Summary
Background
Dehydroepiandrosterone sulfate (DHEAS) is important for its association with immune system function and health outcomes. The characterization of the genetic and environmental contributions to daily DHEAS concentrations is thus important for understanding the genetics of health and aging.
Methods
Saliva was collected from 783 middle-aged men (389 complete pairs and 5 unpaired twins) as part of the Vietnam Era Twin Study of Aging. Samples were taken at multiple specified time points across two non-consecutive days in the home and one day at the study sites. A twin modeling approach was used to estimate genetic and environmental contributions for time-specific and average DHEAS concentrations.
Results
There was a consistent diurnal pattern for DHEAS concentrations in both at-home and day-of-testing (DOT) measures, which was highest at awakening and decreased slightly throughout the day. Heritability estimates were significant for measures at 10am, 3pm and bedtime for the in-home days and at 10am and 3pm on the DOT, ranging between 0.37 and 0.46.
Conclusions
The significant heritability estimates later in the day reflect time-specific genetic effects for DHEAS, compared with prior twin and family designs studies which frequently used averaged morning-only measures. Additive genetic influences on DHEAS concentrations were consistent between at-home and DOT measures.
Keywords: DHEAS, diurnal concentrations, genetics, twin study, aging, men
Introduction
Dehydroepiandrosterone (DHEA) and its sulfated metabolite (DHEAS) are the most abundant products secreted by the zona reticularis of the adrenal cortex (Orentreich, N., Brind, J. L., Rizer, R. L., & Vogelman, J. H., 1984). Like cortisol, DHEA and DHEAS are secreted from the adrenal cortex in response to adrenocorticotrophin (ACTH) stimulation (Pavlov, E. P., Harman, S. M., Chrousos, G. P., Loriaux, D. L., & Blackman, M. R., 1986; Parker, C. R., Jr., Azziz, R., Potter, H. D., & Boots, L. R., 1996). DHEA is the precursor for approximately 50% of the androgens produced in adult men (Brown, R. C., Liu, Y., & Papadopoulos, V., 2002), underscoring the importance of this hormone in the production of sex steroids. DHEA is converted to the more stable DHEAS by DHEA sulfotransferase (HST, SULT2A1) and DHEAS easily becomes DHEA via steroid sulfatase (STS) (Kroboth, P. D., Salek, F. S., Pittenger, A. L., Fabian, T. J., & Frye, R. F., 1999). DHEA is considered to be a biologically active hormone, and many studies focus on the association between DHEA and health-related outcomes. However, DHEAS may have a distinct role from DHEA in the etiology of disease, particularly in the regulation of the immune system (Radford, D. J. et al., 2010) and in neuroprotection (Maninger, N., Wolkowitz, O. M., Reus, V. I., Epel, E. S., & Mellon, S. H., 2009). Further, DHEAS is thought to be the circulating storage pool for DHEA because DHEAS has a half-life of 10–20 hours, while the half-life of DHEA is 1–3 hours (Rosenfeld, R. S., Rosenberg, B. J., Fukushima, D. K., & Hellman, L., 1975). Similarly, the clearance rate of DHEAS is much slower than that of DHEA (Longcope, C., 1996). There is a need to study the causes of the individual variation of diurnal DHEAS production, which is anticipated to be caused by genetic and environmental influences because it is the most abundant steroid hormone in the body, it is necessary for sex hormone synthesis, and is associated with diseases related to aging.
The Roles between DHEAS in the Etiology of Chronic Disorders Related to Aging
Low DHEAS concentrations have been associated with coronary artery disease, cardiovascular disease, non-insulin dependent diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosis, pemiphigoid/pemphigus, and HIV/AIDS, indicating the relationship between DHEAS concentrations and the immune system (Chen, C. C. & Parker, C. R., Jr., 2004). Both DHEAS and cortisol modulate the immune system, although DHEAS generally acts to enhance while glucocorticoids suppress immune function (Butcher, S. K. et al., 2005; Chen, C. C. & Parker, C. R., Jr., 2004). DHEAS has recently been reported to have immunostimulatory effects, increasing superoxide generation in primed human neutrophils in response to pathogens (Radford, D. J. et al., 2010). Additionally, DHEAS has anti-inflammatory effects through the inhibition of NF-κB activation (Iwasaki, Y. et al., 2004).
Lower concentrations of salivary DHEAS have been associated with depression (Barrett-Connor, E., von Muhlen, D., Laughlin, G. A., & Kripke, A., 1999; Corpechot, C., Robel, P., Axelson, M., Sjovall, J., & Baulieu, E-E, 1981; Fabian, T. J. et al., 2001; Goodyer, I. M. et al., 1996; Goodyer, I. M., Herbert, J., Tamplin, A., & Altham, P. M., 2000; Takebayashi, M. et al., 1998; Michael, A., Jenaway, A., Paykel, E. S., & Herbert, J., 2000). Further, DHEAS has anxiolytic, anti-convulsant and sedative-hypnotic actions (Zinder, O. & Dar, D. E., 1999). DHEAS is a “neurosteroid” and as such is synthesized in the central nervous system de novo (Baulieu, E-E, 1981; Majewska, M. D., 1995). Increases in synaptic concentrations of DHEAS inhibit neuronal GABA-induced currents and results in excitatory neurotransmission (Kroboth, P. D., Salek, F. S., Pittenger, A. L., Fabian, T. J., & Frye, R. F., 1999; Debonnel, G., Bergeron, R., & de Montigny, C., 1996). Modulation of neurotransmission is related to brain function, which in turn contributes to different neuropsychological states (Majewska, M. D., 2002).
DHEAS concentrations rise throughout childhood and peak in early adulthood followed by an age-dependent decline (Rainey, W. E. & Nakamura, Y., 2008). The lowest concentrations of DHEAS occur between the ages of 65–70, pointing the possible relevance of these hormones in age-related illness (Maninger, N., Wolkowitz, O. M., Reus, V. I., Epel, E. S., & Mellon, S. H., 2009). Few studies have tested associations between genes related to DHEAS/DHEA function and disease. Further, of the studies testing the relationships between genes related to DHEAS concentrations and disease, no significant associations have been reported (Boger-Megiddo, I., Weiss, N. S., Barnett, M. J., Goodman, G. E., & Chen, C., 2008; Karlson, E. W. et al., 2009). This may be due to an incomplete understanding of the relative importance of the role of genetic and environmental effects on diurnal regulation of DHEAS concentrations, particularly in aging adults.
Genetic Epidemiology of DHEAS Production
To date, seven family studies have estimated the impact of genetic and environmental factors on DHEAS (Table 1). The family study design takes advantage of the familial correlations between individuals across generations (ie: parent-child, siblings, spouses, and grandparent-grandchild) to estimate the degree to which a trait is due to familial aggregation, which includes additive genetic effects and those due to the shared (family) environment. In the absence of additional information, nuclear family data and sibling-only samples are unable to resolve familial resemblance into genetic and environmental effects since family members share both genetic and familial environments (Kendler, K. S. & Neale, M. C., 2009; Rice, T. & Borecki, I. B., 2001). Consequently, heritability estimates from these types of studies refer to the maximal effect of genes on a trait, also known as maximal heritability. One nuclear family study of 184 families reported an average heritability of 66% in families of African American descent and 58% in families of European American descent for baseline DHEAS concentrations (An, P. et al., 2001). Another study of 348 families, reported a pooled, maximal heritability of 45% for DHEAS concentrations, unadjusted for sex differences. Maximal heritability estimates were found to differ by gender in this sample, with estimates of 29% and 74% in men and women respectively (Rice, T. et al., 1993). A study of women with polycystic ovary disease and their sisters (N = 62 sister pairs) reported a heritability 43% for probands and their sisters (Yildiz, B. O., Goodarzi, M. O., Guo, X., Rotter, J. I., & Azziz, R., 2006).
Table 1.
Summary of Heritability Studies on DHEAS
| Author/Date | Study Design |
Sample | Age Range |
Ethnicity | Collection | Specimen | Adjusted Covariates |
Estimated Heritability |
|---|---|---|---|---|---|---|---|---|
| Rotter JI et al. (1985) | F | N = 178 Families = 26 Males = 82 Females = 96 |
10–90 | EA | One sample in hospital | blood serum |
age, sex | 65% |
| Rice T et al (1993) | F | N = 1880 Families= 348 Males = 1320 Females = 560 |
NR | EA | One sample in clinic | blood serum |
age, sex, effects of luteal phase |
Males = 29% Females=74% Pooled = 45% |
| Jaquish et al. (1996) | F | N = 564 Families = 25 Males = 228 Females = 336 |
16–82 | MA | One morning sample in clinic after 12-hour fast |
blood serum |
age, sex, contraceptive use, menopausal status, serum levels of sex hormone binding globulin, % protein ingested/week, %EtOH ingested/week, BMI, waist-hip ratio,subscapular to triceps ratio, HDL3, triglycerides, ApoB |
39% |
| Mitchell et al. (1996) | F | N = 1236 Families = 42 Males = 494 Females = 742 |
16–82 | MA | One morning sample in clinic after 12-hour fast |
blood serum |
29% | |
| Jaquish et al. (1996) | F | N = 635 Families = 27 Males = 250 Females = 385 |
2–78 | MA | One morning sample in clinic after 12-hour fast |
whole blood |
age, sex | 43% |
| An P et al. (2001) | F | N = 768 Families = 184 |
17–65 | AA EA |
Two morning samples in lab after 12-hour fast |
blood serum |
age | 66% (AA) 58% (EA) |
| Yildiz BO (2006) | F | N = 131 (all female) Sister Groups = 62 |
NR | EA | One morning sample between 8–10:30am in clinic after fast during follicular phase of menstrual cycle |
blood serum |
BMI | 43% (proband + all sisters) |
| Miekle AW et al. (1988) | T | 20 MZM pairs 20 DZM pairs |
20–60 | EA | Three pooled samples collected between 8– 9:30am |
blood plasma |
age, smoking, alcohol use, exercise, BMI |
58% |
| Miekle AW et al. (1997) | T | 126 MZM pairs 88 DZM pairs |
25–75 | EA | Three pooled samples collected between 8 – 10:30 am |
blood plasma |
none | 26% |
| Nestler JE et al. (2002) | T | 438 MZF pairs 160 MZM pairs 224 DZF pairs 84 DZM pairs 223 DZO pairs |
NR | EA | One sample with time sample was collected |
blood serum |
age, sex | 57% (<50 yr) 60% (50+ yr) |
F- Family Design, T- Twin Design
EA- European American Ethnicity, MA- Mexican American Ethnicity
MZM- Monozygotic Males, MZF- Monozygotic Females, DZM- Dizygotic Males, DZF- Dizygotic Females, DZO- Opposite Sex
Twin Pair, NR- Not Reported
Heritability estimates, or the degree to which additive genetic influences contribute to DHEAS concentrations can be estimated from extended (across several generations) family and twin studies. Extended family studies of DHEAS ranging in size from 25–42 families reported heritability estimate of DHEAS from 29% to 65% (Jaquish, C. E., Blangero, J., Haffner, S. M., Stern, M. P., & Maccluer, J. W., 1996; Jaquish, C. E. et al., 1996; Mitchell, B. D. et al., 1996; Rotter, J. I., Wong, F. L., Lifrak, E. T., & Parker, L. N., 1985). In addition to estimating heritability, twin studies are also able to estimate and test for the relative importance of both common (often due to shared familial environments) environmental and unique environmental effects (Eaves, L. J., 1982). To date, only three twin studies ranging in size from 40 to 1129 pairs have reported heritability estimates of 26% to 60% for DHEAS concentrations (Meikle, A. W., Stringham, J. D., Woodward, M. G., & Bishop, D. T., 1988; Meikle, A. W., Stephenson, R. A., Lewis, C. M., Wiebke, G. A., & Middleton, R. G., 1997; Nestler, J. E. et al., 2002). Further, these studies report moderate variance due to unique environmental effects.
Previous studies estimating the heritability of DHEAS have generally relied on a single measurement at any point throughout the day since the half-life of DHEAS can range from 7–20 hours and DHEAS concentrations are expected to have low daily variation (Goodyer, I. M. et al., 1996; Rosenfeld, R. S., Rosenberg, B. J., Fukushima, D. K., & Hellman, L., 1975). Most studies have utilized one (Jaquish, C. E., Blangero, J., Haffner, S. M., Stern, M. P., & Maccluer, J. W., 1996; Jaquish, C. E. et al., 1996; Mitchell, B. D. et al., 1996) (Rice, T. et al., 1993; Rotter, J. I., Wong, F. L., Lifrak, E. T., & Parker, L. N., 1985) or two (An, P. et al., 2001) measures of DHEAS concentrations early in the day because they are highest in the morning (Nieschlag, E. et al., 1973; Rosenfeld, R. S., Rosenberg, B. J., Fukushima, D. K., & Hellman, L., 1975). Two studies took multiple measures from each participant across a small window in the morning, usually ranging from 8–10:30 am and used the pooled samples for analysis (Meikle, A. W., Stringham, J. D., Woodward, M. G., & Bishop, D. T., 1988; Meikle, A. W., Stephenson, R. A., Lewis, C. M., Wiebke, G. A., & Middleton, R. G., 1997). To date, no known studies of DHEAS concentrations have estimated the magnitude of genetic effects on DHEAS concentrations over multiple samples throughout the day. Therefore, measures at a single time point or over a small period of time as in previous studies may be inadequate to understand the genetic and environmental contributions on DHEA/DHEAS and chronic disorders.
Prior genetic studies of DHEAS have used blood plasma to estimate heritability. However, salivary measurement of DHEAS provides a unique opportunity to estimate genetic and environmental contributions to DHEAS for comparison against prior studies using blood. Additionally, the ability to systematically measure salivary DHEAS concentrations across the day is advantageous in large-scale data collection and does not alter HPA function compared to intravenous sampling methods. DHEAS is a charged molecule, and is actively transported through the neutral lipid membranes of the salivary cells using a large family of organic anion transport polypeptides (Konttinen, Y. T. et al., 2010; Pomari, E. et al., 2009). Despite low concentrations of salivary DHEAS compared to plasma (Vining, R. F., McGinley, R. A., & Symons, R. G., 1983), these amounts are high enough for use as a result of high DHEAS concentrations in blood (Kroboth, P. D., Salek, F. S., Pittenger, A. L., Fabian, T. J., & Frye, R. F., 1999). Prior studies comparing salivary and plasma concentrations of DHEAS have shown strong associations between saliva and blood (r = 0.738, p < 0.0001) (Lac, G., Marquet, P., Chassain, A. P., & Galen, F. X., 1999).
In order to inform future studies of DHEAS and chronic disorders related to aging as well as to improve genetic association studies using DHEAS measures, it is important to estimate the genetic and environmental effects contributing to the individual differences of diurnal DHEAS concentrations. The present study measured salivary DHEAS concentrations across two non-consecutive days in the home and one day in a testing facility in a large twin sample of middle-aged men. Five samples were collected at specific times on each day. This design allows for the examination of differences in the genetic and environmental influences on DHEAS both within and across days.
Methods
Research participants
The present study consists of 783 individuals comprising 193 MZ and 196 DZ pairs (389 complete pairs) and 5 unpaired twins who provided saliva samples for hormone measures as part of the Vietnam Era Twin Study of Aging (VETSA); the VETSA has been described in detail elsewhere (Kremen et al., 2006). VETSA participants are men enrolled in the Vietnam Era Twin (VET) Registry which comprises a sample of MZ and DZ twin pairs who served in the United States military during the Vietnam era (1965 to 1975), although the majority did not serve in combat or in Vietnam (Eisen et al., 1987, Henderson et al., 1990). The VETSA twin pairs were randomly selected from a pool of 3322 VET Registry twin pairs who had participated in a study of psychological health in 1992.
The VETSA sample consisted of 1237 twins between the ages of 51 and 59 at the time of recruitment and both members of a pair had to agree to participate. Twins traveled either to University of California, San Diego or Boston University for a day-long series of interviews and physical and cognitive assessments. In cases in which a twin could not travel (N = 26 individuals, 3.5%) research assistants conducted assessments at a facility close to the twin’s home. The VETSA Hormone Study was later initiated to examine the role of hormonal regulation and its association with cognitive aging in participants in the primary VETSA study and consists of a smaller sub-sample of participants (N = 783) (Franz, C. E. et al., 2010).
Participants completed two days of saliva collection at home, prior to the day of testing and then sent the saliva samples via overnight mail to the University of California, Davis to be assayed. In addition to the two days of saliva collection at home, participants provided saliva samples during the day of testing. IRB approval was obtained at both sites, and all participants provided signed informed consent. A combination of DNA testing, previously obtained questionnaire and blood group methods was used to determine zygosity (Eisen et al., 1989, Nichols and Bilbro, 1966, Peeters et al., 1998).
Procedures
Saliva collection
Participants were contacted six weeks prior to the day of testing (DOT) to establish the two “typical” working days separated by one day (preferably Tuesday/Thursday to avoid sampling on the beginning and end of the work week) on which they would provide the at-home saliva samples. At-home sample collection took place two to three weeks before the DOT in order to avoid the disruption of schedules that can be caused by travel. Participants were asked the time they usually woke up in the morning in order to individualize their schedules and to set times on reminder watches. Saliva kits were mailed to participants and participants were called the day prior to starting sampling to ensure that the reminder watch was turned on, instructions were understood, and the sampling kit was placed by their bed for the morning sample. The saliva kit included all supplies: labeled 4.5 ml Cryotube saliva vial, Trident original sugarless gum, straws to facilitate drooling into each vial, tissues, instructions, a daily log, pen, a reminder watch, and a storage container with a MEMS 6™ (Aardex) track cap for detecting compliance with the protocol. All materials coming in contact with saliva, including the gum, have been checked for the presence of hormones or compounds that could compromise the assays under the supervision of SPM.
Participants provided samples at wake-up, 30 minutes after wake-up, 10:00 am, 3:00 pm, and 9:00 pm or bedtime. Times were selected on the basis of appropriate times for the measurement of cortisol, which was also measured as part of the VETSA Hormone Study. Diurnal DHEAS is secreted synchronously with cortisol, although the patterns of hormone concentrations differ throughout the day. The highest concentrations of cortisol occur 30–60 minutes after awakening, while the highest concentrations of DHEAS occuring at awakening followed by a gradual decline throughout the day (Hucklebridge, F., Hussain, T., Evans, P., & Clow, A., 2005; Nieschlag, E. et al., 1973). At the specified time, participants selected the appropriate vial and provided approximately 2.25 ml of saliva. If necessary, the participant chewed original Trident gum to stimulate saliva and removed the gum prior to providing the sample. Previous testing by one of the investigators (SPM) showed that this particular gum did not alter DHEAS concentrations. Once a saliva sample was provided, participants were instructed to close the vial tightly, place it in the storage bottle, and complete the log entry for that time period. Each opening of the storage bottle was automatically logged by the track cap. Participants were asked to keep the samples refrigerated and were provided with an insulated lunch bag to keep the supplies together. The reminder watch was programmed to go off at each scheduled time; however, the time protocol was carefully explained to participants (verbally and in writing) to allow for normal variations in schedules. Reminder watch times were individualized so that all participants provided samples at equivalent times (typical awakening, awake plus 30 minutes, awake plus 4 hours, awake plus 9 hours, and bedtime).
Saliva samples were also collected on the DOT. Subjects received their supplies when they arrived at the hotel the day before. As in the at-home sampling, the first sample was collected at awakening the morning of the day of testing at the hotel, then half an hour after awakening at hotel, then approximately 10:00 AM and 3:00 pm in the laboratory, and bedtime at the hotel. Test day protocols were standardized across sites. Participants completed log entries following each sample and provided a detailed description of what they ate for lunch.
DHEAS assays
Samples were centrifuged at 3000 rpm for 20 min to separate the aqueous component from mucins and other suspended particles. Salivary concentrations of DHEAS were estimated in duplicate using commercial radioimmunoassay kits (Diagnostics Systems Laboratories, Webster, TX) according to manufacturer’s instructions. Assay sensitivity (least detectable dose) was 0.0206 ng/ml. Intra- and inter-assay coefficients of variation were 5.36% and 5.82%, respectively. Samples where the DHEAS concentration was greater than 3 standard deviations from the mean were considered to be outliers and coded as missing (N = 49 samples). All 17 samples from each participant were analyzed in the same assay; one to three individuals were included in the same assay batch. Assays were performed without knowledge of the zygosity of the participant.
Scores were imputed for missing values only if the participant had no more than one missing value on a day. Missing data were imputed by calculating the full samples’ mean DHEAS change between the time point with the missing value and the adjacent time point. For all time points except awakening, the time point prior to the missing value was used. The mean DHEAS change for those two points was then added (or subtracted) from the individual participant’s non-missing time point to get the imputed value for the missing time point in question. For example, if a participant was missing a value for the 3pm measure, the full samples’ mean change cortisol from 10am to 3pm hours was calculated. This value was then subtracted from the participant’s own 10am value to obtain the imputed 3pm value. The distributions of DHEAS values were skewed, and as such were natural log transformed prior to data analysis in order to produce normal distributions.
At-home DHEAS concentrations were averaged at corresponding times on day one and day two to create a single value for analysis; which was supported by the high intercorrelations observed between daily measures of the same time point, which ranged from 0.73 to 0.78 (p < 0.0001). Correlations between DHEAS samples at each time point and age were also small, but some were significant; these correlations ranged from r = −0.02 (p = 0.42) to r = −0.15 (p <0.0001). Further, there were no significant associations between salivary DHEAS concentrations and common covariates including BMI, current insulin use, current smoking, or current use of hypertensive medication. Subsequent analyses adjusted for the effect of age on the mean concentrations of DHEAS.
Data Analysis
Determination of Study Sample Representativeness
The participants included in this study were compared with the participants in the VETSA who were not included to determine whether the sub-sample used was representative of the larger VETSA population. Age, body mass index, and educational attainment were compared using a Wilcoxon rank-sum test. Current smoking, current self-reported depression, ever receiving a depression diagnosis by a physician, and current use of any prescribed medications were compared using the χ2-test.
Use of a Markov Chain Monte Carlo Approach to Estimate Genetic and Environmental Effects in the Presence of Batch and Age Effects
The classic twin study is based on the comparison of similarities between monozygotic (MZ) and dizygotic (DZ) twin pairs. MZ twins are expected to be more similar compared to DZ pairs because MZ twins share 100% of their genes, while dizygotic (DZ) twins share on average 50% of their genes. Therefore, a greater difference in measures of similarity between MZ and DZ pairs suggests the presence of additive genetic effects on a trait. Twin modeling approaches estimate proportions of the total variance due to genetic and environmental sources, specifically: (1) additive genetic influences (A); (2) common environmental influences (C) which represents life experiences shared between twin pairs, making them more alike; and (3) unique environmental influences (E) which represents experiences that make siblings different (e.g., having a spouse die). The estimate of unique environmental influences also includes measurement error.
Estimation of Genetic and Environmental Contributions
We controlled for the possibility of extraneous effects that can arise in laboratory measures due to instances of members of a twin pair that were assayed simultaneously on the same assay instead of randomly across all assays. In the context of twin studies, these so-called “batch” effects will increase within pair similarity as a consequence of more similar laboratory conditions and can be mistakenly attributed to the effects of the shared environment. The pattern of correlations between unrelated individuals in the same batch, and related individuals (twin pairs) in different batches was used to estimate the random effects of differences between batches. Correlations for MZ and DZ twin pairs could then be estimated without the confounded effects of differences between batches. We also controlled for the effect of age on DHEAS concentrations since increasing age was significantly associated with lower DHEAS concentrations for some time points.
The simultaneous estimation of the components of variances due to batch and age differences and differences between and within twin pairs was done within a Bayesian framework using Markov Chain Monte Carlo (MCMC) methods in the freely available package WinBUGS (Speigelhalter, D., Thomas, A., Best, N., & Lunn, D., 2004). We denote the estimated DHEAS concentration of the jth twin of the ith pair as Yijk, where the subscript k indicates the batch in which the value was assayed such that assays in the same batch have the same value of k.
We then let: Yijk = µ+ τij + αi + βk where µ represents the measured mean DHEAS concentration, τij represents the random differences between twins, conceivably correlated between pairs, and αi represents the random differences across ages between pairs, and βk represents the random differences between batches. For each type of twin (MZ and DZ) we assume that the twin effects τij, are bivariate normal with standard deviation στ and intraclass correlation ρMZ for MZ and ρDZ for DZ pairs, respectively. Batch and age effects are assumed to have a normal distribution (N[0,σ2βκ] and N[0,σ2ατl]).
When the MCMC algorithm converges, it yields successive samples from the posterior distribution that may be used to estimate summary statistics such as confidence intervals of model parameters. We assumed a relative uninformative normal prior for µ. We assumed broad uniform priors on στ and σβ,, following a suggestion of Spiegelhalter et al. (Spiegelhalter, D. J., Thomas, A., & Best, N. G., 2003). The twin correlations for MZ and DZ pairs were sampled initially from a uniform prior distribution over the range 0–0.9.
Random effects were simulated for each pair (πi), age (αi) and batch (βk) using the appropriate between-pair component of variance for each twin type. Individual twin effects (τij) were simulated to have a normal distribution (N[πI, σ2τw]), where the intra-pair variance is σ2τw = σ2τ (1-ρ), ρ being the intraclass correlation for MZ or DZ twins as appropriate. Ancillary summary statistics were computed from successive samples of ρMZ for and ρDZ together with their confidence intervals. These statistics include estimates of broad sense heritability (H), an indicator of the proportion of total population variance that is due to genetic variation and the shared environment (C), an estimate of the relative contribution of the variance shared between twins. Heritability was estimated using Holzinger’s H, H=2(ρMZ - ρDZ) and the contribution of the shared environment was estimated as C=2ρDZ - ρMZ. Samples from the posterior distribution of H and C allowed for the estimation of their confidence intervals. Note that estimates C may be negative if there are large non-additive genetic effects. Similarly, estimates of H may be negative when no significant genetic effects are present. Thus, this approach to the estimation of H and C avoids the biases inherent in constraining either to be greater than zero in the more familiar maximum likelihood components of variance approach. A copy of the WinBUGS code, together with illustrative data structure and initial values may be obtained from the first author.
Results
Sample Representativeness
As expected from the later start of the VETSA Hormone Study compared to the full VETSA sample, the average age of participants included in this sub-sample was older (55.92; SD = 2.58) compared to VETSA participants who were not in the neuroendocrine study (54.63; SD = 2.05); this is a small, but significant difference (p < 0.0001). There were no significant differences for BMI (p = 0.15) or educational attainment (p = 0.21) between those who were part of the sub-sample and larger VETSA sample. Further, there were no significant differences between the groups for the prevalence of any prescribed drug use (p = 0.40), current smoking (p = 1.00), current self-reported depression (p = 0.63), or any depression diagnosed by physician (p = 0.77).
Summary Statistics
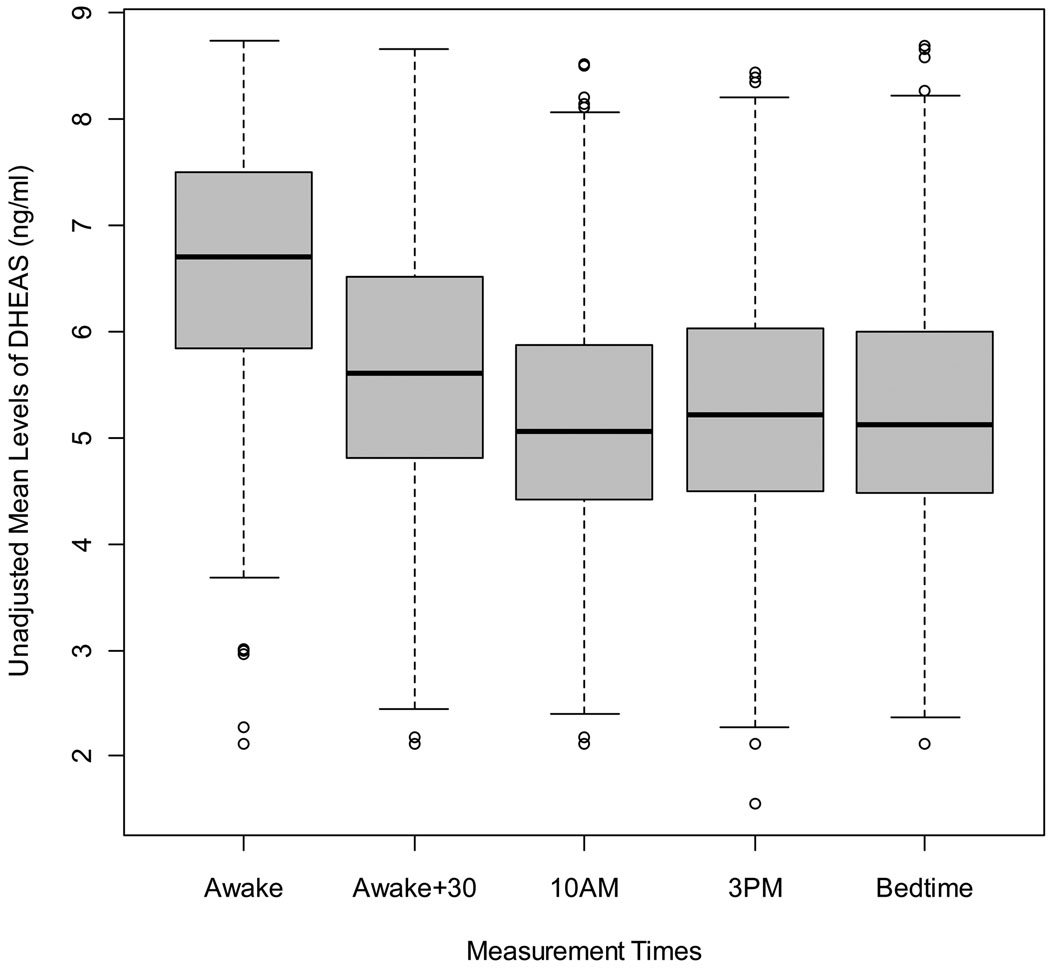
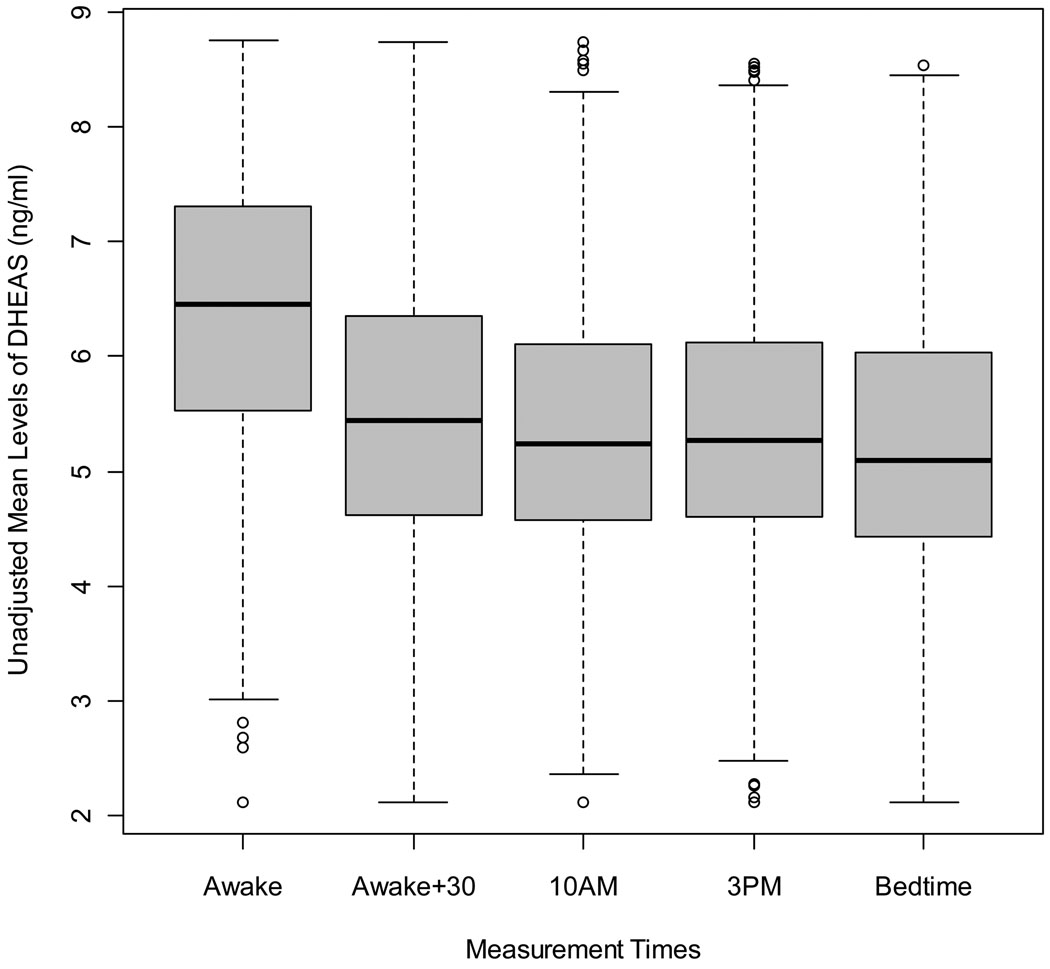
There was a consistent diurnal pattern for salivary DHEAS concentrations in both at-home and DOT measures (Figures 1 and 2). Repeated-measures contrasts were used to compare average unadjusted concentrations of DHEAS as well as age-adjusted concentrations from each time point to the next (ie: awake vs. awake +30, awake +30 vs. 10am, etc…). DHEAS concentrations were significantly higher at awakening and at awake plus 30 minutes (p <0.0001) for both unadjusted and age-adjusted measures. Measures taken between10am and bedtime did not differ significantly from one another. DHEAS concentrations between at-home and DOT measures across specific times were generally consistent, ranging from 0.57 to 0.83 (p < 0.0001) in unadjusted measures and 0.61 to 0.75 (p < 0.0001) for age-adjusted measures.
Figure 1.
Unadjusted Mean Concentrations of Log-Transformed DHEAS Across At-Home Day Measures
Figure 2.
Unadjusted Mean Concentrations of Log-Transformed DHEAS Across Testing Day Measures
Univariate Genetic Analysis of DHEAS Concentrations
There was significant heritability for the 10 am, 3pm and overall daily mean concentrations of unadjusted DHEAS during the at-home days (Table 2) and for the DOT (Table 3). Heritability estimates for these measures ranged between 0.32 and 0.47, with 95% confidence intervals as low as 0.01 to as high as 0.81. Significant additive genetic effects were detected for the 10 am, 3pm and bedtime age-adjusted measures during the at-home days (Table 4). Significant heritability was detected for the 10am and 3pm age-adjusted measures on the DOT (Table 5). Heritability estimates for these measures ranged between 0.37 and 0.46, with 95% confidence intervals as low as 0.01 to as high as 0.78. The heritability estimates of mean DHEAS concentrations on both at-home days and for DOT were no longer significant after adjusting for age.
Table 2.
Summary Statistics and Variance Components Estimates of Genetic and Environmental Effects for Unadjusted At-Home Testing Measures of DHEAS
| Days 1 and 2 | Mean DHEAS Levels in ng/ml (SD) |
Mean Time of DHEAS Collection (SD) |
rMZ | 95% CI | rDZ | 95% CI | A | 95% CI | C | 95% CI | E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Awake | 6.54 (0.05) | 0631h (2.25) | 0.32 | (0.18; 0.44) | 0.27 | (0.14; 0.40) | 0.09 | (−0.26; 0.44) | 0.22 | (−0.07; 0.51) | 0.68 |
| Awake+30 | 5.59 (0.05) | 0684h (2.26) | 0.39 | (0.27; 0.50) | 0.28 | (0.12; 0.42) | 0.22 | (−0.12; 0.59) | 0.17 | (−0.16; 0.46) | 0.61 |
| 10 AM | 5.12 (0.05) | 1019h (1.56) | 0.47 | (0.36; 0.57) | 0.31 | (0.18; 0.44) | 0.32 | (0.01; 0.63) | 0.15 | (−0.12; 0.41) | 0.53 |
| 3 PM | 5.21 (0.05) | 1502h (1.66) | 0.46 | (0.34; 0.56) | 0.22 | (0.08; 0.36) | 0.47 | (0.12; 0.81) | −0.01 | (−0.31; 0.27) | 0.54 |
| Bedtime | 5.19 (0.05) | 2040h (3.63) | 0.35 | (0.22; 0.47) | 0.19 | (0.06; 0.33) | 0.31 | (−0.05; 0.66) | 0.04 | (−0.25; 0.33) | 0.65 |
| Home Overall | 5.53 (0.05) | 0.48 | (0.36; 0.58) | 0.29 | (0.14; 0.41) | 0.38 | (0.07; 0.71) | 0.1 | (−0.20; 0.35) | 0.52 |
rMZ = Monozygotic twin correlation; rDZ = Dizygotic twin correlation
A = Estimate of broad sense heritability, 2(rMZ - rDZ)
C = Estimate of shared environmental effects, 2*rMZ - rDZ
E = Estimate of unique environmental effects 1 – rMZ
Table 3.
Summary Statistics and Variance Components Estimates of Genetic and Environmental Effects for Unadjusted Day of Testing Measures of DHEAS Adjusted for Age
| Test Day | Mean DHEAS Levels in ng/ml (SD) |
Mean Time of DHEAS Collection (SD) |
rMZ | 95% CI | rDZ | 95% CI | A | 95% CI | C | 95% CI | E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Awake | 6.39 (0.05) | 0589h (0.78) | 0.38 | (0.24; 0.50) | 0.26 | (0.09; 0.40) | 0.24 | (−0.13; 0.62) | 0.14 | (−0.19; 0.43) | 0.62 |
| Awake+30 | 5.54 (0.05) | 0644h (0.87) | 0.39 | (0.27; 0.50) | 0.27 | (0.14; 0.40) | 0.23 | (−0.10; 0.58) | 0.16 | (−0.14; 0.43) | 0.61 |
| 10 AM | 5.37 (0.05) | 0973h (0.58) | 0.35 | (0.22; 0.46) | 0.16 | (0.03; 0.30) | 0.37 | (0.02; 0.73) | −0.03 | (−0.32; 0.27) | 0.65 |
| 3 PM | 5.37 (0.05) | 1493h (0.98) | 0.39 | (0.27; 0.51) | 0.20 | (0.08; 0.34) | 0.38 | (0.02; 0.72) | 0.01 | (−0.26; 0.31) | 0.61 |
| Bedtime | 5.25 (0.05) | 2058h (4.67) | 0.32 | (0.15; 0.45) | 0.16 | (0.02; 0.32) | 0.31 | (−0.05; 0.69) | 0.01 | (−0.30; 0.31) | 0.68 |
| Test Overall | 5.59 (0.04) | 0.45 | (0.33; 0.55) | 0.26 | (0.13; 0.38) | 0.38 | (0.05; 0.69) | 0.07 | (−0.19; 0.33) | 0.55 |
rMZ = Monozygotic twin correlation; rDZ = Dizygotic twin correlation
A = Estimate of broad sense heritability, 2(rMZ - rDZ)
C = Estimate of shared environmental effects, 2*rMZ - rDZ
E = Estimate of unique environmental effects 1 – rMZ
Table 4.
Summary Statistics and Variance Components Estimates of Genetic and Environmental Effects for At-Home Testing Measures of DHEAS Adjusted for Age
| Measure | Mean DHEAS Levels in ng/ml (SD) |
Mean Time of DHEAS Collection (SD) |
rMZ | 95% CI | rDZ | 95% CI | A | 95% CI | C | 95% CI | E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Waking | 6.63 (0.05) | 0631h (2.25) | 0.33 | (0.19; 0.45) | 0.30 | (0.16; 0.41) | 0.07 | (−0.29; 0.42) | 0.26 | (−0.03; 0.52) | 0.67 |
| Awake +30 | 5.71 (0.06) | 0684h (2.26) | 0.39 | (0.27; 0.5) | 0.27 | (0.13; 0.41) | 0.24 | (−0.11; 0.59) | 0.15 | (−0.14; 0.44) | 0.61 |
| 10am | 5.24 (0.05) | 1019h (1.56) | 0.44 | (0.31; 0.55) | 0.23 | (0.04; 0.37) | 0.41 | (0.07; 0.78) | 0.03 | (−0.33; 0.32) | 0.56 |
| 3pm | 5.33 (0.05) | 1502h (1.66) | 0.44 | (0.32; 0.55) | 0.21 | (0.09; 0.34) | 0.46 | (0.13; 0.78) | −0.02 | (−0.29; 0.25) | 0.56 |
| Bedtime | 5.32 (0.05) | 2040h (3.63) | 0.35 | (0.21; 0.47) | 0.16 | (0.05; 0.29) | 0.37 | (0.01; 0.7) | −0.02 | (−0.28; 0.26) | 0.65 |
| Mean | 5.93 (0.05) | 0.45 | (0.33; 0.56) | 0.29 | (0.11; 0.42) | 0.31 | (−0.02; 0.71) | 0.14 | (−0.24; 0.41) | 0.55 |
Bold values indicate significant estimate
rMZ = Monozygotic twin correlation; rDZ = Dizygotic twin correlation
A = Estimate of broad sense heritability, 2(rMZ - rDZ)
C = Estimate of shared environmental effects, 2*rMZ - rDZ
E = Estimate of unique environmental effects 1 – rMZ
Table 5.
Summary Statistics and Variance Components Estimates of Genetic and Environmental Effects for Day of Testing Measures of DHEAS Adjusted for Age
| Measure | Mean DHEAS Levels in ng/ml (SD) |
Mean Time of DHEAS Collection (SD) |
rMZ | 95% CI | rDZ | 95% CI | A | 95% CI | C | 95% CI | E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Waking | 6.41 (0.05) | 0589h (0.78) | 0.35 | (0.22; 0.47) | 0.22 | (0.09; 0.35) | 0.26 | (−0.10; 0.61) | 0.09 | (−0.20; 0.37) | 0.65 |
| Awake +30 | 5.57 (0.06) | 0644h (0.87) | 0.41 | (0.28; 0.52) | 0.27 | (0.13; 0.38) | 0.28 | (−0.04; 0.61) | 0.12 | (−0.15; 0.38) | 0.59 |
| 10am | 5.40 (0.05) | 0973h (0.58) | 0.34 | (0.21; 0.46) | 0.16 | (0.03; 0.29) | 0.37 | (0.02; 0.69) | −0.03 | (−0.28; 0.26) | 0.66 |
| 3pm | 5.42 (0.05) | 1493h (0.98) | 0.38 | (0.24; 0.5) | 0.19 | (0.06; 0.33) | 0.38 | (0.03; 0.71) | 0 | (−0.27; 0.29) | 0.62 |
| Bedtime | 5.31 (0.05) | 2058h (4.67) | 0.34 | (0.19; 0.46) | 0.18 | (0.05; 0.31) | 0.32 | (−0.05; 0.67) | 0.01 | (−0.26; 0.30) | 0.66 |
| Mean | 5.75 (0.05) | 0.42 | (0.3; 0.53) | 0.26 | (0.12; 0.39) | 0.32 | (−0.02; 0.66) | 0.10 | (−0.19; 0.37) | 0.58 |
Bold values indicate significant estimate
rMZ = Monozygotic twin correlation; rDZ = Dizygotic twin correlation
A = Estimate of broad sense heritability, 2(rMZ - rDZ)
C = Estimate of shared environmental effects, 2*rMZ - rDZ
E = Estimate of unique environmental effects 1 – rMZ
For both unadjusted and age-adjusted DHEAS concentrations, the heritability estimates for awake and awake plus 30 minute measures were not significant either at-home or for DOT measures. The variance due to the shared environment was not significant for any at-home or DOT measures. The majority of the variance of diurnal DHEAS concentrations was due to unique environmental influences (0.52 – 0.68).
Discussion
This is the first study to characterize the diurnal concentrations of salivary DHEAS across multiple salivary time-points in both at-home and in-lab settings in a community-based sample of middle-aged men. The pattern and variation of DHEAS concentrations is consistent across days, with significantly higher concentrations occurring at awakening and awake plus 30 minutes compared to later in the day. This has been reported in similar studies of humans using blood with smaller sample sizes (Nicolau, G. Y. et al., 1985; Rosenfeld, R. S., Rosenberg, B. J., Fukushima, D. K., & Hellman, L., 1975; Carlström, K., Karlsson R., & von Schoultz, B., 2002; Zhao, Z. Y. et al., 2003) as well as non-human primates (Maninger, N., Capitanio, J. P., Mason, W. A., Ruys, J. D., & Mendoza, S. P., 2010). Additionally, high correlations across the days indicated stable and constant concentrations of diurnal DHEAS. Consequently, salivary concentrations may reliably reflect patterns of DHEAS in blood and in turn provide insight into the steroidogenic activity of the zona reticularis region of the adrenal cortex.
To date, this is the largest population-based twin study to estimate the genetic and environmental contributions across multiple salivary measures of diurnal DHEAS concentrations using multiple days and environmental settings. Salivary DHEAS is a heritable measure, with genetic effects accounting for 37%–46% of the total variance for the late morning (10am) and afternoon (3pm) age-adjusted measures on at-home and DOT. These estimates and their 95% confidence intervals fall within the range of those previously reported from family and twin studies of DHEAS (26% – 66%) (Meikle, A. W., Stringham, J. D., Woodward, M. G., & Bishop, D. T., 1988; Nestler, J. E. et al., 2002). These heritability estimates are expected to reflect the role genetic effects in an aging population which may differ across development. For example, in a small pilot study of adult twin men with an average age of 32, Meikle and colleagues reported an estimate of heritability of 58% (Meikle, A. W., Stringham, J. D., Woodward, M. G., & Bishop, D. T., 1988). In a follow-up study of a larger sample of twin men with average age of approximately 54.5 years, heritability was estimated at 26% (Meikle, A. W., Stephenson, R. A., Lewis, C. M., Wiebke, G. A., & Middleton, R. G., 1997).
The consistency of mean DHEAS concentrations between at-home and DOT days as well as for the magnitudes of the genetic and environmental effects for 10 am and 3pm measures suggests that DHEAS is not responsive to mild stressors similar to those experienced during the day of testing. These mild stressors included study involvement in a laboratory setting and the long-distance travel required to participate. DHEAS has historically been considered a good marker of individual adrenal cortex function and is expected to reflect chronic rather than acute response to the environment (Baulieu, E. E., 1996). Recent studies have reported significant differences in DHEAS concentrations in individuals exposed to chronic stressors such as burnout and caring for patients with Alzheimer’s disease (Jeckel, C. M. et al., 2010). Most studies of the HPA axis have focused on cortisol secretion. However, DHEAS concentrations may also be an indicator of HPA axis activity in response to chronic stressors. A recent study of rhesus monkeys found that DHEAS concentrations increased in response to a chronic (repeated) stressor (Maninger, N., Capitanio, J. P., Mason, W. A., Ruys, J. D., & Mendoza, S. P., 2010). The current results may reflect the genetic and environmental effects of the response related to exposure to chronic stressors and may help to identify additional candidate genes related to HPA axis activity.
The significant heritability estimates for the afternoon rather than morning measures or the average daily concentrations suggests that some aspects of DHEAS production may be under greater genetic control than others. DHEAS concentrations are not expected to be highly variable throughout the day (Kroboth, P. D., Salek, F. S., Pittenger, A. L., Fabian, T. J., & Frye, R. F., 1999). Given the demonstrated diurnal rhythm of DHEAS in this and other studies as well as significant heritability estimates for afternoon measures, the use of multiple measures across the day may better identify important time-specific genetic and environmental effects related to daily concentrations of this hormone for use in genetic association studies rather than single or morning-only measures (Boger-Megiddo, I., Weiss, N. S., Barnett, M. J., Goodman, G. E., & Chen, C., 2008; Karlson, E. W. et al., 2009). The variation associated with diurnal DHEAS concentrations and associated time-specific genetic effects identify a need to study the patterns of DHEAS concentrations across the day rather than only for a specific time-point. Additionally, evaluation of diurnal regulation of DHEAS across development may also be an important risk factor for disease. Prior studies of DHEAS across the lifespan indicated that lifetime trajectories of DHEAS concentrations, rather than baseline DHEAS concentrations alone were associated with higher mortality in older adults (Cappola, A. R. et al., 2009) as well as cardiovascular disease (Sanders, J. L. et al., 2010). Consequently, these results encourage the characterization diurnal DHEAS profiles and the estimation of genetic and environmental contributions to profiles across the lifespan.
These results should be evaluated in the light of the following limitations. First, this is a sample of middle-aged Caucasian men and these findings may not generalize to women or other ethnic populations. Additionally, because DHEAS concentrations are age-specific, these results may not generalize to other periods of development. Nevertheless, the range of these moderate heritability estimates and their 95% confidence intervals suggests that they are within the range of other studies with participants across greater age ranges and consisting of men and women. Further, these estimates serve as a baseline for the genetic and environmental effects on DHEAS concentrations throughout later adulthood because this population has been collected as part of a study of aging. Second, no adjustments were made to account for associations between DHEAS and current affected status for common chronic diseases since there were no significant associations between DHEAS concentrations and common risk factors of chronic disease such as BMI, current insulin use, current smoking, or current use of hypertensive medication. However, it is expected that those with a current diagnosis may have altered concentrations of DHEAS. Third, chewing gum was recently reported to have an effect on concentrations of salivary testosterone, estradiol and immunoglobulin A assays although the mechanism by which this effect is not understood (van Anders, S. M., 2010). Two possibilities explaining this effect were suggested: (1) chewing gum stimulates analyte production and (2) chewing gum interacts with assay results. No data was collected on this aspect of hormone production and consequently the effects of chewing gum on mean concentrations of DHEAS were not included. However, chewing gum represents non-systematic error and its effects would be included in the estimate of unique environmental effects. Further, it is unlikely that genetic influences on chewing gum will bias estimates on the variance due genetic effects for salivary DHEAS. Fourth, no blood DHEAS concentrations were collected for comparison against the salivary measures. Consequently, these results will require replication in other populations with both blood and salivary samples for to compare estimates of genetic and environmental effects.
These results have demonstrated patterns between salivary samples of diurnal DHEAS concentrations to be similar to those of blood. DHEAS obtained from saliva is heritable which is anticipated to encourage future population-based studies of DHEAS. Further, the heritability of DHEAS is higher for mid-morning and early afternoon measures, suggestive of time-specific genetic influences on DHEAS. Future research of DHEAS has the potential to improve understanding of disease etiology and may span across several concentrations of inquiry including: (1) the use of cross-sectional data to determine associations of diurnal DHEAS concentrations with specific outcomes as well as the presence of genetic variance that is common to both the outcome and DHEAS, (2) the analysis of longitudinal data to determine whether the genetic contributions of DHEAS concentrations vary across age and (3) the analysis of DHEAS concentrations and its relationship with functionally-related hormones such as cortisol and testosterone to understand the biological pathway between DHEAS, other sex hormones and related outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An P, Rice T, Gagnon J, Hong Y, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Race differences in the pattern of familial aggregation for dehydroepiandrosterone sulfate and its responsiveness to training in the HERITAGE Family Study. Metabolism. 2001;50:916–920. doi: 10.1053/meta.2001.24926. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, von Muhlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr.Soc. 1999;47:685–691. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Baulieu E-E. Steroid hormones in the brain: several mechanisms. In: Fuxe K, Gustafson JA, Wetterberg L, editors. Steroid hormone regulation of the brain. Elmsford, NY: Pergamon; 1981. pp. 3–14. [Google Scholar]
- Baulieu EE. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin.Endocrinol.Metab. 1996;81:3147–3151. doi: 10.1210/jcem.81.9.8784058. [DOI] [PubMed] [Google Scholar]
- Boger-Megiddo I, Weiss NS, Barnett MJ, Goodman GE, Chen C. V89L polymorphism of the 5alpha-reductase Type II gene (SRD5A2), endogenous sex hormones, and prostate cancer risk. Cancer Epidemiol.Biomarkers Prev. 2008;17:286–291. doi: 10.1158/1055-9965.EPI-07-0238. [DOI] [PubMed] [Google Scholar]
- Brown RC, Liu Y, Papadopoulos V. DHEA: biosynthesis, regulation and function in the central nervous system. In: Morfin R, editor. DHEA and the Brain. Paris: Taylor and Francis; 2002. pp. 65–80. [Google Scholar]
- Butcher SK, Killampalli V, Lascelles D, Wang K, Alpar EK, Lord JM. Raised cortisol:DHEAS ratios in the elderly after injury: potential impact upon neutrophil function and immunity. Aging Cell. 2005;4:319–324. doi: 10.1111/j.1474-9726.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- Cappola AR, O'Meara ES, Guo W, Bartz TM, Fried LP, Newman AB. Trajectories of dehydroepiandrosterone sulfate predict mortality in older adults: the cardiovascular health study. J Gerontol.A Biol.Sci Med.Sci. 2009;64:1268–1274. doi: 10.1093/gerona/glp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlström K, Karlsson R, von Schoultz B. Diurnal rhythm and effects of oral contraceptives on serum dehydroepiandrosterone sulfate (DHEAS) are related to alterations in serum albumin rather than to changes in adrenocortical steroid secretion. Scandinavian Journal of Clinical Laboratory Investigation. 2002;62:361–368. doi: 10.1080/00365510260296519. [DOI] [PubMed] [Google Scholar]
- Chen CC, Parker CR., Jr Adrenal androgens and the immune system. Semin.Reprod.Med. 2004;22:369–377. doi: 10.1055/s-2004-861553. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu E-E. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci USA. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debonnel G, Bergeron R, de Montigny C. Potentiation by dehydroepiandrosterone of the neuronal response to N-methyl-D-aspartate in the CA3 region of the rat dorsal hippocampus: an effect mediated via sigma receptors. J.Endocrinol. 1996;150 Suppl:S33–S42. [PubMed] [Google Scholar]
- Eaves LJ. The Utlity of Twins. In: Anderson VE, Hauser WA, Penry JK, Sing CF, editors. Genetic Basis of the Epilepsies. New York: Raven Press; 1982. pp. 249–276. [Google Scholar]
- Fabian TJ, Dew MA, Pollock BG, Reynolds CF, III, Mulsant BH, Butters MA, Zmuda MD, Linares AM, Trottini M, Kroboth PD. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biol.Psychiatry. 2001;50:767–774. doi: 10.1016/s0006-3223(01)01198-2. [DOI] [PubMed] [Google Scholar]
- Franz CE, York TP, Eaves LJ, Mendoza SP, Hauger RL, Hellhammer DH, Jacobson KC, Levine S, Lupien SJ, Lyons MJ, Prom-Wormley E, Xian H, Kremen WS. Genetic and Environmental Influences on Cortisol Regulation Across Days and Contexts in Middle-Aged Men. Behav.Genet. 2010 doi: 10.1007/s10519-010-9352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PME, Pearson J, Secher SM, Shiers HM. Altered diurnal rhythms in salivary cortisol and dehydroepiandosterone (DHEA) at presentation. Psychological Medicine. 1996;26:245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PM. First-episode major depression in adolescents. Affective, cognitive and endocrine characteristics of risk status and predictors of onset. Br.J Psychiatry. 2000;176:142–149. doi: 10.1192/bjp.176.2.142. [DOI] [PubMed] [Google Scholar]
- Hucklebridge F, Hussain T, Evans P, Clow A. The diurnal patterns of the adrenal seroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Nakashima N. Dehydroepiandrosterone-sulfate inhibits nuclear factor-kappaB-dependent transcription in hepatocytes, possibly through antioxidant effect. J Clin.Endocrinol.Metab. 2004;89:3449–3454. doi: 10.1210/jc.2003-031441. [DOI] [PubMed] [Google Scholar]
- Jaquish CE, Blangero J, Haffner SM, Stern MP, Maccluer JW. Quantitative genetics of dehydroepiandrosterone sulfate and its relation to possible cardiovascular disease risk factors in Mexican Americans. Hum.Hered. 1996;46:301–309. doi: 10.1159/000154368. [DOI] [PubMed] [Google Scholar]
- Jaquish CE, Mahaney MC, Blangero J, Haffner SM, Stern MP, Maccluer JW. Genetic correlations between lipoprotein phenotypes and indicators of sex hormone levels in Mexican Americans. Atherosclerosis. 1996;122:117–125. doi: 10.1016/0021-9150(96)05796-6. [DOI] [PubMed] [Google Scholar]
- Jeckel CM, Lopes RP, Berleze MC, Luz C, Feix L, Argimon II, Stein LM, Bauer ME. Neuroendocrine and immunological correlates of chronic stress in 'strictly healthy' populations. Neuroimmunomodulation. 2010;17:9–18. doi: 10.1159/000243080. [DOI] [PubMed] [Google Scholar]
- Karlson EW, Chibnik LB, McGrath M, Chang SC, Keenan BT, Costenbader KH, Fraser PA, Tworoger S, Hankinson SE, Lee IM, Buring J, De VI. A prospective study of androgen levels, hormone-related genes and risk of rheumatoid arthritis. Arthritis Res.Ther. 2009;11:R97. doi: 10.1186/ar2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. "Familiality" or heritability. Arch.Gen.Psychiatry. 2009;66:452–453. doi: 10.1001/archgenpsychiatry.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen YT, Stegaev V, Mackiewicz Z, Porola P, Hanninen A, Szodoray P. Salivary glands - "an unisex organ"? Oral Dis. 2010;16:577–585. doi: 10.1111/j.1601-0825.2010.01669.x. [DOI] [PubMed] [Google Scholar]
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J.Clin.Pharmacol. 1999;39:327–348. doi: 10.1177/00912709922007903. [DOI] [PubMed] [Google Scholar]
- Lac G, Marquet P, Chassain AP, Galen FX. Dexamethasone in resting and exercising men. II. Effects on adrenocortical hormones. J Appl.Physiol. 1999;87:183–188. doi: 10.1152/jappl.1999.87.1.183. [DOI] [PubMed] [Google Scholar]
- Longcope C. Dehydroepiandrosterone metabolism. J Endocrinol. 1996;150 Suppl:S125–S127. [PubMed] [Google Scholar]
- Majewska MD. Neuronal actions of dehydroepiandrosterone. Possible roles in brain development, aging, memory, and affect. Ann.N.Y.Acad.Sci. 1995;774:111–120. doi: 10.1111/j.1749-6632.1995.tb17375.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD. DHEA: hormone of youth and resilience- still a maverick. In: Morfin R, editor. DHEA and the Brain. Paris: Taylor and Francis; 2002. pp. 65–80. [Google Scholar]
- Maninger N, Capitanio JP, Mason WA, Ruys JD, Mendoza SP. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle AW, Stephenson RA, Lewis CM, Wiebke GA, Middleton RG. Age, genetic, and nongenetic factors influencing variation in serum sex steroids and zonal volumes of the prostate and benign prostatic hyperplasia in twins. Prostate. 1997;33:105–111. doi: 10.1002/(sici)1097-0045(19971001)33:2<105::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Meikle AW, Stringham JD, Woodward MG, Bishop DT. Heritability of variation of plasma cortisol levels. Metabolism. 1988;37:514–517. doi: 10.1016/0026-0495(88)90164-3. [DOI] [PubMed] [Google Scholar]
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol.Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- Mitchell BD, Kammerer CM, Blangero J, Mahaney MC, Rainwater DL, Dyke B, Hixson JE, Henkel RD, Sharp RM, Comuzzie AG, VandeBerg JL, Stern MP, Maccluer JW. Genetic and environmental contributions to cardiovascular risk factors in Mexican Americans. The San Antonio Family Heart Study. Circulation. 1996;94:2159–2170. doi: 10.1161/01.cir.94.9.2159. [DOI] [PubMed] [Google Scholar]
- Nestler JE, Whitfield JB, Williams TY, Zhu G, Condon J, Kirk KM, Heath AC, Montgomery GW, Martin NG. Genetics of serum dehydroepiandrosterone sulfate and its relationship to insulin in a population-based cohort of twin subjects. J.Clin.Endocrinol.Metab. 2002;87:682–686. doi: 10.1210/jcem.87.2.8240. [DOI] [PubMed] [Google Scholar]
- Nicolau GY, Haus E, Lakatua DJ, Bogdan C, Sackett-Lundeen L, Popescu M, Berg H, Petrescu E, Robu E. Circadian and circannual variations of FSH, LH, testosterone, dehydroepiandrosterone-sulfate (DHEA-S) and 17-hydroxy progesterone (17 OH-Prog) in elderly men and women. Endocrinologie. 1985;23:223–246. [PubMed] [Google Scholar]
- Nieschlag E, Loriaux DL, Ruder HJ, Zucker IR, Kirschner MA, Lipsett MB. The secretion of dehydroepiandrosterone and dehydroepiandrosterone sulphate in man. J.Endocrinol. 1973;57:123–134. doi: 10.1677/joe.0.0570123. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J.Clin.Endocrinol.Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Parker CR, Jr, Azziz R, Potter HD, Boots LR. Adrenal androgen production in response to adrenocorticotropin infusions in men. Endocr.Res. 1996;22:717–722. doi: 10.1080/07435809609043767. [DOI] [PubMed] [Google Scholar]
- Pavlov EP, Harman SM, Chrousos GP, Loriaux DL, Blackman MR. Responses of plasma adrenocorticotropin, cortisol, and dehydroepiandrosterone to ovine corticotropin-releasing hormone in healthy aging men. J Clin.Endocrinol.Metab. 1986;62:767–772. doi: 10.1210/jcem-62-4-767. [DOI] [PubMed] [Google Scholar]
- Pomari E, Nardi A, Fiore C, Celeghin A, Colombo L, Dalla VL. Transcriptional control of human organic anion transporting polypeptide 2B1 gene. J Steroid Biochem.Mol.Biol. 2009;115:146–152. doi: 10.1016/j.jsbmb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Radford DJ, Wang K, McNelis JC, Taylor AE, Hechenberger G, Hofmann J, Chahal H, Arlt W, Lord JM. Dehdyroepiandrosterone sulfate directly activates protein kinase C-beta to increase human neutrophil superoxide generation. Mol.Endocrinol. 2010;24:813–821. doi: 10.1210/me.2009-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey WE, Nakamura Y. Regulation of the adrenal androgen biosynthesis. J Steroid Biochem.Mol.Biol. 2008;108:281–286. doi: 10.1016/j.jsbmb.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice T, Borecki IB. Familial resemblance and heritability. In: Rao DC, Province MA, editors. Genetic Dissection of Complex Traits. London: Academic press; 2001. pp. 35–43. [Google Scholar]
- Rice T, Sprecher DL, Borecki IB, Mitchell LE, Laskarzewski PM, Rao DC. The Cincinnati Myocardial Infarction and Hormone Family Study: family resemblance for dehydroepiandrosterone sulfate in control and myocardial infarction families. Metabolism. 1993;42:1284–1290. doi: 10.1016/0026-0495(93)90126-9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld RS, Rosenberg BJ, Fukushima DK, Hellman L. 24-Hour secretory pattern of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J Clin.Endocrinol.Metab. 1975;40:850–855. doi: 10.1210/jcem-40-5-850. [DOI] [PubMed] [Google Scholar]
- Rotter JI, Wong FL, Lifrak ET, Parker LN. A genetic component to the variation of dehydroepiandrosterone sulfate. Metabolism. 1985;34:731–736. doi: 10.1016/0026-0495(85)90023-x. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Boudreau RM, Cappola AR, Arnold AM, Robbins J, Cushman M, Newman AB. Cardiovascular disease is associated with greater incident dehydroepiandrosterone sulfate decline in the oldest old: the cardiovascular health study all stars study. J Am Geriatr.Soc. 2010;58:421–426. doi: 10.1111/j.1532-5415.2010.02724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speigelhalter D, Thomas A, Best N, Lunn D. WinBUGS (Version 1.4.1) [Computer software] 2004 [Google Scholar]
- Spiegelhalter DJ, Thomas A, Best NG. WinBUGS version 1.4 user manual. 2003 [Google Scholar]
- Takebayashi M, Kagaya A, Uchitomi Y, Kugaya A, Muraoka M, Yokota N, Horiguchi J, Yamawaki S. Plasma dehydroepiandrosterone sulfate in unipolar major depression. Short communication. J Neural Transm. 1998;105:537–542. doi: 10.1007/s007020050077. [DOI] [PubMed] [Google Scholar]
- van Anders SM. Chewing gum has large effects on salivary testosterone, estradiol, and secretory immunoglobulin A assays in women and men. Psychoneuroendocrinology. 2010;35:305–309. doi: 10.1016/j.psyneuen.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vining RF, McGinley RA, Symons RG. Hormones in saliva: mode of entry and consequent implications for clinical interpretation. Clin.Chem. 1983;29:1752–1756. [PubMed] [Google Scholar]
- Yildiz BO, Goodarzi MO, Guo X, Rotter JI, Azziz R. Heritability of dehydroepiandrosterone sulfate in women with polycystic ovary syndrome and their sisters. Fertil.Steril. 2006;86:1688–1693. doi: 10.1016/j.fertnstert.2006.05.045. [DOI] [PubMed] [Google Scholar]
- Zhao ZY, Xie Y, Fu YR, Li YY, Bogdan A, Touitou Y. Circadian rhythm characteristics of serum cortisol and dehydroepiandrosterone sulfate in healthy Chinese men aged 30 to 60 years. A cross-sectional study. Steroids. 2003;68:133–138. doi: 10.1016/s0039-128x(02)00167-8. [DOI] [PubMed] [Google Scholar]
- Zinder O, Dar DE. Neuroactive steroids: their mechanism of action and their function in the stress response. Acta Physiol Scand. 1999;167:181–188. doi: 10.1046/j.1365-201x.1999.00579.x. [DOI] [PubMed] [Google Scholar]