Abstract
Human butyrylcholinesterase (BChE) can scavenge and thereby provide protection against various toxic esters, including organophosphate-based chemical warfare agents and the recreational drug cocaine. It is currently being used in molecular evolution studies to generate novel enzymes with improved ability to hydrolyze toxic ester compounds. Currently, the most commonly used purification strategies for recombinant BChE enzymes involve using affinity resins based on small molecule interactions with the enzyme’s substrate binding site. However, as BChE variants are discovered and developed, a generic purification protocol that is insensitive to amino acid substitutions is necessary. In the current manuscript, an expression vector encoding a C-terminal truncation and His6-tag was designed for BChE and used to express recombinant “wild-type” enzyme and two variants (i.e., G117H BChE and G117H/E197Q BChE). All three His6-tagged enzymes were successfully purified via metal-affinity columns using similar procedures with good recovery. Steady-state kinetic parameters were determined for each enzyme, and values were compared to those obtained with the corresponding non-truncated non-His6-tagged enzymes. Rates of inhibition by echothiophate, a model compound for organophosphate-based pesticides, and rates of oxime-mediated reactivation after inhibition with a nerve agent model compound were also determined for selected enzymes. Rates of spontaneous reactivation from ETP inhibition were determined for G117H variants. In all instances examined, truncation of the C-terminus of BChE and introduction of a His6-tag had no significant effects on the observed kinetic parameters, making this a highly useful construct for in vitro characterization of wild-type and variant BChEs.
Keywords: Butyrylcholinesterase, Protein Purification, Recombinant Protein, Metal Ion Chromatography, His6-tag, Kinetic Characterization
Introduction
Human butyrylcholinesterase (BChE), historically referred to plasma or serum cholinesterase, has been found in nearly every tissue in humans. Although its physiological role is unknown, the enzyme’s substrate and inhibitor selectivities largely overlap with the more thoroughly characterized acetylcholinesterase (AChE). BChE can scavenge and therefore provide protection against administered or inhaled poisons that target AChE and similar physiological targets. In animal studies, treatment with BChE has provided protection from exposure of up to 5 × LD50 of chemical nerve agents that target AChE [1], and in 2010, FDA approval was given to develop the BChE as a therapeutic drug for prophylactic treatment against nerve agent exposure [2]. In addition, BChE variants are currently being pursued by various laboratories attempting to generate novel enzymes with enhanced ability to hydrolyze organophosphate ester (OP)-based nerve agents and other toxic compounds, including cocaine [3–8]. As the diversity and application of novel BChE variants develops, so does the need for a robust purification protocol independent of a given variant’s primary structure. Currently, commonly used purification methods rely on BChE affinity for procainamide, a small molecule that binds the enzyme’s substrate binding site [9]. Some variants however, including the previously characterized G117H/E197Q BChE [10], have little affinity for procainamide, and therefore are not efficiently purified using a procainamide column (Lockridge, personal communication). In light of the above noted efforts to develop novel BChE variants, there is a need for a more robust purification method that is insensitive to changes within the enzymes primary structure. Affinity tags are a logical solution.
Addition of affinity tags to BChE is potentially complicated by posttranslational processing of the recombinant protein. The BChE gene encodes an N-terminal sequence targeting the enzyme for secretion from mammalian cells. The N-terminal sequence of BChE is post-translationally cleaved to generate a mature enzyme, making N-terminal affinity tags problematic. Similarly, Blong et al. (1997) documented a significant amount of post-translational proteolysis of the C-terminus during recombinant expression. Preliminary attempts to utilize C-terminal tags provided only 30% recovery of enzymatic functional activity during protein purification with affinity columns (unpublished results). Studies have shown that the C-terminus of BChE is involved in tetramerization and is not essential for catalysis [11, 12]. As many as 50 amino acids can be removed from the C-terminus of wild-type BChE in the cloning stage without large changes in the observed kinetic parameters after expression [11, 13]. One study reported the successful use of a C-terminal His6-tag on truncated BChE enzyme for metal-chelate interaction chromatography (MIC)-based purification [14]. However, the MIC step was applied after significant purification was achieved by ammonium sulfate precipitation and procainamide affinity chromatography, and the recovery efficiency was not reported. To date, no other His-tagged monomeric BChE variants have been reported in the literature. In the current manuscript, a truncated His6-tagged construct generating W541H6Δ BChE variants was used for expression of wild-type and two previously reported BChE variants: G117H and G117H/E197Q BChE [10, 15, 16]. The latter enzyme is known to have poor affinity for conventional procainamide resins. The enzymes were purified via MIC methods and characterized for functional hydrolase activity. Enzymes (wild-type and G117H/E197Q) were further characterized for the rate of inhibition with the OP echothiophate (ETP), the rate of spontaneous reactivation (G117H), and the rate of reactivation with pyridine-2-aldoxime methiodide (2-PAM) or MMB4 after inhibition by a nerve agent model compound (wild-type).
Material and Methods
Cell lines and reagents
CHO-cTA-CAR suspension cells were kindly provided by Dr. R. Gilbert (Biotechnology Research Institute, National Research Council, Canada). 293A cells, cell culture medium, and media supplements were purchased from Life Technologies (Carlsbad, CA). FastStart High Fidelity polymerase and Pwo SuperYield DNA polymerase (Roche, Germany) were both utilized for PCR amplification. Procainamide-sepharose resin was prepared following previously published methods [9, 17]. Ni-NTA Superflow Resin was purchased from Qiagen Inc. (Valencia, CA). Butyrylthiocholine iodide (BTC), 5, 5’-dithiobis(2-nitrobenzoic acid) (DTNB), and 2-PAM were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO). Buffers and solvents were purchased from VWR Scientific, Inc. (San Diego, CA) in the highest purity commercially available. Ecothiophate iodide (ETP) was a generous gift from Dr. O. Lockridge (University of Nebraska Medical Center, Omaha, NE). The sarin analogue SpGBc was synthesized as previously reported [18].
Plasmid construction and protein expression
Construction of expression vectors for full length non-tagged (FL) and truncated his-tagged (W541H6Δ) BChE proteins can be found in the supporting information. Plasmids encoding W541H6Δ BChE have a His6-stop coding sequence immediately following the codon for E540, generating W541H6Δ BChE variants. Transfection of 293A cells and subsequent production, amplification, and quantitation of virus was conducted as detailed in the ViraPower Adenoviral Expression System manual (Life Technologies, Carlsbad, CA). CHO-cTA-CAR suspension cells (typically 50 mL at 5 × 105 cells mL−1) were infected using 100 viral-infection units/cell, and cultured at 37 °C, in either CD-CHO media supplemented with glutamine and dextran sulfate or DMEM:F12 1:1 media supplemented with glutamine and 10% fetal bovine serum. The level of enzyme expression was assessed by monitoring catalyzed rates of BTC hydrolysis using an Ellman assay [19]. Once a plateau level of activity was reached (typically 7–10 days), expression cultures were centrifuged at 30,000 × g for 15 min and the clarified expression medium was used for protein purification.
Protein purification
Highly purified FL wild-type BChE was generously provided by Dr. Lockridge. FL G117H BChE enzyme was purified using a procainamide-sepharose resin. The clarified expression media was dialyzed against 20 mM sodium phosphate pH 7.2 for a minimum of 1 h prior to loading onto a procainamide-sepharose resin equilibrated in dialysis buffer. The column was washed with PBS and protein was eluted from the column using phosphate buffer pH 7.4 containing 1M KCl. The FL G117H/E197Q BChE variant previously showed very low affinity for procainamide-sepharose, and was therefore not purified. Instead, clarified expression media was concentrated and the sample was washed with PBS, as described below for purified enzyme samples.
W541H6Δ BChE enzymes were purified using a batch purification strategy. Briefly, to 50 mL of clarified expression media, 1 mL of Ni-NTA Superflow Resin (50% slurry) was added, and samples were rotated end-over-end in sealed conical tubes overnight at 4 °C. The tubes were centrifuged gently to pellet the resin, and the majority of the liquid was decanted. The remaining mixture (approximately 5 mL supernatant and 0.5 mL resin) was resuspended and transferred into a fritted glass column. The resin was washed with 5 mL of PBS, and bound protein was eluted with two 5 mL washes of 100 mM imidazole, 50 mM potassium phosphate, pH 7.
Purified FL and W541H6Δ BChE enzymes were concentrated with an Amicon Ultra-15 centrifuge 10 kDa MWCO filter (Millipore, Billerica, MA), diluted with PBS pH 7.4 containing 0.025% NaN3, and concentrated a second time prior to storage at 4 °C.
Enzyme kinetics
Data analysis
All data analysis, including statistical comparisons via the sum-of-squares F test, was conducted using GraphPad Prism version 5.01 (GraphPad Inc., San Diego, CA).
Butyrylthiocholine dependence
Enzymatic hydrolysis of BTC was monitored using the Ellman assay [19] in a 96 well-plate format. Substrate dependences for wild-type FL and W541H6Δ BChE were determined using a 60% dilution series providing an assay concentration range of 5 μM to 11 mM BTC in PBS pH 7.4 at room temperature. The substrate dependency of other enzymes was determined using a 62% dilution series over the range of 50 μM to 10 mM. Assays contained 10 to 20 Units per L (where 1 Unit cleaves 1 μmole of substrate per min in PBS pH 7.4 with 1 mM BTC at room temperature). Observed rates of catalysis were fit to Eq. 1, which describes activation (when b > 1) of the enzyme at high BTC concentrations from binding of substrate to a secondary binding site [20, 21].
| Eq. 1 |
BChE active site titration
The specific activity (vobs/[E]) of BChE was determined by an active site titration of the desired enzyme. Two concentrations each of wild-type FL and W541H6Δ BChE were incubated with a serial dilution of ETP overnight at room temperature in PBS, pH 7.4 containing 50 μg/mL β-lactoglobulin (βLG) carrier protein to help stabilize the mixture. Fourteen ETP concentrations were used from 86 pM to 8.6 nM. The remaining enzyme activity was then assessed using an Ellman assay, as described above. The observed rates were fit to the Morrison Equation [22] as a function of the concentration of ETP during an overnight incubation . This equation yielded best-fit parameters for the enzyme concentration, the activity per enzyme concentration (vobs/E), and an apparent KI value for ETP binding. Due to the time-dependent nature of irreversible inhibition by ETP, the apparent KI values were not true dissociation constants, but approached zero with increasing incubation times. This may have resulted in overestimates of the enzyme concentration if sufficient time was not allowed for equilibrium to occur. As both the wild-type FL and W541H6Δ BChE were treated identically with ETP overnight, the comparison of vobs/E values was valid.
Rate of BChE inhibition by ETP
Wild-type FL or W541H6Δ enzyme (20 to 40 Units per L) was incubated with PBS pH 7.4 containing 50 ng/μL BLG and 200 to 900 nM ETP for 1 to 7 min prior to dilution 2-fold with solution containing 2 mM BTC. The rates of BTC hydrolysis were then measured using an Ellman assay as described above. The competitive nature between the BTC substrate and ETP inhibitor prevented detectable inhibition of the enzyme from occurring during the time course of the Ellman assay (i.e., 3–5 min). The observed rates of BTC hydrolysis were fit to a single-phase decay as a function of the incubation time with ETP to yield apparent rates of inhibition (kinhib). The G117H/E197Q double variants were treated similarly with an incubation concentration of 100 μM ETP.
In a second experiment, solutions were prepared containing 1 mM BTC, 0.2 mM DTNB, 10 ng/μL βLG and various ETP concentrations (20 to 80 μM) in PBS pH 7.4. To 1 mL of solution, 5 or 10 μL of wild-type FL or W541H6Δ BChE was added for an assay concentration of 10 to 20 Units L−1, and the absorbance at 412 nm was recorded for 3 to 5 min. Two inhibition studies were run per ETP concentration per enzyme. The resulting absorbance changes were fit to a single-phase time-dependent association to determine the apparent rate of inhibition (kapp). Rates of inhibition were then fit as a linear function of the ETP concentration to yield an apparent bimolecular rate constant.
Spontaneous reactivation of ETP-G117H BChE
G117H (FL or W541H6Δ) was incubated in PBS pH 7.4 with 100 μM ETP and 50 ng/μL BLG for a minimum of 10 min prior to dilution 20 to 80 fold into PBS pH 7.4 with 50 ng/μL BLG, 0.5 mM DTNB, and 1 mM BTC. Absorbance values were measured at 412 nm continuously for 6 min and the data were fit to eq. 2 [23] to determine the rate of spontaneous reactivation (kr). Eq. 2 describes the approach to steady state turnover, where Abs0 is the absorbance at the start of the measurement, v1 is the extrapolated rate of absorbance change at t = 0 min, and v2 is the extrapolated final (maximal) rate of absorbance change.
| Eq. 2 |
Oxime-mediated reactivation of SpGB-BChE
Wild-type FL and W51H6Δ BChE were inhibited with 1.7 μM SpGBc over approximately 20 min to achieve greater than 90% inhibition. Excess SpGBc was then removed via repeated concentration and dilution steps using a polyether sulfone microfuge filter with a 10 kDa MWCO (VWR Scientific, San Diego, CA). The estimated dilution factor for residual SpGBc was greater than 2,000-fold. Recovered inhibited enzyme was added to a solution containing 2-PAM (5 concentrations from 0.04 to 0.2 mM) and incubated at room temperature. At regular intervals (approximately 6 min), aliquots were assayed for esterase activity using a modified Ellman assay with 1 mM BTC in PBS pH 7.4, as described above. Observed rates of hydrolysis (vobs) were plotted against reactivation time, and data points determined for each enzyme were simultaneously fit to a single-phase association as a function of the reactivation time (t) and the oxime concentration ([Ox]) to determine the bimolecular reactivation rate constant (kr; Eq. 3). In Eq. 2, v0 and v∞ represented the initial and extrapolated final level of esterase activity, respectively.
| Eq. 3 |
In a similar experiment, wild-type FL and W541H6Δ enzymes inhibited with 2 μM SpGBc were diluted 500-fold into PBS pH 7.4 containing 50 ng/μL BLG, 1 mM BTC, 0.5 mM DTNB and 0.1 mM MMB4. The rate of oxime-mediated reactivation was determined by monitoring the absorbance at 412 nm and fitting the data to equation 2, as described for the spontaneous reactivation of ETP-G117H. Assays contained 3–6 Units per L of enzyme.
Results and Discussion
Protein expression
A viral based-expression system was used to produce wild-type and variant BChE enzymes. Viral production, amplification, and quantitation was conducted as detailed in the ViraPower Adenoviral Expression System manual (Life Technologies, Carlsbad, CA). The level of enzyme expression was assessed by monitoring esterase activity using an Ellman assay [19]. Early studies suggested maximal expression levels could be obtained using 100 viral-infection units/cell (data not shown). However, increased virus concentrations did not yield deleterious effects. Typical final expression levels for wild-type, G117H, and G117H/E197Q variants were in the range of 2000, 400, and 200 Units/L, respectively. No significant differences were found in expression levels comparing FL and W541H6Δ enzymes. Also, the expression levels were comparable for the CD-CHO and DMEM:F12 medias used in this study. However, preliminary data suggested that these expression levels were achieved only when the CHO-cTA-CAR suspension cells were continuously shaken during incubation. The mechanism behind this observation has not been pursued.
Protein purification
A viral based-expression system was used to produce W541H6Δ-wild-type, G117H, and G117H/E197Q BChE enzymes. All three enzymes were successfully purified via metal-affinity column chromatography using similar procedures. Representative data from purification of G117H/E197Q/W541H6Δ is shown in Table 1. Purification of this variant using a standard procainamide resin typically produced very poor yields (unpublished data, O. Lockridge, personal communication; T. Mor, personal communication). However, with the approach described herein for the W541H6Δ variants, over 80% of the functional activity was recovered using metal affinity chromatography, showing that the metal affinity chromatography was functional and generally insensitive to the amino acid substitutions of the enzyme.
Table 1.
Protein recovery levels for G117H/E197Q, W541H6Δ.
| DMEM-F12 1:1 | CD-CHO | |||
|---|---|---|---|---|
| Unitsa | P. D.b | Units | P. D. | |
| Clarified media | 9.1 | 100 | 2.7 | 100 |
| Flow through + Wash | 0.1 | 10 | 0.3 | 10 |
| Elution | 8.7 | 95 | 2.3 | 86 |
G117H/E197Q, W541H6Δ was expressed in two different media supplemented with FBS and Gln, and purified as described in SI above. The units and percent distribution (P. D.) were recorded for each step.
A unit is defined as the amount of enzyme required to hydrolyze 1 μmole BTC min−1 at room temperature in PBS pH 7.4 containing 1 mM BTC.
P. D. was calculated by dividing the total Units in a given sample by the total Units in the clarified media and multiplying by 100.
Due to the small-scale expression system used in this study, protein concentrations could not readily be determined using a standard Bradford assay. Therefore, to assess the purity of the His6-tagged enzymes, the Units/mg of W541H6Δ BChE was assessed and compared to the literature value for pure enzyme. An absorbance coefficient of 1.8 cm−1 mg−1 mL at 280 nm was used to estimate the protein concentration [24]. After MIC-based purification and concentration using a size-exclusion filter, the W541H6Δ wt enzyme purity was estimated at 27% (i.e., 193 ± 2 Units/mg) using a literature value of 720 Units/mg for “pure” wild-type enzyme [24], or 56% using the value of 340 Units/mg determined by active site titration (see below). In the literature, wild-type enzyme has most commonly been purified from plasma. This is in contrast to the recombinant expression used in the current study and for literature reports of other BChE variants. Comparison of BChE relative purity levels after MIC to native enzyme purified by procainamide-sepharose from plasma is therefore inappropriate. However, purification of a monomeric, low-glycosylated form of enzyme was reported in sufficient detail as to allow for a more direct comparison. The enzyme was recombinantly expressed in CHO cells, and expression media was collected and loaded onto a procainamide-sepharose column [13]. This initial chromatography step yielded enzyme that was 21% pure. Although there are significant differences in the scale of expression and purification procedures, comparison of the current results to the 2002 study data tentatively suggests that purification of the His6-tagged enzyme via MIC resulted in comparable levels of purity to purification of enzyme via procainamide-affinity chromatography. Moreover, introduction of the His6-tag allowed for all the BChE variants examined to be purified using the MIC method. The same was not true for procainamide-based chromatography techniques. In future experiments, the MIC method could likely be optimized through gradient-based elution procedures, alternative metals for chelating, or other methods to improve the resulting levels of purity. Once established, the optimized method should remain insensitive to variations in the enzyme’s primary structure.
Steady-state kinetic parameters with butyrylthiocholine substrate
Steady-state kinetic parameters with BTC substrate were determined for each enzyme using an Ellman assay, and the kinetic parameters were listed in Table 2. Data were fit to the Webb equation (Eq. 1), which described an initial hyperbolic substrate dependence for hydrolysis with a normal Michaelis constant (KM) followed by kinetic activation (when b>1) upon saturation of the peripheral anionic site with an apparent disassociation constant of KSS. For W541H6Δ and FL wild-type enzymes, substrate-dependent activation of kinetic activity was readily apparent. However, initial attempts at fitting the observed G117H and G117H/E197Q kinetic data to Eq. 1 afforded ambiguous kinetic parameters. Therefore, literature values were assigned to the analysis. For G117H BChE, the averaged literature b value of 2.2 [15, 16] was assigned as a constant. For G117H/E197Q BChE, the literature indicated high substrate-dependent activation but low affinity for the peripheral anionic site, with b and KSS values of 5 and 120 mM, respectively [10]. Both values were assigned prior to kinetic analysis. In all instances, the observed kinetic parameters were in reasonable agreement with the reported literature values, although the G117H/E197Q double variant KM value was slightly higher than expected [10, 11, 15, 16, 25]. Most importantly, the fitting parameters did not significantly differ between the corresponding FL and W541H6Δ enzymes for any tested enzyme pairs, indicating that replacement of the tetramerization domain with a His6-tag did not affect normal BChE catalysis.
Table 2.
Kinetic parameters of FL and W541H6Δ BChE enzymes using BTC as a substrate
| Enzyme | p valuea | KM, μM | KSS, mM | b |
|---|---|---|---|---|
| Wild-type | 0.3 | 27 ± 5 | 1.06 ± 0.15 | 2.6 ± 0.2 |
| W541H6Δ | 40 ± 6 | 1.16 ± 0.17 | 2.3 ± 0.2 | |
|
| ||||
| G117H | 0.5 | 170 ± 12 | 1.2 ± 0.2 | 2.2b |
| G117H, W541H6Δ | 181 ± 8 | 1.3 ± 0.1 | 2.2 | |
|
| ||||
| G117H/E197Q | 0.9 | 710 ± 20 | 120c | 5c |
| G117H/E197Q, W541H6Δ | 710 ± 10 | 120c | 5c | |
Enzymatic BTC hydrolysis was monitored at room temperature in PBS, pH 7.4, and the observed rates of hydrolysis were fit to Eq. 1. The listed best fit values and fitting errors resulted from a single determination for each enzyme (18 to 24 data points).
The indicated p values resulted from comparison of the kinetic data sets for the indicated pairs of enzymes using the sum-of-squares F test.
The b value was constrained at a value of 2.2.
The indicated parameters were assigned based on the report by Millard et al., 1998 [9].
Active site titration of FL andW541H6Δ wild-type BChE was done to determine the specific activity (i.e., vobs/[E]) of the enzyme preparations. Selective functional activity remaining was assessed using an Ellman assay with BTC substrate after active-site titration with ETP and data analysis was shown in SI Fig. 1. Two enzyme concentrations were used for each enzyme, differing by approximately 2-fold in concentration. The different concentrations gave similar specific activities for each enzyme, which indicated that sufficient time was provided to reach equilibrium with ETP. Also, the literature value for spontaneous reactivation of wt enzyme from ETP (i.e., .005 h−1) [26] suggests less than 10% of the enzyme would reactivate during the time course of the incubation (~16 h). Finally, statistical analysis of this data using a sum-of-squares F-test suggested that the specific activities for the FL and W541H6Δ enzymes were not statistically different (p = 0.051) with a shared value of 8.8 mAU per min per nM enzyme (i.e., 22 × 103 Units per nmole).
Transient kinetic parameters: rates of organophosphate-based inhibition of BChE and reactivation
Wild-type BChE is rapidly inhibited by OPs such as ETP and this affords a mechanism to scavenge them from the biological milieu and thereby provide protection to other sensitive targets such as AChE. In the current study, the rate of inhibition for the FL and W541H6Δ wild-type enzymes by ETP was measured in the absence and presence of BTC (SI Fig 2 and 3), and the resulting data were analyzed for the apparent bimolecular rate constant, kI app. In the absence of BTC substrate, analysis of 4 ETP concentrations resulted in overlapping rate constants of 1.34 ± 0.05 and 1.36 ± 0.05 min−1 μM−1 for FL and W541H6Δ, respectively. These values are in excellent agreement with previously determined rate constants [18, 27] and showed that the His6-tag and truncation did not affect the rate of ETP inhibition.
In a similar experiment, enzyme was inhibited in the presence of BTC. By including BTC, the observed rates of inhibition (40 ± 1 and 37 ± 1 min−1 μM−1 for FL and W541H6Δ BChE, respectively) were dependent upon both the rate of enzyme inhibition and the relative affinity for the ETP inhibitor and the BTC substrate. No attempt was made to correct the apparent rate constants for the competitive nature between BTC and ETP, but care was taken to assay the FL and W541H6Δ enzyme under identical conditions so as to allow for a meaningful comparison of the data. Although statistical analysis suggested the values are statistically different (p = 0.006), the apparent rate constants differed by less than 10% for FL and W541H6Δ enzymes, giving further support to the similar kinetics for substrate like BTC (shown in Table 2) and semi-substrate or irreversible inhibitors such as ETP.
One possible desired function from BChE variants is an enhanced ability to hydrolyze OP-based nerve agents. Two well characterized enzymes derived towards this end are the G117H and G117H/E197Q BChE variants. The G117H variant remains the most active BChE enzyme reported in the literature for hydrolysis of ETP, with a maximal rate constant (i.e., kcat) of 0.4 to 0.8 min−1 [15, 28] Although this represents a dramatic improvement over the rate of hydrolysis by the wild-type enzyme, this activity is still very low compared to G117H-catalyzed BTC hydrolysis. To assess the ability of the FL and W541H6Δ G117H enzymes to turnover ETP, we took advantage of the large difference in turnover values. Enzyme was incubated with sufficient ETP concentrations to ensure that the majority of enzyme was in the ETP-labeled form. Enzyme was then diluted into solution containing BTC, and the rate at which enzyme returned to the fully active form was determined by monitoring the time-dependent increase in the rate of BTC hydrolysis (SI Fig 4). Analysis of the data using Eq. 2 gave overlapping rate constants of 0.74 ± 0.08 and 0.74 ± 0.12 min−1 for FL and W541H6Δ G117H, respectively, in reasonable agreement with the literature values.
The G117H/E197Q BChE double variant does not turnover ETP as rapidly as the G117H single variant. In our hands, the enzyme reactivates from the ETP-labeled form with a rate constant < 0.01 min−1 (data not shown). Therefore, the rate of ETP-inhibition could be determined in a manner similar to that described above for the wild-type enzymes. The FL and W541H6Δ double variants were incubated with ETP in the absence of substrate, and the time-dependent loss of BTC hydrolytic ability was monitored using an Ellman assay (SI Fig 5). As with the wild-type enzymes, analysis produced overlapping rate constants of 0.15 ± 0.01 and 0.14 ± 0.01 min−1 (p = 0.7) for the FL and W541H6Δ enzymes, respectively.
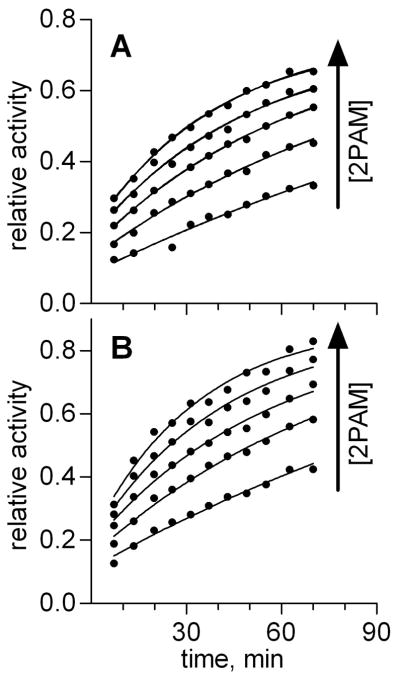
Although wild-type BChE does not independently turnover OP-based toxicants with appreciable rates, BChE can be reactivated by small molecule oximes such as 2-PAM or MMB4 after inhibition by many OP-based warefare agents. The use of chemical warfare agents is strictly regulated. Therefore, model compounds serve as a useful means to test for oxime-mediated reactivation. BChE enzyme inhibited with the sarin model compound SpGBc afforded the same BChE adduct as that inhibited with authentic sarin [29]. FL or W541H6Δ wild type enzyme inhibited with SpGBc was incubated with 40 to 200 μM 2-PAM, and aliquots of the incubation sample were assayed for BTC hydrolytic activity at various time points. The data were normalized to a non-inhibited control enzyme sample and plotted as a function of the reactivation time (Fig. 1). The data were fit to Eq. 2 to provide the oxime-mediated bimolecular rate of reactivation (kr). Independent determinations yielded nearly identical rate constants of 0.12 ± 0.01 and 0.15 ± 0.02 min−1 mM−1 for SpGB-W541H6Δ and SpGB-FL BChE enzymes, respectively. Subsequent analysis using a sum-of-squares F test indicated that neither data set showed statistically significant deviation from the average kr value of 0.13 min−1 mM−1 (p value > 0.3). Furthermore, these values were in excellent agreement with the value of 0.15 min−1 mM−1 derived from a recent report where plant-expressed recombinant human BChE was reactivated with 0.1 mM 2PAM [30].
Fig. 1. 2-PAM oxime-mediated reactivation of SpGB-W541H6Δ (A) and SpGB- FL (B) BChE enzymes.
Enzyme was inhibited with SpGBc, then reactivated with various concentrations of 2-PAM, as described in Materials and Methods. At various time points during the reactivation period, aliquots of enzyme were assessed for their ability to hydrolyze BTC, and the resulting rates of hydrolysis were plotted as a function of the amount of time enzyme incubated with 2-PAM. For clarity, the rates of hydrolysis were normalized to non-inhibited control samples. Data points for the five 2-PAM concentrations were simultaneously fit to Eq. 3 to determine the bimolecular reactivation rate constant (kr) for each enzyme.
In a similar experiment, SpGB-W541H6Δ and SpGB-FL BChE enzymes were incubated in solution containing 1 mM BTC and 0.1 mM MMB4. The rate at which the enzyme returned to its active form was monitored as described above for ETP-G117H reactivation. Analysis of three incubations per enzyme gave overlapping apparent rate constants of 0.064 ± 0.005 and 0.064 ± 0.008 per min−1 for the FL and W541H6Δ enzymes, respectively. Together, the data strongly suggested that removal of the C-terminus and addition of the His6-tag did not affect the observed kinetic behavior for any of the enzymes in this study.
Conclusion
BChE variants are currently being developed for various catalytic purposes, from detoxication of OP-based nerve agents to treatment of cocaine overdose. One well- characterized example is the G117H/E197Q BChE variant that was developed for the purpose of degrading OP-based poisons [10]. Currently, this variant represents the most promising BChE enzyme candidate for catalytic hydrolysis of soman. However, this variant cannot be readily purified utilizing procainamide affinity-based chromatography methods that are utilized in nearly all modern BChE purification schemes. Therefore, this variant serves as but one example underscoring the fact that a purification protocol that is insensitive to amino acid substitutions in the enzyme’s active or peripheral binding sites is currently lacking from the literature.
The current study shows that removing the C-terminal tetramerization domain from BChE and subsequent addition of a C-terminal His6-tag enables robust and rapid purification with good yields independent of the enzymes primary structure, using well-established metal-affinity protocols. The achieved level of purity was comparible to that obtained using procainamide-affinity chromatography, and the purified enzymes were kinetically indistinguishable from their FL counterparts. Therefore, the truncated and His-tagged BChE constructs and purification strategy presented in this report could greatly assist future enzyme kinetic and mechanism studies.
Supplementary Material
Highlights.
A C-terminal truncation and His6-tag addition were utilized to purify recombinant butyrylcholinesterase (BChE) variants with good recovery levels.
Characterization of the purified enzymes showed the truncation and His6-tag did not alter steady-state kinetics, rates of inhibition by organophosphate-based inhibitors, or rates of reactivation (either spontaneous reactivation for ETP-G117H or oximes-mediated reactivation)
The C-terminal truncation and His6-tag is therefore a useful construct for in vitro characterizations of recombinant BChE enzymes.
Acknowledgments
This work was funded by the National Institutes of Health CounterACT Program through the National Institute of Neurological Disorders and Stroke award #U54NS058183 Project 2 to J.Z. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. The authors sincerely thank Mary MacDonald for synthesis of the procainamide-sepharose resin and SpGBc, and Tom Carlson, Adam Kaldor, Snighda Poddar, and Beilin Wang for their technical assistance with molecular biology manipulations and preliminary studies.
Abbreviations
- BChE
butyrylcholinesterase
- PBS
phosphate buffered saline pH 7.4
- βLG
β-lactoglobulin
- DTNB
5,5’-dithiobis(2-nitrobenzoic acid)
- ETP
echothiophate
- 2-PAM
pyridine-2-aldoxime methyliodide
- BTC
butyrylthiocholine
- MIC
metal-ion chromatography
Footnotes
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saxena A, Sun W, Luo C, Myers TM, Koplovitz I, Lenz DE, Doctor BP. Bioscavenger for protection from toxicity of organophosphorus compounds. J Mol Neurosci. 2006;30:145–148. doi: 10.1385/jmn:30:1:145. [DOI] [PubMed] [Google Scholar]
- 2.Lenz DE, Clarkson ED, Schulz SM, Cerasoli DM. Butyrylcholinesterase as a therapeutic drug for protection against percutaneous VX. Chem Biol Interact. 2010;187:249–252. doi: 10.1016/j.cbi.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Xue L, Ko MC, Tong M, Yang W, Hou S, Fang L, Liu J, Zheng F, Woods JH, Tai HH, Zhan CG. Design, Preparation and Characterization of High-Activity Mutants of Human Butyrylcholinesterase Specific for Detoxification of Cocaine. Mol Pharmacol. 2010 doi: 10.1124/mol.110.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W, Xue L, Fang L, Chen X, Zhan CG. Characterization of a high-activity mutant of human butyrylcholinesterase against (-)-cocaine. Chem Biol Interact. 2010;187:148–152. doi: 10.1016/j.cbi.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng F, Yang W, Ko MC, Liu J, Cho H, Gao D, Tong M, Tai HH, Woods JH, Zhan CG. Most efficient cocaine hydrolase designed by virtual screening of transition states. J Am Chem Soc. 2008;130:12148–12155. doi: 10.1021/ja803646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang W, Pan Y, Fang L, Gao D, Zheng F, Zhan CG. Free energy perturbation simulation on transition states and high-activity mutants of human butyrylcholinesterase for (-)cocaine hydrolysis. J Phys Chem B. 2010;114:10889–10896. doi: 10.1021/jp104989b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masson P, Nachon F, Broomfield CA, Lenz DE, Verdier L, Schopfer LM, Lockridge O. A collaborative endeavor to design cholinesterase-based catalytic scavengers against toxic organophosphorus esters. Chem Biol Interact. 2008;175:273–280. doi: 10.1016/j.cbi.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Zheng F, Yang W, Xue L, Hou S, Liu J, Zhan CG. Design of high-activity mutants of human butyrylcholinesterase against (-)-cocaine: structural and energetic factors affecting the catalytic efficiency. Biochemistry. 2010;49:9113–9119. doi: 10.1021/bi1011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ralston JS, Main AR, Kilpatrick BF, Chasson AL. Use of procainamide gels in the purification of human and horse serum cholinesterases. Biochem J. 1983;211:243–250. doi: 10.1042/bj2110243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millard CB, Lockridge O, Broomfield CA. Organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase: synergy results in a somanase. Biochemistry. 1998;37:237–247. doi: 10.1021/bi972057c. [DOI] [PubMed] [Google Scholar]
- 11.Blong RM, Bedows E, Lockridge O. Tetramerization domain of human butyrylcholinesterase is at the C-terminus. Biochem J. 1997;327(Pt 3):747–757. doi: 10.1042/bj3270747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altamirano CV, Lockridge O. Association of tetramers of human butyrylcholinesterase is mediated by conserved aromatic residues of the carboxy terminus. Chem Biol Interact. 1999;119–120:53–60. doi: 10.1016/s0009-2797(99)00013-7. [DOI] [PubMed] [Google Scholar]
- 13.Nachon F, Nicolet Y, Viguie N, Masson P, Fontecilla-Camps JC, Lockridge O. Engineering of a monomeric and low-glycosylated form of human butyrylcholinesterase: expression, purification, characterization and crystallization. Eur J Biochem. 2002;269:630–637. doi: 10.1046/j.0014-2956.2001.02692.x. [DOI] [PubMed] [Google Scholar]
- 14.Chilukuri N, Duysen EG, Parikh K, Sun W, Doctor BP, Lockridge O, Saxena A. Adenovirus-mediated gene transfer of human butyrylcholinesterase results in persistent high-level transgene expression in vivo. Chem Biol Interact. 2008;175:327–331. doi: 10.1016/j.cbi.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Lockridge O, Blong RM, Masson P, Froment MT, Millard CB, Broomfield CA. A single amino acid substitution, Gly117His, confers phosphotriesterase (organophosphorus acid anhydride hydrolase) activity on human butyrylcholinesterase. Biochemistry. 1997;36:786–795. doi: 10.1021/bi961412g. [DOI] [PubMed] [Google Scholar]
- 16.Millard CB, Lockridge O, Broomfield CA. Design and expression of organophosphorus acid anhydride hydrolase activity in human butyrylcholinesterase. Biochemistry. 1995;34:15925–15933. doi: 10.1021/bi00049a007. [DOI] [PubMed] [Google Scholar]
- 17.Grunwald J, Marcus D, Papier Y, Raveh L, Pittel Z, Ashani Y. Large-scale purification and long-term stability of human butyrylcholinesterase: a potential bioscavenger drug. J Biochem Biophys Methods. 1997;34:123–135. doi: 10.1016/s0165-022x(97)01208-6. [DOI] [PubMed] [Google Scholar]
- 18.Barakat NH, Zheng X, Gilley CB, MacDonald M, Okolotowicz K, Cashman JR, Vyas S, Beck JM, Hadad CM, Zhang J. Chemical synthesis of two series of nerve agent model compounds and their stereoselective interaction with human acetylcholinesterase and human butyrylcholinesterase. Chem Res Toxicol. 2009;22:1669–1679. doi: 10.1021/tx900096j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 20.Webb JL. Enzyme and metabolic inhibitors. Academic Press; New York: 1963. Kinetics of some complex enzyme reaction types; pp. 32–48. [Google Scholar]
- 21.Radic Z, Pickering NA, Vellom DC, Camp S, Taylor P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry. 1993;32:12074–12084. doi: 10.1021/bi00096a018. [DOI] [PubMed] [Google Scholar]
- 22.Copeland RA. Evaluation of enzyme inhibitors in drug discovery. A guide for medicinal chemists and pharmacologists. Methods Biochem Anal. 2005;46:1–265. [PubMed] [Google Scholar]
- 23.Copeland RA. Enzymes : a practical introduction to structure, mechanism, and data analysis. Wiley; New York: 2000. [Google Scholar]
- 24.Masson P, Lockridge O. Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior. Arch Biochem Biophys. 2010;494:107–120. doi: 10.1016/j.abb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masson P, Xie W, Froment MT, Lockridge O. Effects of mutations of active site residues and amino acids interacting with the Omega loop on substrate activation of butyrylcholinesterase. Biochim Biophys Acta. 2001;1544:166–176. doi: 10.1016/s0167-4838(00)00217-x. [DOI] [PubMed] [Google Scholar]
- 26.Bartling A, Worek F, Szinicz L, Thiermann H. Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology. 2007;233:166–172. doi: 10.1016/j.tox.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Masson P, Froment MT, Bartels CF, Lockridge O. Importance of aspartate-70 in organophosphate inhibition, oxime re-activation and aging of human butyrylcholinesterase. Biochem J. 1997;325(Pt 1):53–61. doi: 10.1042/bj3250053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng X, Okolotowicz K, Wang B, Macdonald M, Cashman JR, Zhang J. Direct detection of the hydrolysis of nerve agent model compounds using a fluorescent probe. Chem Biol Interact. 2010;187:330–334. doi: 10.1016/j.cbi.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gilley C, MacDonald M, Nachon F, Schopfer LM, Zhang J, Cashman JR, Lockridge O. Nerve agent analogues that produce authentic soman, sarin, tabun, and cyclohexyl methylphosphonate-modified human butyrylcholinesterase. Chem Res Toxicol. 2009;22:1680–1688. doi: 10.1021/tx900090m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geyer BC, Kannan L, Garnaud PE, Broomfield CA, Cadieux CL, Cherni I, Hodgins SM, Kasten SA, Kelley K, Kilbourne J, Oliver ZP, Otto TC, Puffenberger I, Reeves TE, Robbins N, 2nd, Woods RR, Soreq H, Lenz DE, Cerasoli DM, Mor TS. Plant-derived human butyrylcholinesterase, but not an organophosphorous-compound hydrolyzing variant thereof, protects rodents against nerve agents. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1009021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.