Abstract
Insulin resistance is a major characteristic of obesity and type 2 diabetes and develops in multiple organs, including the heart. Compared to other organs, the physiological role of cardiac insulin resistance is not well understood. The heart uses lipid as a primary fuel, but glucose becomes an important source of energy in ischemia. The impaired ability to utilize glucose may contribute to cell death and abnormal function in the diabetic heart. Recent discoveries on the role of inflammation, mitochondrial dysfunction, and ER stress in obesity have advanced our understanding of how insulin resistance develops in peripheral organs. This review will apply these findings to the diabetic heart to provide new insights into the mechanism of cardiac insulin resistance.
Facts about type 2 diabetes and obesity
The prevalence of diabetes is increasing at an alarming rate, and the current world-wide diabetic population of 285 million is expected to almost double by the year 2030 [1]. In the U.S., diabetes affects 26 million people, accounting for more than 8% of the U.S. population. This disturbing trend is partly due to an epidemic increase in obesity, which is a major cause of type 2 diabetes. Recent data from the Centers for Disease Control and Prevention indicate that 68% of American adults are overweight. Daily consumption of food high in calories, along with a sedentary lifestyle, has led to the obesity epidemic. Thus, type 2 diabetes and obesity are intimately linked, and together they increase the risk of cardiovascular events, a leading cause of death in diabetic subjects [2]. Despite this apparent epidemiological evidence, how type 2 diabetes and obesity affect the heart remains poorly understood.
Insulin resistance is a major characteristic of type 2 diabetes, and similar to other metabolic organs, the diabetic heart develops insulin resistance. As we begin to understand how insulin resistance develops in peripheral organs and the underlying role of obesity, inflammation, and ER stress in this process, it is reasonable to ask whether these causal events of peripheral insulin resistance underlie cardiac insulin resistance. This article reviews recently discovered mechanisms of peripheral insulin resistance and applies them to the diabetic heart to provide new insights into etiology of diabetic heart disease.
Multi-faceted characteristics of the diabetic heart
The human heart is a challenging organ in which to investigate, diagnose, and treat anomalies and disease states. When cardiac abnormality is phenotypically apparent or when patients are symptomatic, heart disease has often progressed to an advanced stage with limited therapeutic options. There are numerous abnormalities that can be detected in the hearts of diabetic and obese subjects. Structural changes are observed in the diabetic heart of humans and animal models. Concentric left ventricular (LV) hypertrophy, with increases in LV wall thickness and LV mass index, dilated cardiomyopathy, and extracellular fibrosis are found in the diabetic heart [3]. Functional abnormalities affecting LV systolic and diastolic function are also seen in the diabetic heart [4]. Tissue Doppler and flow analysis suggests that diastolic dysfunction may precede significant systolic disorder affecting ejection fraction and cardiac output in type 2 diabetes [5]. Further, there are metabolic changes in the diabetic heart such as increased lipid oxidation and intramyocardial accumulation of triglyceride [6]. The diabetic heart is also characterized by a reduced capacity to utilize glucose and insulin resistance [7]. Lastly, the diabetic heart manifests cellular changes including oxidative stress with increased generation of reactive oxygen species (ROS), mitochondrial dysfunction, and apoptosis [8]. With such multi-faceted abnormalities in the diabetic heart, it is difficult to discern which of these events is causally associated with type 2 diabetes and which events predispose the diabetic heart for failure.
Metabolic Processes and Regulation of the Normal Heart
Energy demand of the working heart
Normal cardiac function is dependent on a constant rate of ATP synthesis by mitochondrial oxidative phosphorylation and to a much lesser extent, on glycolysis. Under physiological conditions, lipid oxidation is responsible for 60~80% of cardiac energy demand with the remainder provided by glucose metabolism [9]. The main source of lipid for cardiac metabolism is supplied by free fatty acids (FFA) bound to albumin and by fatty esters present in chylomicrons and very-low-density lipoproteins. Fatty acids can be taken up by cardiomyocytes passively via diffusion across the cell membrane as well as by a protein-mediated mechanism involving fatty acid transport proteins (FATPs) and CD36 [10]. FATP1 is a 646-amino acid integral plasma membrane protein that transports long-chain fatty acids and is highly expressed in tissues with active lipid metabolism, such as the heart, adipose tissue, and skeletal muscle [10]. CD36 is a transmembrane protein that transports long-chain fatty acids and is also highly expressed in heart, adipose tissue, and skeletal muscle [11]. In addition to fatty acid transport across the cell membrane, fatty acid binding proteins (FABPs) such as adipocyte FABP (aP2) and keratinocyte FABP (mal1) are abundant low-molecular weight cytoplasmic proteins that are involved in intracellular transport and metabolism of fatty acids [12]. Fatty acid carriers play an important role in lipid uptake into cardiomyocytes because Cd36 deletion markedly reduces myocardial lipid metabolism in mice [13].
Although mitochondrial lipid oxidation is the principal energy source for the normal heart, maintenance of cardiac glucose metabolism is important for normal cardiac function [14]. The heart is very similar to skeletal muscle in that both organs express GLUT4, the major insulin-responsive glucose transporter, and GLUT1. GLUT4 and GLUT1 account for 60% and 40% of total glucose carriers, respectively [15]. Glucose metabolism is at least 4-fold greater in heart than in skeletal muscle and adipose tissue, which may be attributed to a greater expression of GLUT4 proteins in the heart than in other organs [16]. Insulin further promotes glucose uptake into cardiomyocytes by binding to the insulin receptor on the cell-surface and activating intracellular signaling proteins. This involves auto-phosphorylation of the insulin receptor, tyrosine phosphorylation of insulin receptor substrate (IRS), and activation of phosphatidyl-inositol-3 kinase (PI 3-kinase), phosphoinositide-dependent kinase 1 (PDK1), Akt/protein kinase B (PKB), and protein kinase C (PKC)-λ/ζ [17]. Activation of insulin signaling leads to the translocation of glucose transporters (GLUT4) from an intracellular pool to the cell surface and increases glucose transport into cells [17]. Insulin also redistributes GLUT1 from an intracellular site to the surface of cardiomyocytes, but the effect of insulin on GLUT1 is smaller than its effect on GLUT4 [18].
The importance of glucose metabolism is demonstrated by findings from mice with heart-specific ablation of Slc2a4 (GLUT4; G4H−/−). These mice develop major morphological alterations in the heart and exhibit cardiac hypertrophy [19]. Insulin-stimulated glucose uptake is also completely abolished in the heart of G4H−/− mice, but basal cardiac glucose metabolism is elevated. Despite preserved cardiac contractile performance, ischemia-associated stress causes profound and irreversible systolic and diastolic dysfunction in G4H−/− mice [20]. Furthermore, in mice with cardiomyocyte-selective deletion of the insulin receptor (CIRKO) basal glucose transport in isolated cardiomyocytes and insulin action on glucose uptake and glycolysis in isolated working hearts are significantly diminished [21]. The hearts of CIRKO mice are smaller due to reduced cardiomyocyte size [21]. In contrast to the G4H−/− mice, the CIRKO heart shows a global impairment in cardiac function, affecting cardiac output and power, ventricular fractional shortening, and ejection fraction [21]. While these findings implicate cardiac insulin resistance in the pathogenesis of diabetic heart disease, the underlying mechanism remains unknown.
AMPK is a major regulator of cardiac metabolism
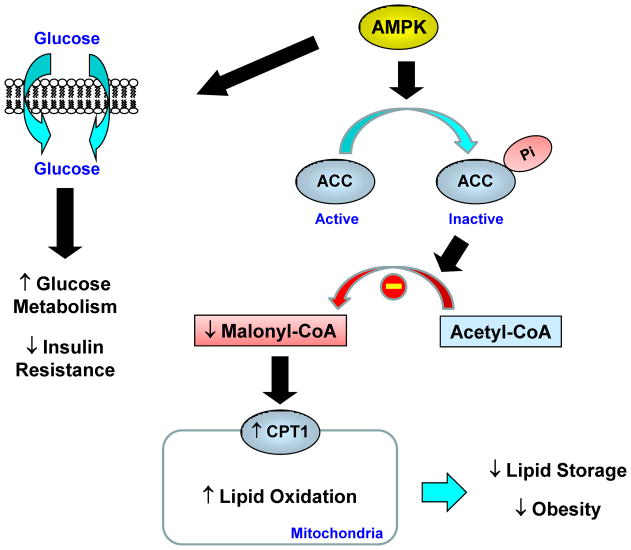
5′AMP-activated protein kinase (AMPK), a serine-threonine kinase, is an important regulator of cardiac energy metabolism [22]. AMPK is a heterotrimer of an α catalytic subunit and β and γ regulatory subunits. Activation of AMPK is mediated by phosphorylation of the Thr172 residue located within the α1 and α2 catalytic subunits, and this process is regulated by upstream kinases (AMPK-activating protein kinases) such as LKB1 [23]. AMPK is activated during myocardial ischemia in response to an increased AMP/ATP ratio and to stimulation by hormones, such as leptin and adiponectin [24,25]. AMPK regulates lipid metabolism by phosphorylating and inactivating acetyl-CoA carboxylase (ACC) [26]. ACC is a biotin-dependent enzyme and catalyzes the synthesis of malonyl CoA, which is an essential substrate for fatty acid synthase and a potent inhibitor of carnitine palmitoyl-CoA transferase-I (CPT-I) [27]. Overall, AMPK acts as an important regulator of myocardial lipid oxidation by inactivating ACC and reducing malonyl CoA levels, which subsequently increase CPT-I activity and mitochondrial lipid oxidation (Figure 1).
Fig. 1. Regulation of lipid and glucose metabolism by AMPK.
AMP-activated protein kinase (AMPK) regulates lipid metabolism by phosphorylating and inactivating acetyl CoA carboxylase (ACC). This leads to a reduced malonyl CoA level that relieves its inhibition of carnitine:palmitoyl-CoA transferase-1 (CPT1), a rate-controlling step in mitochondrial fatty acid oxidation. AMPK activation also increases glucose metabolism and insulin action.
In addition to the regulatory role of AMPK on lipid metabolism, AMPK also modulates myocardial glucose utilization (Figure 1). AMPK acutely stimulates glucose transport into cells and chronically increases the expression of genes associated with glucose metabolism (e.g., GLUT) [29]. AMPK promotes translocation of glucose transporters from an intracellular pool to the plasma membrane, similar to the effects of insulin, and stimulates glycolytic enzymes such as 6-phosphofructo-2-kinase [29,30]. Further, AMPK-mediated increases in myocardial GLUT4 expression were shown to involve activation of PKC isoforms, possibly PKC-ε [31]. These metabolic effects of AMPK play a crucial role in providing energy via a non-oxidative pathway (i.e., glycolysis) in the ischemic heart when oxidative metabolism of glucose and fatty acids is impaired due to reduced oxygen supply. Consistent with this notion, increased AMPK activity by 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside increases myocardial glucose transport activity and reduces cardiomyocyte apoptosis during ischemia [29,30]. In contrast, transgenic mice expressing a kinase dead or dominant negative form of AMPK show blunted myocardial glucose metabolism and increased cardiomyocyte apoptosis in response to ischemia [32]. These findings clearly indicate an important role for AMPK in cardiac energy metabolism.
AMPK effects on energy metabolism also involve the insulin signaling pathway. In the heart, insulin stimulates ACC activity by inhibiting AMPK phosphorylation, and this accounts for insulin-mediated suppression of mitochondrial lipid oxidation [28]. Insulin’s inhibitory effect on AMPK is dependent on Akt activation which may involve Akt-mediated phosphorylation of AMPKα subunits on Ser485 or Ser491, that block LKB1-mediated Thr172 phosphorylation and activation of AMPK [33]. In this regard, the contribution of insulin resistance in enhanced lipid oxidation by the diabetic heart is currently unknown. Whereas insulin antagonizes AMPK action in the heart, insulin and AMPK signaling coordinately regulate glucose metabolism in skeletal muscle, liver, and adipocytes [33].
mTOR regulates protein synthesis and metabolism in the heart
The mammalian target of rapamycin (mTOR), a serine-threonine kinase, is a major regulator of protein synthesis, glucose and lipid metabolism, and cell growth [34]. mTOR is comprised of two multiprotein complexes: mTOR complex 1 (mTORC1) consists of regulatory-associated protein of mTOR (Raptor) and mTOR complex 2 (mTORC2) consists of rapamycin-insensitive companion of mTOR (Rictor). Insulin activation of mTORC1 causes a phosphorylation of ribosomal protein S6 kinase 1 (S6K1) and eukaryotic translation initiation factor 4E (eIF-4E) binding protein 1 (4E-BP1) which promotes mRNA translation and protein synthesis [34]. A negative feedback regulation is provided by S6K1 which increases an inhibitory serine phosphorylation of IRS-1, leading to downregulation of insulin signaling [35]. Consistent with this notion, S6K1 deficiency enhances insulin sensitivity in diet-induced obese mice [36]. The mTORC2 plays a key role in insulin activation of Akt by phosphorylating Ser473 which primes Akt for Thr308 phosphorylation by PDK1 [34]. The Rictor plays an important role in this process since adipocyte ablation of rictor results in a loss of insulin-mediated Akt phosphorylation and dysregulated glucose and lipid metabolism [37].
In the heart, mTOR is regulated by exercise as high-intensity treadmill running increases mTOR activity via Akt activation, and promotes a physiological hypertrophic growth in mice [38]. Other studies using rapamycin found that mTOR inhibition rescues cardiac hypertrophy induced by pressure overload, suggesting that mTOR also mediates pathological remodeling of the heart [39]. In contrast, cardiac-specific overexpression of mTOR was recently shown to protect against pressure overload-induced cardiac dysfunction that involved mTOR-mediated attenuation of interstitial fibrosis and inflammation [40]. These findings support an important role for inflammation in heart failure and suggest that mTOR is an endogenous suppressor of the inflammatory response. Further, inducible, cardiac-specific ablation of mTOR impaired the hypertrophic response and accelerated heart failure in response to pressure overload that was associated with increased expression of 4E-BP1 [41]. Combined deletion of mTOR and 4E-BP1 markedly improved heart function and cardiomyocyte survival following pressure-overload stress [41]. Thus, while it is clear that hypertrophic growth, a salient feature of the diabetic heart, involves increased protein synthesis, the underlying mechanism by which mTOR affects cardiac remodeling in the diabetic heart is unknown.
Abnormal Regulation of Energy Metabolism in the Diabetic Heart
Altered energy metabolism in the diabetic heart
A growing body of evidence indicates that perturbations in cardiac metabolism and insulin resistance are among the earliest diabetes-induced alterations in the myocardium, preceding both functional and pathological changes [42]. Studies using isolated perfused-heart preparations, cultured cardiomyocytes, and positron emission tomography (PET) have uniformly demonstrated insulin resistance in human and animal models of the diabetic heart [43,44]. Cardiac insulin resistance is associated with type 2 diabetes independent of coronary artery disease, hypertension, and changes in coronary blood flow [45]. In fact, insulin resistance develops in the heart of C57BL/6 mice as early as after 10 days of high-fat feeding, before the onset of insulin resistance in peripheral organs (i.e., skeletal muscle and liver) which occurs following 3 weeks of high-fat feeding [46]. Cardiac insulin resistance at this stage involved reductions in glucose uptake, Akt activity, and GLUT4 protein levels [46]. These findings indicate that diet-induced cardiac insulin resistance develops independent of alterations in systemic glucose metabolism and hyperinsulinemia. Further, cardiac insulin resistance in the early stage of obesity may be a physiological event when the excess lipid supply promotes increased lipid utilization and reduced glucose metabolism in the heart. However, a chronic state of insulin resistance and dysregulated metabolism may induce a pathological event involving cardiac remodeling and systolic dysfunction, which were observed in C57BL/6 mice after 20 weeks of high-fat feeding [46]. Similar observations were made in two commonly used genetic mouse models of obesity, leptin-deficient ob/ob mice and leptin receptor-deficient db/db mice, which showed increased lipid oxidation, reduced glucose oxidation and insulin resistance at 4 weeks of age [47]. These metabolic abnormalities were associated with decreases in myocardial efficiency and left ventricular systolic function at 10 weeks of age in db/db mice [47].
A prolonged state of high lipid oxidation in the diabetic heart may lead to functional derangements related to the accumulation of lipid intermediates, mitochondrial or peroxisomal generation of ROS, or excessive oxygen consumption [48]. Recent studies indicate that increased lipid oxidation may be causally associated with a reciprocal reduction in glucose metabolism in the diabetic heart [49]. The peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors that belong to the nuclear receptor superfamily, and of the three identified mammalian PPAR subtypes (α, γ, and δ), PPARα regulates nuclear expression of genes involved in lipid metabolism in the heart [50]. Transgenic mice with heart-selective overexpression of PPARα show increased lipid oxidation and concomitantly reduced glucose metabolism in the heart [51]. Heart-selective PPARα expressing mice also develop cardiac insulin resistance, and these metabolic derangements are associated with structural and functional changes resembling those of the diabetic heart [51,52]. These findings support a causal link between increased lipid oxidation and reduced glucose metabolism in the diabetic heart.
Insulin resistance affects different cellular processes in individual organs
Insulin resistance, defined as the impaired ability of insulin to stimulate glucose utilization, is an early and requisite event in the development of type 2 diabetes [53]. Insulin resistance is also widely considered to be one of the main risk factors for cardiovascular disease. Accumulating evidence points to a causal role for obesity in the initiation and progression of insulin resistance, but the underlying mechanism remains in debate. It is, however, clear that in the obese, insulin resistant state, the heart receives an increased supply of nutrients (i.e., fatty acids and glucose) that challenge the metabolic capacity and efficiency of the working heart.
In skeletal muscle, insulin resistance involves reduced glucose transport and glycogen synthesis that results in blunted clearance of glucose following a meal (54). In the liver, insulin resistance causes excess production of glucose through enhanced gluconeogenesis and glycogen breakdown which contribute to fasting hyperglycemia, a hallmark of type 2 diabetes (55). In adipose tissue, insulin resistance results in excess breakdown of stored triglyceride into fatty acids (i.e., lipolysis) which is responsible for hyperlipidemia in the obese state (55). Insulin resistance in these organs is associated with defects in the insulin signaling pathway involving IRS-1, IRS-2, PI 3-kinase, and Akt [56]. In the heart, insulin resistance involves defects in insulin signaling, glucose transport, and glycogen storage in cardiomyocytes [46,57]. In this regard, cardiac insulin resistance is comparable to insulin resistance in skeletal muscle, which is not surprising given the similarities between cardiac muscle and skeletal muscle. Obesity-mediated insulin resistance in both organs has also been attributed to Randle’s glucose-fatty acid cycle [58]. However, it is widely believed that other mechanisms exist because defects in insulin signaling cannot be explained by Randle’s hypothesis.
What then causes insulin resistance in the heart? A good starting point is to examine how insulin resistance develops in other organs.
Dyslipidemia and lipotoxicity as a cause of cardiac insulin resistance
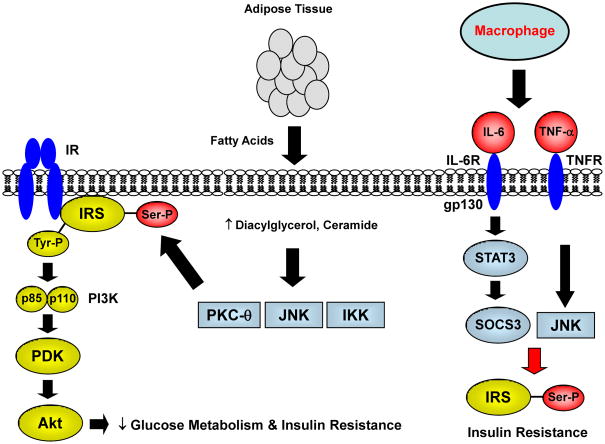
Adipose tissue affects cardiac insulin sensitivity by releasing FFAs into the circulation, providing excess lipid for myocardial utilization, and leading to intracellular accumulation of triglyceride and lipid-derived metabolites [59] (Figure 2). Mice with muscle-specific overexpression of lipoprotein lipase, the rate-determining enzyme in the hydrolysis of triglyceride [60], develop insulin resistance that is associated with increases in intramuscular lipid and lipid-derived metabolites [61]. The underlying mechanism involves activation of a cohort of serine kinases including protein kinase C (PKC)-θ, IκB kinase-β (IKK-β), cJun NH2-terminal kinase (JNK), and S6-kinase, that promote serine phosphorylation of IRS proteins [62–64]. These serine kinases may be activated by lipid-derived metabolites (e.g., fatty acyl CoAs, diacylglycerol, ceramide) [54,65]. Serine phosphorylation of IRS-1 impairs insulin-stimulated IRS-1 tyrosine phosphorylation and PI 3-kinase activity. The inhibitory action of TNF-α on insulin signaling has been shown to involve TNF-α mediated serine phosphorylation of IRS-1 [66] (Figure 2). In a recent study using a diet-induced obese porcine model, cardiac insulin resistance was due to increased Ser307 phosphorylation of IRS-1 and reduced PI3-kinase and Akt activation [67]. In contrast, cardiac insulin signaling was not altered despite reduced glucose metabolism in early obesity in mice [68]. Despite these discrepant findings, excess myocardial lipid has been associated with systolic and diastolic dysfunction in obese animals and humans [69,70]. While these results support a deleterious effect of excess lipid on cardiac insulin action, the role of serine kinases in the diabetic heart is unknown.
Fig. 2. Fatty acid-mediated insulin resistance.
In obesity, insulin resistant adipose tissue releases excess fatty acids that promote increased lipid uptake and accumulation of lipid-derived metabolites, such as diacylglycerol, ceramide and fatty acyl CoAs. Lipid metabolites may activate serine kinases including protein kinase C (PKC)-θ, IκB kinase-β (IKK-β), cJun NH2-terminal kinase (JNK), and S6-kinase that causes a serine phosphorylation (Ser-P) of insulin receptor substrate (IRS). This in turn reduces insulin-mediated tyrosine phosphorylation (Tyr-P) of IRS, subsequent downstream insulin signaling proteins, such as phosphatidyl-inositol-3 kinase (PI3K), phosphoinositide-dependent kinase (PDK), and Akt, and glucose metabolism.
In obesity, adipose tissue inflammation also contributes to systemic insulin resistance. Macrophages and adipocytes secrete inflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor (TNF)-α, that bind to respective receptors (IL-6R and TNFR) on target cell surface. This leads to activation of signal transducer and activator of transcription 3 (STAT3) which increases the expression of suppressor of cytokine signaling (SOCS)-3 that targets IRS proteins for ubiquitine-mediated degradation. These cytokines also activate JNK which promotes a serine phosphorylation of IRS proteins to cause insulin resistance.
Mitochondrial dysfunction as a cause of cardiac insulin resistance
Mitochondrial dysfunction has recently been linked to insulin resistance in obesity and aging [71]. Cardiac abnormality in obese mice is also associated with a reduction in mitochondrial oxidative capacity and increased mitochondrial uncoupling, an event that raises mitochondrial O2 consumption without parallel increases in energetics [72]. Obesity-mediated alterations in mitochondria may involve impaired insulin signaling, excess generation of ROS, and activation of uncoupling proteins (UCPs) [73]. The UCPs are mitochondrial inner membrane proteins that regulate the mitochondrial membrane potential necessary for ATP synthesis. The UCPs dissipate the proton gradient by transporting the protons from the space between the inner and outer mitochondrial membranes back into the mitochondrial matrix [74]. Of the 5 identified UCP homologs, UCP2 and UCP3 are expressed in the heart, and there is evidence for their involvement in mitochondrial uncoupling [75]. Studies have shown that UCP activity and mitochondrial uncoupling are enhanced, possibly by superoxides, but cardiac energetics and efficiency remain impaired in the diabetic heart [76]. Insulin resistance and reduced myocardial insulin signaling also contribute to fatty acid-mediated mitochondrial uncoupling, as cardiac fibers isolated from mice with a cardiomyocyte-specific deletion of the insulin receptor show increased mitochondrial uncoupling upon treatment with fatty acids [73].
Mitochondrial dysfunction affects cardiomyocytes in multiple ways. Because mitochondria control energy production, mitochondrial dysfunction may affect cardiomyocyte energetics and contractility [74]. Imbalance between mitochondrial uncoupling and lipid oxidation may enhance ROS generation and induce oxidative stress [75]. Myocardial uptake of fatty acids is increased as a result of an increased lipid supply in obesity. If mitochondrial oxidative capacity is reduced, possibly due to impaired insulin signaling, the myocardium may accumulate excess lipid and lipid intermediates that exacerbate insulin resistance and oxidative stress. Some studies have shown increased mitochondrial biogenesis in the diabetic heart, which may be a compensatory response to reduced mitochondrial function in cardiomyocytes [76]. On the other hand, myocardial activity of AMPK, which regulates mitochondrial biogenesis through activation of PGC-1α, is reduced in the heart of diet-induced obese mice [77]. Further, a recent study found that mitochondrial dysfunction may be a consequence rather than cause of insulin resistance [78]. Thus, while mitochondrial dysfunction clearly affects cardiac energetics and function, it remains unclear whether mitochondrial dysfunction plays a role in the etiology of insulin resistance in the diabetic heart.
Inflammation and cytokines as a cause of cardiac insulin resistance
Circulating levels of inflammatory cytokines, such as IL-6 and TNF-α, are elevated in obese, diabetic subjects, and the notion that type 2 diabetes has an inflammatory component is becoming widely accepted [79]. Adipose tissue was long considered to function mainly as a lipid storage organ. Recent evidence indicates that adipose tissue is an active endocrine organ capable of secreting hormones and cytokines (termed “adipokines”) that modulate energy balance, glucose and lipid homeostasis, and inflammation [79] (Figure 3). In obesity, macrophages infiltrate adipose tissue in response to local chemokines, such as monocyte chemoattractant protein (MCP)-1 [80]. Macrophages are also recruited to adipose tissue in response to apoptosis and form a distinctive crown-like structure surrounding dead or dying adipocytes in obesity [81]. Further, adipose tissue macrophages play a pivotal role in obesity and inflammation-mediated insulin resistance [82] (Figure 3). Mice with adipocyte-specific overexpression of MCP-1 develop insulin resistance associated with increased macrophage infiltration in adipose tissue [80]. In contrast, mice deficient in C-C motif chemokine receptor-2 (CCR-2), which binds to MCP-1 and regulates macrophage recruitment, show increased insulin sensitivity with reduced macrophage levels in adipose tissue [83].
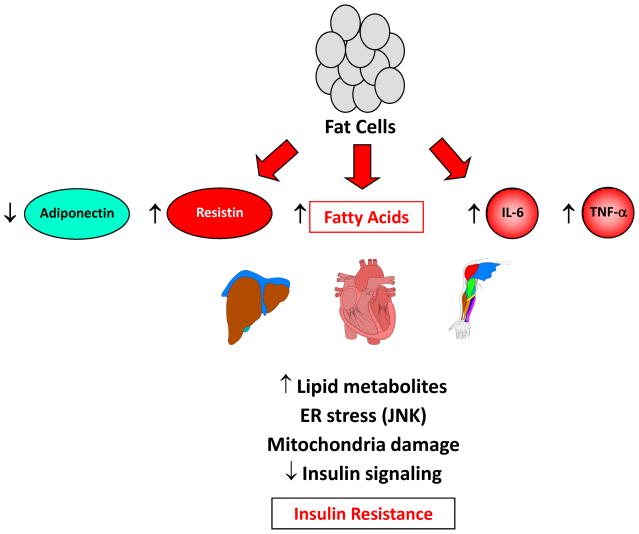
Fig. 3. Adipose tissue is a major endocrine organ that produces hormones and cytokines.
In obesity, adipose tissue causes insulin resistance by releasing excess fatty acids that lead to intracellular accumulation of lipid metabolites, ER stress, and mitochondrial dysfunction. This results in impaired insulin signaling and insulin resistance. Adipose tissue further contributes to insulin resistance by altered production of hormones (reduced adiponectin and increased resistin) that regulate AMPK, insulin signaling and glucose metabolism in other organs. In addition to macrophages, obese adipocytes also produce inflammatory cytokines, such as IL-6 and TNF-α, which cause insulin resistance.
TNF-α was the first inflammatory cytokine to be identified as a link between obesity and insulin resistance. Adipose mRNA expression of TNF-α was shown to be increased in several rodent models of obesity, and neutralization of TNF-α using a soluble TNF-α receptor-IgG chimeric protein improved insulin sensitivity in obese fa/fa rats [84]. TNF-α is shown to cause insulin resistance by suppressing IRS-associated insulin signaling and glucose transport activity in skeletal muscle [84].
IL-6 is a multi-functional cytokine that is produced and released by a wide variety of cell types, including monocytes/macrophages, fibroblasts, and endothelial cells in response to infections or injuries, and it plays an important role in the regulation of the immune system. Recent studies have shown that a significant amount of IL-6 is produced in metabolically-active organs including the heart and adipose tissue, and the degree of obesity is strongly correlated with plasma IL-6 levels in humans [85]. The biological activities of IL-6 involve the recruitment of signal-transducing molecules, such as SHP-2 and signal transducer and activator of transcription 3 (STAT3), leading to the expression of suppressor of cytokine signaling (SOCS)-3. In addition to kinases of the JAK family, IL-6 activates multiple serine/threonine kinases including JNK, p38 mitogen-activated protein (MAP) kinase, and PKC-δ. IL-6 causes insulin resistance by reducing IRS-associated insulin signaling and glucose metabolism [86]. The underlying mechanism involves IL-6 induced intracellular expression of SOCS-3 and subsequent inhibition of the insulin signaling network [87] (Figure 4). In this regard, mRNA expression of Socs3 is elevated in the adipocytes of obese, diabetic mice, and IL-6 stimulates SOCS-3 expression in adipocytes [88].
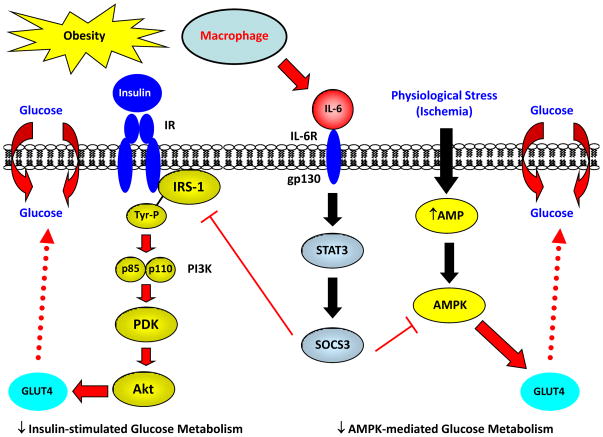
Fig. 4. Obesity induces local inflammation in heart and causes insulin resistance.
In obesity, a local inflammation in heart increases macrophage and cytokine levels, such as IL-6, that affect myocardial glucose metabolism. IL-6 activates STAT3-SOCS3 signaling pathway which suppresses IRS-associated insulin signaling and insulin-mediated glucose metabolism (insulin resistance) in heart. Inflammation and IL-6 also inhibit AMPK activity that reduces basal glucose metabolism in heart. Impaired capacity to utilize glucose during a physiological stress, such as ischemia, may contribute to insufficient cardiomyocyte energetics, cell death, cardiomyopathy, and diabetic heart failure.
In obesity, inflammation also develops in liver and skeletal muscle and may play a role in insulin resistance in these organs [89–91]. Fatty acids have been shown to activate IKKβ and nuclear factor-κB in hepatocytes, increase circulating levels of MCP-1 and cytokines, and cause insulin resistance in rat liver [89]. Diet-induced insulin resistance in skeletal muscle is associated with increased macrophage infiltration and local cytokine production in skeletal muscle, and these effects are reversed by the anti-inflammatory cytokine, IL-10 [86,92]. Skeletal muscle insulin resistance is also associated with inflammation in estrogen receptor α-deficient mice following a high-fat diet [93]. Obesity-mediated inflammation further develops in pancreatic islets and affects glucose-induced insulin secretion [94].
These observations lead to an obvious question: is there an inflammatory event in the heart in obesity? In this regard, a recent study reported profound inflammation in the obese heart, with marked increases in macrophages, cytokines, and SOCS3 levels in cardiomyocytes following high-fat feeding [95]. Diet-induced inflammation was associated with reduced glucose metabolism in the heart [95]. These deleterious effects of inflammation on cardiac metabolism were mediated by IL-6, which was shown to promote local inflammation and cause insulin resistance in the heart [95]. This is consistent with a recent study showing JNK-mediated regulation of adipocyte IL-6 secretion and hepatic insulin resistance in mice [96]. Furthermore, obesity-mediated inflammation and insulin resistance are associated with defects in myocardial activity of AMPK, a critical sensor of energy metabolism in the heart [22]. Altogether, these findings implicate a potential role of inflammation and cytokines in cardiac insulin resistance (Figure 4).
ER stress and stress kinase signaling as a cause of cardiac insulin resistance
The endoplasmic reticulum (ER) is a specialized perinuclear organelle involved in the synthesis of secreted and membrane-targeted proteins. ER stress results from an imbalance between protein load and folding capacity that leads to the unfolded protein response (UPR) [97]. The UPR activates 3 major ER signaling pathways including the PKR-like endoplasmic reticulum kinase, the inositol requiring-1 (IRE-1), and the activating transcription factor 6 pathways [98]. In the obese state, intracellular lipid accumulation activates IRE-1 and stress kinase signaling by factors such as JNK1 [99]. Treatment with chemical chaperones such as 4-phenyl butyric acid or tauroursode-oxycholic acid has been shown to attenuate ER stress and to improve insulin sensitivity in diet-induced obese mice [100]. These observations implicate an important role of ER homeostasis in obesity and insulin resistance.
The 78-kDa glucose regulated protein, GRP78, also known as BiP (immunoglobulin heavy-chain binding protein) or HSPA5, is a key rheostat in regulating ER homeostasis [101]. GRP78 regulates ER function via protein folding and assembly, targeting misfolded protein for degradation, ER Ca2+ binding, and controlling the activation of transmembrane ER stress sensors [101]. Mice with a heterozygous deletion of Grp78 were recently shown to be resistant to diet-induced obesity, which was due to enhanced energy expenditure [102]. Grp78-deficient mice were also more insulin sensitive following high-fat feeding [102]. The underlying mechanism involves activation of an adaptive UPR in response to obesity stress, which resulted in improved ER homeostasis in adipose tissue [102]. Furthermore, a molecular scaffold, kinase suppressor of Ras 2 (KSR2) was recently shown to regulate energy balance and glucose homeostasis, which was mediated by KSR2 regulation of AMPK [103]. In both cell culture and animal models, KSR2 deficiency results in impaired energy expenditure, reduced glucose and lipid metabolism, obesity, and insulin resistance [103]. These findings implicate an important role for ER stress and ER homeostasis in glucose metabolism.
The JNK signaling pathway is involved in the pathogenesis of obesity, insulin resistance, and type 2 diabetes [62]. In the obese condition generated by chronic high-fat feeding or genetic manipulation, JNK1 is activated and mediates downstream signaling events that target glucose metabolism [99]. JNK1 is known to promote the serine phosphorylation of IRS-1, and to inhibit insulin signaling transduction leading to insulin resistance [99]. Consistent with this, mice with muscle-selective deletion of JNK1 are protected from diet-induced insulin resistance and show increased Akt activation and glucose metabolism in skeletal muscle [104]. However, JNK1 plays a different role in liver as hepatocyte-selective deletion of JNK1 causes insulin resistance and hepatic steatosis [105]. In the brain, JNK1 is shown to regulate the hypothalamic-pituitary-thyroid axis as nervous system-selective JNK1 deletion causes a positive energy balance and enhances insulin sensitivity by increasing serum thyroid hormone levels [106]. These observations indicate that JNK1 exerts cell-autonomous effects on glucose metabolism.
Concluding Remarks
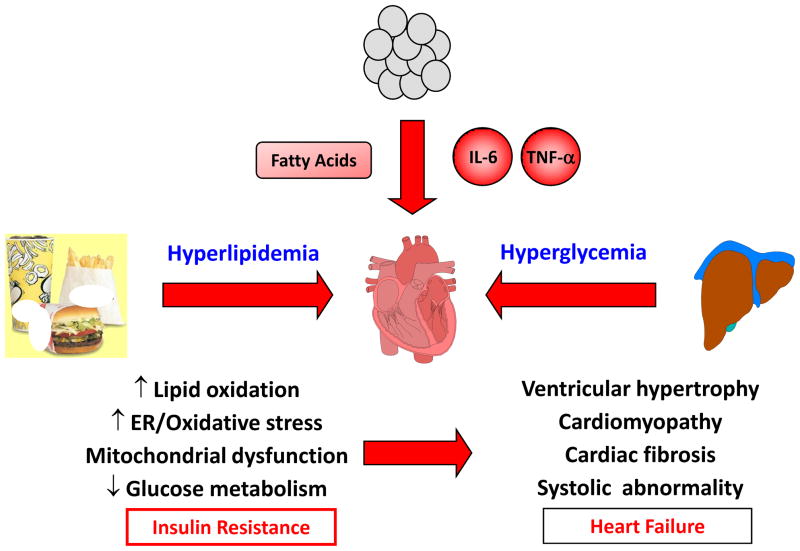
With insulin resistance playing such a significant role in the pathogenesis of type 2 diabetes and related complications that affect the heart, it is important that we understand the underlying mechanism by which cardiac insulin resistance develops. Although the heart primarily utilizes lipid for energy, glucose becomes a critical energy source in the oxygen-deficient state, such as in ischemia. Because endothelial dysfunction, atherosclerosis, and myocardial ischemia are characteristic features of type 2 diabetes [107,108], an impaired capacity to utilize glucose, as in the case of insulin resistance, may affect myocardial energy states and promote localized cell death. These effects may be exacerbated by obesity-induced inflammation and activation of stress kinase signaling. Indeed, the diabetic heart faces numerous stresses from hyperlipidemia, hyperglycemia, and inflammation, where insulin resistance may be a major intracellular event that predisposes the diabetic heart for its ultimate fate (Figure 5). Thus, identifying new therapeutic targets to improve insulin resistance in the heart may be an important step toward treatment of diabetic heart disease.
Fig. 5. Diabetic heart faces many stresses.
Meals high in calories and fat contribute to hyperlipidemia, and insulin resistance in liver causes hyperglycemia. Obesity-mediated inflammation increases serum cytokine levels, and obese adipose tissue releases excess fatty acids. These nutrient stress induce cellular events including increased lipid oxidation and ER/oxidative stress, mitochondrial dysfunction, reduced glucose metabolism, and insulin resistance in heart. Diabetic heart with impaired energy states is predisposed to ischemia-mediated injury that may lead to structural and functional abnormalities and heart failure.
Acknowledgments
Dr. Kim’s work is supported by grants from the National Institutes of Health (R01-DK80756), American Diabetes Association (7-07-RA-80), and American Heart Association (0855492D). Dr. Gray’s work is supported by NIH grant (R01-DK080742).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wild S, et al. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi K, et al. Association between diabetes mellitus and left ventricular hypertrophy in a multiethnic population. Am J Cardiol. 2008;101:1787–1791. doi: 10.1016/j.amjcard.2008.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 5.Boyer JK, et al. Prevalence of ventricular diastolic dysfunction in asymptomatic normotensive patients with diabetes mellitus. Am J Cardiol. 2004;93:870–875. doi: 10.1016/j.amjcard.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Taegtmeyer H, Passmore JM. Defective energy metabolism of the heart in diabetes. Lancet. 1985;1:139–141. doi: 10.1016/s0140-6736(85)91907-5. [DOI] [PubMed] [Google Scholar]
- 7.Iozzo P, et al. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020–3024. doi: 10.2337/diabetes.51.10.3020. [DOI] [PubMed] [Google Scholar]
- 8.Barouch LA, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 9.Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Prob Cardiol. 1994;19:59–113. doi: 10.1016/0146-2806(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 10.Schaffer JE. Fatty acid transport: the roads taken. Am J Physiol Endocrinol Metab. 2001;282:E239–E246. doi: 10.1152/ajpendo.00462.2001. [DOI] [PubMed] [Google Scholar]
- 11.Coburn CT, et al. Role of CD36 in membrane transport and utilization of long-chain fatty acids by different tissues. J Mol Neurosci. 2001;16:117–121. doi: 10.1385/JMN:16:2-3:117. [DOI] [PubMed] [Google Scholar]
- 12.Glatz JFC, et al. Cellular fatty acid-binding proteins: their function and physiological significance. Prog Lipid Res. 1996;35:243–282. doi: 10.1016/s0163-7827(96)00006-9. [DOI] [PubMed] [Google Scholar]
- 13.Coburn CT, et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. Proc Natl Acad Sci USA. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 14.Stanley WC, et al. Regulation of energy substrate metabolism in the diabetic heart. Cardiovascular Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 15.Fischer Y, et al. Insulin-induced recruitment of glucose transporters GLUT4 and GLUT1 in isolated rat cardiac myocytes. Evidence for the existence of different intracellular GLUT4 vesicle populations. J Biol Chem. 1997;272:7085–7092. doi: 10.1074/jbc.272.11.7085. [DOI] [PubMed] [Google Scholar]
- 16.James DE, et al. Insulin-regulatable tissues express a unique insulin-sensitivei glucose transport protein. Nature. 1988;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 17.White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- 18.Fischer Y, et al. Action of metformin on glucose transport and glucose transporter GLUT1 and GLUT4 in heart muscle cells from healthy and diabetic rats. Endocrinology. 1995;136:412–420. doi: 10.1210/endo.136.2.7835271. [DOI] [PubMed] [Google Scholar]
- 19.Abel ED, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–1714. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian R, Abel ED. Responses of GLUT4-deficient hearts to ischemia underscore the importance of glycolysis. Circulation. 2001;103:2961–2966. doi: 10.1161/01.cir.103.24.2961. [DOI] [PubMed] [Google Scholar]
- 21.Belke DB, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young LH, et al. AMP-activated protein kinase: a key stress signaling pathway in the heart. Trends Cardiovasc Med. 2005;15:110–118. doi: 10.1016/j.tcm.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Koh HJ, et al. LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr Opin Clin Nutrition & Metab Care. 2008;11:227–232. doi: 10.1097/MCO.0b013e3282fb7b76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell RR, III, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 26.Kudo N, et al. Characterization of 5′AMP-activated protein kinase activity in the heart and its role in inhibiting acetyl-CoA carboxylase during reperfusion following ischemia. Biochim Biophys Acta. 1996;1301:67–75. doi: 10.1016/0005-2760(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Elheiga L, et al. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 28.Witters LA, Kemp BE. Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J Biol Chem. 1992;267:2864–2867. [PubMed] [Google Scholar]
- 29.Russell RR, et al. Translocation of myocardial GLUT4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 30.Marsin AS, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischemia. Curr Biol. 2000;10:1247–1255. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 31.Nishino Y, et al. Ischemic preconditioning activates AMPK in a PKC-dependent manner and induces GLUT4 up-regulation in the late phase of cardioprotection. Cardiovasc Res. 2004;61:610–619. doi: 10.1016/j.cardiores.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Xing Y, et al. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem. 2003;278:28372–28377. doi: 10.1074/jbc.M303521200. [DOI] [PubMed] [Google Scholar]
- 33.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 34.Zoncu R, et al. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Um SH, et al. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 37.Kumar A, et al. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes. 2010;59:1397–1406. doi: 10.2337/db09-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kemi OJ, et al. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J Cell Physiol. 2008;214:316–321. doi: 10.1002/jcp.21197. [DOI] [PubMed] [Google Scholar]
- 39.McMullen JR, et al. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation. 2004;109:3050–3055. doi: 10.1161/01.CIR.0000130641.08705.45. [DOI] [PubMed] [Google Scholar]
- 40.Song X, et al. mTOR attenuates the inflammatory response in cardiomyocytes and prevents cardiac dysfunction in pathological hypertrophy. Am J Physiol Cell Physiol. 2010;299:C1256–C1266. doi: 10.1152/ajpcell.00338.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D, et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120:2805–2816. doi: 10.1172/JCI43008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stanley WC, et al. Regulation of energy substrate metabolism in the diabetic heart. Cardiovascular Res. 1997;34:25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 43.Kolter T, et al. Molecular analysis of insulin resistance in isolated ventricular cardiomyocytes of obese Zucker rats. Am J Physiol. 1997;273:E59–E67. doi: 10.1152/ajpendo.1997.273.1.E59. [DOI] [PubMed] [Google Scholar]
- 44.Ohtake T, et al. Myocardial glucose metabolism in noninsulin-dependent diabetes mellitus patients evaluated by FDG-PET. J Nucl Med. 1995;36:456–463. [PubMed] [Google Scholar]
- 45.Iozzo P, et al. Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes. 2002;51:3020–3024. doi: 10.2337/diabetes.51.10.3020. [DOI] [PubMed] [Google Scholar]
- 46.Park SY, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005;54:3530–3540. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 47.Buchanan U, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 48.Zhou YT, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopaschuk GD. Abnormal mechanical function in diabetes: relationship to altered myocardial carbohydrates/lipid metabolism. Coronary Artery Dis. 1996;7:116–123. doi: 10.1097/00019501-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Rosen ED, Spiegelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 51.Finck BN, et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SY, et al. Cardiac-specific overexpression of peroxisome proliferator-activated receptor-α causes insulin resistance in heart and liver. Diabetes. 2005;54:2514–2524. doi: 10.2337/diabetes.54.9.2514. [DOI] [PubMed] [Google Scholar]
- 53.Kahn CR. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 54.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 55.DeFronzo RA. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 56.Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- 57.Boudina S, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Randle PJ, et al. The glucose fatty-acid cycle: its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;281:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 59.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 60.Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996;37:693–707. [PubMed] [Google Scholar]
- 61.Kim JK, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Kim JK, et al. PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim JK, et al. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park TS, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rui L, et al. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107:181–189. doi: 10.1172/JCI10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee J, et al. Multiple abnormalities of myocardial insulin signaling in a porcine model of diet-induced obesity. Am J Physiol Heart Circ Physiol. 2010;298:H310–H319. doi: 10.1152/ajpheart.00359.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright JJ, et al. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res. 2009;82:351–360. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szczepaniak LS, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 70.Christoffersen C, et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144:3483–3490. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 71.Petersen KF, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boudina S, et al. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–2695. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 73.Boudina S, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1271–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laskowski KR, Russell RR. Uncoupling proteins in heart failure. Curr Heart Failure Rep. 2008;5:75–79. doi: 10.1007/s11897-008-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gimeno RE, et al. Cloning and characterization of an uncoupling protein homolog: a potential molecular mediator of human thermogenesis. Diabetes. 1997;46:900–906. doi: 10.2337/diab.46.5.900. [DOI] [PubMed] [Google Scholar]
- 76.Boudina S, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 77.Scheuermann-Freestone M, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107:3040–3046. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 78.Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc Res. 2010;88:229–240. doi: 10.1093/cvr/cvq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duncan JG, et al. Insulin-resistant hearts exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Axelsen LN, et al. Cardiac and metabolic changes in long-term high fructose-fat fed rats with severe obesity and extensive intramyocardial lipid accumulation. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1560–R1570. doi: 10.1152/ajpregu.00392.2009. [DOI] [PubMed] [Google Scholar]
- 81.Hoeks J, et al. Prolonged fasting identifies skeletal muscle mitochondrial dysfunction as consequence rather than cause of human insulin resistance. Diabetes. 2010;59:2117–2125. doi: 10.2337/db10-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wellen KF, Hotamisligil GS. Inflammation, stress, diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanda H, et al. MCP-1 contributes to macropahge infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 85.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weisberg SP, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hotamisligil GS, et al. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 88.Gwechenberger M, et al. Cardiac myocytes produce interleukin-6 in culture and in viable border zone of reperfused infarctions. Circulation. 1999;99:546–551. doi: 10.1161/01.cir.99.4.546. [DOI] [PubMed] [Google Scholar]
- 89.Kim HJ, et al. Differential effects of interleukin-6 and -10 on skeletal muscle and liver insulin action in vivo. Diabetes. 2004;53:1060–1067. doi: 10.2337/diabetes.53.4.1060. [DOI] [PubMed] [Google Scholar]
- 90.Ueki K, et al. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi H, et al. Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem. 2004;279:34733–34740. doi: 10.1074/jbc.M403886200. [DOI] [PubMed] [Google Scholar]
- 92.Boden G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr Diabetes Reports. 2006;6:177–181. doi: 10.1007/s11892-006-0031-x. [DOI] [PubMed] [Google Scholar]
- 93.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 94.Kewalramani G, et al. Muscle insulin resistance: assault by lipids, cytokines, and local macrophages. Curr Opin Clin Nutr & Metab Care. 2010;13:382–390. doi: 10.1097/MCO.0b013e32833aabd9. [DOI] [PubMed] [Google Scholar]
- 95.Hong EG, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–35. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ribas V, et al. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERα-deficient mice. Am J Physiol Endocrinol Metab. 2010;298:E304–E319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nunemaker CS, et al. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol Endocrinol Metab. 2008;295:E1065–1075. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ko HJ, et al. Nutrient stress activates inflammation and reduces glucose metabolism by suppressing AMP-activated protein kinase in heart. Diabetes. 2009;58:2536–46. doi: 10.2337/db08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaufman RJ, et al. The unfolded protein response in nutrient sensing and differentiation. Nat Rev Mol Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 101.Lee AH, et al. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 103.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–10. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 105.Ye R, et al. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Costanzo-Garvey DL, et al. KSR2 is an essential regulator of AMP kinase, energy expenditure, and insulin sensitivity. Cell Metab. 2009;10:366–378. doi: 10.1016/j.cmet.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sabio G, et al. Role of muscle c-Jun NH2-terminal kinase 1 in obesity-induced insulin resistance. Mol Cell Biol. 2010;30:106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sabio G, et al. Prevention of steatosis by hepatic JNK1. Cell Metab. 2009;10:491–498. doi: 10.1016/j.cmet.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sabio G, et al. Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Djaberi R, et al. Non-invasive cardiac imaging techniques and vascular tools for the assessment of cardiovascular disease in type 2 diabetes mellitus. Diabetologia. 2008;51:1581–1593. doi: 10.1007/s00125-008-1062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elkeles RA. Coronary artery calcium and cardiovascular risk in diabetes. Atherosclerosis. 2010;210:331–336. doi: 10.1016/j.atherosclerosis.2009.11.026. [DOI] [PubMed] [Google Scholar]