
Panel C is the irinotecan or SN-38 concentration in A253 or FaDu 2h after treatment with 100mg/kg irinotecan alone or in concurrent combination with MSC (0.2 mg/d).
The figure shows no significant difference in drug concentration after the concurrent combination treatment when compared with irinotecan alone.
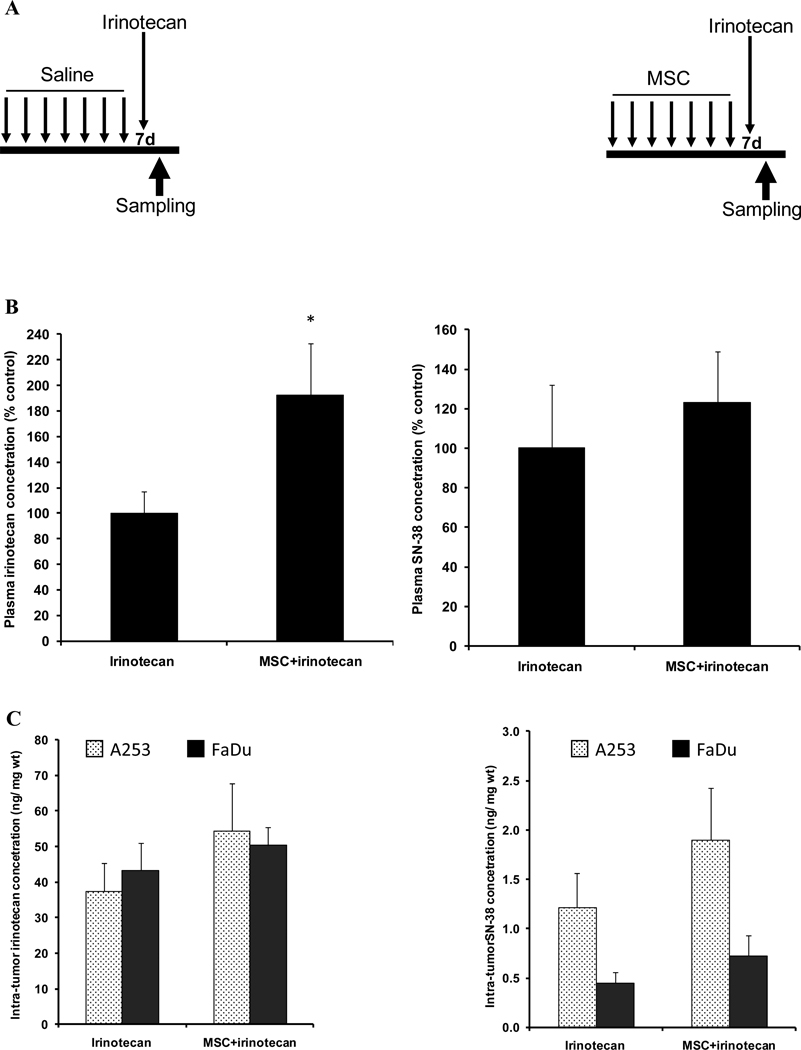
Figure 2. Drug concentrations after the first course treatment.
Panel A is the sequential schedule when samples were analyzed 2h after the first course of treatment (7d) with irinotecan alone or in combination.
Panel B shows plasma irinotecan or SN-38 concentration 2h after treatment with 100mg/kg irinotecan alone or after the first course sequential combination treatment with MSC (0.2 mg/d) and irinotecan.
Panel C shows irinotecan or SN-38 concentrations in A253 or FaDu 2h after treatment with 100mg/kg irinotecan alone or first course sequential combination treatment with MSC (0.2 mg/d) and irinotecan.
When compared with irinotecan alone, the data show a trend of increase of intra-tumor SN-38 concentration (panel C) but it was not significant when compared with irinotecan alone. The only significant change in drug concentrations was an increase in plasma irinotecan concentration in panel B (p<0.05). * denotes p < 0.05 when compared to irinotecan alone.
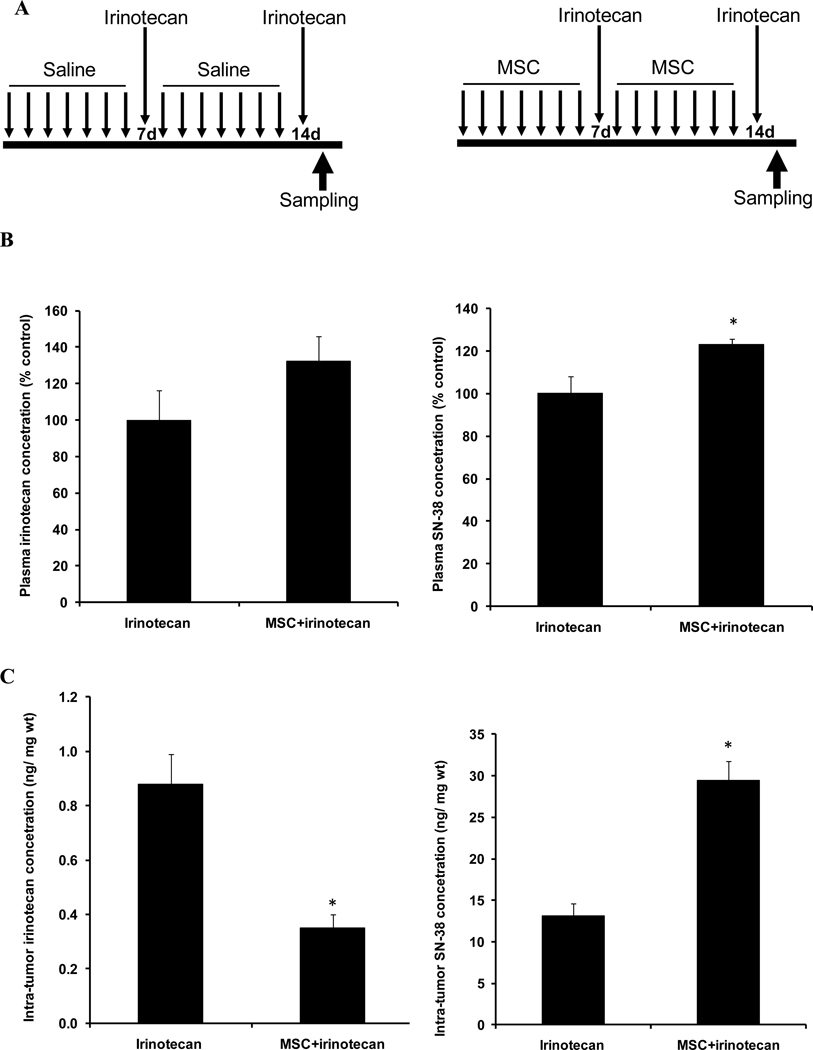
Figure 3. Drug concentrations after the second course treatment.
Panel A is the sequential schedule when samples were analyzed 2h after the second course of treatment (14d) with irinotecan alone or in combination.
Panel B shows plasma irinotecan or SN-38 concentration 2h after treatment with 100mg/kg irinotecan alone or after the second course sequential combination treatment with MSC (0.2 mg/d) and irinotecan.
Panel C is irinotecan concentration or SN-38 concentration in FaDu tumor 2h after treatment with second course of irinotecan alone or in combination with MSC.
After the combination treatment, significant increase (p<0.05) in SN-38 concentrations was observed when compared with irinotecan alone (panel B and C). In addition, irinotecan concentration in FaDu tumors was significantly decreased (p<0.05) after the combination treatment when compared with irinotecan alone (panel C). * denotes p < 0.05 when compared to irinotecan alone.
Treatments were administered to 5 mice per group per experiment and every experiment was repeated twice.
2.3. Tissue preparation for drug measurements using high performance liquid chromatography (HPLC)
Tumors, blood and normal tissue samples were collected 2h after irinotecan alone or in combination. Plasma was obtained at 4°C immediately following the blood collection and subjected to the same solvent extraction procedure and HPLC. Tissue samples were immediately incubated with ice-cold 5ml methanol and acetonitrile (1:1). Samples were homogenized using a Polytron tissue homogenizer (Brinkmann Instruments, Westbury, NY). After centrifugation of the homogenate, the supernatant was evaporated to dry, reconstituted to mobile phase and subjected to HPLC. Protein measurement was performed on the pellet using Bradford protein assay [8]. The lactone forms of irinotecan and its active metabolite, SN-38, were measured using a validated HPLC method with fluorescence detection as described by Warner and Burke [9]. The separation method was carried out on a Waters Nova-Pak C18 column equipped with a µBondapak C18 guard column, with the mobile phase consisting of 20% acetonitrile and 80% triethylamine acetate. Detection was by fluorescence, with excitation at 370nm and emission at 510nm. The limit of quantitation for both was 2.5ng/ml. Quality assurance was maintained by simultaneously assaying the quality control samples prepared in bulk, prior to assay validation.
2.4. Double immunohistochemical staining for endothelial cells and pericytes in tumors
We have developed this method in order to determine the vascular maturation index (VMI), which is a percentage of endothelial cells (detected with MAb CD31) associated with pericytes (detected with MAb for α-smooth muscle actin, α-SMA). Using two chromogens (fast red and DAB), endothelial cells were immunestained red and pericytes were stained brown. The VMI index was determined by image analysis using the double stained (CD31/α-SMA) frozen tissue sections (ten randomly selected microscopic fields x400 of three tumors from each group) using Analyze calculating the total number of CD31+/α-SMA+ areas and areas positive for CD31 alone as published earlier in more details [10].
2.5. Morphological detection of apoptosis in tumors
FaDu xenografts of untreated controls, MSC (0.2mg/d × 7), irinotecan (100mg/kg/d × 7 or 14) and combination were collected 24h after last drug treatments. Tumor xenografts were fixed in 10% neutral-buffered formalin, dehydrated and embedded in paraffin for slides preparation. After conventional hematoxylin-eosin (H&E) staining, the overall histological structure of the tumors and apoptosis were morphologically evaluated. Apoptotic cells were identified by morphological changes of apoptosis such as nuclear fragmentation and condensation. Apoptosis indices were calculated as the percentage of apoptotic tumor cell nuclei among 350–400 tumor cell nuclei in the randomly selected areas of non-necrotic portions of the tumor at ×400 magnification. The presence of apoptotic cells was confirmed using TUNEL immunohistochemical assay on paraffin sections (Apop Tag Plus in Situ Detection Kit, Oncor, Gaithersburg, MD) according to the manufacture’s instruction.
2.6. Statistical Analyses
The average values and standard deviations were calculated. Statistical analysis was performed by comparing the average value of FaDu vs. A253 tumors, as well as the average values after irinotecan alone vs. combination treatment. The p-value was calculated by applying two-tailed distribution unpaired t-test. The result of the comparisons was considered statistically significant when the p-value was less than 0.05.
3. Results
3.1. Enhancement of anti-tumor activity of irinotecan is MSC schedule dependent
To evaluate the effect of MSC on irinotecan efficacy, mice bearing FaDu xenografts were treated daily with MSC alone and in concurrent or sequential combination with irinotecan. Treatment with MSC 0.2mg/d did not result in any enhancement of response rate (table 1). Treatment with irinotecan 100mg/kg resulted in a 35% response that increased to 40% when concurrently combined with irinotecan. However, when irinotecan was combined sequentially with MSC, the response rate increased to 100% (table 1).
Table 1.
In vivo antitumor activity
| Treatments | MSC1 0.2mg/d |
Irinotecan2 100mg/kg |
Concurrent3 Combination |
Sequential4 Combination |
|---|---|---|---|---|
| FaDu response rate5 | 0% | 35% | 40% | 100%*** |
| A253 response rate | 0% | 10% | 10% | 40%*** |
MSC dose (0.2 mg/d × 28)
Irinotecan (100mg/kg/wk × 4)
MSC + irinotecan
MSC 7d prior to and then concurrently with irinotecan
Complete Response (CR): no detectable tumor at the site of transplant for up to 3 months after termination of treatments
p<0.001when compared with other groups
In A253 xenografts, 100mg/kg irinotecan treatment resulted in 10% response (table 1). However, sequential combination of MSC/irinotecan increased this response to 40%.
The data show that, regardless of tumor type, sequential combination treatment is superior to concurrent combination.
3.2. Drug concentrations after concurrent combination schedule
Concurrent combination treatment (figure 1A) increased irinotecan plasma concentration from 1527.38 to 1621.35 ng/ml, a 6% increase. However, concentration of SN-38 decreased from 1000.94 to 988.79 ng/ml, a 1% decrease (figure 1B).
In FaDu tumors after combination treatment, irinotecan and SN-38 concentration decreased from 51.15 to 44.91 and from 2.29 to 1.59 ng/mg wt, a 12% and 31% decrease, respectively (figure 1C).
In A253 tumors, combination treatment decreased irinotecan concentration from 38.97 to 33.93 ng/mg wt, a 13% decrease. SN-38 concentration increased from 1.85 to 2.66 ng/mg wt, a 44% increase (figure 1C).
The data indicates that drug concentrations had not significantly changed after concurrent combination treatment schedule when compared with irinotecan treatment alone.
3.3. Drug concentrations after the first course sequential schedule
First course sequential combination treatment (figure 2A) increased irinotecan plasma concentration from 11820.11 to 22706.51 ng/ml, a significant 92% increase (p=0.03). Plasma concentration of SN-38 also increased from 1038.87 to 1278.03 ng/ml, a 23% increase (figure 2B).
In FaDu tumors after combination treatment, irinotecan and SN-38 concentration increased from 43.34 to 50.35 and from 0.45 to 0.73 ng/mg wt, a 16% and 61% increase, respectively (figure 2C).
In A253 tumors, combination treatment increased irinotecan and SN-38 concentrations from 37.49 to 54.38 and from 1.22 to 1.90 ng/mg wt, a 45% and 56% increase, respectively (figure 2C).
The data indicate that there is a trend of increase in intra-tumor drug concentrations after the first course sequential combination when compared to irinotecan alone. However, the increases in SN-38 concentrations were not statistically significant.
3.4. Drug concentrations after the second course sequential schedule
Second course sequential combination treatment (figure 3A) increased irinotecan plasma concentration from 7696.25 to 10173.35 ng/ml, a 32% increase (figure 3B). Plasma concentration of SN-38 significantly increased from 4357.90 to 5369.34 ng/ml, a significant 23% increase (p= 0.03, figure 3B).
In FaDu tumors after combination treatment, irinotecan significantly decreased from 0.88 to 0.35 ng/mg wt, a significant 60% decrease (p= 0.0002, figure 3C). However, SN-38 concentration significantly increased from 13.12 to 29.40 ng/mg wt, a significant 124% increase (p= 0.00002, figure 3C).
The data indicate that after the second course of sequential combination treatment of MSC/irinotecan there are statistically significant changes in FaDu drug concentrations in favor of increasing SN-38 and decreasing irinotecan concentrations.
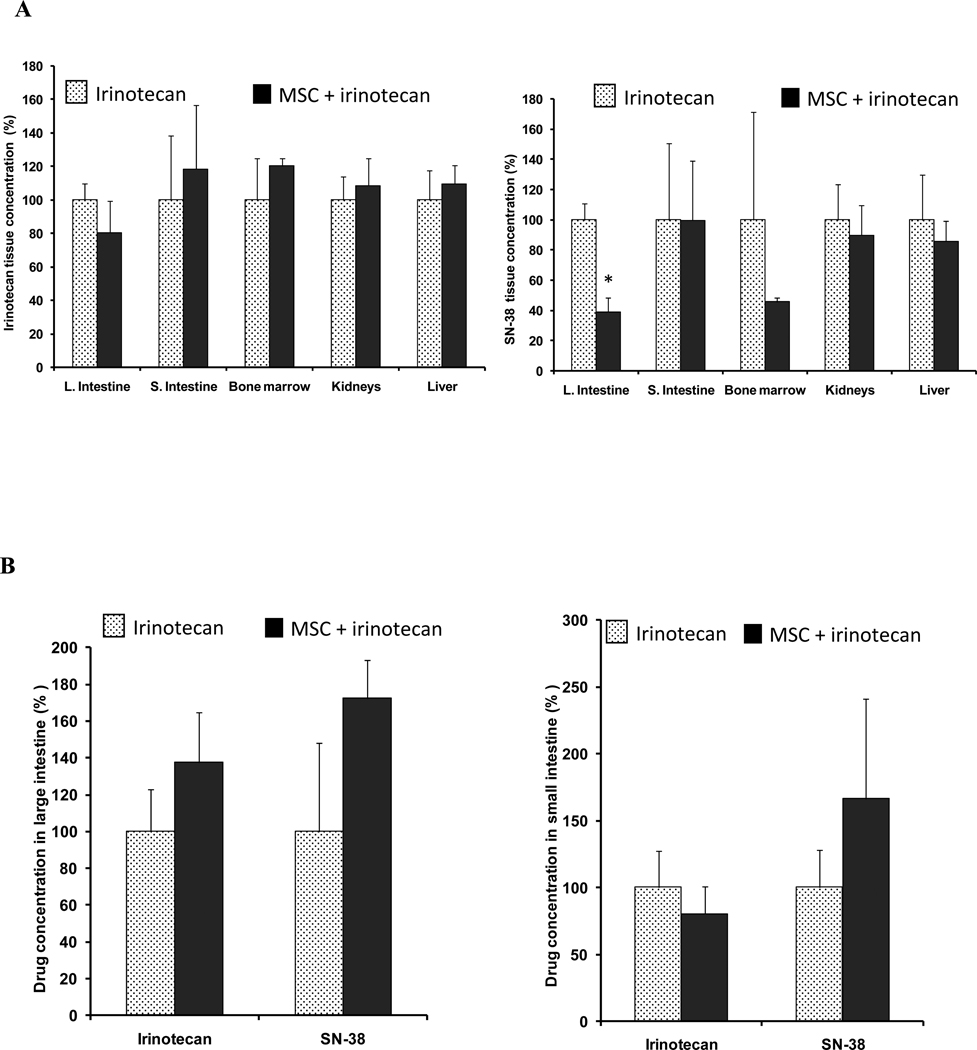
3.5. Drug concentrations in normal tissue after irinotecan alone or in combination schedule
After the concurrent combination treatment (figure 1A), irinotecan concentration decreased in the large intestine from 142.59 to 114.62 ng/mg wt, a 20% decreased (figure 4A). However, irinotecan concentration increased in the kidneys, liver, small intestine and bone marrow ranging from 9 to 20% (figure 4A). SN-38 concentration decreased in all tested normal tissue ranging from 0.3 to 61% (figure 4A). The highest decrease was in the large intestine from 13.68 to 5.34 ng/mg wt, a significant 61% decrease (p= 0.01) followed by a 54% decrease in the bone marrow when compared to irinotecan alone (figure 4A).
Figure 4. Drug concentrations in normal tissue.
Panel A shows irinotecan or SN-38 concentrations in large intestine, small intestine, bone marrow, kidneys and liver 2h after treatment with 100mg/kg irinotecan alone  , or concurrent combination treatment of MSC (0.2 mg/d) and irinotecan
, or concurrent combination treatment of MSC (0.2 mg/d) and irinotecan  .
.
Panel B shows irinotecan and SN-38 concentrations in large intestine or small intestine 2h after the second course of 100mg/kg irinotecan alone  , or in combination with MSC
, or in combination with MSC  .
.
When compared with irinotecan alone, the only significant change in drug concentration was a decrease in large intestine SN-38 concentration in panel A (p<0.05). * denotes p < 0.05 when compared to irinotecan alone.
After the second course of sequential combination treatment (figure 3A), irinotecan and SN-38 concentrations increased in the large intestine by 39 and 69%, respectively. However, no significant difference was observed when compared to irinotecan alone (figure 4B). In the small intestine, the combination treatment decreased irinotecan concentration by 19% and increased SN-38 concentration by 65%. No significant difference was observed when compared with values after irinotecan alone (figures 4B).
The data suggest that adding MSC significantly decreased the SN-38 concentration in large intestine and bone marrow after the first course treatment, but did not significantly change drug concentrations in normal tissue after the second course.
3.6. Sequential combination enhances intra-tumor microvessel maturation
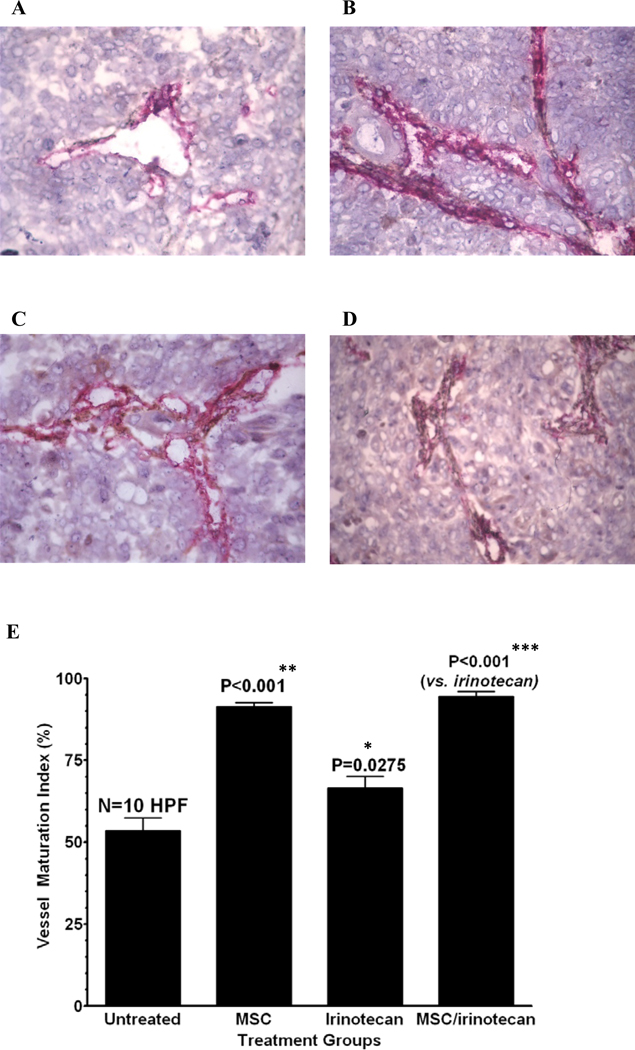
FaDu tumors were studied for micro vessel maturation using CD31/α-SMA double staining. Treatment with 14d MSC increased the pericytes coverage that is associated with endothelial cells, which was evident by the increased brown staining in the photomicrographs when compared to untreated controls (figure 5 panels A and B). The brown staining in the photomicrographs is used as a quantitative measure for vascular maturation and indicated that MSC significantly increased (p<0.001) the percentage of vessel maturation index (VMI) when compared to untreated controls (figure 5 panel E). Irinotecan treatment alone also increased VMI, but less effectively than MSC alone when compared to the control (figure 5 panels C and E). Sequential combination of MSC and irinotecan significantly enhanced VMI (p<0.001) when compared to irinotecan (figure 5 panels D and E).
Figure 5.
Induction of intra-tumor microvessel maturation after the second course of the sequential combination treatment
MSC enhances intra-tumor microvessel maturation as studied on CD31/α-SMA double stained (CD31 for endothelial cells: red, α-SMA for pericytes: brown) frozen sections of FaDu xenografts on day 15 after various treatments (Panel A: untreated, B: MSC, C: irinotecan, D: irinotecan/MSC). The bar graphs show the corresponding vascular maturation index (VMI) which is generally used as a quantitative measure for vascular maturation. The photomicrographs visualize the trend of the change in the pattern of double-immunostaining of the vessels, illustrating that the brown component for pericytes is increasing if MSC treatment is involved (panel B versus A and panel D versus C).
The date of relevant bar graphs from computer image analysis numerically demonstrates that MSC alone and in combination significantly enhanced the VMI as compared to the control or CPT induced VMI, respectively. It proves that MSC treatment increases the proportion of pericytes associated with endothelial cells which is an indication of vessel maturation. CPT treatment alone also increased VMI, but less effective than MSC alone, as compared to the control. * denotes p < 0.05 when compared to untreated controls, ** denotes p < 0.001 when compared to irinotecan alone and *** denotes p < 0.001 when compared to untreated controls.
The data indicate that treatment with MSC alone and in combination significantly enhanced the VMI as compared to the control or irinotecan alone.
3.7. Sequential combination enhances the level of apoptosis in tumor cells
Treatment of FaDu xenografts with 7d MSC alone (figure 6A2, right picture) did not change the incidence of apoptotic tumor cells as compared to untreated control (figure 6A1, left picture). The percentage of apoptosis index was 1.6±0.3 in untreated controls and 2.3±0.3 after 7d MSC treatment (figure 6A3).
Figure 6. Induction of apoptotic cell death after the second course of the sequential combination treatment.
The pictures are representative microphotographs of conventional, formalin-paraffin sections of the tumors after hematoxylin-eosin staining. Apoptotic cells were identified by morphology based on the characteristic nuclear fragmentation and condensation. Apoptotic tumor cell nuclei were counted among 300–400 tumor cell nuclei on H.E. slides in randomly selected, non-necrotic fields (×400) of tumor and expressed as a percentage. Panel A1 illustrates that MSC did not change the incidence of apoptotic tumor cells, as compared to the control (panel A2). Panel B1 visualizes that the first CPT treatment on day 7 induced several apoptotic tumor cells indicated by arrows, but the combination treatment with MSC (panel B2) did not increase the apoptotic incidence significantly. Panel C1 demonstrates that the second irinotecan treatment on day 14 also induces apoptotic tumor cells labeled by arrows and the combination treatment with MSC (panel C2) significantly increased the apoptosis incidence, as seen on the corresponding bar graphs.
The presence of apoptotic cells were confirmed by Tunel immunohistochemical assay. Representative microphotographs of Tunel immunostaining for apoptotic cells (brown) with nuclear counterstaining with haematoxylin (blue). The figure confirms the presence of apoptotic cells in formalin/paraffin sections in FaDu xenografts taken 14 days after the last treatment. Original magnification is ×400 for all panels. Panel D1 shows buffer control for staining specificity (negative control); in panel D2 shows an untreated FaDu xenograft with sporadic apoptotic tumor cells; in panel D3, MSC did not increase the number of apoptotic cells when compared to untreated controls; in panel D4, irinotecan-treated xenograft increased apoptotic tumor cell induction; and in panel D5, the second course of combination treatment resulted in significant increase of apoptotic cell tumor cells (p<0.05) when compared with irinotecan alone.
The corresponding bar graphs show the quantitation of the apoptotic tumor cell fraction (%). * denotes p < 0.05 when compared to irinotecan alone.
After first course treatment, irinotecan induced several apoptotic tumor cells indicated by arrows (figure 6B1, left picture). However, the combination treatment with MSC did not increase the apoptotic incidence significantly (figure 6B2, right picture). The percentage of the apoptosis index increased from 16.66±0.9 after irinotecan alone to 19.66±2.1 after combination treatment (figure 6B3).
After the second course treatment, irinotecan induced apoptotic tumor cells (figure 6C1, left picture) and the combination treatment with MSC significantly increased the apoptosis incidence when compared to irinotecan alone (figure 6C2, right picture). The percentage of the apoptosis index was significantly increased (p=0.03) from 13±1.2 after irinotecan alone to 19.33±1.5 after combination treatment (figure 6C3).
The induction of apoptosis after the second course treatment was confirmed using TUNEL immunohistochemical assay. Figure 6 panels D1-5 show that number of apoptotic cells (brown stained cells). The second course sequential combination (figure 6D5) significantly increased the number of apoptotic tumor cells when compared with all other treatment groups.
The data suggest that the increase in VMI allows more intra-tumor drug concentration, which led to an increase in apoptotic cell death in FaDu xenografts (figure 6).
4. Discussion
Sequential combination treatment of MSC and irinotecan of nude mice bearing FaDu and A253 xenografts, but not the concurrent combination treatment, increased complete response rates (cure rates) from 30% after irinotecan to 100% in FaDu and from 10% to 60% in A253 [5]. We previously reported that intra-tumor concentration of SN-38 increased after the first course of sequential combination treatment of MSC and irinotecan in both xenografts, FaDu and A253 [7]. This study investigates whether the increase in intra-tumor concentrations of SN-38 is observed after multiple courses of therapy, is tumor specific (does not occur in normal tissue) and is associated in part with the enhanced response rates after the sequential combination treatment but not after the concurrent treatment. To confirm this hypothesis, we evaluated the effect of MSC on plasma, intra-tumor and normal tissue drug concentrations after concurrent, first course and second course treatment schedule.
Compared to irinotecan alone, the concurrent treatment schedule did not result in significant differences in drug concentrations except in the large intestine. The percentage change in SN-38 concentrations was decreased in all samples (a −61% decrease in large intestine), except A253 tumors (a 44% increase) (tables 2 and 3). Thus, this could, in part, explain that the concurrent administration of MSC and irinotecan does not enhance irinotecan anti-tumor activity against head and neck human xenografts.
Table 2.
Drug concentrations in normal tissue
| Sample1 | Irinotecan Concentration |
SN-38 Concentration |
|---|---|---|
| Concurrent schedule2 | % change4 | |
| L. Intestine | −20 | −61 |
| S. Intestine | 19 | −0.3 |
| Bone marrow | 20 | −54*** |
| Kidneys | 9 | −10 |
| Liver | 10 | −14 |
| Sequential schedule3 | % change | |
| L. Intestine | 38 | 72 |
| S. Intestine | −19 | 67 |
Samples in concurrent schedule were taken 2h after treatments and 14d after treatment in the sequential schedule
MSC (0.2 mg/mouse) + irinotecan (100mg/kg)
MSC (0.2 mg/mouse/d) 7d prior to and then concurrently with irinotecan (100mg/kg × 2)
Compared to untreated controls
p<0.001 when compared with other groups
Table 3.
Summary of plasma and intra-tumor drug concentrations after treatments
| % change1 | Irinotecan concentration | SN-38 concentration | ||||
|---|---|---|---|---|---|---|
| (combination vs. drug alone) | Plasma | A253 | FaDu | Plasma | A253 | FaDu |
| Concurrent schedule2 | 6 | −13 | −12 | −1 | 44 | −31 |
| Sequential schedule (d7)3 | 92*** | 45 | 16 | 23 | 56 | 61 |
| Sequential schedule (d14)4 | 32 | ND | −60*** | 23*** | ND | 124*** |
Compared to irinotecan alone
MSC (0.2 mg/mouse) + irinotecan (100mg/kg)
MSC (0.2 mg/mouse/d) 7d prior to and then concurrently with irinotecan (100mg/kg×1)
MSC (0.2 mg/mouse/d) 7d prior to and then concurrently with irinotecan (100mg/kg×2)
p<0.001 when compared with irinotecan alone
Previously published results indicate that after the first course treatment, the sequential combination resulted in non-significant increase SN-38 area under the concentration time curve by 20% in FaDu and 62% in A253 [7]. In this study, we confirmed the increase of drug concentration after the first dose of irinotecan (table 3). Although there is a trend of increase in drug concentration, especially the active metabolite SN-38, this trend was not statistically significant when compared to irinotecan alone.
After the second course treatment, adding MSC caused some changes in drug concentration in normal tissue (table 2). However, these changes were not statically significant when compared with irinotecan alone. When compared with the concentrations after the first course treatment, plasma irinotecan concentration dropped by 60% while SN-38 concentration stayed the same at 23% (table 3). This data indicate that the plasma conversion of irinotecan to SN-38 stayed unchanged when MSC is added.
In order to determine the effect of the second course treatment on intra-tumor SN-38 concentration, we studied the sensitive FaDu tumors. Irinotecan concentration was significantly (p<0.05) decreased and the percentage change was −60%, when compared with irinotecan alone (table 3). FaDu SN-38 concentration was significantly (p<0.05) increased and the percentage increase was 124%, when compared with irinotecan alone (table 3). When compared to drug concentrations after the first course treatment, the second course treatment concentrations of irinotecan in FaDu decreased by 76% while SN-38 concentration increased to more than double (table 3). The data demonstrate that the most significant increase of intra-tumor SN-38 concentration is after the second course of sequential combination treatment. This increase was associated with an increased VMI and apoptosis index. As we previously reported and discussed [7] the 20% increase in FaDu SN-38 concentration after the first course of combination treatment was correlated with the increase in carboxyleastrase-1 (irinotecan activation enzyme) and decrease in SN-38 efflux pump (ABCC1). The enhanced intra-tumor SN-38 concentration could be achieved by “enhancement of SN-38 uptake/transport and/or inhibition of enzymes associated with the degradation of SN-38” [7]. To add to this list, sequential combination of MSC and irinotecan caused blood vessel maturation on day 14 but not on day 7 when compared with irinotecan alone. The maturation of vessels is characterized by VMI, which indicates what percentage of endothelial cells is associated with pericytes. MSC, like other antiangiogenic agent, prunes the microvessels by decreasing the number of endothelial cells which is associated with the relative increase of pericytes. In this study, MSC treatment increases the proportion of pericytes associated with endothelial cells which is an indication of vessel maturation. There is no evidence that MSC has a direct affect on pericytes.
Our data using xenografts model system demonstrated a variable increase in complete cure rate of combination therapy of MSC/irinotecan in FaDu (100%) and A253 (60%). However, adding MSC is always associated with an increase in the intra-tumor concentrations of SN-38. In addition, in A253 tumors, adding MSC (daily×14) to a single dose treatment of doxorubicin resulted in higher intra-tumor delivery of doxorubicin [10]. Nevertheless, the increase in SN-38 intra-tumor concentration in A253 improves response rate but it does not guarantee a complete response rate (100% cure rates). This could be due to the involvement of other contributing factors that are needed to achieve the complete cure rates. One of these factors is the distribution pattern of intra-tumor SN-38. In A253 tumors, SN-38 does not reach all tumor cells equally leaving a rim of cells in the well differentiated areas of the tumor unexposed to SN-38, thus protected from drug effect [11].
In brief, this study confirms that SN-38 concentration is increased after the sequential combination treatment with the best and significant increase is observed after the second course combination treatment (day 14). Thus, a possible reason for the enhanced response rate could be that MSC enhances vessel maturation which leads to increase intra-tumor drug concentration and in turn enhances apoptotic tumor cell death.
Acknowledgments
We acknowledge the pharmacokinetics/pharmacodynamics facility at Roswell Park cancer Institute for their help with the measurement of drug concentrations.
This study was supported in part by a Research Grant IRG-02-197-06 form the American Cancer Society and an Institute Comprehensive Cancer Center Support Grant CA16056 from the National Cancer Institute, Bethesda, MD.
Footnotes
Conflict of Interest
None of the authors has any financial or personal conflict of interest.
References
- 1.Rivory LP, Bowles MR, Robert J, Pond SM. Conversion of irinotecan (CPT-11) to its active metabolite, 7-ethyl-10-hydroxycamptothecin (SN-38), by human liver carboxylesterase. Biochemical Pharmacology. 1996;52:1103–1111. doi: 10.1016/0006-2952(96)00457-1. [DOI] [PubMed] [Google Scholar]
- 2.Ma MK, McLeod HL. Lessons learned from the irinotecan metabolic pathway. Current Medicinal Chemistry. 2003;10:41–49. doi: 10.2174/0929867033368619. [DOI] [PubMed] [Google Scholar]
- 3.Rivory LP. Irinotecan (CPT-11): a brief overview. Clinical & Experimental Pharmacology & Physiology. 1996;23:1000–1004. doi: 10.1111/j.1440-1681.1996.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 4.Ip C. Lessons from basic research in selenium and cancer prevention. Journal of Nutrition. 1998;128:1845–1854. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 5.Cao S, Durrani F, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clinical Cancer Resreach. 2004;10:2561–2569. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 6.Azrak RG, Cao S, Slocum HK, Toth K, Durrani FA, Yin MB, Pendyala L, Zhang W, McLeod HL, Rustum YM. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clinical Cancer Research. 2004;10:1121–1129. doi: 10.1158/1078-0432.ccr-0913-3. [DOI] [PubMed] [Google Scholar]
- 7.Azrak RG, Yu J, Pendyala L, Smith PF, Cao S, Li X, Shannon WD, Durrani FA, McLeod HL, Rustum YM. Irinotecan pharmacokinetic and pharmacogenomic alterations induced by methylselenocysteine in human head and neck xenograft tumors. Molecular Cancer Therapeutics. 2005;4:843–854. doi: 10.1158/1535-7163.MCT-04-0315. [DOI] [PubMed] [Google Scholar]
- 8.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Warner DL, Burke TG. Simple and versatile high-performance liquid chromatographic method for the simultaneous quantitation of the lactone and carboxylate forms of camptothecin anticancer drugs. Journal of Chromatography. B, Biomedical Sciences & Applications. 1997;691:161–171. doi: 10.1016/s0378-4347(96)00426-4. [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharya A, Seshardi M, Oven SD, Toth K, Vaughan MM, Rustum YM. Tumor vascular maturation and improved drug delivery induced by methylselenocysteine leads to therapeutic synergy with anticancer drugs. Clinical Cancer Research. 2008;14:3926–3932. doi: 10.1158/1078-0432.CCR-08-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya A, Toth K, Mazurchuk R, Spernyak JA, Slocum HK, Pendyala L, Azrak R, Cao S, Durrani FA, Rustum YM. Lack of microvessels in well-differentiated regions of human head and neck squamous cell carcinoma A253 associated with functional magnetic resonance imaging detectable hypoxia, limited drug delivery, and resistance to irinotecan therapy. Clinical Cancer Research. 2004;10:8005–8017. doi: 10.1158/1078-0432.CCR-04-1306. [DOI] [PubMed] [Google Scholar]