Abstract
Human natural killer (NK) cells are central in immune defense with their ability to lyse tumor cells, and virally infected cells. Increases in tumor formation and viral infection may occur if NK cytotoxic function is disrupted. Ziram (zinc dithiocarbamate) is used as an accelerating agent in the production of latex and to protect various fruits and vegetables from fungal infection. Previously, we have shown that exposure to ziram inhibits NK lytic function. Butyltin (BT) environmental contaminants, which also inhibit NK lytic function, cause rapid activations of mitogen activated protein kinases (MAPKs) and decreases in expression of the cytolytic proteins granzyme B and perforin (after 24 h) in exposed NK cells. MAPKs are important regulators of the lytic response of NK cells and spurious activation of these enzymes by contaminants would leave the NK cells unable to respond to appropriate targets. This study examined effects of ziram exposures on MAPKs (p44/42, p38, and JNK) and on levels of cytolytic proteins. Ten minute to 6 hour exposures of NK cells to ziram caused activation of MAPKs, p44/42 and p38. Exposure to ziram for 24 h caused a decrease in granzyme B and perforin levels. MAPK inhibitors were able to prevent these ziram-induced decreases in granzyme B and perforin. These results suggest that ziram-induced MAPK activation is at least in part responsible for decreased cytolytic function in ziram-exposed NK cells. Further, the results indicate that these changes are in common with other environmental contaminants that have been shown to decrease NK lytic function.
Keywords: NK cells, ziram, MAPK, granzyme, perforin
INTRODUCTION
Ziram is a dithiocarbamate used to treat a variety of fungal diseases in crops around the world such as potatoes, nuts, some fruits, and grain (Caldas et al., 2001). It is also used as an accelerating agent in the production of latex rubber (DeJong et al., 2002). Humans may be exposed to ziram by coming into contact with latex or ingesting treated crops (Caldas et al., 2001; Nettis et al., 2002). It has been shown to cause contact sensitivity in murine local lymph node assays and health care workers have exhibited positive responses to patch test (used to determine potential allergic reactions) of a mixture of latex rubber vulcanizing chemicals including ziram (DeJong et al., 2002). Ziram increases the concanavalin A stimulated production of interferon gamma (INF γ) and Interleukin 4 (IL4) in murine vascular lymph node cells (De Jong et al., 2002).
Natural Killer (NK) cells are lymphocytes that are capable of killing tumor cells, virally infected cells, and antibody-coated cells. NK cells are responsible for limiting the spread of blood-borne metastases as well as limiting the development of primary tumors (Lotzova 1993). These cells are the front line of immune response against tumor and virally infected cells due to their ability to lyse appropriate target cells without prior sensitization (Lotzova 1993; Vivier et al., 2004). Interference with NK cell function by any compound could increase the risk of viral infection and tumor formation.
A cascade of events is triggered by the binding of target cells to NK cells that leads to the release of cytotoxic proteins including granzyme B and perforin (housed in granules) from the NK cells, which lyse the target cells. Upon binding NK-sensitive target cells, non-receptor protein tyrosine kinases (PTKs) such as Syk, ZAP-70, Src and Pyk2 are activated (Gismondi et al., 2000). Through a series of signaling steps (Payne et al., 1991; Derijard et al., 1995; Han et al., 1996), PTK activation ultimately results in activation of mitogen-activated protein kinases (MAPKs) p44/42, p38, and JNK (Vivier et al.2004). Activation of MAPKs appears to be in part responsible for the release of cytotoxic granules as well as other regulatory aspects of NK cells (Trotta et al, 1998, Trotta et al., 2000, Wei et al., 1998).
It has been shown that even brief exposure of human NK cells to the environmental contaminants, tributyltin (TBT) and dibutyltin (DBT) induced irreversible inhibition of the ability of NK cells to destroy tumor targets (lytic function) (Whalen et al., 1999, 2002a, 2002b, Dudimah et al., 2007a, 2007b). This loss of lytic function is accompanied decreased expression of the cytolytic proteins perforin and granzyme (Thomas et al., 2004). TBT and DBT also activate MAPKs in NK cells (Aluoch and Whalen 2005; Aluoch et al., 2006; Aluoch et al., 2007; Odman-Ghazi et al., 2010).
Our previous studies have shown that purified NK cells treated with certain concentrations of ziram are less efficient at killing tumor cells (K562) (Wilson et al., 2004; Whalen et al., 2003). Ziram is effective in blocking the lytic function of highly purified NK cells at concentration as low as 125 nM and these effects increase with time (Wilson et al., 2004; Taylor et al., 2005). Importantly, it was also shown that a brief, 1 h, exposure to ziram ranging from 10-0.5 µM caused a persistent loss of lytic function when the cells were given as long as 6 days in ziram-free media before testing for lytic function (Taylor et al., 2005).
Based on the past studies showing that the most rapid consequence of exposures to TBT and DBT was activation of MAPKs (Aluoch and Whalen 2005; Aluoch et al., 2006; Aluoch et al., 2007; Odman-Ghazi et al., 2010), the current study examined the effects of ziram exposures (that lead to loss of lytic function) (Whalen et al., 2003; Wilson et al., 2004; Taylor et al., 2005) for their ability to alter the activation state of MAPKs in NK cells. NK cells were exposed to 10-0.5 µM Ziram for 10 min, 1 h, and 6 h and then monitored for MAPK activation. Concentrations of 10-2.5 µM ziram have previously been shown to cause a loss of NK lytic function following a 1 h exposure (Whalen et al., 2003; Taylor et al., 2005). Again, based on the effects seen with TBT and DBT, this study examined the NK cytotoxic granule proteins, granzyme B and perforin, to determine if their levels were decreased by exposure to ziram at concentrations and lengths of exposure known to decrease NK lytic function (Whalen et al., 2003; Taylor et al., 2005).
MATERIAL AND METHODS
Isolation of NK cells
Peripheral blood from healthy adult volunteer donors was used for this study. Blood samples (buffy coats) were obtained from a donation facility (American Red Cross, Portland or and Key Biologics, Memphis, TN). Highly purified NK cells were obtained using a rossetting procedure. Buffy coats were mixed with 0.6– 0.8 mL of RossetteSepTM human NK cell enrichment antibody cocktail (Stem Cell Technologies, Vancouver, British Colombia, and Canada) per 45 mL of buffy coat. The mixture was incubated for 25 min at room temperature with periodic mixing. Following the incubation, 6–9 mL of the mixture was layered onto 4 mL Ficoll-Hypaque (1.077 g/mL; Sigma, St. Louis, ML, USA) and centrifuged at 1200g for 20 min. The cell layer was collected and washed twice with phosphate buffered saline, pH 7.2 (PBS) and stored in complete media (RPMI-1640 supplemented with 10% heat-activated BCS, 2mM L-glutamine and 50 U penicillin G with 50 µg streptomycin/ml) at 1×106 cells/mL (Whalen et al., 2002b). The resulting cell preparation was >95% CD16+ and 0% CD3+ according to fluorescence microscopy
Chemical Preparation
Ziram was purchased from Wako Pure Chemicals Industries Limited (Osaka, Japan). Dimethylsulfoxide (DMSO) was obtained from Sigma-Aldrich (St. Louis, MO USA). A stock solutions of the compound was made in DMSO. Ziram was diluted in media to achieve the desired concentrations so that the final concentrations of DMSO did not exceed 0.01%.
Cell Treatment
NK cells (at a concentration of 1.5 million cells/ mL) were exposed in the following ways. 1. Ziram for 10 minutes: Cells were treated with the appropriate control or 10, 5, 2.5, 1, and 0.5 µM Ziram for 10 min at 37°C, 5%CO2. 2. Ziram for 1 hour: Cells were treated with the appropriate control or 10, 5, 2.5, 1, and 0.5 µM Ziram for 1 h at 37°C, 5%CO2. 3. Ziram for 6 hours: Cells were treated with the appropriate control or 10, 5, 2.5, 1, and 0.5 µM Ziram for 6 h at 37°C, 5%CO2. 3. Ziram for 24 hours: Cells were treated with the appropriate control or 2.5, 1, and 0.5 µM Ziram for 24 h at 37°C, 5%CO2. Following the above incubations the cells were washed twice and then lysed as described below.
Cell Viability
Cell viability was determined by trypan blue exclusion (Whalen et al., 2003). Cell number and viability, assessed at the beginning and end of each exposure period, did not vary significantly among the control cells and experimental conditions for any of the concentrations tested.
Cell Lysates
Cell lysates were made using NK cells treated as described in the cell treatment section. Following the above treatments, the cells were centrifuged and the cell pellets were lysed using 500 µL of lysis buffer (Active motif, Carlsbad, CA) per 10 million cells. The cell lysates were stored frozen at −80°C up to the point when they were run on SDS-PAGE. All controls and Ziram-exposed cells for a given experimental set-up (described above) were from an individual donor. Each of the experimental set-ups was repeated a minimum of three times using cells from different donors.
Western Blot
Cell lysates were run on 10% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane. The PVDF was immunoblotted with anti-phospho-p38 (Thr180 and Tyr182), anti-p38, anti-phospho-p44/42 (Thr202/Tyr204), anti-p44/42, anti-phospho-JNK, anti-JNK, and anti-β-actin antibodies (Cell Signaling Technologies, Beverly, MA). Antibodies were visualized using ECL chemiluminescent detection system (Amersham, Piscataway, NJ) and Kodak Image Station (Kodak, Rochester, NY). The density of each protein band was determined by densitometric analysis using the Kodak Image Station analysis software. The settings on the image station were optimized to detect the largest possible signal range and to prevent saturation of the system. Samples from all the experimental setups (listed in the cell treatment section) were run on a separate gel/blot. A given experimental set-up (as described in the cell treatment section) always had its own internal control. Thus, changes in protein expression were determined relative to the internal control. This determination provided relative quantitation by evaluating whether a given treatment changed expression of the specified protein relative to untreated cells. β-actin levels were determined for each condition to verify that equal amounts of protein were loaded. In addition, the density of each protein band was normalized to β-actin to correct for small differences in protein loading among the lanes.
Statistical analysis
Statistical analysis of the data for western blot analyses was carried out using ANOVA followed by pair-wise comparison using Student’s t test. A minimum of three separate determinations were carried out for each experimental set-up (n≥3) and statistical significance was noted at p<0.05.
RESULTS
Effects of 10 minute exposures to ziram on MAPKs in NK cells
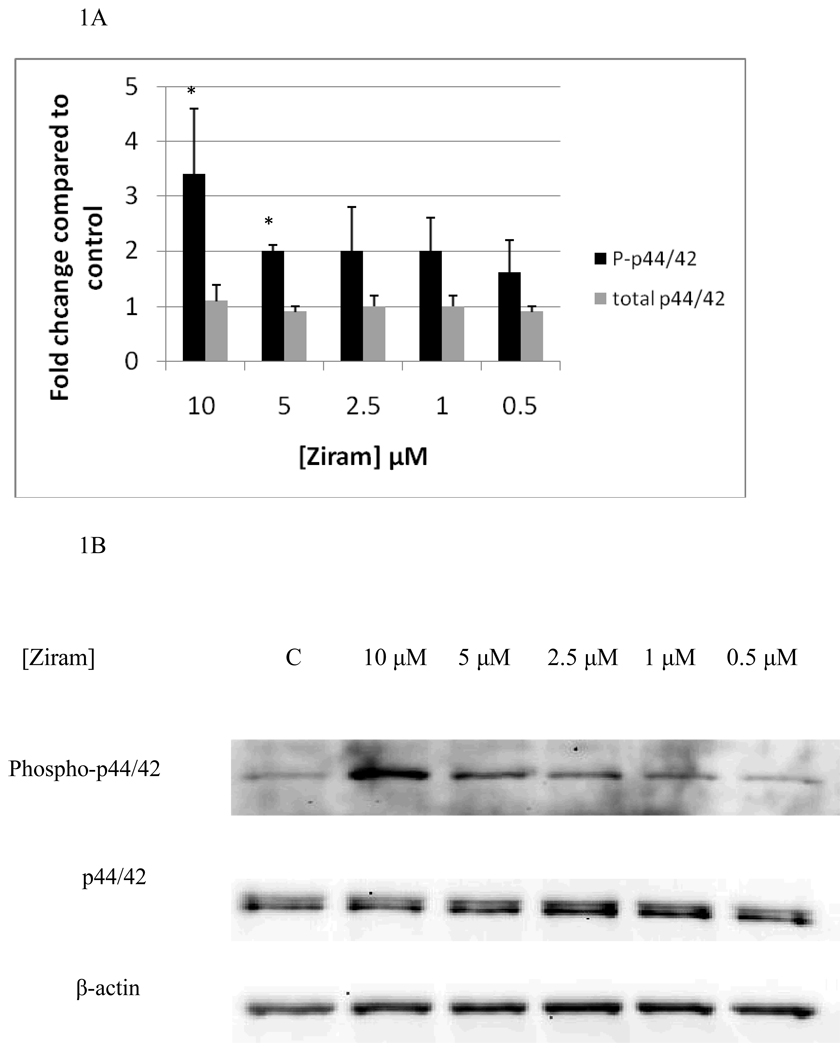
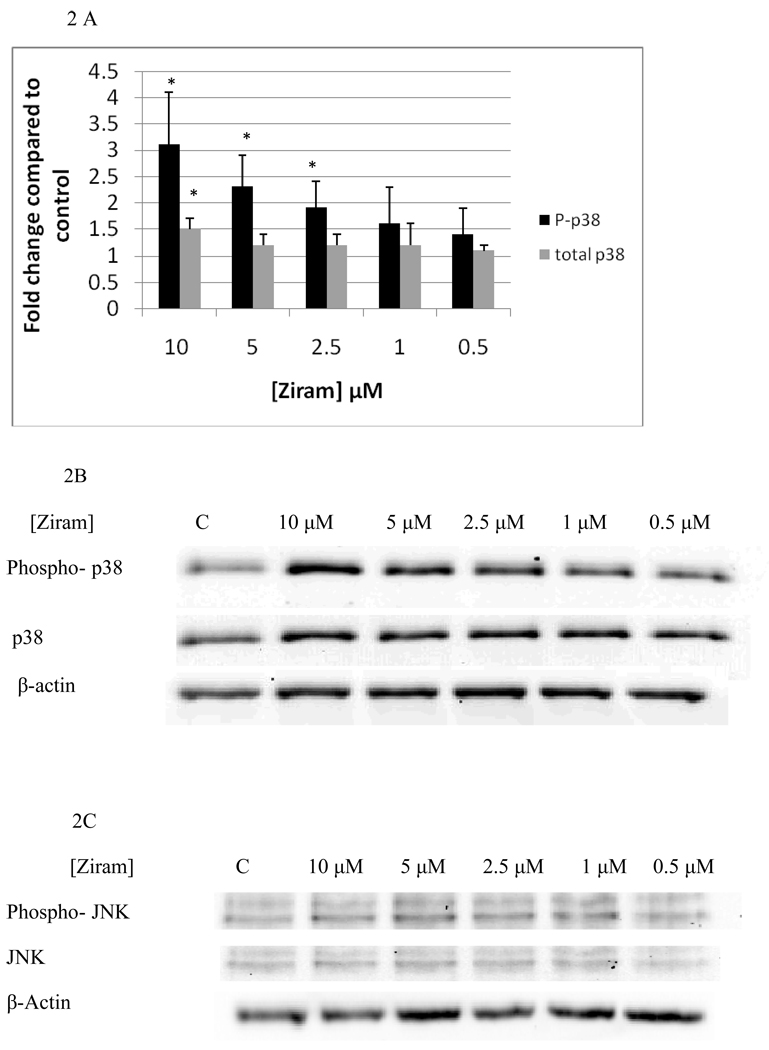
Figure 1 A and B show the effects of 10 minute exposures to 10 µM, 5 µM, 2.5 µM, 1 µM, and 0.5 µM ziram on p44/42. 10 minute exposures to 10 µM ziram caused a 3 fold increase in phosphorylation of p44/42(p<0.05) and 10 minute exposures to 5 µM ziram caused a 2 fold increase in phosphorylation of p44/42 (p<0.05). 10 minute exposures to 2.5 µM, 1 µM, and 0.5 µM ziram caused no significant increase in phosphorylation of p44/42(p>0.05). The total level of p44/42 was not affected by exposure to ziram (Figure 1A and B). 10 minute exposures to 10 µM ziram caused a 3 fold increase in phosphorylation of p38 (p<0.05), while exposures to 5 µM and 2.5 µM ziram caused 2 fold increases in phosphorylation of p38 (p<0.05) (Figure 2A and B). There were no significant changes in either phosphorylated or total JNK (Figure 2C, representative experiment).
Figure 1.
Effects of 10 minute exposures to 10-0.5 µM Ziram on phospho-p44/42 and total p44/42 in NK cells: A) levels of phospho-p44/42 and total p44/42 normalized to control in pure NK. Values are mean ± S.D. from at least three separate experiments using cells from different donors. * indicates significant difference compared to the control (p<0.05). β-actin levels were determined for each condition to verify that equal amounts of protein were loaded. In addition, the density of each protein band was normalized to β-actin to correct for small differences in protein loading among the lanes. B) Blot from a representative experiment.
Figure 2.
Effects of 10 minute exposures to 10-0.5 µM Ziram on phospho-p38 and total p38 and phospho-JNK and total JNK in NK cells: A) levels of phospho-p38 and total p38 normalized to control in pure NK. Values are mean ± S.D. from at least three separate experiments using cells from different donors. * indicates significant difference compared to the control (p<0.05). B) Blot from a representative experiment for phospho-p38 and total p38. C) Blot from a representative experiment for phospho-JNK and total JNK.
Effects of 1 h exposure to ziram on NK cell MAPKs
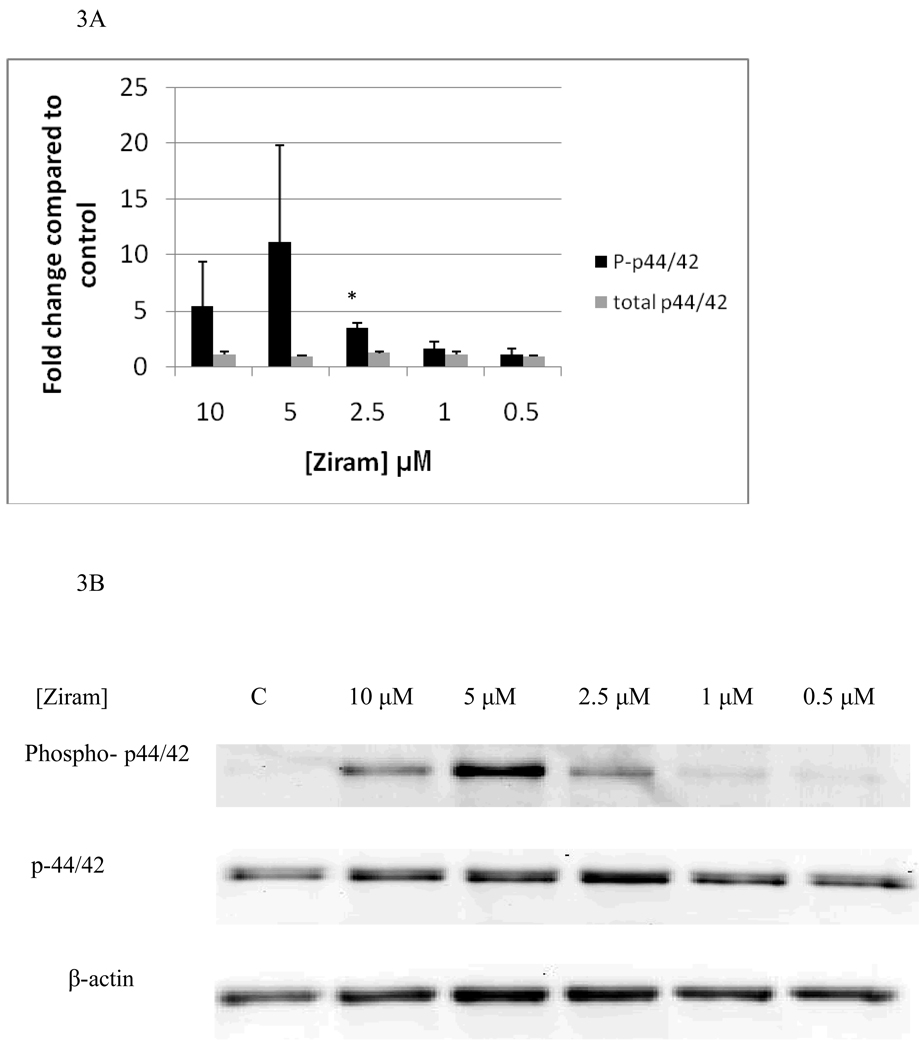
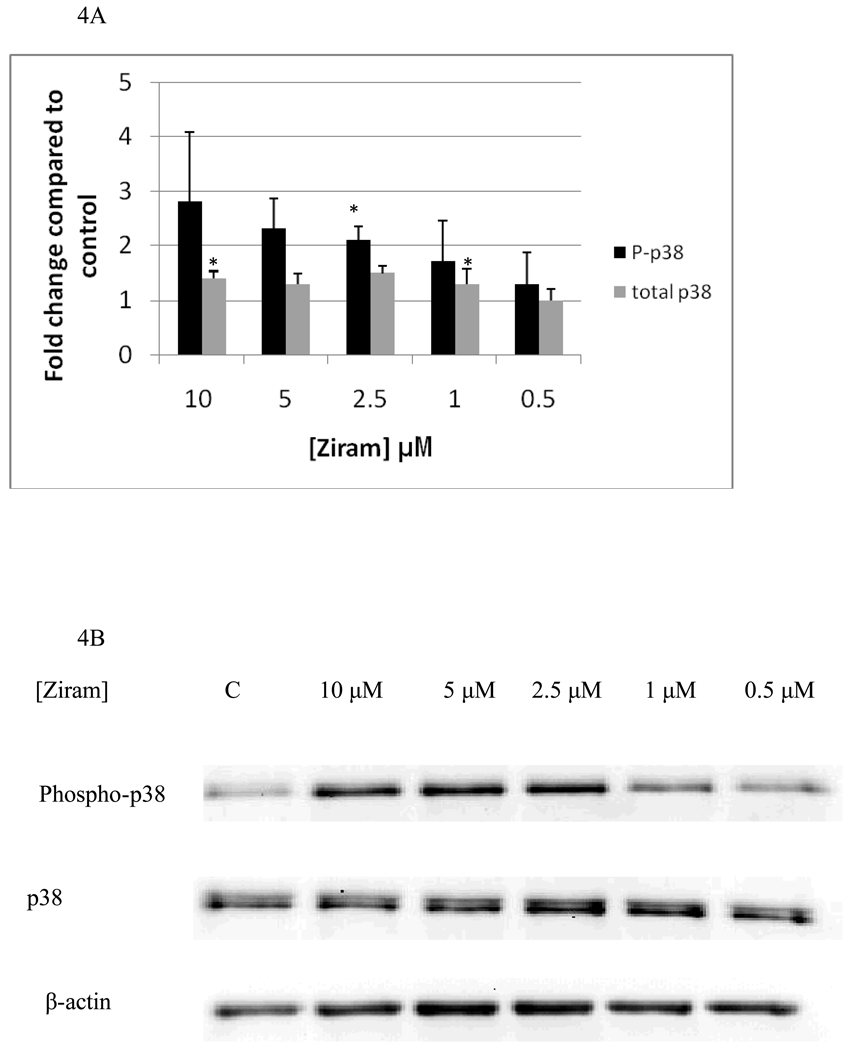
Effects of 1 h exposures to ziram on p44/42 are shown in Figure 3A and B. 1 h exposures to 10 µM and 5 µM ziram always caused increases in phosphorylation of p44/42 in each of the experiments carried out (a minimum of 3 separate experiments). The increases seen at 10 µM ranged from 2.5 fold to 10 fold while those seen at 5 µM ranged from 2 fold to 20 fold. However, the variability in fold increase was such that when the data was combined the increases gave a p value greater than 0.05. 1 h exposures to 2.5 µM ziram caused a 3 fold increase in phosphorylation of p44/42 which was statistically significant (p<0.05), while 1 h exposures to 1 µM and 0.5 µM ziram caused no significant change in phosphorylation of p44/42 (p>0.05). Effects of 1h exposures to 10 µM, 5 µM, 2.5 µM, 1 µM, and 0.5 µM ziram on p38 are shown in Figure 4A and B. 1 h exposures to 10 µM and 5 µM ziram caused increases in p38 phosphorylation in every experiment. The fold increases seen with 10 µM ziram were 1.8 to 4.3 fold and at 5 µM were 1.8–2.9 fold. At these concentrations, the p-value was greater than 0.05 when all experiments were combined due to the variability in the fold increases. A 1 h exposure to 2.5 µM ziram caused a very reproducible 2 fold increase in p38 phosphorylation (p<0.05) and a 1 h exposure to 1 µM, and 0.5 µM ziram caused no significant increase in p38 phosphorylation (p>0.05). As was seen after a 10 min exposure to ziram, there were no significant changes in either phosphorylated or total JNK with 1 h exposures to ziram (data not shown)
Figure 3.
Effects of 1 hour exposures to 10-0.5 µM Ziram on phospho-p44/42 and total p44/42 in NK cells: A) levels of phospho-p44/42 and total p44/42 normalized to control in pure NK. Values are mean ± S.D. from at least three separate experiments using cells from different donors. * indicates significant difference compared to the control (p<0.05). B) Blot from a representative experiment.
Figure 4.
Effects of 1 hour exposures to 10-0.5µM Ziram on phospho-p38 and total p38 in NK cells: A) levels of phospho-p38 and total p38 normalized to control in pure NK. Values are mean ± S.D. from at least three separate experiments using cells from different donors. * indicates significant difference compared to the control (p<0.05). B) Blot from a representative experiment.
Effects of 6 h exposure to ziram on NK cell MAPKs
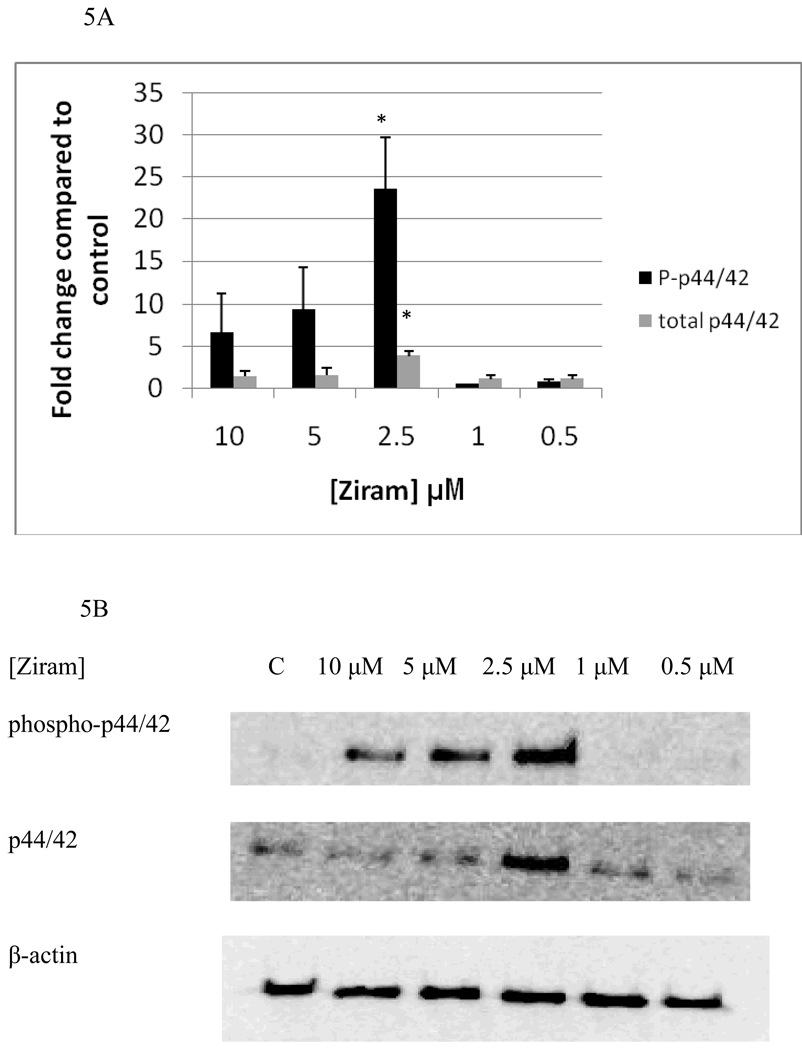
Figure 5A and B show the effects of 6 h exposures to 10 µM, 5 µM, 2.5 µM, 1 µM, and 0.5 µM ziram on p44/42. 6 h exposures to 10 µM ziram caused increases in phosphorylation of p44/42 that ranged from 1.4 to 9.7 fold while increases in phosphorylation of 4 to 13 fold were seen when NK cells were exposed to 5 µM ziram. As was seen with the 1 h exposure at these concentrations of ziram, the variability was such that when data from replicate experiments were combined the p value exceeded 0.05. A 6 h exposure to 2.5 µM ziram caused a 23 fold increase in phosphorylation of p44/42 (p<0.05). A 6 h exposure to 10 µM, 5 µM, 2.5 µM, 1 µM, and 0.5 µM ziram caused no statistically significant increase in p38 phosphorylation (data not shown). Again, there were no significant changes in either phosphorylated or total JNK after 6 h exposures to ziram (data not shown).
Figure 5.
Effects of 6 hour exposures to 10-0.5 µM Ziram on phospho-p44/42 and total p44/42 in NK cells: A) levels of phospho-p44/42 and total p44/42 normalized to control in pure NK. Values are mean ± S.D. from at least three separate experiments using cells from different donors. * indicates significant difference compared to the control (p<0.05). B) Blot from a representative experiment.
Effects of ziram on NK cell cytolytic proteins
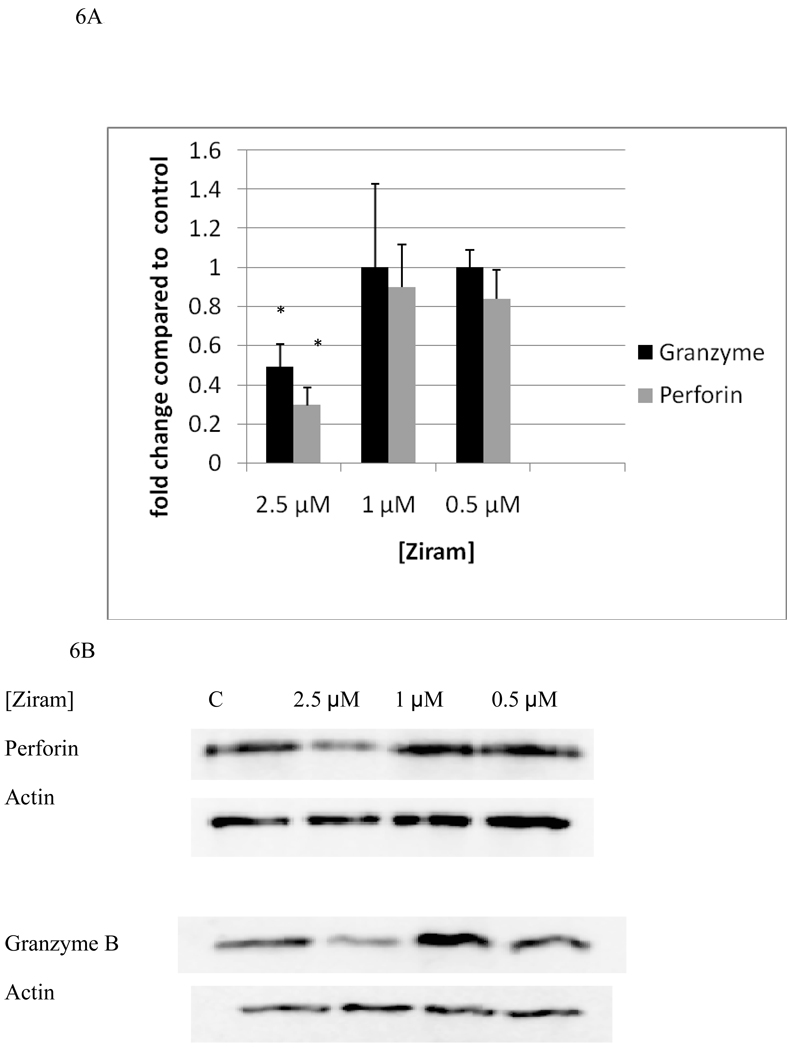
Effects of 2.5 µM, 1 µM, and 0.5 µM ziram on levels of granzyme B and perforin are shown in figure 6A and B. A 24 h exposure to 2.5 µM ziram caused a 51% decrease in granzyme levels (P<0.05) while 24 h exposure to 1 µM and 0.5 µM ziram caused no decreases in NK cell granzyme levels. A 24 h exposure to 2.5 µM ziram caused a 70% decrease perforin levels (P<0.05) a 24 h exposure to 1 µM and 0.5 µM ziram caused no significant decreases in NK cell perforin.
Figure 6.
Effects of 24 hour exposures to 2.5-0.5 µM Ziram on levels of granzyme B and perforin in NK cells: A) levels of granzyme B and perforin normalized to control in pure NK. Values are mean ± S.D. from at least three separate experiments using cells from different donors. * indicates significant difference compared to the control (p<0.05). B) Blots from a representative experiment.
Effects of inhibitors of p44/42 and p38 activation on the decreases in cytolytic proteins seen with 2.5 µM ziram
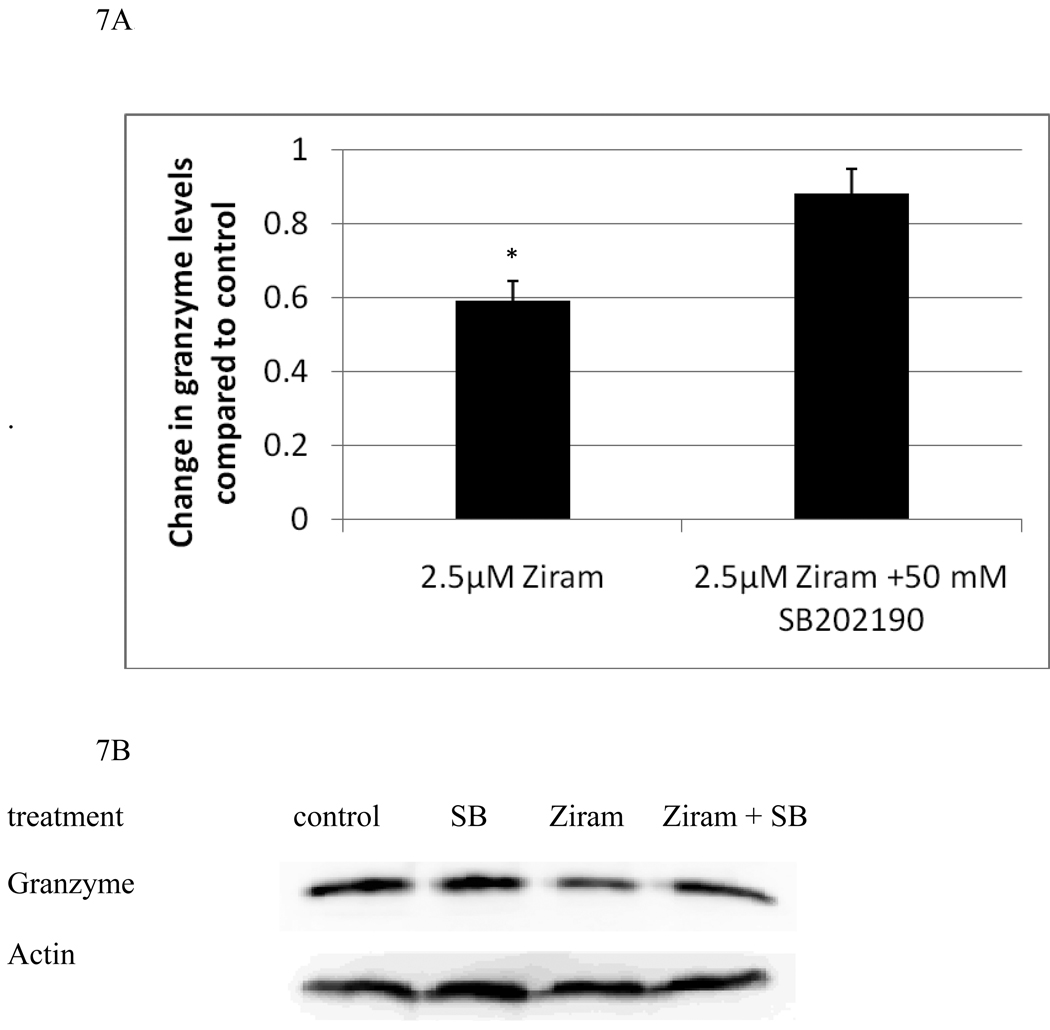
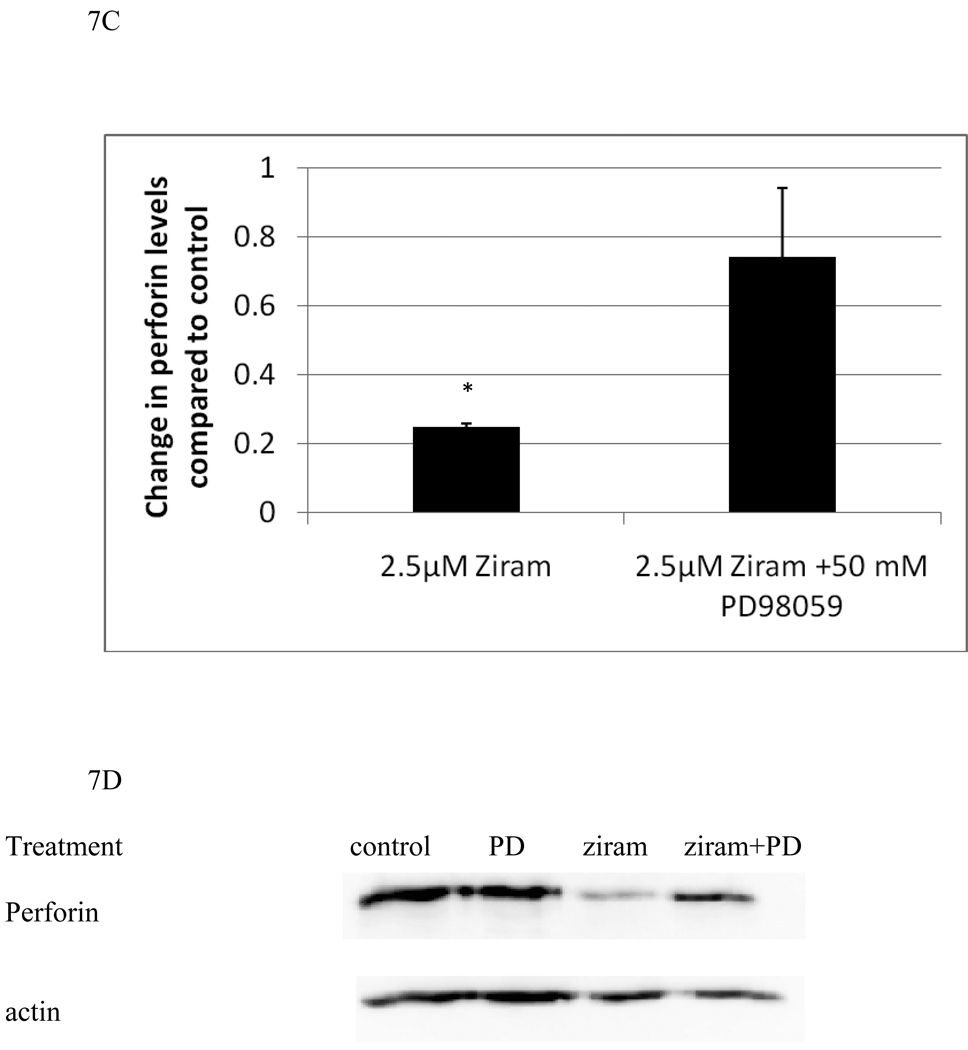
In order to examine whether there was a role for the ziram-induced activation of either p44/42 or p38 in the decreases in granzyme and perforin seen with ziram treatments, we carried out experiments using specific inhibitors of each enzyme. NK cells were treated with 2.5 µM ziram which reliably decreased both granzyme and perforin levels in NK cells after a 24 h exposure. NK cells that were pretreated for 1 h with either 50 mM SB202190 (a selective inhibitor of p38 activation) or 50 mM PD98059 (a selective inhibitor of p44/42 activation) or with control media were then exposed to either control or 2.5 µM ziram for 24 h. The cells were examined for levels of granzyme and perforin using western blot. Figures 7A and 7B show that cells pretreated with SB202190 for 1 h prior to a 24 h exposure to 2.5 µM ziram showed only a slight decrease in granzyme compared to control cells. Thus, inhibition of p38 activation is able to prevent nearly all of the loss of granzyme B that is induced by a 24h exposure to 2.5 µM ziram. However, SB202190 was unable to block the ziram-induced decrease in perforin. Conversely, inhibition of p44/42 by PD98059 was able to block the ziram-induced decrease in perforin but had no effect on ziram-induced decreases in granzyme B (Figures 7C and 7D).
Figure 7.
p38 inhibitor, SB202190, reverses ziram-induced granzyme B decreases and the p44/42 inhibitor, PD98059, reverses ziram-induced perforin decreases in NK cells: A) levels of granzyme B in cells exposed to 2.5 µM ziram in the presence and absence of the p38 inhibitor, SB202190. Values are mean ± S.D. from three separate experiments using cells from different donors. * indicates significant difference compared to the cells treated with ziram in the presence of SB202190 (p<0.05). B) Blots from a representative experiment. C) levels of perforin in cells exposed to 2.5 µM ziram in the presence and absence of the p44/42 inhibitor, PD98059 . Values are mean ± S.D. from three separate experiments using cells from different donors. * indicates significant difference compared to the cells treated with ziram in the presence of PD98059 (p<0.05). D) Blots from a representative experiment.
DISCUSSION
Earlier studies have shown that ziram exposures decrease the lytic function of NK cells (Taylor et al., 2005; Wilson et al., 2004; Whalen et al., 2003). A brief (1h) exposure to ziram induces decreases in lytic function that can persist for at least 6 days in ziram-free media (Taylor et al., 2005). Ziram is not considered mutagenic (Franekic et al., 1994; Zenzen et al., 2001), however, chromosomal changes have been seen in workers exposed to ziram for 3–5 years, indicating some risk of mutations (Edwards et al., 1991). There do not appear to be any studies examining the levels of ziram in human blood. Ziram’s ability to inhibit NK cells (Whalen et al., 2003; Wilson et al., 2004; Taylor et al., 2005), which are involved in preventing tumor formation, may classify it as a promoter of increased cancer risk. Examining the effects of this compound on human NK function is valuable for those who work in the industry of producing this compound, those in agriculture using it as a fungicide and individuals that may live near fields that have been treated with ziram.
The purpose of the current study was to examine the effects of ziram on the activation state of MAPKs in NK cells as well as NK cytolytic protein levels. NK cells were treated with levels of the compounds that have previously been shown to cause a loss of NK function following a 1 h exposure. As blood levels of ziram in humans have not been measured, it is hard to know if the levels at which we see effects would be experienced in exposed individuals. Exposures to another category of environmental contaminant, butyltins (BTs), have been shown to activate MAPKs (Aluoch and Whalen, 2005; Aluoch et al., 2006; Aluoch et al., 2007; Odman-Ghazi et al., 2010) at levels that have been measured in human blood samples (Whalen et al., 1999; Kanan et al, 1999). As many of the other changes seen in NK cells with exposures to BTs, such as persistent loss of NK function with a brief exposure to the compounds and decreased cell-surface protein expression (Whalen et al., 2002; Odman-Ghazi et al., 2003; Dudimah et al., 2007a, b), were also seen with ziram exposures (Wilson et al., 2004; Taylor et al., 2005; Taylor and Whalen, 2009), we examined ziram’s ability to activate MAPKs. Exposures as brief as 10 min showed significant increases in activating phosphorylation of the MAPKs (p44/42 and p38). Overall there was an increase in MAPK activation with dose as well as with time. As was noted in the results section, higher concentrations of ziram (10 µM, and 5 µM) caused increases in activations of p44/42 and p38 which were on average greater at 1 h than those seen at 10 min. However, they were not statistically significant due to the high variability of the increases seen from one experiment to the next. This was also true after a 6 h exposure for p44/42. However, a six hour exposure to 2.5 µM ziram caused a dramatic 23 fold increase in p44/42 phosphorylation, which was much greater than that seen at 10 or 5 µM.
It is of interest that the activation of both p44/42 and p38 seen at higher concentrations (10 and 5 µM) of ziram for 1 h was highly variable but became stable at 2.5 µM. It is possible that when there is such a large activation of the MAPKs at 10 and 5µM that this may induce a feedback inhibitory process attempting to inhibit their activation and leading to significant variability in the measurable activation. The stability of the more moderate activation seen at 2.5 µM may be due to that fact that this level of activation doesn’t cause as robust an activation of this turn-off mechanism . It has been shown (in other mammalian cells) that activation of p38 induces the production of MAPK phosphatase-1 (Staples et al., 2010) which is capable of dephosphorylating and thus inactivating both p44/42 and p38 MAPKs (Dickinson and Keyse, 2006). It is possible that such a p38 induced activation of an MKP-1 may be occurring in NK cells and is an area for further study. It was also possible that NK cell death was occurring at the higher concentrations of Ziram at 1 h and 6 h and thus causing the increased variability in the activations of MAPKs. However, we examined the viability of the NK cells under these conditions and it was not decreased compared to control cells (materials and methods section).
The release of NK cytolytic granules and thus the cytolytic process is in part regulated by MAPKs (Trotta et al, 1998, Trotta et al., 2000, Wei et al., 1998). In normally functioning NK cells, the binding of a target cell will stimulate activation of MAPKs. Exposure of NK cells to ziram activates the p44/42 and p38 MAPKs. The activation of these MAPKs by ziram would then leave the NK cell unable to activate MAPKs in response to target binding as these enzymes would have already been activated by the Ziram exposure and are no longer available to be activated by the target cell’s binding. We have shown that premature activation of p44/42 by phorbol 12-myristate 13-acetate (PMA) leads to inhibition of NK lytic function (Dudimah et al., 2010). Compounds that activate p44/42 to a similar extent to that seen with PMA inhibit NK lytic function to a similar extent (Dudimah et al., 2010).
Previously, we have shown that tributyltin (TBT) was able to activate p44/42, p38, and JNK MAPKs (Aluoch et al., 2006; Aluoch et al., 2007) while dibutyltin (DBT) activated only p44/42 and p38 but not JNK (Odman-Ghazi et al., 2010). The fact that ziram activated only p44/42 and p38 was similar to the results seen with DBT. However, the fold activation seen with Ziram at 10 and 5 µM is greater than that seen with DBT at the same concentrations and lengths of exposure. All three compounds share the ability to activate NK-cell MAPKs however, the pattern and intensity of activation varies depending on the compound. The shared target of MAPKs among these three different compounds may indicate that the MAPK signaling pathway in NK cell (which is known to regulate NK responsiveness) is particularly vulnerable to an array of environmental contaminants.
The current study examined ziram effects on NK cell cytolytic proteins, showing that a 24 h exposure to 2.5 µM ziram caused decreases in granzyme B levels and perforin levels. These decreases would also diminish the capacity of the NK cells to respond to target cells. These results are similar to what has been found with TBT (Thomas et al., 2004) and DBT (Caltin et al., 2005). NK cytolytic protein levels appear to be at least in part regulated by the MAPK dependent transcription regulator AP-1 (Hanson et al., 1993) and thus, the decrease in their expression with longer ziram exposures may be a result of the ziram-induced dysregulation of MAPKs. We investigated this possibility by exposing NK cells to ziram in the presence of an inhibitor that prevents p44/42 activation (PD98059) as well as an inhibitor that prevents p38 activation to see if inhibiting ziram-induced p44/42 or p38 activation could prevent ziram induced decreases in granzyme and perforin. The inhibitors were used at concentrations that prevent activation of these MAPKs in NK cells (Abraha et al. 2009). The results showed that activation of p38 by ziram is needed for ziram-induced losses of granzyme B, while p44/42 activation appears to be needed for ziram–induced decreases in perforin. These data show direct involvement of each of the MAPKs that are activated by ziram exposure in the loss of cytolytic proteins seen with ziram.
Acknowledgements
This research was supported by Grant 2S06GM-08092-35 from the National Institutes of Health.
Footnotes
Declaration of Interest
The Authors report no conflicts of interest. The Authors are alone responsible for the content and writing of the paper.
REFERENCES
- Abraha A, Whalen MM. The role of p44/42 activation in tributyltin-induced inhibition of human natural killer cells: effects of MEK inhibitors. J. Appl. Toxicol. 2009;29:165–173. doi: 10.1002/jat.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Pattern of MAP kinases p44/42 and JNK activation by non-lethal doses of tributyltin in human natural killer cells. Arch. Toxicol. 2007;81:271–277. doi: 10.1007/s00204-006-0155-4. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells. Toxicology. 2006;224:229–237. doi: 10.1016/j.tox.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Whalen MM. Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cells. Toxicology. 2005;209:263–277. doi: 10.1016/j.tox.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Caldas ED, Conceicao MH, Miranda MC, de Souza LCKR, Lima JF. Determination of dithiocarbamate fungicide residues in food by a spectrophotometric method using a vertical disulfide reaction system. J. Agricult. And Food Chem. 2001;49:4521–4525. doi: 10.1021/jf010124a. [DOI] [PubMed] [Google Scholar]
- Catlin R, Shah H, Bankhurst AD, Whalen MM. Dibutyltin exposure decreases granzyme B and perforin in human natural killer cells. Environmental Toxicology and Pharmacology. 2005;20:395–403. doi: 10.1016/j.etap.2005.03.012. [DOI] [PubMed] [Google Scholar]
- De Jong WH, Tentij M, Spiekstra SW, Vandebriel RJ, Van Loveren H. Determination of the sensitizing activity of the rubber contact sensitizers TMTD, ZDMC, MBT and DEA in a modified local lymph node assay and the effect of sodium dodecyl sulfate pretreatment on local lymph node responses. Toxicology. 2002;176:123–134. doi: 10.1016/s0300-483x(02)00131-2. [DOI] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barret T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J. Cell Sci. 2006;119:4607–4615. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Odman-Ghazi SO, Hatcher F, Whalen MM. Effect of tributyltin (TBT) on ATP levels in human natural killer (NK) cells: Relationship to TBT-induced decrease in NK function. J Appl Toxicol. 2007a;27:86–94. doi: 10.1002/jat.1202. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Gibson C, Whalen MM. Effect of Dibutyltin (DBT) on ATP levels in human natural killer cells. Environ. Toxicol. 2007b;22:117–123. doi: 10.1002/tox.20252. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Griffey D, Wang X, Whalen MM. Activation of p44/42 MAPK plays a role in the TBT-induced loss of human natural killer (NK) cell function. Cell Biology and Toxicology. 2010;26:435–444. doi: 10.1007/s10565-010-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards IR, Ferry DG, Temple WA. Fungicides and related compounds. In: Hayes WJ, Laws ET, editors. Handbook of pesticide toxicology. Vol. 3, Classes of pesticides. New York: Acedemic Press; 1991. [Google Scholar]
- Franekic J, Bratulic N, Pavlica M, Papes D. Genotoxicity of dithiocarbamates and their metabolites. Mutation Research. 1994;325:65–74. doi: 10.1016/0165-7992(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Gismondi A, Jacobelli J, Mainiero F, Paolini R, Piccoli M, Frati L, Santoni A. Cutting Edge: Functional Role for Proline-Rich Tyrosine Kinase 2 in NK Cell-Mediated Natural Cytotoxicity. J. Immunol. 2000;164:2272–2276. doi: 10.4049/jimmunol.164.5.2272. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol. Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- Hanson RD, Grisolano JL, Lay TJ. Consensus AP-1 and CRE motifs upstream from the human cytotoxic serine protease B (CSP-B/CGL-1) gene synergizes to activate transcription. Blood. 1993;82:2749–2757. [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP. Occurrence of butyltin compounds in human blood. Environ. Sci. Technol. 1999;33:1776–1779. [Google Scholar]
- Lotzova E. Definition and function of natural killer cells. Nat. Immun. 1993;12:169–176. [PubMed] [Google Scholar]
- Nettis E, Assennato G, Ferrannini A, Tursi A. Type I allergy to natural rubber latex and type IV allergy to rubber chemicals in health care workers with glove-related skin symtoms. Clin. Exp Allergy. 2002;32:441–447. doi: 10.1046/j.1365-2222.2002.01308.x. [DOI] [PubMed] [Google Scholar]
- Odman-Ghazi SO, Hatcher F, Whalen MM. Expression of Functionally Relevant Cell Surface Markers in Dibutyltin-exposed Human Natural Killer Cells. Chemico-Biological Interactions. 2003;146:1–18. doi: 10.1016/s0009-2797(03)00069-3. [DOI] [PubMed] [Google Scholar]
- Odman-Ghazi SO, Abraha A, Isom ET, Whalen MM. Dibutyltin activates MAP kinases in human natural killer cells, in vitro. Cell Biology and Toxicology. 2010 doi: 10.1007/s10565-010-9157-3. in press. NIHMSID#183231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DM, Rossomando AJ, Martino P, Erickson AK, Her JH, Shabanowitz J, Hunt DF, Weber MJ, Sturgill TW. Identification of the regulatory phosphorylation sites in p42/mitogen-activated protein kinase (MAP kinase) EMBO Journal. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples CJ, Owens DM, Maier JV, Cato ACB, Keyse SM. Cross-talk between p38α and JNK MAPK pathways mediated by MAP kinase phosphatase-1 determines cellular sensitivity to radiation. J. Biol. Chem. 2010;285:25928–25940. doi: 10.1074/jbc.M110.117911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TR, Tucker T, Whalen MM. Persistent inhibition of human natural killer cell function by ziram and pentachlorophenol. Environmental Toxicology. 2005;20:418–424. doi: 10.1002/tox.20127. [DOI] [PubMed] [Google Scholar]
- Taylor TR, Whalen MM. Effects of Ziram on tumor-cell-binding capacity, cell-surface marker expression and ATP levels of human natural killer cells. Cell Biology and Toxicology. 2009;25:447–455. doi: 10.1007/s10565-008-9098-2. PMCID: PMC2732751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LD, Shah H, Green S, Bankhurst AD, Whalen MM. Tributyltin exposure causes decreased granzyme B and perforin levels in human natural killer cells. Toxicology. 2004;200:221–233. doi: 10.1016/j.tox.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Trotta R, Puorro KA, Paroli M, Azzoni L, Abebe B, Eisenlohr LC, Perussia B. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-regulated kinases. J. Immunol. 1998;161:6648–6656. [PubMed] [Google Scholar]
- Trotta R, Fettuciari K, Azzoni L, Abebe B, Puorro KA, Eisenlohr LC, Perussia B. Differential role of p38 and c-Jun N-terminal kinase 1 mitogen-activated protein kinases in NK cell cytotoxicity. J. Immunol. 2000;165:1782–1789. doi: 10.4049/jimmunol.165.4.1782. [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Wei S, Gamero AM, Liu JH, Daulton AA, Valkov NI, Trapani JA, Larner AC, Weber MJ, Djeu JY. Control of lytic function by mitogen- activated protein kinase/extracellular regulatory kinase 2 (ERK2) in a human natural killer cell line: identification of perforin and granzyme B mobilization by functional ERK2. J. Exp. Med. 1998;187:1753–1765. doi: 10.1084/jem.187.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of environmentally relevant concentrations of butyltins on human natural killer cells in vitro. Environ. Res. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Green SA, Longanathan BG. Brief Butyltin Exposure Induces Irreversible Inhibition of the Cytotoxic Function on Human Natural Killer Cells, In vitro. Environ Res. 2002a;88:19–29. doi: 10.1006/enrs.2001.4318. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Ghazi S, Loganathan BG, Hatcher F. Expression of CD16, CD18 and CD56 in tributyltin-exposed human natural killer cells. Chemico-Biol. Interact. 2002b;139:159–176. doi: 10.1016/s0009-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Yamashita N, Saito T. Immunomodulation of Human Natural Killer Cell Cytotoxic Function by Triazine and Carbamate Pesticides. Chemico-Biological Interactions. 2003;145:311–319. doi: 10.1016/s0009-2797(03)00027-9. [DOI] [PubMed] [Google Scholar]
- Wilson S, Dzon L, Reed A, Pruitt M, Whalen MM. Effects of Extended, In Vitro, Exposures to Low Levels of Organotin and Carbamate Pesticides on Human Natural Killer Cell Cytotoxic Function. Environmental Toxicology. 2004;19:554–563. doi: 10.1002/tox.20061. [DOI] [PubMed] [Google Scholar]
- Zenzen V, Fauth E, Zankl H, Janzowski C, Eisenbrand G. Mutagenic and cytotoxicity effectiveness of zinc dimethyl and zinc disonolydithiocarbamate in human lymphocyte cultures. Mutation Research. 2001;497:89–99. doi: 10.1016/s1383-5718(01)00238-8. [DOI] [PubMed] [Google Scholar]