Abstract
Background and Purpose
Immune responses to brain antigens occur after stroke, and experimental studies show that the likelihood of developing a detrimental autoimmune response to these antigens is increased by systemic inflammation at the time of stroke. The aim of this study was to determine if patients who developed infection in the post-stroke period would be similarly predisposed to develop autoimmune responses to central nervous system (CNS) antigens.
Methods
We enrolled 114 patients within 72 hours of ischemic stroke. Clinical and demographic data were obtained, and cellular immune responses to a panel of CNS antigens were assessed during the initial week and again at day 90. Outcome was assessed using the modified Rankin Scale.
Results
Patients who developed an infection, especially pneumonia, in the 15 days after stroke were more likely to evidence a TH1(+) response to myelin basic protein (MBP) and glial fibrillary acidic protein (P=0.019 and P=0.039, respectively) at 90 days after stroke. Further, more robust TH1 responses to MBP at 90 days were associated with a decreased likelihood of good outcome, even after adjusting for baseline stroke severity and patient age (Odds Ratio = 0.477, 95% CI = 0.244–0.935; P=0.031).
Conclusion
This study demonstrates that immune responses to brain antigens occur after stroke. And while these responses are likely to be an epiphenomenon of ischemic brain injury, the response to MBP appears to have clinical consequences. The potential role of post-ischemic autoimmune mediated brain injury deserves further investigation.
Keywords: Stroke, Autoimmune, Infection, MBP, Outcome
Following stroke there is a breakdown of the blood-brain barrier (BBB) that allows for encounter of central nervous system (CNS) antigens by the systemic immune system; this encounter can occur in the injured brain as well as in the periphery.1–5 Given that brain antigens are generally sequestered from the systemic immune system, the possibility for developing an autoimmune response to these antigens thus exists. In fact, studies have shown increased titers of circulating antibodies to neurofilaments and a portion of the N-methyl-D-aspartic acid (NMDA) receptor in individuals with a history of stroke.6, 7 Cellular immune responses to myelin associated antigens and other brain antigens are also seen in stroke survivors and are more robust than the responses seen in patients with multiple sclerosis.8–11
In an animal model of severe focal cerebral ischemia, we showed that the predominant lymphocyte response to the brain antigen myelin basic protein (MBP) is best characterized by antigen specific secretion of transforming growth factor (TGF)-β 1, consistent with a regulatory (TREG) response.12 If, however, systemic inflammation was induced during the acute stroke, animals had an increased predisposition to develop an antigen specific inflammatory response characterized by lymphocyte secretion of interferon (IFN)-γ, consistent with a TH1 response.12 Further, those animals with a TH1(+) response to MBP experienced worse one month outcomes.12, 13 Based on these observations, we hypothesized that patients who become infected in the immediate post-stroke period would be more likely to develop a TH1(+) response to brain antigens, and that this response would be associated with worse long term outcome.
Materials and Methods
Research Subjects
Patients with ischemic stroke admitted to Harborview Medical Center from 9/2005 through 5/2009 who were at least 18 years of age were enrolled within 72 hours of symptom onset. Individuals with ongoing therapy for malignancy, known history of HIV, hepatitis B or C, history of brain tumor, anemia (hematocrit<35 on admission), and those taking immunomodulatory drugs were excluded. Blood was drawn as soon as possible after stroke onset and at 72 hours, 7 days, and 90 days after stroke onset. Blood was also drawn from 40 volunteers to determine normative data for cellular immune responses. The study was approved by the Institutional Review Board; all patients or their surrogates as well as control subjects provided informed consent.
Clinical Data
Clinical and demographic data were collected on all patients. Stroke severity was determined by the National Institutes of Health Stroke Scale (NIHSS) score and outcome by the modified Rankin Scale (mRS).14, 15 Details about therapeutic interventions for the treatment of stroke were collected and there was active ascertainment of infection data. Infection was defined as clinical symptoms of an infection (fever and/or pyuria for urinary tract infection [UTI] and fever and/or productive cough and radiographic evidence of consolidation for pneumonia [PNA]) and positive culture data (for both PNA and UTI). Antibiotic therapy (ABX) was used as appropriate to treat infections; prophylactic ABX were not used. Stroke etiology was determined using the TOAST criteria.16 Total infarct volume on initial diffusion weighted MRI imaging was calculated by the ABC/2 method.17
Lymphocyte Responses
Mononuclear cells were isolated over a ficoll gradient and frozen in liquid nitrogen until use. Enzyme linked immunoSPOT (ELISPOT) assays were done to detect the antigen specific secretion of IFN-γ and TGF-β1 (R&D Systems). Cells were cultured for 24 hours in 96-well plates (MultiScreen®-IP; Millipore) at a concentration of 1×106 per mL in media alone or with human MBP (25 μg/mL; Sigma-Aldrich), human PLP (5 μg/mL; ABD Serotec), human NSE (5 μg/mL; Sigma-Aldrich), bovine S100 (5 μg/mL; Fitzgerald), or human glial fibrillary acidic protein (GFAP 5 μg/mL; Calbiochem-EMD BioSci ) and incubated for 24 hours. The response to tetanus toxin (TT 5 μg/mL; Sigma-Aldrich) was also assessed as a control. Experiments were performed in triplicate; spots were counted using a semi-automated system (MetaMorph®). The degree of TH1 response to each antigen is measured as the ratio of the relative increase in the number of cells secreting IFN-γ to the relative increase in the number of cells secreting TGF-β to a given antigen. The median response to each antigen during the first week after stroke was determined for each patient. For the 90 day responses, those that were above the 75th percentile seen in control subjects were considered to represent a TH1(+) response.
Laboratory Studies
Leukocyte counts were determined by the clinical hematology laboratory. The concentrations of high sensitivity C reactive protein (hsCRP) and glucose were determined by the hospital laboratory using standard methods. Additional plasma was immediately frozen at −80°; the concentrations of circulating cytokines (IL-2, IL-6, TNF-α) were later measured with a cytometric bead-based system (Fluorokine MAP®; R&D Systems). The sensitivity of the assays was 2.23 pg/mL, 1.11 pg/mL and 1.50 pg/mL, respectively. Values below the limit of detection are referred to as not detected (nd) and assigned the lowest limit of detection for statistical testing.
Statistics
Descriptive data are presented as median and interquartile range (IQR) for continuous variables and percentages for categorical variables; group comparisons were performed using the Kruskal-Wallis H test, the Mann-Whitney U test or the χ2 test statistic as appropriate. Logistic regression was used to estimate the odds ratio OR and 95% confidence interval CI for the effect of a TH1 immune response to each antigen (as a continuous measure) at day 90 on neurologic outcome at that time point. Given the relatively severe strokes seen in this study, good outcome was defined as independent ambulation (mRS≤3). Logistic regression was also used to estimate the OR and 95% CI for variables that predict the probability of having a TH1(+) immune response to a given antigen at 90 days. Significance was set at P≤0.05.
Results
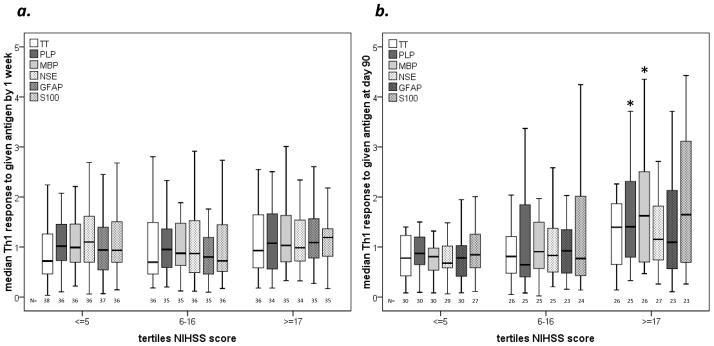
A total of 114 patients were enrolled in CASIS; none had a history of autoimmune disease. The median age was 57 years (44–67) and the median NIHSS score was 11 (4–19). Patients were divided into tertiles based on initial stroke severity (the highest NIHSS score in the first 72 hours); group characteristics are presented in Table 1. The degree of the TH1 response to the panel of brain antigens (as well as to TT) in the first week after stroke onset is depicted in Figure 1a; these responses are similar among all tertiles of stroke severity. Four patients died in the hospital as a direct result of their stroke/withdrawal of support. Among the survivors, the TH1 response to PLP and MBP at 90 days was higher in patients with more severe strokes (Figure 1b). In comparison to the healthy volunteers, the TH1 response to TT was lower in patients with stroke during the first week after symptom onset (0.80 [0.49, 1.79] vs. 1.26 [0.92, 1.62]; P=0.049), but this difference was no longer significant at day 90 (0.88 [0.48, 1.80] vs. 1.26 [0.92, 1.62]; P=0.181). In patients with less severe strokes (NIHSS≤5), however, the response to TT was less than in the control group early after stroke as well as at 90 days after stroke. The responses of each tertile in comparison to the controls are presented in Table 2.
Table 1.
Comparison of stroke patients by initial stroke severity. Data are presented as either the median and interquartile range or the proportion. Statistics are by Kruskal-Wallis H test or χ2 as appropriate.
| NIHSS ≤5 N=39 | NIHSS 6–16 N=36 | NIHSS ≥17 N=39 | P | |
|---|---|---|---|---|
| baseline demographics | ||||
| age (years) | 54 (40–66) | 56 (43–68) | 60 (46–70) | NS |
| female | 11/39 (28%) | 16/36 (44%) | 12/39 (31%) | NS |
| HTN | 19/39 (49%) | 22/36 (61%) | 20/39 (51%) | NS |
| DM | 7/39 (18%) | 8/36 (22%) | 13/39 (33%) | NS |
| CHD | 7/39 (18%) | 10/36 (28%) | 10/39 (26%) | NS |
| CABG | 6/39 (15%) | 7/36 (19%) | 6/39 (15%) | NS |
| HLD | 25/39 (64%) | 26/36 (72%) | 30/39 (77%) | NS |
| AF | 5/39 (13%) | 6/36 (17%) | 5/39 (13%) | NS |
| smoker | 13/39 (33%) | 11/36 (31%) | 19/39 (49%) | NS |
| stroke etiology | ||||
| cardioembolic | 9/39 (23%) | 10/36 (28%) | 12/39 (31%) | NS |
| lacunar | 8/39 (20%) | 3/36 (8%) | 0 | 0.009 |
| atherosclerosis | 3/39 (8%) | 4/36 (11%) | 10/39 (26%) | 0.062 |
| other known cause | 11/39 (28%) | 9/36(25%) | 10/39 (26%) | NS |
| unknown | 8/39 (20%) | 10/36 (28%) | 7/39 (18%) | NS |
| stroke characteristics and treatment | ||||
| infarct volume (mL) | 0.8 (0.3–10.1) | 11.6 (1.8–32.8) | 144.0 (51.1–292.7) | <0.001 |
| IV tPA | 4/39 (10%) | 12/36 (33%) | 12/39 (31%) | 0.037 |
| endovascular therapy | 0/39 | 7/36 (19%) | 8/39 (20%) | 0.011 |
| hemicraniectomy | 0/39 | 1/36 (3%) | 8/39 (20%) | 0.001 |
| complications | ||||
| highest temperature in 1st 72 hrs | 37.3 (37.2–37.8) | 37.6 (37.2–37.9) | 37.8 (37.4–38.6) | 0.003 |
| infection by day 15 | 4/39 (10%) | 4/36 (11%) | 21/38 (55%) | <0.001 |
| PNA by day 15 | 0/39 | 0/36 | 12/38 (32%) | <0.001 |
| concentrations of inflammatory markers | ||||
| hsCRP (per mg/L)* | 2.4 (1.5–6.2) | 10.6 (4.6–40.0) | 66.6 (19.2–112.2) | <0.001 |
| IL-6 pg/mL* | 1.0 (nd-1.9) | 2.2 (0.8–5.8) | 9.5 (4.2–25.6) | <0.001 |
| TNF-α pg/mL* | 1.2 (nd-2.8) | 1.9 (0.7–3.0) | 2.1 (nd-3.7) | NS |
| IL-2 pg/mL* | nd (nd-nd) | nd (nd-nd) | nd (nd-2.6) | 0.005 |
| laboratory variables | ||||
| glucose (mg/dL)* | 119 (107–143) | 123 (107–151) | 143 (129–171) | 0.002 |
| cortisol (μg/dL)* | 9.8 (7.6–13.8) | 13.9 (10.9–16.7) | 20.2 (13.8–27.4) | <0.001 |
| ACTH (pg/mL)* | 19.0 (13.0–28.2) | 20.0 (11.5–40.5) | 22.5 (16.5–32.0) | NS |
| WBCs (thou/mL)* | 8.1 (6.0–10.1) | 10.3 (8.7–13.8) | 12.1 (10.5–15.0) | <0.001 |
| PMNs (thou/mL)* | 3.9 (3.0–5.2) | 5.9 (4.5–7.6) | 8.1 (6.3–9.7) | <0.001 |
| lymphs (thou/mL)† | 1.7 (1.3–2.1) | 1.4 (1.2–1.7) | 1.2 (0.9–1.5) | 0.002 |
HTN=hypertension, DM=diabetes mellitus, CHD=coronary heart disease, HLD=hyperlipidemia, AF=atrial fibrillation, IV tPA=intravenous tissue plasminogen activator, PNA=pneumonia, hsCRP=high sensitivity C reactive protein, IL=interleukin, IL-1ra=interleukin 1 receptor antagonist, TNF=tumor necrosis factor, ACTH=adrenocorticotrophic hormone, PMNs=polymorphonuclear cells, lymphs=lymphocytes.
denotes the highest value in the first 72 hours;
denotes lowest value in the first 72 hours.
Figure 1.
Median TH1 responses (Y-axis) to TT and brain antigens in the first week after stroke onset (a) do not differ based on tertile of initial stroke severity (X-axis). By day 90, the responses to PLP and MBP are more robust in patients with severe strokes (b). Statistics are by Kruskal-Wallis H; *P<0.05, **P<0.001.
Table 2.
Differences between the TH1 response to each antigen for each tertile of stroke severity and controls after stroke onset and at day 90. Data are displayed as median (IQR); statistics are by Mann-Whitney U test (stroke patients versus controls).
| controls N=39 | Tertile 1 NIHSS ≤5 N=38 | P | Tertile 2 NIHSS 6–16 N=36 | P | Tertile 3 NIHSS ≥17 N=36 | P | ||
|---|---|---|---|---|---|---|---|---|
| week st within 1 | TT | 1.26 (0.92, 1.62) | 0.87 (0.47, 1.33) | 0.020 | 0.70 (0.45, 2.09) | 0.073 | 1.05 (0.60, 2.17) | NS |
| PLP | 0.85 (0.63, 1.85) | 1.04 (0.73, 1.45) | NS | 0.95 (0.58, 1.40) | NS | 1.08 (0.56, 1.67) | NS | |
| MBP | 1.02 (0.73, 1.47) | 1.04 (0.71, 1.73) | NS | 0.88 (0.63, 1.50) | NS | 1.04 (0.70, 1.66) | NS | |
| NSE | 0.92 (0.55, 1.38) | 1.12 (0.70, 1.92) | NS | 0.86 (0.44, 1.56) | NS | 0.98 (0.72, 1.55) | NS | |
| GFAP | 1.11 (0.51, 1.46) | 1.00 (0.55, 1.69) | NS | 0.80 (0.45, 1.31) | NS | 1.12 (0.78, 1.57) | NS | |
| S100B | 1.00 (0.60, 1.42) | 0.98 (0.71, 1.60) | NS | 0.76 (0.50, 1.46) | NS | 1.20 (0.82, 1.47) | NS | |
| day 90 | TT | 1.26 (0.92, 1.62) | 0.78 (0.35, 1.88) | 0.033 | 0.83 (0.52, 1.49) | 0.092 | 1.38 (0.52, 1.97) | NS |
| PLP | 0.85 (0.63, 1.85) | 0.89 (0.65, 1.30) | NS | 0.78 (0.43, 1.55) | NS | 1.40 (0.80, 2.31) | 0.115 | |
| MBP | 1.02 (0.73, 1.47) | 0.81 (0.54, 1.06) | 0.060 | 0.88 (0.57, 1.42) | NS | 1.68 (0.71, 2.60) | 0.094 | |
| NSE | 0.92 (0.55, 1.38) | 0.68 (0.57, 1.19) | NS | 0.93 (0.51, 1.41) | NS | 1.00 (0.72, 1.74) | NS | |
| GFAP | 1.11 (0.51, 1.46) | 0.78 (0.40, 1.05) | 0.062 | 0.98 (0.48, 1.44) | NS | 0.96 (0.53, 2.13) | NS | |
| S100B | 1.00 (0.60, 1.42) | 0.86 (0.53, 1.54) | NS | 0.77 (0.45, 1.97) | NS | 1.65 (0.69, 3.43) | 0.079 |
TT = tetanus toxoid, PLP = proteolipid protein, MBP = myelin basic protein, NSE = neuron specific enolase, GFAP = glial acidic fibrillary acidic protein.
The effect of the immune response to each antigen (as a continuous measure) on outcome at day 90 is presented in Table 3. Patients with more robust TH1 responses to MBP are less likely to experience a good outcome (mRS≤3). Further, this effect is independent of initial stroke severity and patient age. There are similar but less robust effects of the immune response to PLP and GFAP on outcome.
Table 3.
The effect of the immune response to each antigen at 90 days (as a continuous variable) on 90 day outcome. The ORs are either unadjusted or adjusted for baseline NIHSS score or baseline NIHSS score and age.
| response to: | 90 day outcome | mRS≤3 | ||
|---|---|---|---|---|
| OR | P | |||
| at 90 days | TT (N=80) | unadjusted | 1.015 (0.866–1.190) | NS |
| adjusted for NIHSS | 1.029 (0.876–1.208) | NS | ||
| adjusted for NIHSS/age | 1.023 (0.868–1.205) | NS | ||
| PLP (N=79) | unadjusted | 0.644 (0.426–0.973) | 0.037 | |
| adjusted for NIHSS | 0.702 (0.460–1.071) | 0.100 | ||
| adjusted for NIHSS/age | 0.677 (0.438–1.045) | 0.078 | ||
| MBP (N=80) | unadjusted | 0.437 (0.251–0.763) | 0.004 | |
| adjusted for NIHSS | 0.469 (0.240–0.919) | 0.027 | ||
| adjusted for NIHSS/age | 0.477 (0.244–0.935) | 0.031 | ||
| NSE (N=80) | unadjusted | 0.833 (0.505–1.373) | NS | |
| adjusted for NIHSS | 1.000 (0.556–1.801) | NS | ||
| adjusted for NIHSS/age | 1.060 (0.590–1.904) | NS | ||
| GFAP (N=77) | unadjusted | 0.669 (0.442–1.012) | 0.057 | |
| adjusted for NIHSS | 0.783 (0.497–1.232) | NS | ||
| adjusted for NIHSS/age | 0.786 (0.494–1.249) | NS | ||
| S100 (N=73) | unadjusted | 1.001 (0.775–1.294) | NS | |
| adjusted for NIHSS | 1.171 (0.829–1.654) | NS | ||
| adjusted for NIHSS/age | 1.168 (0.819–1.665) | NS | ||
TT=tetanus toxoid, PLP=proteolipid protein, MBP=myelin basic protein, NSE=neuron specific enolase, GFAP=glial fibrillary acidic protein, mRS=modified Rankin Scale. NS signifies P≥0.020.
In this study we defined a TH1(+) response to an antigen as a response that was greater than the 75% percentile of the control response to that antigen. Patients with more severe strokes (NIHSS≥17) were more likely to develop TH1(+) responses to PLP (P=0.026) and MBP (P<0.001) than patients with less severe strokes. The primary hypothesis for this study was that infection in the post-stroke period would increase the likelihood of an individual developing a TH1(+) response to brain antigens. Table 4 shows the proportion of patients with and without infection who had evidence of a TH1(+) response to each antigen at 90 days. The median response to PLP at 90 days was also higher among patients who developed PNA in the first 15 days after stroke than those who were not infected (1.56 [1.23–2.08] vs. 0.90 [0.48–1.30]; P=0.025). Stroke severity was much worse among patients who developed an infection compared to those who did not (NIHSS = 21 [12–26] versus 8 [3–16]; P<0.001). Patients with PNA had more severe strokes (NIHSS = 25 [21–30]) than patients with UTI (NIHSS = 18 [9–24]) and patients without infection (NIHSS = 8 [3–16]; P<0.001). The effect of infection on immunologic outcome is lost after controlling for stroke severity
Table 4.
Proportion of patients with a TH1(+) response (response >75th percentile of the control population) to given antigen based on infection history. Statistics are by χ2 and compare patients with infection (any vs. no infection or PNA vs. no infection) to those without infection.
| any infection by day 15? | PNA by day 15? | |||||
|---|---|---|---|---|---|---|
| yes | no | P | yes | no | P | |
| TT | 11/22 (50%) | 14/59 (24%) | 0.023 | 5/10 (50%) | 14/59 (24%) | 0.085 |
| PLP | 6/22 (27%) | 10/58 (17%) | NS | 4/10 (40%) | 10/58 (17%) | 0.100 |
| MBP | 9/22 (41%) | 14/59 (24%) | 0.127 | 6/10 (60%) | 14/59 (24%) | 0.019 |
| NSE | 5/23 (22%) | 14/58 (24%) | NS | 4/11 (36%) | 14/58 (24%) | NS |
| GFAP | 8/22 (36%) | 11/56 (20%) | 0.122 | 5/10 (50%) | 11/56 (20%) | 0.039 |
| S100B | 8/21 (38%) | 18/53 (34%) | NS | 3/9 (33%) | 18/53 (34%) | NS |
PNA = pneumonia, TT = tetanus toxoid, PLP = proteolipid protein, MBP = myelin basic protein, NSE = neuron specific enolase, GFAP = glial fibrillary acidic protein. NS signifies P≥0.200.
Univariate predictors of developing a TH1 response greater than the 75th percentile of the control population at day 90 for each of the antigens studied (a TH1[+] response) are shown in the supplemental Table. Stroke severity was one of the most important predictors of developing a TH1(+) response to most antigens and was more predictive than infarct volume. For each one point increase in the NIHSS score there was nearly a 10% increase in the odds of developing a TH1(+) response to MBP. There were trends towards increased risk of developing TH1(+) response to TT and GFAP among patients undergoing hemicraniectomy; these trends no longer existed after controlling for stroke severity. Curiously, higher numbers of lymphocytes were associated with a decreased risk of developing a TH1(+) response to PLP and GFAP. Not surprisingly, infection (and fever) in the first 15 days after stroke onset was associated with increased risk of developing a TH1(+) response to TT, PLP, MBP, and GFAP. Finally, Gram-positive infections were more likely to be associated with a subsequent TH1(+) response (likely because the preponderance of Gram-positive infections were PNAs).18
Discussion
Following stroke, lymphocytes infiltrate the ischemic brain allowing for contact with CNS antigens from numerous different cell types (neurons, astrocytes, oligodendrocytes) which are normally sequestered from the peripheral immune system.1–3 Further, there is an increase in the concentration of antigens such as MBP, NSE, S100 and GFAP in the systemic circulation following stroke, which allows for lymphocyte encounter with these antigens in peripheral lymphoid organs.5, 19 Given this lymphocyte contact with novel CNS antigens, irrespective of site, it is thus possible for an (auto)immune response to occur to these antigens. Indeed, antibodies and cellular immune responses to brain antigens are documented in stroke survivors.6–11 Our observation that TH1(+) responses to CNS antigens like MBP occur following stroke is thus not novel, but the finding that these responses may have pathological consequences is.
In experimental studies of severe stroke, we showed that TH1(+) responses to MBP were uncommon under usual circumstances; the tendency to develop a TH1(+) response to MBP, however, could be increased by induction of a systemic inflammatory response with lipopolysaccharide (LPS), a component of the Gram negative bacterial cell wall, at the time of stroke.12, 13 The systemic inflammatory response induced by LPS was intended to mimic the response that occurs during infection. Based on our animal data, we hypothesized that patients who became infected in the immediate post-stroke period would be more likely to develop TH1(+) responses to brain antigens. The findings from this study support our hypothesis that infection would increase the likelihood of developing a TH1(+) response to brain antigens given that a greater proportion of patients with post-stroke infection (especially PNA) developed TH1(+) responses to the myelin associated antigens (MBP and PLP) and the glial antigen GFAP. The fact that infections with Gram positive organisms were associated with increased risk for developing TH1(+) responses in this study likely relates to the fact that patients with UTIs, which were caused primarily by Gram negative pathogens, had less severe strokes than patients with PNA, which were predominantly related to Gram positive organisms.18
Potentially the most important observation in this study is that the more robust the immune response to MBP (and to a lesser extent, PLP and GFAP) at 90 days, the greater the chances of a poor outcome at this time point. A similar association between a TH1(+) response to MBP and poor outcome following stroke was demonstrated in animal studies.12, 13, 20 MBP is a myelin associated protein found in oligodendrocytes within the CNS, and PLP is the most abundant protein in myelin. Both MBP and PLP are implicated as autoantigens in multiple sclerosis and can induce experimental autoimmune encephalomyelitis.21 We also observed a trend towards worse outcome in patients with more robust responses to GFAP. Both humoral responses and cellular immune responses to GFAP have been described in patients with dementia.22–24 Immune responses to NSE, S100, and TT, on the other hand, were not predictive of outcome.
Given that severe strokes and infections are both associated with the development of more robust immune responses to TT, PLP, MBP, and GFAP, it is possible to argue that the responses to these antigens are merely a marker for patients with severe stroke, and hence worse outcome. The fact that more robust immune responses to TT were not associated with worse outcome, however, tends to dispute this argument, as does the fact that the predictive value of the immune response on outcome, at least for MBP, was largely unaltered after controlling for stroke severity. Further, the fact that the responses to NSE and S100 were not substantially related to stroke severity, infection or outcome suggest that the observed responses to PLP, MBP and GFAP are more than just a non-specific inflammation in patients with severe stroke.
Molecular mimicry and bystander activation are concepts that have been proposed to explain how infection can induce autoimmunity.25 For molecular mimicry, it is believed that the immune response to the pathogen cross reacts with a self antigen; for bystander activation, it is believed that the infection/inflammation leads to the expression of the necessary co-stimulatory molecules (“danger signals”) that activate lymphocytes leading to an immune response to antigens in the vicinity of the “danger signals”. Our previous experimental work showed that administration of LPS at the time of stroke led to upregulation of B7.1, an important costimulatory molecule, in the brain.12 There are also abundant data showing that LPS and other inflammatory stimuli lead to upregulation of B7.1 in monocytes and dendritic tissue in the periphery.26, 27 It is thus plausible that an infection like PNA provokes enough of an inflammatory response to upregulate the expression of costimulatory molecules in either peripheral lymphoid organs or in brain to create a microenvironment that promotes bystander activation of lymphocytes encountering CNS antigens. The finding that increasing lymphocyte numbers were associated with a decreased likelihood of developing a TH1(+) response to PLP and GFAP is counterintuitive, but may be explained by the profound effect of stroke severity on lymphocyte numbers (Table 1) and the fact that stroke severity is such a potent predictor of the TH1(+) response to these antigens.
Limitations of this study include the relatively small sample size and the fact that we assessed responses to only a small number of potential antigens. Further, our control population was not fully characterized and matched to the stroke patients; for the most part, the control subjects had no history or stroke and were largely young and healthy. Larger studies will need to be done to confirm our results and determine whether stroke severity or infection is more important in driving the autoimmune responses to CNS antigens and to establish normative values for larger cohorts of healthy volunteers. These studies could also assess the response to portions of MBP and PLP with defined antigenicity and evaluate the TH17 response as well as the TH1 response to these antigens. Finally, new approaches to identifying novel autoantigens could also be considered in future studies.
In summary, this study again confirms that cellular immune responses to brain antigens occur after stroke. More importantly, we showed that the response to some of these antigens, MBP in particular, predicts long term outcome. The risk of developing a TH1(+) response to MBP is increased by PNA in the first 15 days after stroke, consistent with observations from our animal model. The development of a detrimental autoimmune response to brain after stroke may help to explain why infection is an independent predictor of poor outcome after stroke.
Supplementary Material
Footnotes
Disclosures
The authors have no financial disclosures. This study was funded by NINDS 5R01NS049197.
References
- 1.Schroeter M, Jander S, Witte OW, Stoll G. Local immune responses in the rat cerebral cortex after middle cerebral artery occlusion. J Neuroimmunol. 1994;55:195–203. doi: 10.1016/0165-5728(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 2.Jander S, Kraemer M, Schroeter M, Witte OW, Stoll G. Lymphocytic infiltration and expression of intercellular adhesion molecule-1 in photochemically induced ischemia of the rat cortex. J Cereb Blood Flow Metab. 1995;15:42–51. doi: 10.1038/jcbfm.1995.5. [DOI] [PubMed] [Google Scholar]
- 3.Braun JS, Jander S, Schroeter M, Witte OW, Stoll G. Spatiotemporal relationship of apoptotic cell death to lymphomonocytic infiltration in photochemically induced focal ischemia of the rat cerebral cortex. Acta Neuropathol (Berl) 1996;92:255–263. doi: 10.1007/s004010050516. [DOI] [PubMed] [Google Scholar]
- 4.Campanella M, Sciorati C, Tarozzo G, Beltramo M. Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke. 2002;33:586–592. doi: 10.1161/hs0202.103399. [DOI] [PubMed] [Google Scholar]
- 5.Jauch EC, Lindsell C, Broderick J, Fagan SC, Tilley BC, Levine SR. Association of serial biochemical markers with acute ischemic stroke: The national institute of neurological disorders and stroke recombinant tissue plasminogen activator stroke study. Stroke. 2006;37:2508–2513. doi: 10.1161/01.STR.0000242290.01174.9e. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- 7.Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to n-methyl-d-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- 8.Youngchaiyud U, Coates AS, Whittingham S, Mackay IR. Cellular-immune response to myelin protein: Absence in multiple sclerosis and presence in cerebrovascular accidents. Aust N Z J Med. 1974;4:535–538. doi: 10.1111/j.1445-5994.1974.tb03233.x. [DOI] [PubMed] [Google Scholar]
- 9.Kallen B, Nilsson O, Thelin C. Effect of encephalitogenic protein on migration in agarose of leukocytes from patients with multiple sclerosis. A longitudinal study of patients with relapsing multiple sclerosis or with cerebral infarction. Acta Neurol Scand. 1977;55:47–56. [PubMed] [Google Scholar]
- 10.Wang WZ, Olsson T, Kostulas V, Hojeberg B, Ekre HP, Link H. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88:157–162. doi: 10.1111/j.1365-2249.1992.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocklin RE, Sheremata WA, Feldman RG, Kies MW, David JR. The guillain-barre syndrome and multiple sclerosis. In vitro cellular responses to nervous-tissue antigens. N Engl J Med. 1971;284:803–808. doi: 10.1056/NEJM197104152841501. [DOI] [PubMed] [Google Scholar]
- 12.Becker KJ, Kindrick DL, Lester MP, Shea C, Ye ZC. Sensitization to brain antigens after stroke is augmented by lipopolysaccharide. J Cereb Blood Flow Metab. 2005;25:1634–1644. doi: 10.1038/sj.jcbfm.9600160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zierath D, Thullbery M, Hadwin J, Gee JM, Savos A, Kalil A, Becker KJ. CNS immune responses following experimental stroke. Neurocrit Care. 2010;12:274–284. doi: 10.1007/s12028-009-9270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, Zivin J. Underlying structure of the national institutes of health stroke scale: Results of a factor analysis. NINDS tpa stroke trial investigators. Stroke. 1999;30:2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 16.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanzi P, Cain K, Kalil A, Zierath D, Savos A, Gee JM, et al. Post-stroke infection: A role for IL-1ra? Neurocrit Care. doi: 10.1007/s12028-010-9490-7. epub 2010/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: A comparative analysis of serum concentrations of protein s-100b and glial fibrillary acidic protein. Stroke. 2000;31:2670–2677. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- 20.Gee JM, Kalil A, Thullbery M, Becker KJ. Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke. 2008;39:1575–1582. doi: 10.1161/STROKEAHA.107.501486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannie M, Swanborg RH, Stepaniak JA. Experimental autoimmune encephalomyelitis in the rat. Curr Protoc Immunol. 2009;Chapter 15(Unit 15):12. doi: 10.1002/0471142735.im1502s85. [DOI] [PubMed] [Google Scholar]
- 22.Ishida K, Kaneko K, Kubota T, Itoh Y, Miyatake T, Matsushita M, et al. Identification and characterization of an anti-glial fibrillary acidic protein antibody with a unique specificity in a demented patient with an autoimmune disorder. J Neurol Sci. 1997;151:41–48. doi: 10.1016/s0022-510x(97)00108-1. [DOI] [PubMed] [Google Scholar]
- 23.Mecocci P, Parnetti L, Romano G, Scarelli A, Chionne F, Cecchetti R, et al. Serum anti-GFAP and anti-S100 autoantibodies in brain aging, alzheimer’s disease and vascular dementia. J Neuroimmunol. 1995;57:165–170. doi: 10.1016/0165-5728(94)00180-v. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Takeda M, Tanaka T, Tanaka J, Kato Y, Nishinuma K, et al. Glial fibrillary acidic protein stimulates proliferation and immunoglobulin synthesis of lymphocytes from alzheimer’s disease patients. Methods Find Exp Clin Pharmacol. 1992;14:141–149. [PubMed] [Google Scholar]
- 25.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: Infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmittel A, Scheibenbogen C, Keilholz U. Lipopolysaccharide effectively up-regulates b7–1 (cd80) expression and costimulatory function of human monocytes. Scand J Immunol. 1995;42:701–704. doi: 10.1111/j.1365-3083.1995.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 27.Harris NL, Ronchese F. The role of B7 costimulation in T-cell immunity. Immunol Cell Biol. 1999;77:304–311. doi: 10.1046/j.1440-1711.1999.00835.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.