Abstract
Peripheral serotonin (5HT) has been implicated in migraine and temporomandibular pain disorders in humans and animal models and yet the mechanism(s) by which 5HT evokes pain remains unclear. Trigeminal pain can be triggered by activation of the transient receptor potential V1 channel (TRPV1), expressed by a subset of nociceptive trigeminal ganglia (TG) neurons and gated by capsaicin, noxious heat, and other noxious stimuli. As 5HT is released in the periphery during inflammation and evokes thermal hyperalgesia and TRPV1 is essential for thermal hyperalgesia, we hypothesized that 5HT increases the activity of capsaicin-sensitive trigeminal neurons and that this increase can be attenuated by pharmacologically targeting peripheral 5HT receptors. TG cultures were pretreated with 5HT (10 nM–100 µM), sumatriptan (5HT1B/1D agonist), ketanserin (5HT2A antagonist), granisetron (5HT3 antagonist) or vehicle prior to capsaicin (30 nM – 50 nM). Single-cell accumulation of intracellular calcium was recorded or CGRP release was measured following each treatment. In addition, using in situ hybridization and immunohistochemistry, we detected the co-localization of 5HT1B, 5HT1D, 5HT2A and 5HT3A, but not 5HT2C, mRNA with TRPV1 in TG cells. 5HT pretreatment evoked a significant increase in calcium accumulation in capsaicin-sensitive trigeminal neurons and enhanced capsaicin-evoked CGRP release, but had no significant effect when given alone. Sumatriptan, ketanserin and granisetron treatment attenuated calcium accumulation and 5HT enhancement of capsaicin-evoked CGRP release. Together these results indicate that 5HT increases the activity of capsaicin-sensitive peripheral nociceptors, which can be attenuated by pharmacologically targeting peripheral 5HT receptors, thereby providing a mechanistic basis for peripheral craniofacial pain therapy.
Keywords: calcitonin-gene related peptide, inflammation, pain, 5HT, periphery, craniofacial, orofacial
1. Introduction
Approximately one in four people experience persistent craniofacial pain, such as temporomandibular pain disorders and headache [44; 45] and approximately 10 million Americans experience debilitating migraine [65] making craniofacial pain one of the most common and poorly controlled pain problems [5]. The monoamine neurotransmitter serotonin (5HT) has been implicated in several pain conditions mediated by the trigeminal system [20; 22; 30; 32]. Several therapeutics being examined in clinical trials predominately target the central serotonergic system to treat migraine [13; 24] and trigeminal neuropathy [36]. As 5HT is pronociceptive in the peripheral nervous system where pain is often initiated, it may be effective to target the peripheral serotonergic system as a therapeutic option. However, the mechanism(s) by which 5HT acts on peripheral sensory neurons to modulate pain remain unclear.
The vast majority of 5HT in the mammalian body is located in peripheral tissues where it can be actively absorbed and released by platelets, mast cells, and immune cells [63]. Noxious stimuli trigger these cells to release pro-inflammatory mediators, including 5HT. In the trigeminal system, 5HT levels are increased in human masseter muscle associated with pain and allodynia [22; 23] and following temporomandibular joint movement-evoked pain [39]. Additionally, injection of exogenous 5HT evokes hyperalgesia in both humans [4; 20; 22; 23] and animals [49; 68; 69], which is attenuated by local administration of 5HT receptor antagonists indicating a modulatory role of 5HT in at least certain pain states.
During the inflammatory process, the activation of the transient receptor potential V1 channel (TRPV1), which is expressed on a major set of trigeminal nociceptors, evokes thermal hyperalgesia. The activation of TRPV1 by thermal stimuli [9; 10; 15; 71], oxidized linoleic acid metabolites [53; 56] and inflammatory mediators [11; 58] induce calcium influx in nociceptors resulting in release of inflammatory peptides, primarily calcitonin gene-related peptide (CGRP). Furthermore, sensitization of TRPV1 by inflammatory mediators, such as bradykinin and prostaglandins, can modulate the threshold for activation of trigeminal nociceptors [14; 33]. There is evidence in cultured dorsal root ganglia (DRG) neurons that 5HT potentiates TRPV1 functions during inflammatory states [48; 62] and prolongs nociceptor excitation during inflammation [2; 31]. Although 5HT is a major pro-inflammatory mediator, it is unknown whether 5HT can modulate TRPV1-expressing trigeminal nociceptors.
We hypothesized that 5HT increases the activity of capsaicin-sensitive trigeminal sensory neurons and that this increase can be attenuated by pharmacologically targeting peripheral 5HT receptors. Of the currently known 5HT receptors, the most extensively reported receptors to be involved in peripheral pain processing and expressed in peripheral sensory neurons are the 5HT1B, 5HT1D, 5HT2A, 5HT2C and 5HT3 receptors. Although these receptor subtypes have been implicated in peripheral nociception [12; 37; 47–49; 57; 68; 75], it is not known if they modulate the activity of TRPV1-expressing nociceptors. To address these gaps in knowledge, we first evaluated whether 5HT receptor transcripts were expressed in the TRPV1-expressing subpopulation of trigeminal afferent neurons and then determined whether 5HT and its peripheral receptors modulated calcium signaling and CGRP release from this important class of nociceptors.
2. Materials and Methods
2.1. Animals
Adult (250–350 g) intact male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were used in these experiments. Rats were housed for at least 5 days prior to study with ad libitum access to food and water. These studies were performed in compliance with the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and conform to federal guidelines and guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain.
2.2. Drugs
All drugs (Sigma-Aldrich, St. Louis, MO) were dissolved and stored at 4°C in stock concentration form and diluted in buffered saline (experimental vehicle) immediately prior to use, except serotonin hydrochloride which was both dissolved and diluted immediately prior to each use. Capsaicin (TRPV1 agonist) was dissolved in ethanol stock, with final dilutions containing <0.5% ethanol. Serotonin hydrochloride, sumatriptan succinate (5HT1B/1D receptor agonist), GR 55562 dihydrochloride (5HT1B/1D receptor antagonist) and granisetron hydrochloride (5HT3 receptor antagonist) were dissolved in double-distilled water. Ketanserin (+)-tartrate salt (5HT2A antagonist) was dissolved in dimethyl sulfoxide (DMSO) stock, with final dilutions containing <1% DMSO. All experimental vehicles were made at the same time as and of the same constitution as the experimental drug.
2.3. Primary culture of rat TG neurons
TG were harvested from adult male rats immediately following decapitation. Primary neuronal cultures were prepared using previously described methods [54]. Briefly, TG were suspended in Hanks buffered saline solution (HBSS; Invitrogen, San Diego, CA), disassociated and resuspended in Dulbecco’s modified Eagle’s medium (DMEM) containing penicillin-streptomycin, glutamine, 10% fetal bovine serum, nerve growth factor (NGF, 100ng/ml; Harlan, Indianapolis, IN) and treated with the mitotic inhibitors 5-fluoro-2-deoxyuridine (3 µg/ml; Invitrogen) and uridine (7 µl/ml; Sigma-Aldrich). Cells were directly applied to either 48-well poly-D-lysine-coated plates (6 TGs/plate; BD Biosciences, Bedford, MA) or poly-D-lysine/laminin-coated coverslips and maintained in an incubator at 37°C and 5% CO2.
2.4. Construction of 5HT receptor riboprobes
Riboprobes designed against the 5HT1B, 5HT1D, 5HT2A, 5HT2C and 5HT3A receptors were constructed with primers identified with Primer3 online software [60] (see specifications in Table 1) and custom ordered from Sigma-Genosys. Isolated rat brain cDNA was probed with designed primers by polymerase chain reaction and DNA was cloned with the pGEM-T vector system (Promega; Madison, WI), transformed with TOP10 E. Coli cells (Invitrogen) and extracted for digoxigenin (DIG)-cRNA-labeled riboprobe generation. Product size (>1000 bp) and structural verification was confirmed by sequencing performed at the Nucleic Acids Core Facility at the University of Texas Health Science Center at San Antonio. Corresponding sense probes and additional shorter length probes designed against each subtype (400–600 bp) were also generated to confirm specificity and rat brain cortex was used as a positive control.
Table 1.
Riboprobes for 5HT receptor subtype mRNA localization using in situ hybridization.
| Subtype | Primers | Position (bp) |
Product Size (bp) |
NCBI Accession No. |
|---|---|---|---|---|
| 5HT1B | Forward 5’-TGGAGGAGCAGGGTATTCAG-3’ | 2-21 | 1043 | NM_022225.1 |
| Reverse 5’-GTCAAAAATGGCCATGTGAA-3’ | 1044-1025 | |||
| Forward 5’-TGTTAGCGCTCATCACCTTG-3’ | 152-171 | 443 | ||
| Reverse 5’-GTTCACAAAGCAGTCCAGCA-3’ | 594-575 | |||
| 5HT1D | Forward 5’-CTGCCAAACCAGTCCCTAGA-3’ | 7-26 | 1108 | NM_012852.1 |
| Reverse 5’-TTCGGAAATGGACGACTCTC-3’ | 1114-1095 | |||
| Forward 5’-TCTGTGTCATCGCTCTGGAC-3’ | 377-396 | 493 | ||
| Reverse 5’-GCAGAGATCCTCTTGCGTTC-3’ | 869-850 | |||
| 5HT2A | Forward 5’-AGCTCTGTGCGATCTGGATT-3’ | 496-515 | 1066 | NM_017254.1 |
| Reverse 5’-TTGTTGCAGCCTCCTTATCC-3 | 1561-1542 | |||
| Forward 5’-GCGATCTGGATTTACCTGGA-3’ | 504-523 | 596 | ||
| Reverse 5’-ACGGCCATGATATTGGTGAT-3’ | 1099-1080 | |||
| 5HT2C | Forward 5’-AACTGGCCAGCACTTTCAAT-3’ | 163-182 | 1036 | NM_012765.2 |
| Reverse 5’-TCTGTCGAACAGGAGGCTTT-3’ | 1198-1179 | |||
| Forward 5’-GATGGTGGACGCTTGTTTCAATTCCCGGA-3’ | 124-152 | 612 | ||
| Reverse 5’-TTGACGGCGCAGGACGTAGATCGTTAAGA-3’ | 735-707 | |||
| 5HT3A | Forward 5’-CTTGCTGCCCAGTATCTTCC-3’ | 1091-1110 | 1011 | NM_024394.1 |
| Reverse 5’-GTCTCAGGCACCTAGGCAAG-3’ | 2101-2082 | |||
| Forward 5’-GAGACCATCTTCATTGTGCAGCTGGTGCA-3’ | 904-932 | 415 | ||
| Reverse 5’-ACAGCAGCGTGTCCAGCACATATCCCACC-3’ | 1318-1290 |
2.5. In situ hybridization/immunocytochemistry
For in situ hybridization, 30 µM thick sections of fresh frozen rat TG were fixed with 4% formaldehyde for 30 min, made permeable with 0.25% Triton X-100 and treated with 0.1% diethylpyrocarbonate (DEPC) for 30 min. Tissue was washed with 5X standard saline-sodium citrate (SSC) buffer, pretreated with hybridization buffer (containing 50% formamide, 1x Denhardt’s, 10% dextran sulfate, 500 µg/ml tRNA) then incubated for 12–24 hrs at 58°C with DIG-cRNA-labeled riboprobes. Tissue was then treated with RNase, washed with decreasing concentrations SSC buffer (final wash 0.1X SSC at 55°C) and incubated in anti-digoxigenin-AP, Fab fragments (Roche, Indianapolis, IN). Additional tissue sections were incubated with corresponding sense probes or pretreated with RNase prior to incubation with each probe to confirm RNA specificity. The resulting hybridization was detected using a standard nitro-blue tetrazolium/5-Bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) alkaline phosphatase reaction.
To label TRPV1 protein expression in the same sections, tissue was then treated with 4% normal goat serum and incubated for 12–24 hrs with previously characterized [28] guinea pig anti-TRPV1 C-terminus antibody (1:2000; Neuromics, cat. no. GP14100; Bloomington, MN) or with the TRPV1 antibody preabsorbed with the C-terminus blocking peptide (100 µM; Neuromics) as a peptide blocked control. TRPV1 immunoreactivity was detected by fluorescent-conjugated secondary antibody goat anti-guinea pig Alexa-568 (1:300; Molecular Probes, Eugene, OR). Tissue was coverslipped with Vectashield (Vector Laboratories; Bulringame, CA) and images were acquired with a Nikon Eclipse 90i microscope (Melville, NY) with a Nikon C1si laser confocal imaging system also equipped with a charge-coupled device (CCD) camera. Both fluorescent and brightfield images were taken from the identical field. This protocol does not permit quantification of colocalization of mRNA and protein labeling, but does illustrate transcript signal in corresponding TRPV1-expressing TG cells.
To analyze the diameter of TG cells expressing both 5HT receptor mRNA and TRPV1 signal, images from TG of 3 to 4 different rats per receptor subtype (n=80 cells total) taken under the same gain were processed using NIH ImageJ software (available at http://rsb.info.nih.gov/ij/). The staining intensity was filtered by applying a threshold value based on controls (mean + 6.5 times the standard deviation) [3] to remove low intensity pixels that represent nonspecific/background staining. Cells containing TRPV1 signal and 5HT receptor mRNA were selected and cell size was calculated by averaging the length and width of each cell then averaged across all images. Data are reported as mean±standard deviation (µm) and range of cells with colocalized expression.
2.6. Intracellular calcium imaging
Using protocols previously established to measure intracellular calcium ([Ca2+]i) levels [55], coverslips containing fura-loaded TG cells were placed in a chamber with constant infusion of HBSS and a baseline measure was collected for at least 60 sec. Cells were then bath treated with either 5HT (10 nM – 100 µM) or HBSS for 3 min followed by a 60 sec stimulation with capsaicin (30 nM) in the presence of 5HT delivered locally to the cells to identify capsaicin-sensitive and capsaicin-insensitive populations. This concentration of capsaicin is ~EC50 in this assay and was selected to permit detection of either facilitated or inhibited effects of 5HT on capsaicin-evoked increases in [Ca2+]i. Additional cultures were treated with sumatriptan (40 nM), ketanserin (10 nM) or granisetron (10 nM) or a combination for 5 min prior to capsaicin and 5HT (1 µM) or capsaicin alone. These concentrations were chosen based on the functional CGRP release assay concentration-response curves. Additional cultures were treated with GR 55562 (100 nM) prior to sumatriptan and a separate control group received 5HT (1 µM) prior to capsaicin and 5HT to test for agonist desensitization. Fluorescence images from 340 nm and 380 nm excitation wavelengths were collected every 10 sec and analyzed with MetaFluor software (Universal Imaging, Downingtown, PA). The net change in intracellular calcium, recorded and reported as ratiometric data (ΔF340/380), was determined by subtracting the averaged baseline prior to drug treatment from the peak change in [Ca2+]i after exposure to experimental manipulation. In experiments with multiple treatments, each peak change was calculated from the 60 seconds immediately preceding each drug treatment.
2.7. CGRP release assay
Using a protocol previously described43, TG cultures were washed twice and incubated for 15 min in a 500 µl volume to obtain a basal CGRP release measure. Cultures were then pretreated with either vehicle or 5HT (1µM – 1 mM) for 15 min prior to stimulation by capsaicin (50 µM) in the presence of 5HT for 15 min. Additional cultures were treated with sumatriptan, ketanserin or granisetron (1 nM – 100 µM) or a combination of all three drugs (10 nM, 10 nM, 100 nM; respectively) for 15 min prior to capsaicin and 5HT (100 µM). The concentration range of each drug was chosen based on reported known binding affinities (http://pdsp.med.unc.edu). Separate cultures were also pretreated with GR 55562 (100 nM) prior to sumatriptan. The superfusate was analyzed for CGRP levels by radioimmunoassay. All experiments were conducted in duplicate with n = 6 wells per treatment group for a total of approximately 12 wells per group.
2.8. CGRP radioimmunoassay
Aliquots were incubated for 24 hr at 4°C with a rabbit anti-CGRP antibody (1:1,000,000; donated by Dr. M. Iadarola, NIDCR) as previously described [26]. Following 24 hr, 100 µl [125I]-Tyr0-CGRP28–37 (~23,000 – 28,000 cpm) and 50 µl goat anti-rabbit antisera coupled to ferric beads (PerSeptive Diagnostics, Cambridge, MA) were added to the samples and incubated for 24 hr at 4°C. Bound and free [125I]-Tyr0-CGRP28–37 were then separated by immunomagnetic separation and radioactivity was counted and analyzed against a standard curve of known CGRP standards to estimate amount of CGRP. Actual fmol of CGRP per tube was calculated as 10^[(LN((B/B0 / 1- B/B0))-intercept)/slope]; where B/B0 =(total cpm-non-specific binding)/0 standard). The data were then transformed and reported as percentage of basal release. The minimal detectable level of CGRP in the superfusate is approximately 8 fmol and the 50% displacement is approximately 28 fmol.
2.9. Data analysis
Data were analyzed by using GraphPad Prism software version 5 (GraphPad, San Diego, CA) and data are presented as mean±SEM percentage of basal levels. Statistical analyses were conducted by unpaired t-test or ANOVA and individual groups were compared using Newman-Keuls Multiple Comparison post hoc test. For calcium imaging data, peak values were used for statistical analysis. For CGRP release data, nonlinear regression curves were generated from the concentration-response data. The statistical significance was tested at p<0.05.
3. Results
3.1. 5HT receptor mRNA is coexpressed with TRPV1 in rat trigeminal ganglia
Using combined in situ hybridization and fluorescent immunocytochemistry, we examined whether 5HT1B, 5HT1D, 5HT2A, 5HT2C and 5HT3A receptor subtype mRNA (Table 1) is coexpressed with TRPV1 protein in rat TG. Transcripts complimentary to 5HT1B, 5HT1D, 5HT2A, and 5HT3A, but not 5HT2C, receptor mRNA were detected predominately in TG cells (Figure 1A–D). Complementary expression was observed using a second probe (400–600bp) designed against the same subtypes (Supplementary Figure 1A–D). Probes were verified by positive signal in rat brain cortical tissue and RNA specificity was confirmed by a lack of hybridization signal when tissue was pretreated with RNase and with corresponding sense probes (Supplementary Figure 1). TRPV1 was predominately expressed in small (<20 µM) to medium (20–40 µM) sized TG cells (Figure 1E–H). TRPV1 antibody specificity following in situ hybridization protocols was confirmed by a lack of TRPV1 signal following preabsorption with the TRPV1 blocking peptide (Supplementary Figure 1 O). 5HT1B, 5HT1D, 5HT2A, and 5HT3A receptor transcripts were expressed in a medium diameter subpopulation of these TRPV1-positive cells. The mean size of TRPV1-positive cells that also contained 5HT receptor mRNA is reported per receptor subtype as 5HT1B = 43±15 µm (19 – 63 µm; n=22), 5HT1D = 26±10 µm (16 – 54 µm; n=18), 5HT2A = 31±12 µm (17 – 53 µm; n=17) and 5HT3A = 33±10 µm (19 – 51 µm; n=23).
Figure 1.
Example photomicrographs illustrating in situ hybridization with riboprobes against 5HT1B, 5HT1D, 5HT2A and 5HT3A receptor mRNA (>1000bp probes; A–D) combined with fluorescent immunocytochemistry for TRPV1 (E–H) in rat trigeminal ganglia. Arrows indicate colocalized cells.
3.2. 5HT evokes a concentration-dependent increase in calcium influx in cultured capsaicin-sensitive trigeminal sensory neurons
To test the hypothesis that 5HT increases the activity of capsaicin-sensitive TG cells, we measured changes in [Ca2+]i levels following treatment with 5HT. TG cultures were treated with either 5HT (10 nM – 100 µM) or buffered saline vehicle for 3 min prior to 60 sec stimulation with the TRPV1 agonist capsaicin (30 nM). This approach permits post-hoc analysis of 5HT effects in the capsaicin-responsive and capsaicin-insensitive subpopulations of TG cells. Notably, 5HT treatment triggered increases in [Ca2+]i in the capsaicin-responsive subpopulation of neurons (Figure 2A with example time course in Figure 2B), but not in the capsaicin-insensitive subpopulation (Figure 2C). These 5HT effects were concentration dependent over the range of 10–1,000 nM [F(4,219)=5.0, p<0.05]. There was no change in calcium influx with treatment with vehicle (HBSS) alone (steady at Δ~0.04 as denoted by dotted line). At higher concentrations of 5HT, there was a progressive reduction in calcium accumulation in the capsaicin-sensitive population of TG neurons. In contrast, 5HT produced no concentration-dependent effects in the capsaicin-insensitive cells (Figure 2C), although the 10 nM concentration did trigger a significant increase in [Ca2+]i [F(5,155)=3.4, p<0.05]. The application of capsaicin 30 nM produced a 4–5 fold increase in [Ca2+]i. Capsaicin-evoked calcium influx following 5HT treatment was consistently higher than capsaicin-treatment alone, however, this effect was not significant [F(5,285)=1.316, n.s.] (Figure 2D).
Figure 2.
Effect of 5HT treatment on intracellular calcium accumulation in capsaicin-sensitive (A) and capsaicin-insensitive (C) cultured TG neurons reported as change from baseline (Δ). A representative time course of the effect of 5HT followed by capsaicin on calcium influx (B) reported as actual ratio. Also shown is the response to capsaicin following 5HT (D). Capsaicin-sensitive neurons were identified by a 60 sec application of 30 nM capsaicin following 3 min 5HT (10 nM – 100 µM) treatment. The lower dotted line illustrates the response to initial vehicle (Veh) treatment and the upper dotted line illustrates the response to veh/capsaicin (Cap). Data is presented as mean ± SEM change from vehicle baseline (n=37–76 cells per group). Asterisks denote significance at p<0.05 compared to vehicle.
3.3. 5HT enhances CGRP release from capsaicin-sensitive cultured trigeminal sensory neurons
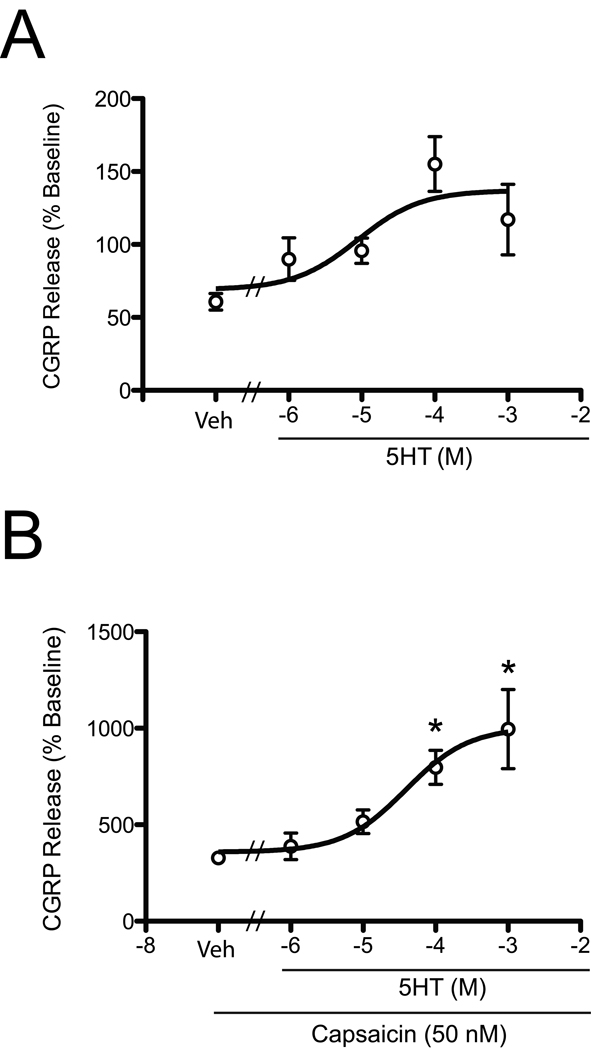
As CGRP release is a functional measure of peptidergic neuronal activity, we next tested the functional effect of 5HT on capsaicin-evoked release of CGRP from cultured TG neurons. Cultures were washed and pretreated with either buffered saline vehicle or 5HT (1 – 1,000 µM) for 15 min prior to stimulation by capsaicin (50 nM) in the presence of 5HT. 5HT treatment alone did not trigger a significant increase in CGRP release (Figure 3A). Following 5HT pretreatment, neurons were stimulated with capsaicin and the effect of 5HT on capsaicin-evoked CGRP release was analyzed (Figure 3B). The application of capsaicin alone produced about a 3-to-4-fold increase in CGRP release, and this was significantly enhanced by pretreatment with 5HT [F(4,76)= 3.738; p<0.05] with an EC50 = 50 µM. The 100 – 1000 µM concentration of 5HT more than doubled the CGRP release evoked by capsaicin alone.
Figure 3.
5HT enhances capsaicin-stimulated CGRP release in cultured rat trigeminal sensory neurons. TG cultures were pretreated with 1 µM, 10 µM, 100 µM or 1 mM 5HT or vehicle for 15 min (A). Following 5HT pretreatment, the cultures were treated with 5HT+capsaicin (50 nM) for 15 min (B). Data are shown as mean±SEM percentage of basal (~10–20 fmol) levels (n=8–12 per group). Asterisks denote significance at p<0.05 compared to vehicle.
3.4. Targeting 5HT receptors on trigeminal neurons attenuates calcium accumulation in capsaicin-sensitive trigeminal neurons
We next examined the effect of sumatriptan (5HT1B/1D agonist), ketanserin (5HT2A antagonist) and granisetron (5HT3 antagonist) on calcium accumulation in capsaicin-sensitive TG cells. Cultures were treated with either 40 nM sumatriptan or 10 nM ketanserin or 10 nM granisetron for 5 min prior to application of 1 µm 5HT for 3 min followed by 60 sec stimulation with 30 nM capsaicin. Following 5HT treatment, cells pretreated with sumatriptan had significantly less calcium accumulation in capsaicin-sensitive cells and ketanserin and granisetron pretreatment attenuated 5HT-induced calcium influx [F(4,245)=2.846; p<0.05] (Figure 4A with example time courses Figure 4D–F). Both ketanserin and granisetron reduced calcium influx by approximately 50%. A similar reduction in the 5HT-enhanced calcium influx in capsaicin-sensitive cells was observed following treatment with a combination of all three drugs (Figure 4A). The attenuation of calcium influx in capsaicin-sensitive cells following sumatriptan was reversed by pretreatment with 5HT1B/1D antagonist GR 55562 (Figure 4C), while GR 55562 alone had no effect on calcium levels compared to vehicle controls (0.77±0.04 vs. 0.71±0.03; p>0.05). The response to 30 nM capsaicin following drug pretreatment was also significantly reduced in all groups [F(4,176)=8.472, p<0.05] (Figure 4B), while there was no significant effect of a combination of drugs versus vehicle on capsaicin alone [t(31)=1.524; n.s.]. Lack of agonist desensitization of the capsaicin response was confirmed by a comparable capsaicin response following repeated 5HT treatment versus vehicle prior to capsaicin [t(14)=0.74; n.s.].
Figure 4.
Targeting peripheral 5HT receptors attenuates calcium accumulation in capsaicin-sensitive cultured TG neurons. Cultures were pretreated with 40 nM sumatriptan, 10 nM ketanserin, 10 nM granisetron, a combination of all 3 drugs or vehicle for 5 minutes followed by treatment with 1 µM 5HT for 3 min (A). Capsaicin-sensitive neurons were identified by stimulation with capsaicin (30 nM) following 5HT treatment. Also shown is the effect of drug treatment on capsaicin-evoked calcium accumulation (B) and pretreatment with GR 55562 prior to sumatriptan and 5HT treatment versus vehicle in capsaicin-sensitive cells (C) reported as change from baseline (Δ). Representative time courses of the effect of 5HT followed by capsaicin on calcium influx following each drug treatment (D–F) reported as actual ratio. Data is shown as mean±SEM change from baseline (n=53–73 per group). Asterisks denote significant reduction at p<0.05 compared to 5HT treatment alone.
3.5. Targeting 5HT receptors on trigeminal neurons attenuates 5HT-induced enhancement of CGRP release
As targeting 5HT receptors on TG neurons reduces calcium accumulation in capsaicin-sensitive cells, we next examined the ability of these pharmacological agents to reduce CGRP release. Cells were pretreated with sumatriptan, ketanserin, or granisetron (1 nM – 100 µM). Cells were then treated with 100 µM 5HT, a concentration that doubles capsaicin-evoked CGRP release (see Figure 3B) and 50 nM capsaicin. The results indicated that sumatriptan (IC50 = 75 nM), ketanserin (IC50 = 50 nM) and granisetron (IC50 = 20 nM) pretreatment significantly reduced 5HT-enhanced CGRP release evoked by capsaicin (Figure 5A, C, D). In contrast, pretreatment with these compounds did not have a significant effect on capsaicin-evoked CGRP release alone [F(3,60)=0.2517, n.s.]. Pretreatment with a combination of sumatriptan, ketanserin and granisetron (10 nM, 10 nM, 100 nM; respectively) significantly reduced CGRP release from 749±74% baseline to 555±45% baseline [F(2,30)=133.7, p<0.05], but not below capsaicin-evoked levels (412%). Sumatriptan significantly reduced CGRP release compared to 5HT pretreatment alone prior to capsaicin, and this attenuation was significantly reversed by pretreatment with the 5HT1B/1D receptor antagonist GR 55562 [F(2,54)=4.103, p<0.05] (Figure 5B), while GR 55562 alone had no effect on CGRP release compared to vehicle controls (99.46±27.12 vs. 116.19±45.63; p>0.05).
Figure 5.
Targeting peripheral 5HT receptors attenuates 5HT enhancement of capsaicin-induced CGRP release from cultured trigeminal neurons. Cultures were pretreated with various concentrations of sumatriptan (A), ketanserin (C) and granisetron (D) for 15 minutes followed by treatment with 5HT (100 µM) for 15 minutes prior to stimulation with 5HT+capsaicin (50 nM) for 15 minutes. Data is shown in concentration-response curves as mean±SEM percentage of basal (~10 fmol) levels (n=8–12 per group). Also shown is the effect of GR 55562 on sumatriptan-induced reduction in CGRP release compared to 5HT alone (B). Asterisk denotes significance at p<0.05 compared to capsaicin treatment (A,C,D) alone or 5HT pretreatment (B).
4. Discussion
Numerous studies have established that peripheral 5HT, released following noxious insult, is a proinflammatory and pronociceptive mediator [63]. However, the mechanism by which 5HT enhances peripheral nociception remains unclear. We hypothesized that 5HT increases activity of capsaicin-sensitive trigeminal neurons and this increase can be attenuated by pharmacologically targeting peripheral 5HT receptors. Here we report three novel findings supporting our hypothesis: (1) 5HT receptor transcripts are coexpressed with TRPV1 in rat TG, (2) 5HT increases [Ca2+]i accumulation in capsaicin-sensitive TG neurons and enhances capsaicin-evoked CGRP release, but does not enhance CGRP release when given alone, and (3) this enhanced nociceptive activity is attenuated by pharmacologically targeting peripheral 5HT receptors.
While it is established that the 5HT1, 5HT2 and 5HT3 receptor classes are expressed in sensory neurons and involved in evoking pain, little is known about their expression in the TRPV1-subpopulation of trigeminal nociceptors. The coexpression of TRPV1 with 5HT2A receptor protein and 5HT3A receptor mRNA has been reported in rat DRG [70; 75], but there are no such reports in the TG. These data demonstrate that 5HT1B, 5HT1D, 5HT2A, and 5HT3A receptor transcripts are present in rat TG, consistent with other reports in TG [41; 51] and DRG [12; 48; 57]. In the present study, 5HT2C mRNA was not expressed in TG cells in agreement with some studies in the DRG [48; 74]. Importantly, we report a novel finding that 5HT1B, 5HT1D, 5HT2A, and 5HT3A receptor mRNA are coexpressed in a subpopulation of TRPV1-positive TG cells that were on average 33 µm in diameter (855 µm2 in area). These data are consistent with other data reporting that TRPV1-expressing neurons are typically 10–30 µm in diameter [10; 29; 38], while 5HT receptor-expressing sensory neurons are 20–40 µm in diameter [34; 43; 49; 70; 75]. Together, these results provide evidence of 5HT receptor transcript expression in TRPV1-expressing TG neurons and provide a molecular framework for evaluating 5HT modulation of activities of the capsaicin-sensitive subclass of nociceptors. Further, our anatomical data suggests that any potential modulation of TRPV1-expressing trigeminal ganglion neurons may be more pronounced in medium diameter TRPV1 cells.
Nociception is often evoked by activation of TRPV1 by chemical irritants, thermal stimuli [9; 10; 15; 71] and inflammatory mediators [11; 58] released peripherally following noxious insult. Activation of these peptidergic neurons, in turn, evokes calcium influx resulting in the release of proinflammatory peptides, primarily CGRP, which has been implicated in migraine [7]. TRPV1 is highly coexpressed with CGRP in TG neurons [6; 35; 42; 49]. Concurrently, approximately 3.5 µg 5HT is released locally following thermal injury [61], while inflammation induces a 4-fold increase [16; 17; 46; 51]. Genetic deletion of the 5HT transporter reduces peripheral 5HT tissue levels with a corresponding attenuation of thermal hyperalgesia [50], indicating that increased sensitivity to pain may be linked to increased peripheral 5HT. One mechanism by which 5HT may be driving peripheral nociception is by increasing calcium influx and CGRP release in the TRPV1-expressing population of trigeminal nociceptors. Here we report that 5HT increases calcium influx in capsaicin-sensitive TG cells and enhances capsaicin-evoked CGRP release.
Interestingly, there was a concentration dependent effect of 5HT on calcium influx at lower concentrations (10 – 1000 nM), which also increased calcium influx to capsaicin, whereas this effect was attenuated at higher concentrations (10–100 µM). These data may indicate that multiple 5HT receptors with distinct signaling systems are present on TRPV1-expressing sensory neurons, as supported by our anatomical data, and thus differential signaling pathways may be engaged at higher concentrations of 5HT. Alternatively, 5HT at concentrations over 10 µM has an affinity for other inhibitory G protein coupled receptors, including the adrenergic α2 and β2 as well as dopamine D2 receptors (http://pdsp.med.unc.edu). The observed attenuated effects at higher concentrations of 5HT may represent involvement of non-5HT receptor systems.
In parallel to the increase in calcium influx in the capsaicin-sensitive population of TG cells, we found a concentration-dependent increase in capsaicin-evoked CGRP release indicating that 5HT may be acting at the TRPV1 population of trigeminal nociceptors. This appears to occur via a sensitization mechanism, as 5HT alone does not evoke a significant increase in CGRP release by itself; instead, it requires the application of capsaicin. Other studies have reported 5HT is more effective as a nociceptive agent during states of injury [2; 64] or when coadministered with another pronociceptive mediator, such as bradykinin [1; 63]. Since the present study demonstrates an extremely rapid onset for enhanced 5HT effects, it is possible that facilitation is mediated by cell signaling pathways rather than transcriptional events. It is unclear why 5HT produced a maximal effect on calcium accumulation at 1 µM with declining calcium levels thereafter, while CGRP release continued to be enhanced at 10 – 100 µM. The difference may be in the onset of 5HT effects on calcium accumulation (measured at 3 min) and the subsequent release of CGRP (measured over a 15 min period), however, further studies are required to examine this possibility.
Repeated capsaicin administration leads to desensitization of TRPV1, which is currently being examined as a therapeutic tool. Alternatively, as inflammatory mediators sensitize TRPV1 and 5HT receptors engage cell-signaling pathways that are known to alter TRPV1 activities, it is also possible to block sensitization of TRPV1. The 5HT1 receptor subtypes are inhibitory G protein coupled leading to a decrease in cAMP signaling, which could reduce nociceptive neurotransmission, while the 5HT2 receptor subtypes are excitatory leading to an increase in PLC/PKC signaling, which could sensitize TRPV1. These differences have also been illustrated behaviorally [24; 49; 75]. We found that 5HT2A antagonism attenuated calcium signaling and peptidergic activity in cultured TG cells, indicating that ketanserin may be attenuating 5HT sensitization of TRPV1. Interestingly, 100 µM 5HT-evoked enhancement of CGRP release was significantly inhibited by 1 nM ketanserin. This finding is consistent with the observation that others have reported regarding full antagonism of 5HT effects with low nM ketanserin concentrations [40] and a similar dose-response curve at similar concentrations [18]. Alternatively, the 5HT3 receptor is an ionotropic receptor that directly excites nociceptors [25; 75]. Concentrations of granisetron over 100 nM blocked the ability of 5HT to enhance capsaicin-evoked CGRP release. This is consistent with previous literature in humans and animal models identifying that 5HT3 receptor activation mediates 5HT-induced sensitization of trigeminal pain processing [21; 67].
The calcium influx following 5HT and capsaicin was significantly attenuated in TG cells treated with sumatriptan (5HT1B/1D agonist). This attenuation was not due to agonist desensitization as repeated 5HT treatment did not reduce the capsaicin response compared to vehicle. A corresponding attenuation of CGRP release was also observed, with maximal inhibition occurring with as low as 10 nM sumatriptan. Others have also reported inhibitory effects of sumatriptan at low nM concentrations [8]. Our results are similar to results previously reporting that sumatriptan attenuates CGRP release likely via increased phosphatase activity mediated by elevated intracellular calcium [19]. Pretreatment with the 5HT1B/1D receptor antagonist GR 55562 prevented sumatriptan-evoked attenuation of calcium influx and CGRP release, while GR 55562 alone had no significant effect. This provides a cellular mechanism for the report that peripherally administered 5HT1 receptor agonists significantly reduce inflammatory nociceptive behavior in the rat hindpaw, which can be reversed by GR 55562 [27].
A reduction in 5HT- and capsaicin-evoked calcium influx and CGRP release was observed when cells were treated with a combination of sumatriptan, ketanserin and granisetron, although this reduction was not significantly greater than any drug given alone. While these drugs did not have a significant effect on capsaicin-evoked calcium influx or CGRP release alone, they appear to be attenuating the sensitizing effects on 5HT on the capsaicin response. Together, our data indicate that the net effect of 5HT on capsaicin-sensitive nociceptors is likely due to an integrated response mediated by multiple 5HT receptors and associated signaling pathways. In addition, other 5HT receptors may play a role in this effect. It was recently reported that CGRP release may be reduced by administration of a selective 5HT7 receptor antagonist in an animal model of experimental migraine [72]. Further studies analyzing the anatomical expression and therapeutic relevance of the more recently reported 5HT receptors to be involved in peripheral pain processing are warranted.
In summary, our findings that 5HT receptor mRNA is coexpressed with TRPV1 and 5HT evokes increased nociceptive function in peripheral sensory neurons provide further evidence that 5HT is driving nociception in the periphery and may be enhancing cell signaling and proinflammatory processes via the TRPV1-expressing population of nociceptors. Importantly, there is a high prevalence of trigeminal pain disorders in women compared to men. The present study was limited to male rats for comparison to the current literature, however, future studies expanding these studies in females are warranted. As 5HT has not only been implicated in craniofacial pain disorders, but also fibromyalgia [73] and irritable bowel syndrome [52; 59; 66], these results may have relevance to other pain conditions. The present results provide further evidence for a role on 5HT on TRPV1-mediated nociception and provides a mechanistic basis for identifying the peripheral 5HT system as a therapeutic target.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the technical assistance of Mayur Patil, Paul Chen, Mei Li and the Nucleic Acids Core Facility at the University of Texas Health Science Center at San Antonio. The authors would also like to acknowledge Drs. Bill Clarke, Kelly Berg and Jill Fehrenbacher for helpful discussions on experimental design. Receptor binding affinity data was provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP).
Grant Support: NIH grants R01 NS72890, U54RR02438 (KMH), T32 DE14318, F32 DE021309 (DRL), and R01 DE015576 (MAH)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
References
- 1.Abbott FV, Hong Y, Blier P. Activation of 5-HT2A receptors potentiates pain produced by inflammatory mediators. Neuropharmacology. 1996;35(1):99–110. doi: 10.1016/0028-3908(95)00136-0. [DOI] [PubMed] [Google Scholar]
- 2.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20(12):4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarado LT, Perry GM, Hargreaves KM, Henry MA. TRPM8 Axonal expression is decreased in painful human teeth with irreversible pulpitis and cold hyperalgesia. J Endod. 2007;33(10):1167–1171. doi: 10.1016/j.joen.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babenko V, Svensson P, Graven-Nielsen T, Drewes AM, Jensen TS, Arendt-Nielsen L. Duration and distribution of experimental muscle hyperalgesia in humans following combined infusions of serotonin and bradykinin. Brain Res. 2000;853(2):275–281. doi: 10.1016/s0006-8993(99)02270-2. [DOI] [PubMed] [Google Scholar]
- 5.Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71(8):559–566. doi: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- 6.Bonaventure P, Voorn P, Luyten WH, Leysen JE. 5HT1B and 5HT1D receptor mRNA differential co-localization with peptide mRNA in the guinea pig trigeminal ganglion. Neuroreport. 1998;9(4):641–645. doi: 10.1097/00001756-199803090-00015. [DOI] [PubMed] [Google Scholar]
- 7.Brain SD. Calcitonin gene-related peptide (CGRP) antagonists: blockers of neuronal transmission in migraine. Br J Pharmacol. 2004;142(7):1053–1054. doi: 10.1038/sj.bjp.0705806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carmichael NM, Charlton MP, Dostrovsky JO. Activation of the 5-HT1B/D receptor reduces hindlimb neurogenic inflammation caused by sensory nerve stimulation and capsaicin. Pain. 2008;134(1–2):97–105. doi: 10.1016/j.pain.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 11.Cesare P, Moriondo A, Vellani V, McNaughton PA. Ion channels gated by heat. Proc Natl Acad Sci U S A. 1999;96(14):7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JJ, Vasko MR, Wu X, Staeva TP, Baez M, Zgombick JM, Nelson DL. Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J Pharmacol Exp Ther. 1998;287(3):1119–1127. [PubMed] [Google Scholar]
- 13.Chen LC, Ashcroft DM. Meta-analysis of the efficacy and safety of zolmitriptan in the acute treatment of migraine. Headache. 2008;48(2):236–247. doi: 10.1111/j.1526-4610.2007.01007.x. [DOI] [PubMed] [Google Scholar]
- 14.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 15.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 16.Doak GJ, Sawynok J. Formalin-induced nociceptive behavior and edema: involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience. 1997;80(3):939–949. doi: 10.1016/s0306-4522(97)00066-3. [DOI] [PubMed] [Google Scholar]
- 17.Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75(2):125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 18.Drozdov I, Kidd M, Gustafsson BI, Svejda B, Joseph R, Pfragner R, Modlin IM. Autoregulatory effects of serotonin on proliferation and signaling pathways in lung and small intestine neuroendocrine tumor cell lines. Cancer. 2009;115(21):4934–4945. doi: 10.1002/cncr.24533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durham PL, Russo AF. Regulation of calcitonin gene-related peptide secretion by a serotonergic antimigraine drug. J Neurosci. 1999;19(9):3423–3429. doi: 10.1523/JNEUROSCI.19-09-03423.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernberg M, Hedenberg-Magnusson B, Kurita H, Kopp S. Effects of local serotonin administration on pain and microcirculation in the human masseter muscle. J Orofac Pain. 2006;20(3):241–248. [PubMed] [Google Scholar]
- 21.Ernberg M, Lundeberg T, Kopp S. Effect of propranolol and granisetron on experimentally induced pain and allodynia/hyperalgesia by intramuscular injection of serotonin into the human masseter muscle. Pain. 2000;84(2–3):339–346. doi: 10.1016/s0304-3959(99)00221-3. [DOI] [PubMed] [Google Scholar]
- 22.Ernberg M, Lundeberg T, Kopp S. Pain and allodynia/hyperalgesia induced by intramuscular injection of serotonin in patients with fibromyalgia and healthy individuals. Pain. 2000;85(1–2):31–39. doi: 10.1016/s0304-3959(99)00233-x. [DOI] [PubMed] [Google Scholar]
- 23.Ernberg M, Voog U, Alstergren P, Lundeberg T, Kopp S. Plasma and serum serotonin levels and their relationship to orofacial pain and anxiety in fibromyalgia. J Orofac Pain. 2000;14(1):37–46. [PubMed] [Google Scholar]
- 24.Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001;358(9294):1668–1675. doi: 10.1016/S0140-6736(01)06711-3. [DOI] [PubMed] [Google Scholar]
- 25.Fozard JR. Neuronal 5-HT receptors in the periphery. Neuropharmacology. 1984;23(12B):1473–1486. doi: 10.1016/0028-3908(84)90091-1. [DOI] [PubMed] [Google Scholar]
- 26.Garry MG, Richardson JD, Hargreaves KM. Sodium nitroprusside evokes the release of immunoreactive calcitonin gene-related peptide and substance P from dorsal horn slices via nitric oxide-dependent and nitric oxide-independent mechanisms. J Neurosci. 1994;14(7):4329–4337. doi: 10.1523/JNEUROSCI.14-07-04329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granados-Soto V, Arguelles CF, Rocha-Gonzalez HI, Godinez-Chaparro B, Flores-Murrieta FJ, Villalon CM. The role of peripheral 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E and 5-HT1F serotonergic receptors in the reduction of nociception in rats. Neuroscience. 2010;165(2):561–568. doi: 10.1016/j.neuroscience.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11(3):946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 29.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. The European journal of neuroscience. 1999;11(3):946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 30.Hargreaves RJ, Shepheard SL. Pathophysiology of migraine--new insights. Can J Neurol Sci. 1999;26 Suppl 3:S12–S19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 31.Herbert MK, Schmidt RF. Activation of normal and inflamed fine articular afferent units by serotonin. Pain. 1992;50(1):79–88. doi: 10.1016/0304-3959(92)90115-R. [DOI] [PubMed] [Google Scholar]
- 32.Herken H, Erdal E, Mutlu N, Barlas O, Cataloluk O, Oz F, Guray E. Possible association of temporomandibular joint pain and dysfunction with a polymorphism in the serotonin transporter gene. Am J Orthod Dentofacial Orthop. 2001;120(3):308–313. doi: 10.1067/mod.2001.115307. [DOI] [PubMed] [Google Scholar]
- 33.Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- 34.Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain research. 2001;909(1–2):112–120. doi: 10.1016/s0006-8993(01)02645-2. [DOI] [PubMed] [Google Scholar]
- 35.Hou M, Kanje M, Longmore J, Tajti J, Uddman R, Edvinsson L. 5-HT(1B) and 5-HT(1D) receptors in the human trigeminal ganglion: co-localization with calcitonin generelated peptide, substance P and nitric oxide synthase. Brain Res. 2001;909(1–2):112–120. doi: 10.1016/s0006-8993(01)02645-2. [DOI] [PubMed] [Google Scholar]
- 36.Kanai A, Saito M, Hoka S. Subcutaneous sumatriptan for refractory trigeminal neuralgia. Headache. 2006;46(4):577–582. doi: 10.1111/j.1526-4610.2006.00405.x. discussion 583-574. [DOI] [PubMed] [Google Scholar]
- 37.Kayser V, Elfassi IE, Aubel B, Melfort M, Julius D, Gingrich JA, Hamon M, Bourgoin S. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A−/−, 5-HT1B−/−, 5-HT2A−/−, 5-HT3A−/− and 5-HTT−/− knock-out male mice. Pain. 2007;130(3):235–248. doi: 10.1016/j.pain.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. The Journal of comparative neurology. 2005;493(4):596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 39.Kopp S. The influence of neuropeptides, serotonin, and interleukin 1beta on temporomandibular joint pain and inflammation. J Oral Maxillofac Surg. 1998;56(2):189–191. doi: 10.1016/s0278-2391(98)90867-9. [DOI] [PubMed] [Google Scholar]
- 40.Lu R, Alioua A, Kumar Y, Kundu P, Eghbali M, Weisstaub NV, Gingrich JA, Stefani E, Toro L. c-Src tyrosine kinase, a critical component for 5-HT2A receptor-mediated contraction in rat aorta. The Journal of physiology. 2008;586(16):3855–3869. doi: 10.1113/jphysiol.2008.153593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma QP. Co-localization of 5-HT(1B/1D/1F) receptors and glutamate in trigeminal ganglia in rats. Neuroreport. 2001;12(8):1589–1591. doi: 10.1097/00001756-200106130-00015. [DOI] [PubMed] [Google Scholar]
- 42.Ma QP, Hill R, Sirinathsinghji D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur J Neurosci. 2001;13(11):2099–2104. doi: 10.1046/j.0953-816x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- 43.Ma QP, Hill R, Sirinathsinghji D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. The European journal of neuroscience. 2001;13(11):2099–2104. doi: 10.1046/j.0953-816x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- 44.Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV. Predictors of outcome for orofacial pain in the general population: a four-year follow-up study. J Dent Res. 2004;83(9):712–717. doi: 10.1177/154405910408300911. [DOI] [PubMed] [Google Scholar]
- 45.Macfarlane TV, Blinkhorn AS, Davies RM, Ryan P, Worthington HV, Macfarlane GJ. Orofacial pain: just another chronic pain? Results from a population-based survey. Pain. 2002;99(3):453–458. doi: 10.1016/S0304-3959(02)00181-1. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima K, Obata H, Ito N, Goto F, Saito S. The nociceptive mechanism of 5-hydroxytryptamine released into the peripheral tissue in acute inflammatory pain in rats. Eur J Pain. 2009;13(5):441–447. doi: 10.1016/j.ejpain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 47.Nikai T, Basbaum AI, Ahn AH. Profound reduction of somatic and visceral pain in mice by intrathecal administration of the anti-migraine drug, sumatriptan. Pain. 2008;139(3):533–540. doi: 10.1016/j.pain.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohta T, Ikemi Y, Murakami M, Imagawa T, Otsuguro K, Ito S. Potentiation of transient receptor potential V1 functions by the activation of metabotropic 5-HT receptors in rat primary sensory neurons. J Physiol. 2006;576(Pt 3):809–822. doi: 10.1113/jphysiol.2006.112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okamoto K, Imbe H, Morikawa Y, Itoh M, Sekimoto M, Nemoto K, Senba E. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain. 2002;99(1–2):133–143. doi: 10.1016/s0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- 50.Palm F, Mossner R, Chen Y, He L, Gerlach M, Bischofs S, Riederer P, Lesch KP, Sommer C. Reduced thermal hyperalgesia and enhanced peripheral nerve injury after hind paw inflammation in mice lacking the serotonin-transporter. Eur J Pain. 2008;12(6):790–797. doi: 10.1016/j.ejpain.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 51.Parada CA, Tambeli CH, Cunha FQ, Ferreira SH. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102(4):937–944. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- 52.Pata C, Erdal E, Yazc K, Camdeviren H, Ozkaya M, Ulu O. Association of the -1438 G/A and 102 T/C polymorphism of the 5-Ht2A receptor gene with irritable bowel syndrome 5-Ht2A gene polymorphism in irritable bowel syndrome. J Clin Gastroenterol. 2004;38(7):561–566. doi: 10.1097/00004836-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120(5):1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25(39):8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci U S A. 2006;103(30):11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106(44):18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pierce PA, Xie GX, Levine JD, Peroutka SJ. 5-Hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: a polymerase chain reaction study. Neuroscience. 1996;70(2):553-–559. doi: 10.1016/0306-4522(95)00329-0. [DOI] [PubMed] [Google Scholar]
- 58.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;(179):155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 59.Qin HY, Luo JL, Qi SD, Xu HX, Sung JJ, Bian ZX. Visceral hypersensitivity induced by activation of transient receptor potential vanilloid type 1 is mediated through the serotonin pathway in rat colon. Eur J Pharmacol. 2010;647(1–3):75–83. doi: 10.1016/j.ejphar.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 60.Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. Totowa, NJ: Humana Press; 2000. [DOI] [PubMed] [Google Scholar]
- 61.Sasaki M, Obata H, Kawahara K, Saito S, Goto F. Peripheral 5-HT2A receptor antagonism attenuates primary thermal hyperalgesia and secondary mechanical allodynia after thermal injury in rats. Pain. 2006;122(1–2):130–136. doi: 10.1016/j.pain.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 62.Simonetti M, Fabbro A, D'Arco M, Zweyer M, Nistri A, Giniatullin R, Fabbretti E. Comparison of P2X and TRPV1 receptors in ganglia or primary culture of trigeminal neurons and their modulation by NGF or serotonin. Mol Pain. 2006;2:11. doi: 10.1186/1744-8069-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sommer C. Serotonin in pain and analgesia: actions in the periphery. Mol Neurobiol. 2004;30(2):117–125. doi: 10.1385/MN:30:2:117. [DOI] [PubMed] [Google Scholar]
- 64.Song XJ, Zhang JM, Hu SJ, LaMotte RH. Somata of nerve-injured sensory neurons exhibit enhanced responses to inflammatory mediators. Pain. 2003;104(3):701–709. doi: 10.1016/S0304-3959(03)00167-2. [DOI] [PubMed] [Google Scholar]
- 65.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267(1):64–69. [PubMed] [Google Scholar]
- 66.Sugiuar T, Bielefeldt K, Gebhart GF. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J Neurosci. 2004;24(43):9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sung D, Dong X, Ernberg M, Kumar U, Cairns BE. Serotonin (5-HT) excites rat masticatory muscle afferent fibers through activation of peripheral 5-HT3 receptors. Pain. 2008;134(1–2):41–50. doi: 10.1016/j.pain.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 68.Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience. 1992;48(2):485–490. doi: 10.1016/0306-4522(92)90508-y. [DOI] [PubMed] [Google Scholar]
- 69.Tokunaga A, Saika M, Senba E. 5-HT2A receptor subtype is involved in the thermal hyperalgesic mechanism of serotonin in the periphery. Pain. 1998;76(3):349–355. doi: 10.1016/S0304-3959(98)00066-9. [DOI] [PubMed] [Google Scholar]
- 70.Van Steenwinckel J, Noghero A, Thibault K, Brisorgueil MJ, Fischer J, Conrath M. The 5-HT2A receptor is mainly expressed in nociceptive sensory neurons in rat lumbar dorsal root ganglia. Neuroscience. 2009;161(3):838–846. doi: 10.1016/j.neuroscience.2009.03.087. [DOI] [PubMed] [Google Scholar]
- 71.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430(7001):748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- 72.Wang X, Fang Y, Liang J, Yin Z, Miao J, Luo N. Selective inhibition of 5-HT7 receptor reduces CGRP release in an experimental model for migraine. Headache. 2010;50(4):579–587. doi: 10.1111/j.1526-4610.2010.01632.x. [DOI] [PubMed] [Google Scholar]
- 73.Wolfe F, Russell IJ, Vipraio G, Ross K, Anderson J. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J Rheumatol. 1997;24(3):555–559. [PubMed] [Google Scholar]
- 74.Wu S, Zhu M, Wang W, Wang Y, Li Y, Yew DT. Changes of the expression of 5-HT receptor subtype mRNAs in rat dorsal root ganglion by complete Freund's adjuvant-induced inflammation. Neurosci Lett. 2001;307(3):183–186. doi: 10.1016/s0304-3940(01)01946-2. [DOI] [PubMed] [Google Scholar]
- 75.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002;22(3):1010–1019. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.