Abstract
Recent evidence suggests that cocaine addiction may involve progressive drug-induced neuroplasticity of the dorsal striatum. Here, we examined the effects of a) dorsolateral caudate putamen (dlCPu) lesions on cocaine self-administration, extinction of responding, and subsequent reinstatement to cocaine-seeking, and b) reversible inactivation of the dlCPu with GABA receptor agonists (baclofen and muscimol) immediately prior to reinstatement testing. Male, Sprague-Dawley rats self-administered cocaine (0.2 mg/50 μl infusion, i.v.) along an FR1 schedule in daily 2 hr sessions for 10 days, whereby lever presses resulted in cocaine infusions and presentation of a paired light-tone stimulus complex. After 14 days of abstinence, animals were returned to the self-administration chamber and lever responding was recorded, but had no programmed consequences (relapse test). Animals then underwent daily extinction, followed by reinstatement tests in the presence of the conditioned cues, after a cocaine priming injection (10 mg/kg), or cues + cocaine prime. Lesions of the dlCPu failed to affect responding during self-administration, extinction, relapse, or cued-induced reinstatement. However, lesioned animals showed reduced cocaine-seeking during cocaine-primed reinstatement as compared to sham controls. Furthermore, reversible inactivation of the dlCPu significantly impaired both cocaine-primed and cocaine-primed + cue-induced reinstatement. These results demonstrate the critical involvement of the dlCPu in cocaine-primed reinstatement, perhaps via chronic drug-induced changes in the interoceptive effects of cocaine that impact drug-seeking.
Keywords: cocaine, dorsal striatum, rat, reinstatement, relapse, self-administration
1. Introduction
Relapse to drug-seeking following months or years of abstinence presents a significant problem for treatment of drug-dependent individuals. Drugs or drug associated stimuli can trigger drug craving, despite protracted abstinence in human drug addicts (Carter and Tiffany, 1999; Childress et al., 1988; Childress et al., 1993; Ehrman et al., 1992; Volkow et al., 2006). Animal models investigating relapse have focused on several factors that can reinstate responding following withdrawal and extinction. Such factors include exposure to previously drug associated cues, or exposure to a priming injection of the drug itself (Carter and Tiffany, 1999; de Wit and Stewart, 1981; Meil and See, 1996; See, 2002; Shaham et al., 2003).
The circuitry of both cocaine-primed and cue-induced reinstatement to cocaine-seeking has been extensively investigated. Cocaine-primed reinstatement has been found to primarily involve dopaminergic projections from the ventral tegmental area (VTA) to the dorsal prefrontal cortex (dPFC), which sends glutamatergic projections to the nucleus accumbens (for review see McFarland et al., 2004; Schmidt et al., 2005). Reversible inactivation of the VTA, the dPFC, or the nucleus accumbens (NAc) impairs cocaine-primed reinstatement (McFarland and Kalivas, 2001). Similarly, administration of either dopamine (DA) or cocaine in the dPFC or NAc reinstates cocaine-seeking (Cornish and Kalivas, 2000; McFarland and Kalivas, 2001; Park et al., 2002). Cue-induced reinstatement has been found to additionally require the specific involvement of the basolateral amygdala (BLA), as conditioned cue-induced reinstatement of cocaine-seeking is impaired following both excitotoxic lesions (Meil and See, 1997) and pharmacological inactivation (Grimm and See, 2000) of the BLA.
Brain imaging studies using fMRI or PET in human cocaine addicts have demonstrated increased metabolic activity (Garavan et al., 2000) and enhanced DA release (Volkow et al., 2006) within the dorsal striatum (i.e., caudate-putamen) in response to cocaine associated cues. In rhesus monkeys with extensive cocaine self-administration experience, glucose utilization in the striatum showed progressive changes, with more pronounced effects in the dorsal striatum seen following greater duration of cocaine intake (Porrino et al., 2004). Further evidence in rat models has indicated a key role for the dorsolateral caudate putamen (dlCPu) in cocaine-seeking. Reversible inactivation of the dlCPu during a discrete cue-induced reinstatement or contextual relapse test following abstinence significantly impaired cocaine-seeking (Fuchs et al., 2006; Pacchioni et al., 2011; See et al., 2007). Rats trained on a second order schedule of reinforcement showed increased DA release in the dlCPu in response to presentations of cocaine-contingent cues (Ito et al., 2002). Additionally, intra-dorsal striatum administration of DA receptor antagonists (Vanderschuren et al., 2005) or disconnection between the ventral striatum and dlCPu through DA receptor antagonism in the contralateral dlCPu to the unilaterally lesioned NAc core (Belin and Everitt, 2008) impaired cocaine-seeking maintained by a conditioned reinforcer.
Unlike the NAc, (Grimm and See, 2000; Roberts et al., 1977; Roberts et al., 1980; Robledo et al., 1992; Zito et al., 1985), the dlCPu has generally not been shown to be involved in the primary reinforcing effects of cocaine (Caine et al., 1995; Ikemoto, 2003). Caine et al. (1995) found that DA D1 receptor antagonism in the dorsal striatum only increased cocaine self-administration after diffusion out of the dorsal striatum into other areas, such as the NAc and central amygdala. Moreover, in contrast to the ventral striatum, rats do not self-administer cocaine into the dorsal striatum (Ikemoto, 2003). While a recent study showed that electrolytic lesions of either dorsal or ventral portions of the striatum reduced responding for cocaine under a progressive ratio schedule of reinforcement (Suto et al., 2011), examination of the effects of dorsal striatum lesions on cocaine self-administration, and the subsequent extinction and reinstatement of cocaine-seeking have been surprisingly lacking. Despite prior investigations into the circuitry underlying reinstatement to cocaine-seeking (McFarland and Kalivas, 2001), the dlCPu has not been investigated with respect to cocaine-primed reinstatement. Given the increasing evidence of the role of the dorsal striatum in drug-seeking behavior (Belin and Everitt, 2008; Fuchs et al., 2006; Ito et al., 2002; Pacchioni et al., 2011; Porrino et al., 2004; Vanderschuren et al., 2005), the current study examined the impact of permanent excitotoxic lesions of the dlCPu on cocaine-taking and responding during cocaine self-administration, extinction, and reinstatement testing, as well as the effects of reversible dlCPu inactivation on cocaine-primed and cue-induced + cocaine-primed reinstatement.
2. Results
2.1. Histology
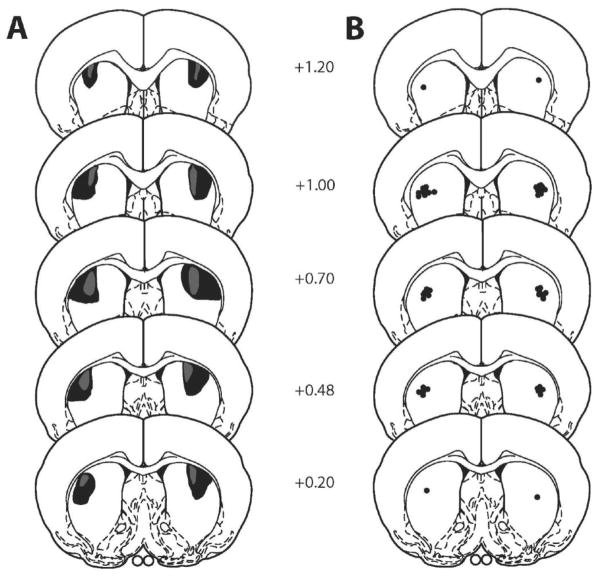
Schematic representations of the extent of the excitotoxic lesions and the most ventral point of the dlCPu infusion cannulae are indicated in Fig. 1. Infusion cannulae locations within the dlCPu ranged from +1.20 to +0.20 anterior from bregma. Animals with one or both infusion cannulae tracks located outside of the target regions were not included in the data analyses. The final N/group for Experiment 1: dlCPu lesion, N = 10, and dlCPu sham, N = 8. Final N for Experiment 2: dlCPu intracranial infusions of baclofen/muscimol (B/M), N = 23.
Fig. 1.
Schematic diagram of coronal sections (anterior in mm from bregma) of excitotoxic lesions (panel A) and infusion cannulae placements (panel B) for the dorsolateral caudate putamen (dlCPu). Shaded areas represent the maximum (black) and minimum (grey) extent of the lesions for rats included in the analyses (adapted from Paxinos and Watson, 1997).
2.2. Experiment 1: dlCPu lesion
Animals with dlCPu lesions readily acquired cocaine self-administration and showed stable responding and cocaine intake during the self-administration period (Fig. 2). While dlCPu lesioned animals showed a modestly lower intake pattern, no significant differences were found between lesion and sham groups for active lever responding (Fig. 2A) or for daily cocaine intake (Fig. 2B). Inactive lever responding across all sessions was routinely very low and did not show any apparent trends or difference between groups (mean ± SEM for inactive lever responses/day across the last 5 days of self-administration: lesion = 1.54 ± 0.26, sham = 1.38 ± 0.47).
Fig. 2.
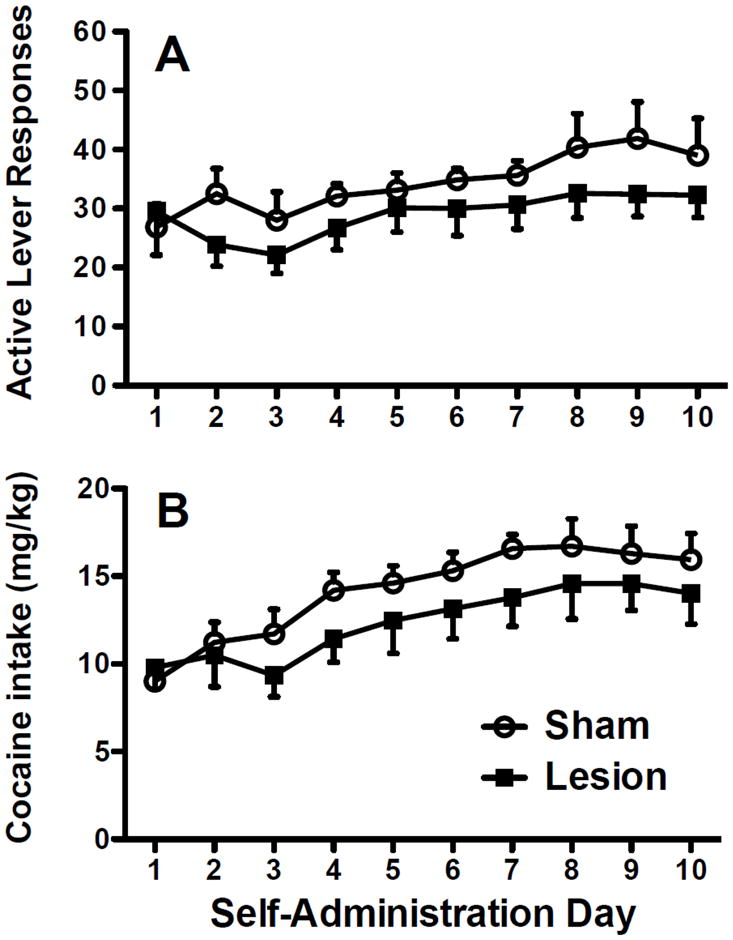
Active lever responses (panel A) and cocaine intake in mg/kg (panel B) during cocaine self-administration following excitotoxic or sham dlCPu lesions.
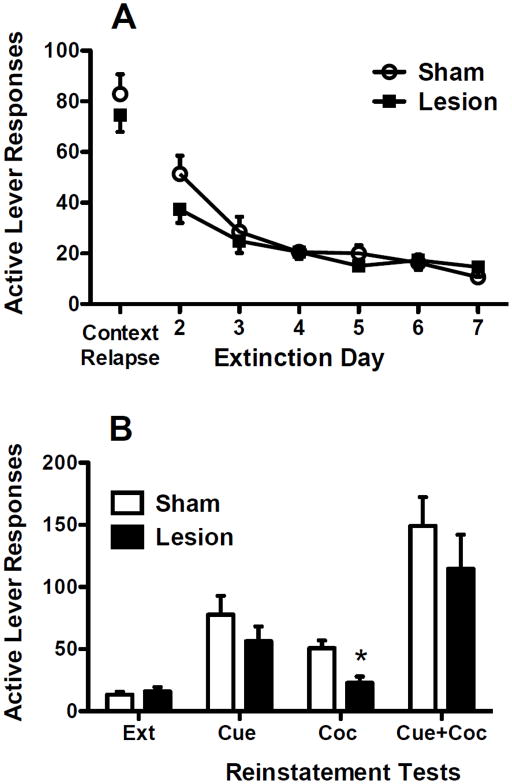
Following 14 days of abstinence, both lesion and sham groups showed robust responding on the context relapse test (Fig. 3A), with no differences between groups. Additionally, lesion and sham groups did not differ in lever responding across the subsequent seven days of extinction (Fig. 3A). Reinstatement tests showed robust cocaine-seeking under the different conditions (Fig. 3B). Separate two-way group × test ANOVAs were conducted to determine group differences between reinstatement tests and whether reinstatement tests differed from extinction responding. For cue-induced reinstatement and the combination of cue-induced + cocaine-primed reinstatement, both lesion and sham groups reinstated to cues (F1,16=31.53, P<0.001) and to cues + cocaine prime (F1,17=45.50, P<0.001), with no significant differences between the two groups. In contrast, for cocaine-primed reinstatement alone, lesion animals showed attenuated cocaine-seeking. Significant main effects for group (F1,16=7.64, P<0.05) and test (F1,16=22.77, P<0.001) were found, as well as a significant interaction (F1,16=10.95, P<0.01). Post-hoc tests indicated that dlCPu lesioned animals responded significantly less than sham animals during cocaine-primed reinstatement (Tukey P<0.05). Additionally, while sham animals showed significant cocaine-primed reinstatement over extinction levels (Tukey P<0.05), lesion animals did not significantly differ from extinction responding.
Fig. 3.
A) Active lever responses during the context relapse test and subsequent extinction sessions in animals with excitotoxic or sham dlCPu lesions. B) Active lever responses during the last two days of extinction (Ext) and cue-induced (Cue), cocaine-primed (Coc), and cue-induced + cocaine-primed (Cue+Coc) reinstatement. Significant differences are indicated as compared to sham (*P<0.05).
2.3 Experiment 2: dlCPu inactivation
Animals with dlCPu guide cannulae readily acquired cocaine self-administration and showed stable responding and cocaine intake during the 10-day self-administration period (data not shown). At the completion of extinction, animals were split into groups based on treatment received during the first reinstatement test (B/M or Veh). Each animal received the opposite treatment on the subsequent reinstatement test and the groups are designated by order as B/M-Veh and Veh-B/M. Across the last 5 days of self-administration, the groups did not differ in responding (Active lever responses/day: B/M-Veh = 36.73 ± 0.99, Veh-B/M = 36.27 ± 0.93; Inactive lever responses/day: B/M-Veh = 0.5 ± 0.40, Veh-B/M = 1.25 ± 0.78), or for cocaine intake (B/M-Veh = 16.37 ± 0.34 mg/kg/day, Veh-B/M = 14.83 ± 0.29 mg/kg/day). Additionally, there were no differences between groups for active lever responding across extinction sessions (final day of extinction: B/M-Veh = 13.58 ± 1.61, Veh-B/M = 16.08 ± 2.11).
Cocaine priming injections (Fig. 4A) produced significant reinstatement above extinction level responding after vehicle infusions into the dlCPu (t10=2.76, P<0.05), an effect that was completely blocked by reversible B/M inactivation of the dlCPu. Inactivation also significantly reduced cocaine-seeking when compared to vehicle infusions (t21=2.36, P<0.05). For cue-induced + cocaine-primed reinstatement (Fig. 4B), significant reinstatement over extinction levels occurred after both B/M (t10=3.22, P<0.01) and vehicle infusions (t11=3.46, P<0.01). However, B/M inactivation of the dlCPu significantly impaired reinstatement responding when compared to vehicle control (t22=1.77, P<0.05).
Fig. 4.
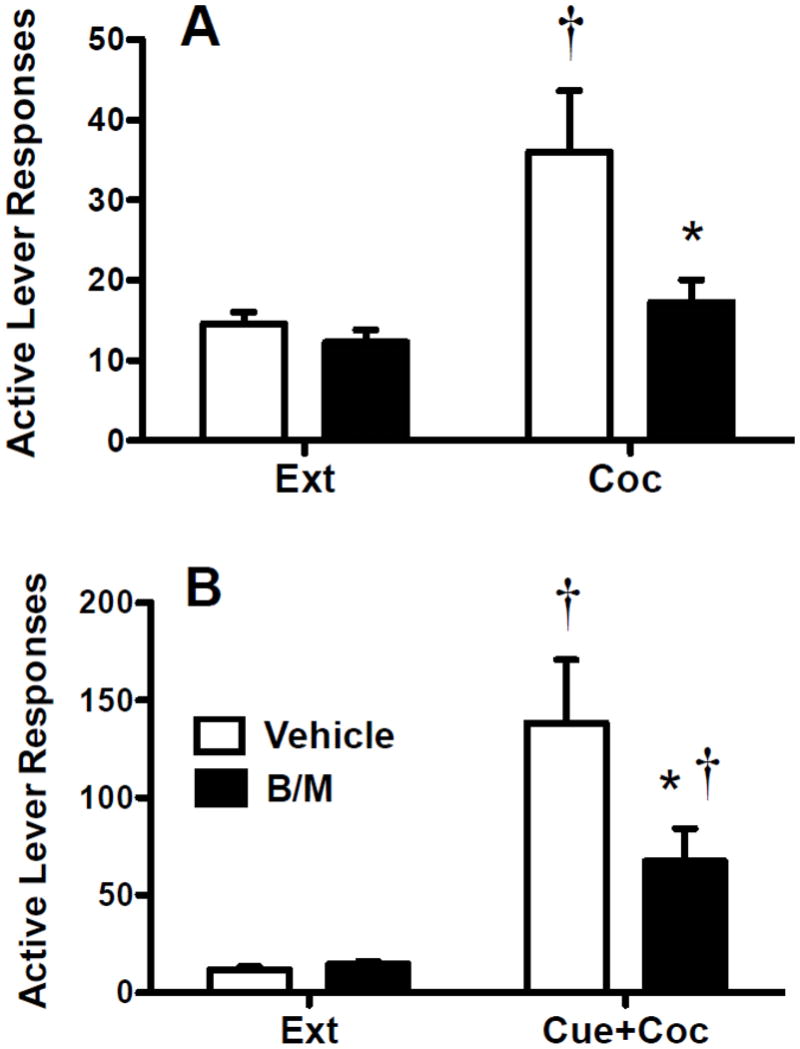
Active lever responses during the last two days of extinction and the cocaine-primed reinstatement test (panel A) or cue-induced + cocaine-primed reinstatement test (panel B). Animals received bilateral intra-dlCPu infusions of vehicle or B/M immediately prior to each reinstatement test. Significant differences are indicated as compared to extinction levels (†P<0.05) or vehicle (*P<0.05).
3. Discussion
Previous research using self-administration models has demonstrated a significant role for the NAc in the primary reinforcing effects of cocaine (Grimm and See, 2000; Roberts et al., 1977; Roberts et al., 1980; Robledo et al., 1992; Zito et al., 1985). Kainic acid lesions (Zito et al., 1985), as well as 6-OHDA lesions (Roberts et al., 1977; Roberts et al., 1980) of the NAc impaired cocaine self-administration, and both DA D1 and D2 antagonism in the NAc increased cocaine self-administration (Robledo et al., 1992). The current results indicate that excitotoxic lesions of the dlCPu prior to cocaine self-administration did not affect self-administration in terms of lever responding, lever discrimination, or cocaine intake. Our findings are congruent with prior studies suggesting that intact dorsal striatum function is not necessary for primary reinforcement with cocaine (Caine et al., 1995; Ikemoto, 2003), including a recent report that lidocaine inactivation of the lateral striatum had no effect on subsequent responding or cocaine intake (Kantak et al., 2009). In contrast, a recent study found that electrolytic lesions of either dorsal or ventral striatal regions reduced the breakpoint on a progressive ratio schedule for cocaine reinforcement (Suto et al., 2011). However, lesions in the Suto et al study encompassed both medial and lateral regions of the dorsal striatum and the lesions occurred after acquisition and extensive training with the progressive ratio schedule. Indeed, studies have also demonstrated the involvement of dlCPu in cocaine-seeking using a second order schedule of reinforcement, which involves a substantial amount of behavioral output (Belin and Everitt, 2008; Ito et al., 2002; Vanderschuren et al., 2005). These findings indicate a role of the dlCPu during cocaine self-administration under more demanding reinforcement schedules, such as progressive ratio or second order schedules.
We have previously demonstrated that B/M inactivation of the dlCPu following abstinence attenuates cocaine-seeking during context relapse (Fuchs et al., 2006; Pacchioni et al., 2011; See et al., 2007). However, permanent lesions prior to self-administration may lead to compensatory changes, whereby the dlCPu is no longer necessary to sustain responding, and structures such as the BLA and dorsal hippocampus fully maintain context-mediated cocaine-seeking (Crombag et al., 2008; Fuchs et al., 2005; Fuchs et al., 2007). Given the lack of effects of dlCPu lesions on cocaine self-administration and responding during the context relapse test (which serves as the first day of extinction), it is not surprising that lesions had no further effects on the rate and magnitude of extinction across trials.
While dlCPu lesions slightly reduced cue-induced and cue-induced + cocaine-primed reinstatement, the only significant effect occurred with cocaine-primed reinstatement. The importance of the dlCPu in cocaine-primed reinstatement was reinforced by the ability of reversible inactivation to completely block cocaine-primed reinstatement. Post-lesion compensatory changes may mitigate the role of the dlCPu in cue-induced reinstatement, which is likely maintained by the BLA and associated structures (Grimm and See, 2000; Kantak et al., 2002a; Meil and See, 1997). However, it is striking that the dlCPu plays such an ubiquitous role in cocaine-primed reinstatement. The cocaine priming injection likely acts as a conditioned interoceptive stimulus that drives drug-seeking (Wise et al., 2008), potentially through dlCPu mediated stimulus-response learning. In the absence of intact dlCPu function during the time of daily cocaine self-administration, the cocaine-priming stimulus is no longer able to initiate subsequent reinstatement to drug-seeking.
While this presumably involves enhanced DA efflux in the dlCPu, other mechanisms may be at work as well, including chronic cocaine-induced changes in striatal GLU signaling as previously characterized in the ventral striatum (Kalivas et al., 2009). Dorsal striatum mediation of cocaine-seeking is likely regulated by dopaminergic inputs from the midbrain and glutamatergic inputs from the cortex onto medium spiny neurons in the striatum (Smith and Bolam, 1990), and DA-GLU interactions have been shown to be necessary for long-term synaptic changes in the dorsal striatum (for review, see Centonze et al., 2001). DA modulates the strength of GLU inputs to medium spiny neurons, as seen with cocaine-stimulated increases in phosphorylation of AMPA GluR1 subunit through activation of striatal DA D1 receptors (Snyder et al., 2000). Further, as GLU dysregulation in the NAc may be responsible for addictive behaviors by increasing reliance on the motor output circuit (Kalivas, 2009), cocaine induced changes in GLU signaling that drive cocaine-primed reinstatement likely occur in both the dorsal and ventral striatum. Indeed, neuroplastic changes in the dorsal striatum have been demonstrated in studies utilizing similar cocaine self-administration parameters. Following 10 days of cocaine self-administration and 14 days of abstinence, a significant increase in GluR2 surface expression and a significant decrease in GluR1 surface expression were found in the dorsal striatum (Hearing et al., 2011). Similarly, increases in immediate early gene expression in the dorsal striatum were seen following 10 days of cocaine self-administration and 22 h or 14 days of abstinence (Hearing et al., 2008).
The current results and previous studies (Fuchs et al., 2006; Pacchioni et al., 2011; See et al., 2007) have together shown that dlCPu reversible inactivation with baclofen/muscimol impairs context relapse following abstinence, as well as cue-induced, cocaine-primed, and cue-induced + cocaine-primed reinstatement, suggesting that the dorsal striatum plays a role across various forms of reinstatement. However, conflicting studies exist regarding the involvement of the dlCPu in cue-induced reinstatement. Lidocaine inactivation of the dlCPu did not impair cue-induced reinstatement in rats trained under a second order schedule of reinforcement (Kantak et al., 2002b). However, lidocaine additionally affects fibers of passage, which may account for some of the differences between studies. Moreover, in the second order schedule used in this study, the conditioned cue was presented with every fifth active lever response, thus giving animals proportionally far fewer cue presentations during a one hour session despite very high behavioral output. In a study by Di Ciano et al. (2008), rats were initially trained to nose poke for cocaine paired with a light cue and then received extensive training to maintain a lever response for the light cue alone. Under these conditions, dlCPu inactivation via baclofen/muscimol did not impair cue-induced reinstatement of the initial nose poke response (Di Ciano et al., 2008). However, dlCPu inactivation did impair lever responding for the light cue alone, demonstrating dlCPu involvement in responding for a conditioned reinforcer (Di Ciano et al., 2008). Further, dlCPu inactivation attenuated cue-induced reinstatement after a history of self-administration along an FR1 schedule, whereby each active lever response resulted in both a cocaine infusion and the cue presentation (Fuchs et al., 2006), indicating that the role of the dlCPu in cue-induced reinstatement may directly relate to the relative number of pairings between conditioned cues and responding during self-administration. These results are consistent with findings that dlCPu dependent stimulus-response learning is acquired incrementally over time (Knowlton et al., 1996; Mishkin and Petri, 1984; Packard and Knowlton, 2002).
Cocaine-primed reinstatement has previously been shown to depend upon the VTA-dPFC-NAc circuitry (McFarland and Kalivas, 2001), and intra-dPFC or intra-NAc administration of either DA or cocaine reinstates cocaine-seeking (Cornish and Kalivas, 2000; McFarland and Kalivas, 2001; Park et al., 2002). Inactivation of the dPFC (i.e., anterior cingulate and prelimbic cortex) impairs cue-induced, stress-induced, and cocaine-primed reinstatement (Capriles et al., 2003; McFarland and Kalivas, 2001; McLaughlin and See, 2003). Additionally, both the BLA and VTA have projections to the dPFC (Groenewegen et al., 1990; Koob, 1992), indicating their involvement in integrating information from limbic regions. The glutamatergic projections from the dPFC to the NAc core have been suggested to act as a final common output pathway for reinstatement (for review, see Kalivas and McFarland, 2003). However, the dorsal striatum also receives inputs from the dorsal anterior cingulate (Kunishio and Haber, 1994; McFarland and Haber, 2000). Both primate (Shima and Tanji, 1998) and human studies (Bush et al., 2002) have indicated the involvement of the dorsal anterior cingulate in reward based decision making. Therefore, the projections from the anterior cingulate to the dorsal striatum may be involved in motor control motivated by drug-seeking. Additionally, the “spiraling loop circuitry” between the ventral and dorsal striatum (Haber et al., 2000; Haber, 2003) has been implicated in cocaine-seeking (Belin and Everitt, 2008). Given the projections from the anterior cingulate and the NAc, it follows that the dlCPu also plays a significant role in the motor output pathway for reinstated drug-seeking. This pathway appears to be common across drugs, as studies using heroin self-administration have demonstrated dlCPu involvement in heroin-primed and cue-induced reinstatement (Rogers et al., 2008), and context-induced reinstatement (Bossert et al., 2009) to heroin-seeking. Future exploration of the role of the dlCPu in drug-seeking is needed to identify the molecular and cellular mechanisms altered by chronic drug use and guide the development of appropriate intervention strategies for addiction.
4. Experimental procedures
4.1. Subjects
Male, Sprague-Dawley rats (Charles-River; 250–275 g) were individually housed on a 12:12 hr reverse light-dark cycle, with lights off from 06:00–18:00. All animals received standard rat chow (Harlan, Indianapolis, IN, USA) and water ad libitum for the duration of the experiment. Housing and care of the rats were carried out in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council) and the MUSC IACUC approved all experimental procedures.
4.2. Apparatus
Testing was conducted in standard self-administration chambers (30×20×20 cm, Med-Associates, St Albans, VT, USA) linked to a computerized data collection program (MED PC). Each chamber was equipped with two retractable levers, a white stimulus light, a tone generator (ENV-223HAM, Med Associates), and a house light on the wall opposite the levers. Each chamber was contained within a sound-attenuating cubicle equipped with a ventilation fan.
4.3. Surgery
Animals were anesthetized using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg, respectively, IP), followed by equithesin (0.5 ml/kg with a solution of 9.72 mg/ml pentobarbital sodium, 42.5 mg/ml chloral hydrate, and 21.3 mg/ml magnesium sulfate heptahydrate dissolved in a 44% propylene glycol, 10% ethanol solution, IP). Ketorolac (2.0 mg/kg, IP) was administered immediately prior to surgery for analgesia. For jugular catheter implantation, an indwelling catheter (Silastic tubing; 0.51 mm i.d. and 0.94 mm o.d.; Dow Corning, Midland, MI, USA) was inserted into the right jugular vein and securely sutured. The other end was led subcutaneously to a back incision, where it was connected to an external silicone harness (Plastics One, Roanoke, VA). Stylets were inserted into the catheters when the rats were not connected to infusion pumps. Immediately following catheter surgery, animals were placed into a stereotaxic frame (Stoelting, Wood Dale, IL, USA) for either excitotoxic lesions or guide cannulae implantation.
For five days following surgery, catheters were flushed daily with 0.1 ml each of 70 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, NJ, USA) and an antibiotic solution cefazolin (10 mg/0.1 ml, Schein Pharmaceuticals, Florham Park, NJ, USA) to maintain catheter patency. During the entire self-administration period, rats received an infusion of 10 U heparinized saline immediately prior to each session, and the cefazolin and 70 U/ml heparinized saline regimen following the session. Catheter patency was assessed occasionally by administration of 2% methohexital sodium (10.0 mg/ml IV; Eli Lilly and Co., Indianapolis, IN, USA), a short-acting barbiturate that produces rapid and reversible muscle flaccidity.
4.3.1. Experiment 1: Excitotoxic lesions
For excitotoxic lesions, 28 gauge cannulae were directed towards the dlCPu using the appropriate coordinates in mm (dlCPu: AP +0.7, ML 3.7, DV −5.0) and 0.6 μl/side of NMDA (0.12 M, Sigma) or vehicle was bilaterally infused into the dlCPu over 3 min and the cannulae were left in place for an additional 5 min. These coordinates were based on previous studies utilizing NMDA lesions of the dlCPu to disrupt habitual learning (Yin et al., 2004).
4.3.2. Experiment 2: Cannulae implantation
For cannulae implantation, bilateral stainless steel guide cannulae (26 gauge; Plastics One, Roanoke, VA) were directed towards the dlCPu using the appropriate coordinates in mm (dlCPu: AP +0.7, ML 3.7, DV −3.0). Guide cannulae were secured to the skull using steel jeweler’s screws and dental acrylic. Following surgery, stylets were placed into the guide cannulae to prevent blockage.
4.4. Cocaine self-administration
Five days following surgery, rats began cocaine self-administration. Infusion tubing for cocaine administration was enclosed in a wire coil and screwed to the external catheter mount on the rat’s back. A weighted swivel apparatus (Instech, Plymouth Meeting, PA, USA) allowed for free movement within the chamber. Cocaine hydrochloride (National Institute on Drug Abuse, Research Triangle Park, NC, USA) was mixed in 0.9% sterile saline and filtered (0.45 μm) prior to self-administration, with infusions (0.2 mg/50 μl bolus, i.v.) delivered by syringe pumps located outside the cubicle. The house light signaled the initiation of the session and remained illuminated throughout the session. Cocaine reinforcement was available along a fixed-ratio 1 (FR-1) schedule for daily 2-h sessions. During each session, a response on the active lever resulted in a 2-s cocaine infusion and a 5-s compound cue presentation, consisting of activation of a white stimulus light above the active lever and a tone generator (78 dB, 10 kHz), followed by a 20-s time-out period. Responding during the time-out or on the inactive lever was recorded, but resulted in no programmed consequences. Daily self-administration sessions progressed until animals reached a criterion of 10 sessions with at least 10 cocaine infusions per session. Upon reaching criterion, animals were placed in abstinence.
4.5. Abstinence and extinction
Following completion of cocaine self-administration, animals underwent a 14 day abstinence period during which animals remained in their home cages after self-administration for days 1–7. Daily handling and placement in an alternate environment were conducted on days 8–14, whereby rats were placed for 2 h/day in a room distinctly different from the self-administration testing room (alternate environment) in plastic holding chambers (27 × 16 × 20 cm high, Allentown Caging, Allentown PA). On day 15, animals underwent a context relapse test in the self-administration chamber for a single 2-h session, during which lever responses were recorded, but had no programmed consequences. This test was followed by daily 2-h extinction sessions, during which both the active and inactive lever responses were recorded, but had no programmed consequences. Conditioned cues were not present during either the relapse test or subsequent extinction sessions. Once extinction criterion was reached (a minimum of 7 sessions with at least 2 consecutive sessions of ≤25 active lever responses), animals underwent reinstatement testing.
4.6. Experiment 1: Reinstatement testing
Reinstatement testing for experiment 1 consisted of a cue-induced reinstatement test (5-s presentation of the light-tone stimulus in the absence of cocaine reinforcement), a cocaine-primed reinstatement test (10 mg/kg, i.p. given immediately before testing), and a combined cue-induced + cocaine-primed reinstatement test. We focused only on the cocaine-primed and cue-induced + cocaine-primed reinstatement to cocaine-seeking, as it has already been demonstrated that cue-induced reinstatement alone is attenuated by dlCPu inactivation (Fuchs et al. 2006). Between reinstatement tests, animals underwent daily 2-h extinction sessions until reaching a criterion of 2 consecutive sessions of ≤25 active lever responses. The order of reinstatement tests was counterbalanced across animals.
4.7. Experiment 2: Intracranial infusions
To assess the effects of acute and reversible inactivation of the dlCPu during cocaine-primed and cue-induced + cocaine-primed reinstatement, rats received intra-dlCPu infusions of either baclofen/muscimol (B/M, 1.0 and 0.1 mM, respectively) or phosphate buffered saline (PBS) vehicle immediately prior to the test session. Injection cannulae (33 gauge; Plastics One) were inserted 2 mm below the tip of the guide cannulae. Bilateral infusions (0.6 μl/side) lasted 2 min, and the cannulae remained for an additional min after the infusion to allow for diffusion. Previous studies have shown that this concentration of B/M and respective bolus amounts impairs reinstatement of cocaine-seeking when infused in the dlCPu just prior to context relapse, but has no effects on general locomotor activity (Fuchs et al., 2006; See et al., 2007). Between reinstatement tests, animals underwent daily 2-h extinction sessions until reaching a criterion of at 2 consecutive sessions of ≤25 active lever responses. The order of reinstatement tests was counterbalanced across animals.
4.8. Histology and data analysis
Following testing, animals were anesthetized and transcardially perfused with PBS and 10% formaldehyde solution. Brains were coronally sectioned at a thickness of 75 μm, and stained with cresyl violet. The sections were examined under light microscopy to determine lesion or cannulae placement and the most ventral point of each cannula track mapped onto schematics from a rat brain atlas (Paxinos and Watson, 1997). Analyses of active and inactive lever responding and cocaine intake during self-administration, and lever responses during extinction and reinstatement testing were conducted using one- or two-way repeated measures analysis of variance (ANOVA) and one-way ANOVA or t tests, where appropriate. Tukey tests were used for post hoc comparisons. Planned comparisons (t tests) were conducted (experiment 2) to determine the effects of reversible inactivation on cocaine-primed and cue-induced + cocaine-primed reinstatement tests. All data were analyzed using GraphPad Prism 5 (GraphPad Software Inc. La Jolla, CA). Data points that were two standard deviations above the mean were excluded from the analyses. One sham lesion rat from experiment 1 and one rat from experiment 2 were excluded based on acquisition data determined to be outliers.
Highlights.
Excitotoxic lesions of the dorsolateral caudate putamen (dlCPu) failed to affect cocaine self-administration.
dlCPu lesions blocked cocaine-primed, but not cue-induced reinstatement to cocaine-seeking.
Reversible inactivation of the dlCPu reduced both cocaine-primed and cocaine-primed + cue-induced reinstatement.
These data expand the circuitry of cocaine-primed reinstatement to drug-seeking to include dorsal, as well as the previously characterized ventral striatum.
Acknowledgments
The authors thank Shannon Ghee, Clifford Chan, and Andrew Novak for excellent technical assistance. This research was supported by National Institute on Drug Abuse Grant DA10462 (RES) and NIH grants T32007288 and C06 RR015455.
Abbreviations
- BLA
basolateral amygdala
- B/M
baclofen/muscimol
- DA
dopamine
- dlCPu
dorsolateral caudate putamen
- dPFC
dorsal prefrontal cortex
- FR1
fixed ratio 1
- GABA
γ-Aminobutyric acid
- GLU
glutamate
- NAc
nucleus accumbens
- NMDA
N-methyl-D-aspartate
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belin D, Everitt B. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Bossert J, Wihbey K, Pickens C, Nair S, Shaham Y. Role of dopamine D1-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Childress A, Ehrman R, McLellan AT, O’Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. NIDA Res Monogr. 1988;81:74–80. [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct. 2008;213:215–227. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacology. 2011;14:784–795. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S. Involvement of the olfactory tubercle in cocaine reward: Intracranial self-administration studies. J Neurosci. 2003;23:9305–9311. doi: 10.1523/JNEUROSCI.23-28-09305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002a;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Stimulus-response functions of the lateral dorsal striatum and regulation of behavior studied in a cocaine maintenance/cue reinstatement model in rats. Psychopharmacology (Berl) 2002b;161:278–287. doi: 10.1007/s00213-002-1036-z. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Mashhoon Y, Silverman DN, Janes AC, Goodrich CM. Role of the orbitofrontal cortex and dorsal striatum in regulating the dose-related effects of self-administered cocaine. Behav Brain Res. 2009;201:128–136. doi: 10.1016/j.bbr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Kunishio K, Haber SN. Primate cingulostriatal projection: Limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350:337–356. doi: 10.1002/cne.903500302. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J Neurosci. 2000;20:3798–3813. doi: 10.1523/JNEUROSCI.20-10-03798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee BL, Meshul CK. Time-dependent changes in extracellular glutamate in the rat dorsolateral striatum following a single cocaine injection. Neuroscience. 2005;133:605–613. doi: 10.1016/j.neuroscience.2005.02.020. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: an animal model of relapse. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Petri HL. Memories and habits: some implications for the analysis of learning and retention. In: Squire LR, Butters N, editors. Neuropsychology of Memory. Guilford; New York: 1984. pp. 287–296. [Google Scholar]
- Pacchioni AM, Gabriele A, See RE. Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res. 2011;218:296–300. doi: 10.1016/j.bbr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Corcoran ME, Fibiger HC. On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacol Biochem Behav. 1977;6:615–620. doi: 10.1016/0091-3057(77)90084-3. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Robledo P, Maldonado-Lopez R, Koob GF. Role of dopamine receptors in the nucleus accumbens in the rewarding properties of cocaine. Ann N Y Acad Sci. 1992;654:509–512. doi: 10.1111/j.1749-6632.1992.tb26015.x. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC. Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol. 2005;526:65–76. doi: 10.1016/j.ejphar.2005.09.068. [DOI] [PubMed] [Google Scholar]
- See RE. Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav. 2002;71:517–529. doi: 10.1016/s0091-3057(01)00682-7. [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Wise RA, Vezina P. Dorsal as well as ventral striatal lesions affect levels of intravenous cocaine and morphine self-administration in rats. Neurosci Lett. 2011;493:29–32. doi: 10.1016/j.neulet.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Wang B, You ZB. Cocaine Serves as a Peripheral Interoceptive Conditioned Stimulus for Central Glutamate and Dopamine Release. PLoS ONE. 2008;3:e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Zito KA, Vickers G, Roberts DCS. Disruption of cocaine and heroin self-administration following kainic acid lesions of the nucleus accumbens. Pharmacol Biochem Beh. 1985;23:1029–1036. doi: 10.1016/0091-3057(85)90110-8. [DOI] [PubMed] [Google Scholar]