Abstract
Background
The biological mechanisms by which acute stress increases alcohol consumption are unclear. One potential mechanism is that stress acts by altering the pharmacological and subjective effects of alcohol. Acute stress produces a cascade of physiological and psychological effects, each with a distinctive time course. In this study, we investigated whether different phases of response to an acute stress alter the subjective effects of intravenous alcohol, by administering the drug at two different times after the stress.
Methods
Healthy men (N=25) participated in two sessions; one with the Trier Social Stress Test, the other with a non-stressful control task, each followed by infusions of intravenous alcohol (targeting 40mg% in 5 min) and placebo. One group of participants received alcohol within 1 min of completing the tasks (Alc0, N=11), followed by placebo 30 min later. In the other group (Alc30, N=14), the order of alcohol and placebo infusions was reversed. Subjective effects (i.e., Anxiety, Stimulation, Want more) and physiological measures (heart rate, blood pressure, salivary cortisol) were measured before and at repeated intervals after the tasks and infusions.
Results
Stress did not change the subjective effects of alcohol in either group. However, when individual differences in alcohol responses were considered, stress differentially altered the stimulant-like and sedative effects of alcohol. Among individuals who exhibited predominantly stimulant responses to alcohol in the non-stressful condition, stress decreased the stimulant-like effects of alcohol and ‘wanting more’. By contrast, among participants who did not report stimulation after alcohol in the control session, stress decreased the sedative effects and increased ‘want more’. In addition, alcohol administered immediately after the TSST dampened cortisol responses yet prolonged negative subjective responses to the stress.
Conclusions
These findings demonstrate that there are bi-directional relationships between alcohol and stress. Alcohol influences responses to stress, and stress changes reactions to alcohol, depending on an individual's pattern of response to alcohol. This study highlights the fact that stress-alcohol interactions vary among individual drinkers, suggesting that the effects of stress on motivation to drink alcohol may also differ between individuals.
Keywords: Acute stress, Trier Social Stress Test, Alcohol, Anxiety, Cortisol
Introduction
Acute stress is thought to be an important precipitant of alcohol drinking. Epidemiological studies demonstrate links between stressful life events and both drinking rates or risk for alcohol use disorder (Jose et al., 2000; Lemke et al., 2008; Richman et al., 1996; Rospenda et al., 2000). There is also evidence that acute stress increases alcohol consumption in both nonhumans (Blanchard et al., 1993; Lynch et al., 1999; Miczek et al., 1994a, Miczek et al., 1994b; Pohorecky, 2010, 1990; Racz et al., 2008; Siegmund et al., 2005; Vengeliene et al., 2003; Volpicelli et al., 1990; Werme et al., 2002; Yang et al., 2008) and humans (Higgins and Marlatt 1975; Hull and Young, 1983; Pelham et al., 1997).
The mechanisms by which stress influences alcohol consumption are not understood. Acute stress could influence alcohol intake in several ways. For example, alcohol intake may increase because the drug reduces or dampens the negative subjective effects of stress (Conger, 1956). In fact, there is some evidence to suggest that alcohol does reduce anxiety (Blokland et al., 1992; Gallate et al., 2003; Pohorecky et al., 2008; Spanagel et al., 1995) and that it is used by anxious individuals as a method of self-medication (Marquenie et al., 2007; Merinkangas et al., 1998; Terra et al., 2006; Thomas et al., 2003). However, the specific anxiety-reducing effects of alcohol after stress have been difficult to demonstrate in the laboratory among healthy individuals (Levenson et al., 1987; Sinha et al., 1998; Zack et al., 2007; Zimmerman et al., 2004). An alternative process by which stress may increase alcohol intake is by altering the pharmacological or subjective effects of alcohol, thereby influencing its reinforcing properties. We (de Wit et al., 2003; Soderpalm and de Wit, 2002) have examined the effects of alcohol consumed immediately after an acute stress procedure, and found that acute stress decreased the subjective stimulant effects and increased the sedative effects of ingested alcohol. Although acute stress did not change self-reported desire for more drug, it did increase consumption of both a placebo and an alcohol-containing beverage. One limitation of our previous study (Soderpalm and de Wit, 2002) was that by the time the orally administered alcohol was fully absorbed, the effects of the stress had declined. Thus, in the present study we aimed to investigate the influence of acute stress on subjective responses to alcohol administered intravenously. In this way, we were able to examine the immediate effect of stress on alcohol response (zero delay), as well as the delayed effect of stress on alcohol response (30 min delay).
Acute stress produces a cascade of physiological and psychological effects, each of which has a distinctive time course of onset and decline after stress exposure. Soon after the initiation of stress there is an increase in heart rate and blood pressure and in psychological arousal and discomfort, and these remain high for the duration of the stressor. Approximately 10 min after stressor onset, plasma levels of adrenocorticotrophic hormone and noradrenaline peak (Childs et al., 2010a; Kudielka et al., 2004), followed 10-20 min later by plasma levels of cortisol, progesterone and allopregnanolone (Childs and de Wit, 2008; Childs et al., 2010b). Any of these components of the stress response could interact with alcohol in distinct ways to alter its pharmacological and subjective effects and/or motivation to consume alcohol. Thus, the effect of acute stress on responses to alcohol may depend on when the drug is experienced after stressor onset. For example, acute stress may reduce the sedative effects if alcohol is ingested at the time of peak stress-induced arousal. Alternatively, stress may potentiate its sedative effects if it is administered later, at a time when levels of allopregnanolone, a neurosteroid with sedative effects (Bitran et al., 1995; Khisti et al., 2000; Reddy and Kulkarni, 1997; Zimmerberg and Kajunski, 2004), are elevated. Therefore, in the current study we aimed to investigate whether acute stress differentially influences subjective responses to alcohol when it is administered immediately after the stressor or 30min later.
The effects of stress on alcohol responses may also depend on the individual's response to alcohol under normal (i.e., non-stressful) conditions. There are stable individual differences in subjective responses to alcohol; some individuals experience pleasant, stimulant-like effects whereas others report mainly unpleasant, sedative effects, regardless of the dose or time since administration (de Wit et al., 1987; 1989; Freed, 1978; Holdstock and de Wit, 1998; King et al., 2002). Acute stress also increases stimulation and arousal. Thus, acute stress may potentiate the positive stimulant-like effects of alcohol among those individuals who are sensitive to these alcohol effects. In this study we also aimed to investigate stress-alcohol interactions between individuals who did or did not report stimulation after alcohol during the non-stressful control session.
The present study was designed to investigate interactions between acute stress and alcohol as a function of the time interval between stress exposure and alcohol administration and in relation to individual differences in subjective responses to alcohol. For the acute stressor, we used a standardized public speaking stress procedure, the Trier Social Stress Test (TSST; Kirschbaum et al, 1993a). Participants completed two sessions, one with the TSST and one with a non-stressful control task, followed by administration of intravenous alcohol (targeting 40mg% in 5 min) or placebo. One group of participants received alcohol infusions beginning within 1 min of completing the tasks (group Alc0, N=11) and the other group received alcohol infusions beginning 30min after completing the tasks (group Alc30, N=14). Placebo infusions were administered to control for expectancies. We hypothesized that acute stress would potentiate the stimulant-like effects of alcohol administered immediately after the task but that it would potentiate the sedative effects of alcohol administered 30min later. In secondary analyses, we examined individual differences in the stress-alcohol interaction, based on whether participants reported mainly sedative or stimulant effects from the alcohol on the non stress session. We hypothesized that acute stress would potentiate the stimulant-like effects of alcohol among Stimulant Responders. Finally, since alcohol was administered only after performance of the tasks, we could assess the effect of alcohol on the responses to stress. We hypothesized that alcohol administered immediately after the task would dampen the magnitude or duration of stress responses.
Materials and Methods
Design
This study used a mixed within- (Stress vs. No stress) and between-subjects (alcohol administered immediately or 30 min after stress) design. Volunteers participated in two experimental sessions in which they underwent the TSST or a control task in randomized order, and received an intravenous infusion of alcohol (raising breath alcohol concentration, BrAC, to 40mg% in 5 min) either immediately after (Group Alc0, N=11) or 30min after the tasks (Group Alc30, N=14) were completed. Group Alc0 also received a placebo infusion at 30 min and Group Alc30 received a placebo infusion immediately after the tasks. Participants were randomly assigned to the two groups and were blinded to the content of infusions. The sessions were conducted between noon and 4pm at least 48 hours apart.
Participants
Healthy male social drinkers (less than 4 drinks per day; n=25) aged 18-32 years were recruited from the University and surrounding community without regard to race or ethnicity. Exclusion criteria included habitual tobacco use, regular use of medications, a current or prior diagnosis of Axis I disorder (APA, 1994) including drug abuse or dependence, less than a high school education (for the purposes of completing standardised questionnaires), a body mass index outside of 19-26 kg/m2, high or low blood pressure (as defined by the U.S. Centers for Disease Control and Prevention), an abnormal electrocardiogram, or night shift work. Cigarette smokers were excluded since smoking alters responses to the TSST (al'Absi et al., 2003; Childs and de Wit, 2009; Kirschbaum et al., 1993b).
Procedure
The University of Chicago Hospital's Institutional Review Committee for the use of human subjects approved the protocol. Participants signed a consent form which stated that the aim of study was to examine the effects of drugs on mood, performance and physiology. For blinding purposes, they were told that they might receive a stimulant, a sedative, a steroid hormone, a cannabinoid, alcohol or placebo. They were also told that they would complete short verbal tasks during each session but were not told the specific nature or purpose of the tasks until they had completed the study. Participants were instructed to refrain from eating for two hours prior to arrival at the laboratory for the sessions.
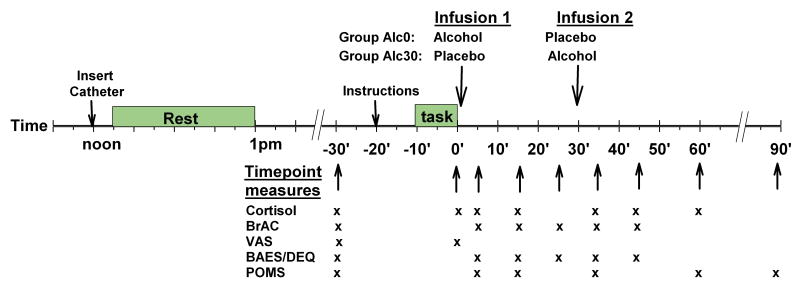
Participants were tested individually in the Human Behavioral Pharmacology Laboratory (HBPL) and in the University of Chicago Hospital's General Clinical Research Center (GCRC). Figure 1 shows the sequence of procedures during the sessions. Upon arrival at noon, participants provided breath and urine samples to detect recent drug and alcohol use; no one tested positive. An indwelling catheter was inserted into an anticubital vein for delivery of infusions, after which participants rested for 1h and ate a granola bar. Before testing procedures began, participants received an intravenous infusion of placebo in order to familiarise them with the sounds and sensations of the procedures. Fifteen minutes later, baseline dependent measures i.e., salivary cortisol, subjective mood, heart rate and blood pressure, were collected. Ten minutes later, instructions for the stress task to be performed during that session were read to the participant. The TSST and control tasks were conducted based upon standardised procedures as previously described (Childs and de Wit, 2009). Briefly, the TSST involved 5 min of public speaking followed by 5 min of mental arithmetic (serial subtraction) performed in front of two examiners, who were unknown to the participant, and a video camera (participants were told that their performances were videotaped, although they were not). Throughout the TSST, participants could see their own image portrayed on a television monitor. During the non-stressful control task (also 10min), participants described their favourite book or film, etc., to the research assistant for 5 min, then played a computer game (solitaire), without evaluation or a video camera. Participants were given 10min to prepare for each task. They were escorted to an adjacent room to perform the tasks which always began at 2pm. Once the task was complete, participants were escorted back to the patient room where they immediately completed a brief VAS scale to assess subjective mood (approx. 10s to complete) and provided another saliva sample before the infusion procedure began. All participants received two infusions; one beginning immediately (within 1min) and the other beginning 30min after completing the task. Participants in group Alc0 received alcohol in the first infusion and placebo in the second, whereas the order was reversed in group Alc30. Cardiovascular, hormonal and subjective measures and BrAC were obtained at repeated intervals after the tasks (see Figure 1 for details). At the end of each session after the final measures had been collected, participants completed a questionnaire on which they rated and commented on their experiences overall and indicated which drug they thought they had received. They were allowed to leave provided BrAC was below 20mg%. At the end of the second session, participants were fully debriefed about the specific purposes of the study and received payment (a $200 cheque).
Figure 1.
Schematic showing the timing of tasks, infusions and collection of cardiovascular, hormonal and subjective measures during the experimental sessions.
Intravenous Infusion Procedure
Alcohol infusions contained 5% alcohol in 5% dextrose (B Braun Medical, Inc, Bethlehem, PA) and placebo infusions contained 5% dextrose in water (Hospira Inc., Lake Forest, IL). Infusates were administered via a manually controlled infusion pump for 5 min at a constant rate that was pre-computed to raise the individual's BrAC to 40 mg%. Each individual's rate was based on an algorithm (O'Connor S, unpublished) derived from a physiologically based pharmacokinetic (PBPK) model of alcohol distribution and elimination, with parameters based on each subject's age, height and weight (Plawecki et al., 2008). The average volume delivered across subjects was 175 ml (7.2g ethanol).
Dependent measures
Measures of responses to stress included cardiovascular, hormonal and subjective assessments. Heart rate was measured continuously using a Polar chest band (Mini-Logger® system) and blood pressure was measured at repeated intervals using a monitor (Critikon Dinamap® Plus Vital Signs Monitor). Saliva samples for cortisol levels were collected using Salivette® cotton wads and were assayed by the GCRC Core Laboratory at The University of Chicago (Saliva=Salimetrics LLC, sensitivity=0.003ug/dL). Changes in subjective mood were measured using the Profile of Mood States (POMS; McNair and Droppleman, 1971) and Visual Analogue Scales (VAS; Folstein and Luria, 1973). The VAS consisted of four 100mm lines labelled with Anxious, Calm, Relaxed and Jittery anchored at one end with “not at all” and at the other by “very much so”.
Measures of responses to alcohol included subjective assessments and breath alcohol levels. Subjective drug effects were measured using the Biphasic Alcohol Effects Scale (BAES, Martin et al. 1993) and the Drug Effects Questionnaire (DEQ, Johanson and Uhlenhuth, 1980). BrAC was measured using a Breathalyser (Alco-sensor III, Intoximeters, St. Louis MO).
Statistical Analysis
Several control comparisons were made to ensure that the two groups were comparable. First, demographic characteristics and substance use data were compared between groups using Independent Samples t-test and Chi-squared analysis (for continuous and categorical variables respectively). The groups differed significantly in BMI (see Results section). Individualised infusion rates compensated for individual differences in BMI yielding the same BrAC in all subjects, nevertheless BMI was included in later analyses as a covariate (but dropped if there were no significant effects or interactions). The groups were also compared for differences in the dependent measures at baseline before each task and before the infusions using Independent Samples t-tests; there were no significant differences in pre-task and pre-infusion baselines between the groups or sessions. We compared responses to stress (the pre- to post- task difference) before alcohol was administered between the two groups, using two-factor (Task × Group) repeated measures analysis of variance (ANOVA). The effects of session order i.e., stress first or second, upon the measures were also analysed by including Session Order as a between-subjects factor. Finally, the groups were compared on the subjective and physiological effects of alcohol during the control task session, testing the assumption that the groups would not differ in their responses to alcohol in the absence of stress. For this analysis, we compared the pre- to post-infusion difference for infusion 1 in Alc0 with infusion 2 in Alc30 upon the subjective measures using univariate ANCOVA with BMI as covariate. BMI did not significantly influence the results, thus we report comparisons using Independent Samples t-test. We also compared the effects of alcohol relative to placebo by comparing the pre- to post-infusion difference for infusion 1 between the two groups (i.e., alcohol for Alc0, placebo for Alc30 using Independent Sample t-tests. We did not compare the effects of alcohol infusions to placebo infusions among all subjects because in group Alc0, placebo was always administered after alcohol, thus there may have been carryover effects, even though BrAC at the beginning of the placebo infusion in group Alc0 averaged less than 10mg%. BrAC declines rapidly after intravenous administration because arterial, venous and body water alcohol concentrations equilibrate rapidly once the pumps are turned off.
The effects of stress upon subjective responses to alcohol were assessed in two ways. First, we compared responses to alcohol infusions in the Alc0 and Alc30 groups between the TSST and control conditions. The pre- to post-infusion change was compared using two-factor (Group × Task) repeated measures ANOVA. Second, we assessed the effects of stress upon subjective responses to alcohol infusions for volunteers identified as predominantly stimulant responders i.e., those who reported an increase in BAES Stimulation (Stimulant Responders, SRs; N=10), or those who exhibited no change or a decrease in BAES Stimulation scale scores (Non-Stimulant Responders, NSRs; N=15), during the non-stressful condition using two-factor (Responder × Task) repeated measures ANOVA. Individual differences in other subjective and physiological responses to alcohol and stress were also compared between the Stimulant and Non-Stimulant Responders using two-factor (Responder × Task) repeated measures ANOVA.
Finally, the effects of alcohol vs. placebo administration immediately after the TSST upon responses to stress were analysed by comparing changes in subjective and physiological measures using two-factor (Group × Time) repeated measures ANOVA upon data obtained during the stress condition.
Results
Demographic Characteristics
Most participants were European-Americans in their mid twenties. They reported drinking on average 4 alcoholic drinks per week in the last month (range 0-14). The groups differed only in BMI (Table 1) which was included in later analysis as a covariate. There were no significant effects or interactions of the covariate in these analyses. Other demographic characteristics and substance use history did not differ between participants in the Alc0 and Alc30 groups.
Table 1.
Demographic characteristics and substance use history of participants. Asterisks indicate a significant difference between the groups (Independent Samples t-test, p=0.001)
| Group Alc0 | Group Alc30 | |
|---|---|---|
| N | 11 | 14 |
| Age (mean ± SD) | 25.7 ± 2.7 | 26.4 ± 3.8 |
| Body Mass Index (kg/m2)* | 23.0 ± 1.6 | 25.6 ± 1.8 |
| Race (N / %): | ||
| European-American | 7 / 64 | 7 / 50 |
| African-American | 3 / 27 | 4 / 29 |
| Asian-American | 0 / 0 | 1 / 7 |
| Other | 1 / 9 | 2 / 14 |
| Current Drug Use (mean ± SD) | ||
| Alcoholic drinks/wk | 4.6 ± 4.6 | 4.4 ± 3.3 |
| Caffeine cups/wk | 6.8 ± 4.2 | 5.4 ± 5.0 |
| Cigarettes/wk | 0.0 ± 0.0 | 0.1 ± 0.3§ |
| Marijuana times/month | 0.2 ± 0.4 | 0.9 ± 1.5 |
| Lifetime Drug Use (N / % ever used) | ||
| Stimulants | 2 / 18 | 4 / 29 |
| Sedatives | 1 / 9 | 1 / 7 |
| Hallucinogens | 3 / 27 | 4 / 29 |
| Opiates | 1 / 9 | 4 / 29 |
| Marijuana | 8 / 73 | 12 / 86 |
| Inhalants | 2 / 18 | 2 / 14 |
| Full Time Student (N / %) | 2 / 18 | 2 / 14 |
One participant smoked one cigarette per week in the Alc30 group.
The number of participants classified as predominantly Stimulant Responders or predominantly Non-Stimulant Responders (see later Individual Differences section) did not differ between the Alc0 and Alc30 groups, and these subsets of participants also did not differ on demographic characteristics or substance use history.
Effects of stress before infusions
The TSST significantly increased heart rate [F(1,20)=13.5 p<0.01], systolic blood pressure [F(1,23)=11.6 p<0.01], diastolic blood pressure [F(1,23)=16.6 p<0.001], and VAS Anxiety [F(1,23)=18.0 p<0.001] and Jitteriness [F(1,23)=17.5 p<0.001] and decreased ratings of Calm [F(1,23)=21.8 p<0.001] and Relaxed [F(1,23)=24.4 p<0.001, Table 2] compared to the control task. As expected, the TSST did not significantly increase levels of salivary cortisol immediately after the task [F(1,22)=1.8 p>0.1], although cortisol levels were significantly elevated at later times after the stress (see Figure 4 and related text). The foregoing effects of stress did not differ significantly between the two alcohol administration groups or between subsets of participants i.e., Stimulant and Non-Stimulant Responders.
Table 2.
Effects of Stress upon Subjective and Physiological Measures. Data indicate mean±SEM change from pre-task baseline to the first post-task time point i.e., immediately after the TSST and control tasks for all participants (there were no significant differences between the groups). Asterisks indicate a significant main effect of task (Repeated measures ANOVA, *** p<0.001)
| Mean pre-task Baseline |
Change from baseline | ||
|---|---|---|---|
| TSST | Control | ||
| Anxiety (mm) | 9.2 ± 1.7 | 16.1 ± 4.5 | -2.7 ± 1.6*** |
| Relaxed (mm) | 70.8 ± 3.6 | -26.0 ± 5.5 | 3.8 ± 2.9*** |
| Calm (mm) | 70.1 ± 3.6 | -25.1 ± 5.8 | 2.4 ± 1.9*** |
| Jittery (mm) | 8.4 ± 2.1 | 20.3 ± 4.5 | -1.6 ± 2.0*** |
| Heart rate (bpm) | 69.2 ± 2.3 | 12.3 ± 2.2 | 3.4 ± 0.9*** |
| Systolic (mmHg) | 117.0 ± 1.9 | 16.8 ± 2.5 | 5.5 ± 1.6*** |
| Diastolic (mmHg) | 64.7 ± 1.1 | 8.7 ± 1.2 | 3.4 ± 0.8*** |
| Cortisol (ug/dL) | 0.18 ± 0.02 | 0.02 ± 0.02 | -0.01 ± 0.2 |
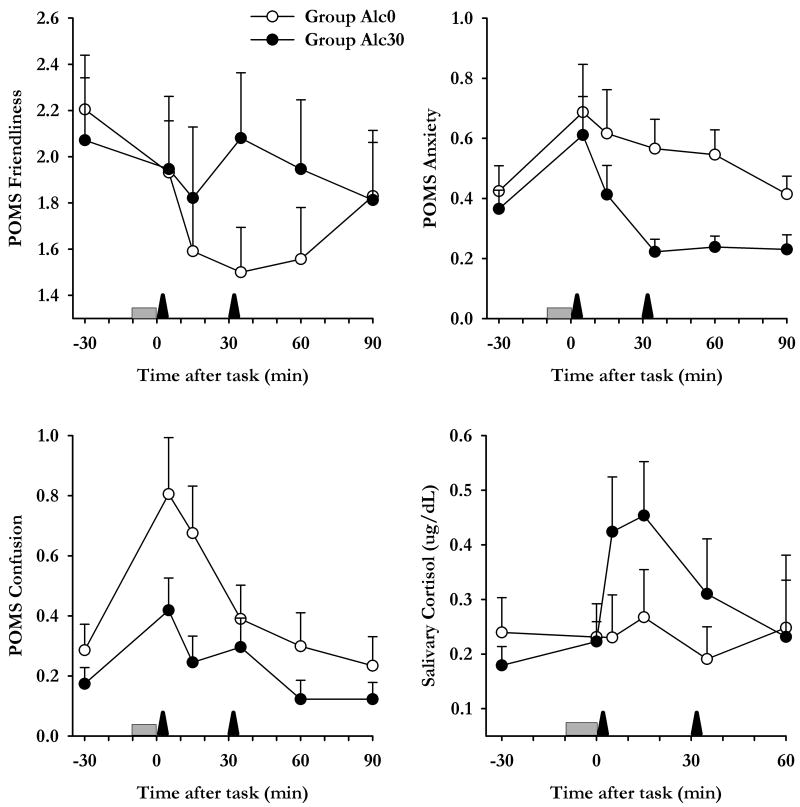
Figure 4.
Effects of intravenous alcohol upon responses to acute stress. Data indicate mean±SEM subjective and cortisol responses to stress as a function of time among participants in group Alc0 (open symbols) and Alc30 (closed symbols). The shaded bar indicates when tasks were performed and arrows indicate when infusions were delivered.
Effects of alcohol, compared to placebo, during the non-stressful session
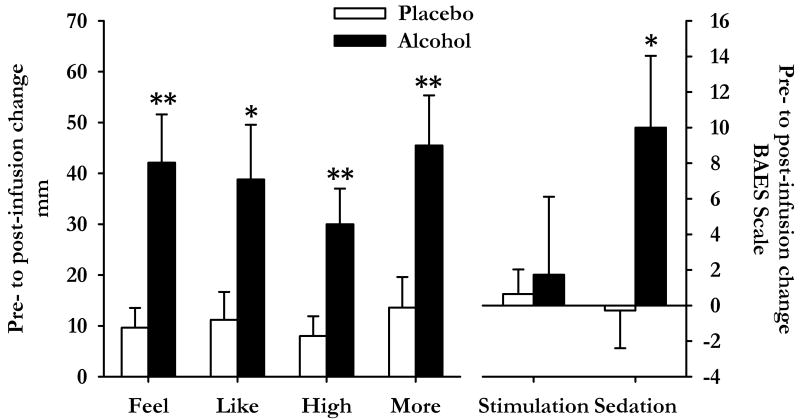
Alcohol infusions increased breath alcohol concentrations to 35±3 mg% and 36±2 mg% in precisely 5 min among participants in the Alc0 and Alc30 groups respectively. The subjective effects of alcohol did not differ significantly between the two groups (Infusion 1 for group Alc0 vs. Infusion 2 for Group Alc30). Comparisons of the effects of Infusion 1 between Alc0 and Alc30 participants showed that alcohol significantly increased ratings of “I feel some drug effects” [t(23)=3.4 p<0.01], “I feel high” [t(23)=2.9 p<0.01], “I like the effects I am feeling” [t(23)=2.4 p<0.05], “I want more drug” [t(23)=2.9 p<0.01] and scores on the BAES Sedation (Negative Effects) scale [t(23)=2.4 p<0.05, see Figure 2] in comparison to placebo.
Figure 2.
Subjective responses to intravenous alcohol and placebo after the control task. The graphs show responses to infusion 1 during the control session among participants in group Alc0 (N=11, alcohol, filled bars) and group Alc30 (N=14, placebo, open bars). Data indicate mean±SEM change from pre-infusion baseline for the DEQ (left hand side) and BAES (right hand side). Asterisks indicate a significant difference between the groups (Student's independent samples t-test, *p<0.05 **p<0.01).
Effects of alcohol after acute stress, compared to no stress
Stress did not affect subjective responses to alcohol regardless of whether alcohol was administered immediately after the task or 30 min later (data not shown). Stress also did not alter peak breath alcohol concentrations (Stress=36±2mg%, No Stress=37±1mg%) in either group.
Individual differences in responses to alcohol compared to placebo
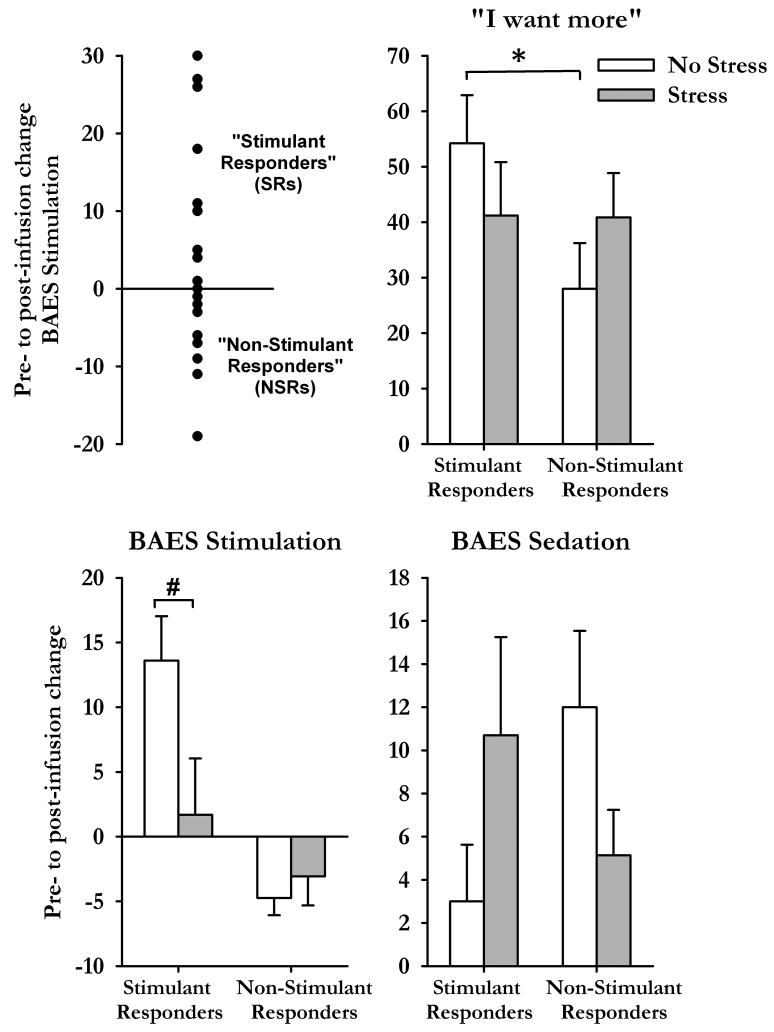
During the non-stressful session, some participants (N=10) reported increases in BAES Stimulation scale scores i.e., classified as Stimulant Responders (SRs; Figure 3, top left), whereas others (N=15) did not (called Non-Stimulant Responders, NSRs). SRs and NSRs did not differ on demographic characteristics, including recent drinking history, or peak BrAC and were equally distributed between group Alc0 and Alc30. However, SRs exhibited higher ratings of “I want more” after alcohol than NSRs [t(23)=2.1 p<0.05, Figure 3, top right].
Figure 3.
Individual differences in response to alcohol after the TSST and non-stressful tasks. Top left graph shows scores of BAES Stimulation for all participants after the non-stressful task and classification of subsets of participants. Bar charts illustrate changes (from pre-infusion baseline, mean±SEM) in subjective responses to alcohol after the TSST (shaded bars) and control task (open bars) for participants classified as Stimulant or Non-Stimulant Responders. Asterisks indicate a significant difference between the groups (Student's independent samples t-test, p<0.05) and # indicates a significant difference between the tasks (Student's Paired t-test, p<0.05).
Individual differences in the influence of stress on responses to alcohol
The TSST differentially influenced scores of Stimulation [Task × Responder F(1,23)=11.8 p<0.01], Sedation [Task × Responder F(1,23)=4.7 p<0.05] and ratings of “I want more drug” [Task × Responder F(1,23)=5.2 p<0.05] among SRs and NSRs (Figure 3). Stress significantly attenuated the stimulant-like effects of alcohol among SRs [t(9)=2.8 p<0.05, Student's paired t-test] but not in NSRs [t(14)=-1.0 p>0.1]. Stress produced opposite effects upon ratings of sedation among SRs and NSRs; it increased the sedative effects of alcohol among SRs [t(9)=-1.4 p>0.1] yet attenuated these effects among NSRs [t(14)=1.7 p>0.1]. Finally, stress abolished the differences in ratings of “I want more drug” between the SR and NSR groups that were evident after the non-stressful task [Non-stressful task t(23)=2.1 p<0.05; TSST t(23)=0.03 p>0.1, Independent Samples t-test]. That is, stress decreased ratings of wanting more among SRs whereas it increased want more ratings among NSRs.
Effects of alcohol administration, compared to placebo, upon stress responses
In the group who received alcohol immediately after the TSST, alcohol blocked the cortisol responses to stress [Group*Time F(5,110)=3.4 p<0.01, Figure 4]; Group Alc30 exhibited significant increases in levels of cortisol in response to stress [Time F(4,48)=7.2 p<0.001] but Group Alc0 did not [Time F(4,40)=0.5 p>0.1]. Alcohol administered immediately after the task also potentiated negative subjective responses to stress; it prolonged stress-induced decreases in Friendliness [Group*Time F(5,115)=4.4 p=0.001], increased Anxiety [Group F(1,23)=4.4 p<0.05] and tended to prolong increases in Confusion [Group*Time F(5,115)=2.2 p=0.06].
Discussion
This study aimed to investigate interactions between acute stress and low doses of alcohol in healthy male social drinkers. We examined stress-alcohol interactions in relation to the time between the stressor and alcohol administration, and in relation to individual differences in subjective responses to alcohol. There were several interesting findings. First, when all subjects were considered together, acute stress did not influence subjective responses to alcohol, whether it was administered immediately after stress or 30 min later. However, when subjects were separated into those who reported predominantly positive stimulant-like or predominantly negative sedative responses to alcohol (in the non-stressful control condition), stress differentially influenced the subjective effects of alcohol in each group. Among participants who reported mainly stimulant-like effects from alcohol on the non-stress session, stress blunted ratings of stimulation, and wanting more drug, and increased sedation, which was in contrast to our hypothesis. Conversely, among participants who did not report stimulation from alcohol, stress decreased alcohol-induced sedation and increased ratings of “want more drug”. We also found that alcohol administered immediately after stress blocked the stress-induced increase in cortisol, yet prolonged negative subjective responses. The latter finding may have implications for the interpretation of the stress-dampening effect of alcohol. Overall, the findings provide evidence of bi-directional interactions between alcohol and stress, which could influence drinking under stressful conditions.
This study is among the first to investigate stress-drug interactions using alcohol delivered intravenously. Although the intravenous alcohol administration procedure is usually employed to maintain steady breath alcohol concentrations over a prolonged period, the alcohol “clamp” (O'Connor et al., 2000), in this study we administered the drug in a single, relatively brief dose. We used a model-based algorithm of alcohol distribution and elimination to calculate the infusion rates that would yield the same target concentration of BrAC within a set interval in all subjects. Then, the pumps were turned off once the prescribed peak BrAC was achieved, allowing BrAC levels to decline immediately. Our rationale for i.v, alcohol administration was fourfold. First, we wanted to be able to control when peak BrACs were achieved in relation to the stress response (e.g., when responses were near maximal immediately after the task, or during recovery approximately 30min later). Second, intravenous infusion avoids the delay of approximately 30min to peak BrACs when alcohol is ingested orally, thus we were able to administer alcohol immediately after the stressor and achieve peak BrAC in 5 minutes to coincide with near maximal stress responses. Alcohol administered orally before the stress confounds the effects of alcohol on perception of the stress task from its effect on the response to the stress task, and alcohol administered orally after stress might miss the peak time of stress effects. Third, the method avoided the substantial inter-individual variation in absorption kinetics of alcohol when it is administered orally. Finally, we wanted to blind participants to the drug that was being administered in order to minimize expectancy effects. Responses to the questionnaire given at the end of each session indicated that blinding was moderately effective; only 12 of 25 subjects correctly identified the drug as alcohol during one of the sessions, and only 36% of all drug identifications were correct.
There are both commonalities and differences in the effects of alcohol administered by the oral and intravenous route. We and others (O'Connor et al., 1998; Zoethout et al., 2009) have found that the subjective effects of alcohol are very similar whether it is administered orally or intravenously. However, the clearance kinetics of alcohol after short high rate intravenous infusions differ from the clearance after oral administration. In our study, there was a rapid drop in BrAC immediately after the infusion was terminated as arterial, venous and body water alcohol concentrations equilibrated, which was followed by a longer pseudolinear decline at the same rate as that after oral administration. Despite the differences in kinetics, however, the subjective responses in this study were similar to those reported after oral administration paradigms (O'Connor et al., 1998; Zoethout et al., 2009). Another difference between the administration procedure used here and oral administration is that complete post-hepatic administration of the alcohol dose (approximately 2 standard drinks) was achieved in 5min, whereas after oral administration only approximately 10% of participants completely absorb an equivalent dose of alcohol (Ramchandani et al., 2009). There is little evidence that this difference in speed of onset influenced the subjective or physiological responses to the drug. Thus, we believe that the intravenous route used was the most appropriate and controlled way to address the question under study.
An important finding in this study was that stress attenuated the stimulant-like effects and increased the sedative effects of alcohol among participants who exhibited stimulant-like responses to alcohol in the control condition. While these findings are in contrast to our original hypotheses, they are in line with two previous studies (de Wit et al., 2003; Soderpalm and de Wit, 2002) which reported that acute stress blunted the positive stimulant effects and increased the negative sedative effects of orally administered alcohol. Stress also decreased ratings of “want more drug” among stimulant responders, which is in accordance with evidence that stimulant-like effects of alcohol are positively related to alcohol consumption and liking. It is not clear how the finding that stress dampened the stimulant effects of alcohol explains stress-induced alcohol drinking. Possibly, people may drink more alcohol to overcome the attenuated effect. Alternatively, they may drink to reduce unpleasant feelings of stress or simply for the purposes of “pleasant” sedation. By contrast, among participants who did not report pleasant stimulant-like responses to alcohol during the non-stressful condition, stress attenuated the negative sedative effects of alcohol and increased ratings of want more. Thus, these individuals may experience less negative subjective effects from alcohol, and so may consume more during stress. Although this result is consistent with the expected increase in alcohol drinking after stress, it was only reported in participants who normally experience relatively negative sedative effects of alcohol. These findings suggest that stress differentially influences subjective responses to alcohol depending upon whether individuals usually experience predominantly stimulant effects of alcohol or not under normal conditions and therefore, stress-induced changes in alcohol drinking may be mediated differently among subsets of drinkers.
Another important finding in this study was that administration of alcohol immediately after stress blocked the expected increase in cortisol after stress. Previously others have shown that alcohol administered prior to stress exposure blunts ACTH and cortisol responses to stress (Dai et al., 2002; Zimmerman et al., 2004), but in those studies, alcohol may have affected the perception of the stressful situation, effectively reducing its strength. Here, we extend these findings to show that alcohol can inhibit the cortisol stress responses even if it is administered after exposure to the stressful stimulus. It has been proposed that alcohol inhibits stress-induced activation of the hypothalamic pituitary adrenal (HPA) axis. Several mechanisms have been proposed including actions upon excitatory and inhibitory afferents to corticotrophic releasing hormone (CRH) containing neurons of the paraventricular nucleus (PVN) (De Waele et al., 1992; Gianoulakis 1990; Wand et al., 1998), actions upon GABA receptors expressed by CRH containing neurons of the PVN (Jessop et al., 1999) or by inhibiting vasopressin which is co-released with CRH and which synergizes to enhance adrenocorticotrophic hormone (ACTH) release (Antoni, 1986; Watabe et al., 1988). However, our results suggest that the inhibitory effects of alcohol may be mediated downstream of the hypothalamus since alcohol was administered after stressor exposure and presumably after the hypothalamic CRH-containing neurons were stimulated. Indeed, previously it was shown that alcohol blunted ACTH response to exogenous CRH but not cortisol response to exogenous ACTH (Waltman et al., 1993). Limitations of this study include that we did not collect blood samples for ACTH analysis, which would have aided in the interpretation of the effects of alcohol upon the HPA axis, and that we obtained a single cortisol measurement before the tasks, which provides only an approximate indicator of baseline levels. Nevertheless, our results are in line with earlier findings which suggest alcohol may decrease responsivity of pituitary ACTH-containing neurones to CRH.
Paradoxically, although alcohol administered after a stressor dampened cortisol responses, it prolonged negative subjective responses. This is inconsistent with Conger's stress-dampening theory of alcohol consumption (1956) but is in agreement with studies demonstrating that alcohol use can actually increase stress (Pohorecky, 1991). The body's physiological response to an acute stressor is designed to counteract the threat and to minimise perturbations by restoring homeostasis. Therefore, it may follow that if the body's normal physiological response to stress is disrupted e.g., by alcohol, then other responses i.e., psychological, may be similarly disrupted. In effect, although alcohol dampens cortisol responses to acute stress, it interrupts the body's ability to cope effectively and efficiently with a stressor thus increasing overall subjective stress levels. Previously, others reported that alcohol dampened subjective emotional responses in anticipation of a stressor (Conrod et al., 1998; Finn et al., 1990), however it is not clear whether this effect was due to the influence of alcohol upon perception of the stressor (Sayette, 1993). Thus, here we demonstrate that a low dose of alcohol administered after a stressor significantly alters physiological and psychological stress responses among healthy men.
These results also have implications for studies of alcohol effects upon the HPA axis (e.g., Richardson et al., 2008). Interestingly, high doses of alcohol (at least twice as large as that administered in the present study) have been shown to stimulate the HPA axis causing ACTH and cortisol release (McCaul et al., 2001; Inder et al., 1995; Reddy and Sarkar, 1993; Rivier, 1996; Rivier and Vle, 1988; Silveri and Spear 2004). These increases are thought to result from actions of alcohol upon hypothalamic paraventricular CRH neurons or their afferents (Ogilvie et al., 1998; Rivier and Lee, 1996) and may be mediated by local metabolism of alcohol to acetaldehyde (Cannizzaro et al., 2010). In this study, we demonstrate that alcohol can inhibit HPA axis activity and glucocorticoid release after it has been activated by an external stimulus. Thus, as suggested by previous studies (Waltman et al., 1993), it appears that the effects of alcohol upon HPA axis activity and glucocorticoid release i.e., stimulation or inhibition, appear to depend upon the current level of activation. Additionally, interactions between alcohol and the HPA axis may be altered with prolonged activation of the HPA system i.e., with chronic stress.
Together, the findings of this study indicate that there are bi-directional relationships between alcohol and stress. Alcohol influences responses to stress, and stress changes reactions to alcohol, and it does so differently across individuals. Considering the complex nature of these interactions, it is perhaps not surprising that it has been difficult to demonstrate consistent effects of stress upon alcohol drinking in the laboratory. Future studies may seek to investigate stress-alcohol interactions in specific, targeted subsets of drinkers or using paradigms specifically designed to measure motivational aspects of stress-alcohol interactions.
Acknowledgments
We would like to thank Nicholas Van Dam, Anna Asiama, Gina Beguhn, Joshua Shulruff, and Christy Casnar who assisted with data collection and Les Sidney for conducting the TSST interviews. This research was supported by NIDA (DA02812), the University of Chicago Hospital's GCRC (USPHS MO1RR000555) and by NIAAA (P60 AA007611).
References
- al'Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74(2):401–10. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Antoni FA. Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev. 1986;7(4):351–78. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and Statistical Manual of Psychiatry. 4th. Washinton DC: 1994. [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7(3):171–7. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko EB, Blanchard DC. Alcohol, aggression and the stress of subordination. J Stud Alcohol Suppl. 1993;11:146–55. doi: 10.15288/jsas.1993.s11.146. [DOI] [PubMed] [Google Scholar]
- Blokland A, Prickaerts J, Raaijmakers W. Reduced level of anxiety in adult Lewis rats after chronic ethanol consumption. Physiol Behav. 1992;51(2):245–8. doi: 10.1016/0031-9384(92)90137-q. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, La Barbera M, Plescia F, Cacace S, Tringali G. Ethanol modulates corticotropin releasing hormone release from the rat hypothalamus: does acetaldehyde play a role? Alcohol Clin Exp Res. 34(4):588–93. doi: 10.1111/j.1530-0277.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology (Berl) 2009;203(1):1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Van Dam NT, de Wit H. Effects of acute progesterone administration upon responses to acute psychosocial stress in men. Exp Clin Psychopharmacol. 2010a;18(1):78–86. doi: 10.1037/a0018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, Dlugos A, De Wit H. Cardiovascular, hormonal, and emotional responses to the TSST in relation to sex and menstrual cycle phase. Psychophysiology. 2010b;47(3):550–9. doi: 10.1111/j.1469-8986.2009.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem and challenge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17(2):296–305. [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcohol Clin Exp Res. 1998;22(3):585–97. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Response of the hypothalamic-pituitary-adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology. 2002;27(3):442–52. doi: 10.1016/S0893-133X(02)00308-1. [DOI] [PubMed] [Google Scholar]
- De Waele JP, Papachristou DN, Gianoulakis C. The alcohol-preferring C57BL/6 mice present an enhanced sensitivity of the hypothalamic beta-endorphin system to ethanol than the alcohol-avoiding DBA/2 mice. J Pharmacol Exp Ther. 1992;261(2):788–94. [PubMed] [Google Scholar]
- de Wit H, Soderpalm AH, Nikolayev L, Young E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin Exp Res. 2003;27(8):1270–7. doi: 10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Pierri J, Johanson CE. Individual differences in behavioral and subjective responses to alcohol. Alcohol Clin Exp Res. 1987;11(1):52–9. doi: 10.1111/j.1530-0277.1987.tb01263.x. [DOI] [PubMed] [Google Scholar]
- deWit H, Pierri J, Johanson CE. Assessing individual differences in ethanol preference using a cumulative dosing procedure. Psychopharmacology (Berl) 1989;98(1):113–9. doi: 10.1007/BF00442016. [DOI] [PubMed] [Google Scholar]
- Finn PR, Zeitouni NC, Pihl RO. Effects of alcohol on psychophysiological hyperreactivity to nonaversive and aversive stimuli in men at high risk for alcoholism. J Abnorm Psychol. 1990;99(1):79–85. doi: 10.1037//0021-843x.99.1.79. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3(4):479–86. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Freed EX. Alcohol and mood: an updated review. Int J Addict. 1978;13(2):173–200. doi: 10.3109/10826087809039273. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Morley KC, Ambermoon P, McGregor IS. The consequences of beer consumption in rats: acute anxiolytic and ataxic effects and withdrawal-induced anxiety. Psychopharmacology (Berl) 2003;166(1):51–60. doi: 10.1007/s00213-002-1291-z. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol. 1990;180(1):21–9. doi: 10.1016/0014-2999(90)90588-w. [DOI] [PubMed] [Google Scholar]
- Higgins RL, Marlatt GA. Fear of interpersonal evaluation as a determinant of alcohol consumption in male social drinkers. J Abnorm Psychol. 1975;84(6):644–51. doi: 10.1037//0021-843x.84.6.644. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res. 1998;22(9):1903–11. [PubMed] [Google Scholar]
- Hull JG, Young RD. Self-consciousness, self-esteem, and success-failure as determinants of alcohol consumption in male social drinkers. J Pers Soc Psychol. 1983;44(6):1097–109. doi: 10.1037//0022-3514.44.6.1097. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Joyce PR, Wells JE, Evans MJ, Ellis MJ, Mattioli L, Donald RA. The acute effects of oral ethanol on the hypothalamic-pituitary-adrenal axis in normal human subjects. Clin Endocrinol (Oxf) 1995;42(1):65–71. doi: 10.1111/j.1365-2265.1995.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Jessop DS. Stimulatory and inhibitory regulators of the hypothalamo-pituitary-adrenocortical axis. Baillieres Best Pract Res Clin Endocrinol Metab. 1999;13(4):491–501. doi: 10.1053/beem.1999.0039. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacology. 1980;71(3):269–73. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Jose BS, van Oers HA, van de Mheen HD, Garretsen HF, Mackenbach JP. Stressors and alcohol consumption. Alcohol Alcohol. 2000;35(3):307–12. doi: 10.1093/alcalc/35.3.307. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67(1):137–43. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26(6):827–35. [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993a;28(1-2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993b;44(3):527–31. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schommer NC, Hellhammer DH, Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day. Psychoneuroendocrinology. 2004;29(8):983–92. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Lemke S, Schutte KK, Brennan PL, Moos RH. Gender differences in social influences and stressors linked to increased drinking. J Stud Alcohol Drugs. 2008;69(5):695–702. doi: 10.15288/jsad.2008.69.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Oyama ON, Meek PS. Greater reinforcement from alcohol for those at risk: parental risk, personality risk, and sex. J Abnorm Psychol. 1987;96(3):242–53. doi: 10.1037//0021-843x.96.3.242. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kushner MG, Rawleigh JM, Fiszdon J, Carroll ME. The effects of restraint stress on voluntary ethanol consumption in rats. Exp Clin Psychopharmacol. 1999;7(4):318–23. doi: 10.1037//1064-1297.7.4.318. [DOI] [PubMed] [Google Scholar]
- Marquenie LA, Schade A, van Balkom AJ, Comijs HC, de Graaf R, Vollebergh W, van Dyck R, van den Brink W. Origin of the comorbidity of anxiety disorders and alcohol dependence: findings of a general population study. Eur Addict Res. 2007;13(1):39–49. doi: 10.1159/000095814. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17(1):140–6. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Stauffer R, Lee SM, Rohde CA. Naltrexone dampens ethanol-induced cardiovascular and hypothalamic- pituitary-adrenal axis activation. Neuropsychopharmacology. 2001;25(4):537–47. doi: 10.1016/S0893-133X(01)00241-X. [DOI] [PubMed] [Google Scholar]
- McNair D, Droppleman MLL, editors. Profile of Mood States. Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, Aguilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, DeWit DJ, Kolody B, Vega WA, Wittchen HU, Kessler RC. Comorbidity of substance use disorders with mood and anxiety disorders: results of the International Consortium in Psychiatric Epidemiology. Addict Behav. 1998;23(6):893–907. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Miczek KA, DeBold JF, van Erp AM. Neuropharmacological characteristics of individual differences in alcohol effects on aggression in rodents and primates. Behav Pharmacol. 1994a;5(4 And 5):407–421. doi: 10.1097/00008877-199408000-00004. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts E, Haney M, Tidey J. Neurobiological mechanisms controlling aggression: preclinical developments for pharmacotherapeutic interventions. Neurosci Biobehav Rev. 1994b;18(1):97–110. doi: 10.1016/0149-7634(94)90040-x. [DOI] [PubMed] [Google Scholar]
- O'Connor S, Morzorati S, Christian J, Li TK. Clamping breath alcohol concentration reduces experimental variance: application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res. 1998;22(1):202–10. [PubMed] [Google Scholar]
- O'Connor S, Ramchandani VA, Li TK. PBPK modeling as a basis for achieving a steady BrAC of 60 +/- 5 mg% within ten minutes. Alcohol Clin Exp Res. 2000;24(4):426–7. [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Weiss B, Rivier C. Mechanisms mediating the influence of alcohol on the hypothalamic-pituitary-adrenal axis responses to immune and nonimmune signals. Alcohol Clin Exp Res. 1998;22(5 Suppl):243S–247S. doi: 10.1097/00000374-199805001-00005. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Lang AR, Atkeson B, Murphy DA, Gnagy EM, Greiner AR, Vodde-Hamilton M, Greenslade KE. Effects of deviant child behavior on parental distress and alcohol consumption in laboratory interactions. J Abnorm Child Psychol. 1997;25(5):413–24. doi: 10.1023/a:1025789108958. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O'Connor SJ. Physiologically based pharmacokinetic (PBPK) models for ethanol. IEEE Trans Biomed Eng. 2008;55(12):2691–700. doi: 10.1109/TBME.2008.919132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA. Stress and alcohol interaction: an update of human research. Alcohol Clin Exp Res. 1991;15(3):438–59. doi: 10.1111/j.1530-0277.1991.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Psychosocial stress and chronic ethanol ingestion in male rats: effects on elevated plus maze behavior and ultrasonic vocalizations. Physiol Behav. 2008;94(3):432–47. doi: 10.1016/j.physbeh.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Acute novel stressors modify ethanol intake of psychosocially stressed rats. Pharmacol Biochem Behav. 2010;95(4):390–400. doi: 10.1016/j.pbb.2010.02.017. [DOI] [PubMed] [Google Scholar]
- Racz I, Schurmann B, Karpushova A, Reuter M, Cichon S, Montag C, Furst R, Schutz C, Franke PE, Strohmaier J, Wienker TF, Terenius L, Osby U, Gunnar A, Maier W, Bilkei-Gorzo A, Nothen M, Zimmer A. The opioid peptides enkephalin and beta-endorphin in alcohol dependence. Biol Psychiatry. 2008;64(11):989–97. doi: 10.1016/j.biopsych.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, O'Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in healthy volunteers. Alcohol Clin Exp Res. 2009;33(5):938–44. doi: 10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Reddy BV, Sarkar DK. Effect of alcohol, acetaldehyde, and salsolinol on beta-endorphin secretion from the hypothalamic neurons in primary cultures. Alcohol Clin Exp Res. 1993;17(6):1261–7. doi: 10.1111/j.1530-0277.1993.tb05239.x. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res. 1997;752(1-2):61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28(8):1641–53. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JA, Flaherty JA, Rospenda KM. Perceived workplace harassment experiences and problem drinking among physicians: broadening the stress/alienation paradigm. Addiction. 1996;91(3):391–403. [PubMed] [Google Scholar]
- Rivier C. Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res. 1996;20(2):240–54. doi: 10.1111/j.1530-0277.1996.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Lee S. Acute alcohol administration stimulates the activity of hypothalamic neurons that express corticotropin-releasing factor and vasopressin. Brain Res. 1996;726(1-2):1–10. [PubMed] [Google Scholar]
- Rivier C, Vale W. Interaction between ethanol and stress on ACTH and beta-endorphin secretion. Alcohol Clin Exp Res. 1988;12(2):206–10. doi: 10.1111/j.1530-0277.1988.tb00181.x. [DOI] [PubMed] [Google Scholar]
- Rospenda KM, Fujishiro K, Shannon CA, Richman JA. Workplace harassment, stress, and drinking behavior over time: gender differences in a national sample. Addict Behav. 2008;33(7):964–7. doi: 10.1016/j.addbeh.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA. An appraisal-disruption model of alcohol's effects on stress responses in social drinkers. Psychol Bull. 1993;114(3):459–76. doi: 10.1037/0033-2909.114.3.459. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29(7):1139–45. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Characterizing the ontogeny of ethanol-associated increases in corticosterone. Alcohol. 2004;32(2):145–55. doi: 10.1016/j.alcohol.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Sinha R, Robinson J, O'Malley S. Stress response dampening: effects of gender and family history of alcoholism and anxiety disorders. Psychopharmacology (Berl) 1998;137(4):311–20. doi: 10.1007/s002130050624. [DOI] [PubMed] [Google Scholar]
- Soderpalm AH, de Wit H. Effects of stress and alcohol on subjective state in humans. Alcohol Clin Exp Res. 2002;26(6):818–26. doi: 10.1097/00000374-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, Landgraf R. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122(4):369–73. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Terra MB, Barros HM, Stein AT, Figueira I, Jorge MR, Palermo LH, Athayde LD, Goncalves MS, Spanemberg L, Possa MA, Daruy Filho L, Da Silveira DX. Social anxiety disorder in 300 patients hospitalized for alcoholism in Brazil: high prevalence and undertreatment. Compr Psychiatry. 2006;47(6):463–7. doi: 10.1016/j.comppsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Randall CL, Carrigan MH. Drinking to cope in socially anxious individuals: a controlled study. Alcohol Clin Exp Res. 2003;27(12):1937–43. doi: 10.1097/01.ALC.0000100942.30743.8C. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Siegmund S, Singer MV, Sinclair JD, Li TK, Spanagel R. A comparative study on alcohol-preferring rat lines: effects of deprivation and stress phases on voluntary alcohol intake. Alcohol Clin Exp Res. 2003;27(7):1048–54. doi: 10.1097/01.ALC.0000075829.81211.0C. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Ulm RR, Hopson N. The bidirectional effects of shock on alcohol preference in rats. Alcohol Clin Exp Res. 1990;14(6):913–6. doi: 10.1111/j.1530-0277.1990.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Waltman C, Blevins LS, Jr, Boyd G, Wand GS. The effects of mild ethanol intoxication on the hypothalamic-pituitary-adrenal axis in nonalcoholic men. J Clin Endocrinol Metab. 1993;77(2):518–22. doi: 10.1210/jcem.77.2.8393888. [DOI] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55(12):1114–9. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Watabe T, Tanaka K, Kumagae M, Itoh S, Kogure M, Hasegawa M, Horiuchi T, Morio K, Takeda F, Ubukata E, et al. Role of endogenous arginine vasopressin in potentiating corticotropin-releasing hormone-stimulated corticotropin secretion in man. J Clin Endocrinol Metab. 1988;66(6):1132–7. doi: 10.1210/jcem-66-6-1132. [DOI] [PubMed] [Google Scholar]
- Werme M, Lindholm S, Thoren P, Franck J, Brene S. Running increases ethanol preference. Behav Brain Res. 2002;133(2):301–8. doi: 10.1016/s0166-4328(02)00027-x. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang S, Rice KC, Munro CA, Wand GS. Restraint stress and ethanol consumption in two mouse strains. Alcohol Clin Exp Res. 2008;32(5):840–52. doi: 10.1111/j.1530-0277.2008.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M, Poulos CX, Aramakis VB, Khamba BK, MacLeod CM. Effects of drink-stress sequence and gender on alcohol stress response dampening in high and low anxiety sensitive drinkers. Alcohol Clin Exp Res. 2007;31(3):411–22. doi: 10.1111/j.1530-0277.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- Zimmerberg B, Kajunski EW. Sexually dimorphic effects of postnatal allopregnanolone on the development of anxiety behavior after early deprivation. Pharmacol Biochem Behav. 2004;78(3):465–71. doi: 10.1016/j.pbb.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Spring K, Kunz-Ebrecht SR, Uhr M, Wittchen HU, Holsboer F. Effect of ethanol on hypothalamic-pituitary-adrenal system response to psychosocial stress in sons of alcohol-dependent fathers. Neuropsychopharmacology. 2004;29(6):1156–65. doi: 10.1038/sj.npp.1300395. [DOI] [PubMed] [Google Scholar]
- Zoethout RW, Schoemaker RC, Zuurman L, van Pelt H, Dahan A, Cohen AF, van Gerven JM. Central nervous system effects of alcohol at a pseudo-steady-state concentration using alcohol clamping in healthy volunteers. Br J Clin Pharmacol. 2009;68(4):524–34. doi: 10.1111/j.1365-2125.2009.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]