Abstract
Others reported that rats fed a high-fructose diet for 6 months were leptin resistant. We tested peripheral and/or central leptin responses in rats fed fructose for shorter time periods. Rats fed a diet containing 60% energy (% kcal) fructose and 10% kcal fat diet for 21 days had the same serum triglycerides (TG), gained less weight than controls, decreased their food intake and weight gain in response to central injections of 0.5 or 1.0 ug leptin, but were resistant to an i.p. injection of 2.0 mg leptin/kg. An i.p. injection of 1 mg leptin/kg increased phosphorylation of hypothalamic signal transducer and activator of transcription 3 (PSTAT3) implying resistance was not a failure of leptin to cross the blood brain barrier. The effects of dietary fructose were compared with those of dietary fat. Rats fed a 10% kcal fructose and 30% kcal fat diet for 39 days were leptin resistant whereas rats fed a 40% kcal fructose and 30% kcal fat diet responded to i.p. leptin. Another monosaccharide, glucose, replicated the effects of fructose in the 30% kcal fat diet. Surprisingly, none of the rats showed a reliable response to third ventricle leptin and peripheral leptin failed to stimulate hypothalamic PSTAT3 although it did increase PSTAT3 in the brainstem of rats fed the 40% kcal fructose or glucose diets. Thus a high-fructose, low-fat diet induces peripheral leptin resistance in less than 4 weeks, but high dietary concentrations of fructose or glucose prevent peripheral leptin resistance in rats fed a high-fat diet.
Keywords: Food intake, weight gain, triglycerides, dietary fat, STAT3
INTRODUCTION
When leptin was first discovered it was anticipated that this adipose-derived hormone would function as a feedback signal in the regulation of body weight [1]. Leptin receptors are expressed at high concentrations in the arcuate nucleus of the hypothalamus (ARC) [2] an area of the brain that appears critical to the control of food intake and regulation of energy balance [3]. More recently, it has been shown that the effects of leptin on food intake are not limited to the ARC and that leptin receptors in the ventromedial nucleus of the hypothalamus [4], the ventral tegmental area [5] and the hindbrain [6] also contribute to leptin-induced changes in food intake and energy expenditure. It is now well established that leptin inhibits food intake and reduces body fat mass in normal weight animals or in animals that are leptin-deficient [7–9], but that it has a limited effect in animals that are obese [10–11] or that have been adapted to a high-fat diet [12–14]. This lack of response to endogenous or exogenous leptin has been termed “leptin resistance” and has been explained by a failure of leptin to cross the blood brain barrier [15] and activate the central leptin receptors that mediate leptin-induced changes in food intake and energy expenditure. As obesity continues to develop the animals become resistant to both central and peripheral administration of leptin [12]. In these conditions a possible cause of leptin resistance is an increased expression of suppressor of cytokine signaling 3 (SOCS3) [16], which prevents phosphorylation of signal transducer and activator of transcription 3 (STAT3) [17]. STAT3 is a transcription factor that is an essential part of the leptin signaling pathway responsible for modulating energy homeostasis [18]. Others also have suggested that leptin receptor protein may be down-regulated in conditions of leptin resistance [19].
With the increasing incidence of obesity in Western Societies that cannot be attributed to single gene mutations or neuroendocrine disorders, there is a growing awareness of the role of environmental factors, including diet composition, in promoting body fat accumulation [20]. There have been several recent reports of an association between fructose consumption and an increased incidence of obesity [20–22]. Consumption of sweeteners, including sucrose and high-fructose corn syrup, by the general population has steadily increased over the last 30 years [23]. Excess consumption of fructose promotes hepatic lipogenesis and gluconeogenesis [24] and it is this aspect of fructose metabolism that has been associated with the increased risk for metabolic diseases [21–22]. In humans, post-prandial triglyceride (TG) levels are increased after a high-fructose meal [25–26] and fasting TG are increased after several weeks of consuming a high-fructose diet [27–28], with a greater effect in men than women [28]. Banks et al. have shown that elevated circulating TG effectively inhibit leptin transport across the blood brain barrier in rodents [29]. Therefore, it is possible that consumption of a high-fructose diet causes leptin resistance through TG inhibition of leptin transport across the blood brain barrier and that this contributes to the association between fructose intake and obesity.
Recently, Shapiro et al [30] reported that rats fed a high-fructose diet (67% energy) for 6 months were leptin resistant and had elevated serum TG concentrations although they were not obese. Leptin responsiveness was not measured at any earlier time-point in the study and if it were due to elevated blood TG, then the resistance would be expected to occur within a relatively short time frame. Leptin resistance in the Shapiro study [30] was measured as a decrease in 24 hour food intake following an intraperitoneal (i.p.) injection of leptin and no attempt was made to test whether the rats were responsive to central injections of leptin. The objective of the studies described here was to test whether shorter periods of consumption of high levels of dietary fructose resulted in the development of leptin resistance in rats. In the first two experiments we tested the effects of central and peripheral injections of leptin on food intake and weight gain of rats fed a low-fat (10% of energy as fat) diet containing 60% of energy (%kcal) as fructose and determined whether this high fructose diet changed circulating TG concentrations following a meal. In these studies the rats became leptin resistant in less than 4 weeks, but the fructose-fed animals were lighter and thinner than their controls. In order to maintain the body fat content of fructose-fed rats we tested the effects of a high concentration of dietary fructose in a diet containing 30% kcal fat. Surprisingly we found that fructose prevented the development of leptin resistance in these high-fat fed rats. Therefore, the two final experiments tested whether the changes in leptin responsiveness of the rats were fructose-specific or could be replicated by increasing the concentration of a different monosaccharide (glucose) in the diet.
METHODS
Animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Georgia. All animals used in these studies were male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN). They were housed individually in rooms maintained at 23°C with lights on for 12 hours/day from 7.00 a.m. and had free access to food and water unless stated otherwise.
Experiment 1: Response to peripheral and central leptin injections in rats fed a high fructose diet
The objective of this experiment was to test whether rats fed a low-fat, high-fructose diet developed resistance to peripheral and/or central injections of leptin.
Twenty-two rats were each fitted with a third ventricle guide cannula as described previously [31]. Guide cannulae (Plastics One, Roanoke, VA: 22 gauge, 11 mm below the pedestal) were placed using the following coordinates applied to a flat skull: anteroposterior −2.8, lateral 0.0, ventral −8.3 from the bregma. The rats were housed in hanging wire-mesh cages with free access to water and one of the diets described below. Cannula placement was tested one week after surgery by observing drinking behavior after an infusion of 10 ng angiotensin II. The rats were then divided into two weight-matched groups. One was given free access to a low-fructose (LFruc) diet containing 10% kcal fat, 20% protein, 35.5% sucrose, 31% corn starch, 3.5% maltodextrin (D12450B, Research Diets Inc., New Brunswick, NJ). This diet contained approximately 18% kcal fructose and has been used as a control for studies in which leptin resistance is induced by high-fat feeding [12, 32]. The second experimental group was offered a high-fructose (HFruc) diet containing 10% kcal fat, 20% protein, 60% fructose, 10% corn starch (D02022704, Research Diets Inc.). Diets were isocaloric (3.8 kcal/g). Because we anticipated an early onset of leptin resistance, leptin responsiveness was tested after 14 days. The rats were food deprived from 8.00 a.m. and were injected i.p. at 5.00 p.m. with either 2.0 mg leptin/kg in a volume of 2 ml/kg or an equivalent volume of sterile saline. Food was returned to the cage at 6.00 p.m. and intake was measured 2, 14, 24 and 38 hours after injection. Body weight was measured 14, 24, 38 and 62 hours after the injection. Both food intake and weight gain were used as indices of leptin-responsiveness because leptin has been reported to increase energy expenditure [33] in addition to suppressing food intake [8]. HFruc-fed rats were leptin responsive, defined as a significant leptin-induced inhibition of food intake and weight gain, therefore the test was repeated when the rats had been on diet for 21 days, at which time the HFruc-fed rats were leptin resistant. On this day a tail blood sample was collected at the same time as food intake was measured 2 hours after leptin injection for measurement of serum leptin (Rat leptin RIA kit, MP Biomedicals, Solon, OH) and TG (L-Type TG H kit; Wako Chemicals) concentrations.
Four days after demonstration of peripheral leptin resistance (25 day on diet) the central leptin responsiveness of the rats was tested. Food was removed from the cages at 8.00 a.m. At 5.30 p.m. each rat received an icv infusion of 0, 0.5 or 1.0 ug leptin in a 2 ul volume infused over 1 minute. At 6.30 p.m. food was returned to the cages and food intake was recorded 2, 14, 24 and 38 hours after infusion. Body weight was measured 14 and 38 hours after infusion. Each rat was tested in random order with each dose of leptin and there was a period of at least 4 days between tests to ensure that the rats recovered any weight loss before they were tested again. The rats had been on diet for 45 days by the end of the study.
Experiment 2: Changes in serum triglyceride concentrations and hypothalamic phosphorylated STAT3 in rats fed a high fructose diet
Experiment 1 indicated that rats fed HFruc diet were resistant to peripheral, but not central leptin administration. The objective of this study was to determine whether an increase in serum TG could potentially account for this resistance and whether the HFruc diet prevented an increase in phosphorylation of STAT3 in hypothalamic and brainstem tissue of rats receiving i.p. injections of leptin.
Twenty-six rats were adapted to the environment for one week, were divided into two weight-matched groups and were offered the LFruc or the HFruc diet. Body weight was recorded twice each week for the duration of the study. Serum TG were measured after 7 days on diet when all rats would be expected to be leptin responsive and again after 21 days on diet when HFruc-fed rats would be expected to be leptin resistant. On the day of measurement food was removed from the cages at 8.00 a.m. The rats were weighed at 6.00 p.m., a small blood sample was taken by tail bleeding and then the rats were given free access to food. Additional blood samples were collected 2, 4 and 6 hours after food was returned. Food intake was measured during the 2 hours between the first two blood samples.
The effect of peripheral leptin on central PSTAT3 was tested when the rats had been on experimental diet for 26 days. This was a time at which the rats were resistant to peripheral leptin, but responsive to central leptin in Experiment 1. Food was removed from the cages at 10.00 a.m. Starting at 6.30 p.m. each rat received an i.p. injection of either 1 mg leptin/kg or an equivalent volume of PBS. Exactly 20 min after injection the rat was decapitated and the brain was rapidly removed. Blocks of tissue that included the hypothalamus or brain stem were dissected and snap frozen. The optic tract was used as an anterior marker and the interpeduncular fossa as a posterior marker for the hypothalamic section. The block was cut laterally to include the lateral hypothalamus and a dorsal cut was made through the mammillothalamic tract. The anterior cut for the hindbrain included the medial vestibular nucleus and the posterior cut included spinal vestibular nucleus. A ventral cut was made to include the medial nucleus of the solitary tract [34]. The tissue was subsequently homogenized in sucrose buffer (20 mmol/L Tris-HCl, 1 mmol/L EDTA, 255 mmol/L sucrose, pH 7.4), 1% nonidet-P40 containing protease and phosphatase inhibitors (1 μg/mL leupeptin, 1 μg/mL aprotinin, 2 μmol/L phenylmethylsulfonylfluoride, 10 mmol/L sodium fluoride, 2 mmol/L sodium vanadate, 1 mmol/L molybdate). PSTAT3 was detected by Western blot using Phospho-Stat3 (Tyr705) antibody (Cell Signaling Technology Inc., Danvers, MA) and, after stripping the membrane, total STAT3 was detected using Stat3 antibody (Cell Signaling Technology). The blots were developed using chemiluminescence (Perkin Elmer Life Sciences Inc., Boston, MA), exposed to BioMax-MR X-ray films (Eastman Kodak Co., Rochester, NY) and the films were scanned and images digitized using UN-SCAN-IT™ gel version 5.1 software (Silk Scientific, Orem, UT). Data were expressed as the ratio of PSTAT3/STAT3 in arbitrary units. A common sample was tested on each membrane to standardize results.
Experiment 3: Response to peripheral leptin injections in rats fed a high-fructose, high-fat diet
In Experiments 1 and 2 the HFruc diet had a low fat content but a very high fructose content and HFruc-fed rats gained less weight than LFruc-fed animals (see Results). This experiment tested leptin responsiveness in rats fed low- and high-fructose diets that also had a relatively high fat content. The fat content of the diet was increased to prevent the reduction in body weight observed in HFruc-fed rats, but also allowed us to compare the effects of fructose on the development of leptin resistance in rats fed low- and high-fat diets. The fructose content of the diet had to be reduced from that in the HFruc diet (60% kcal fructose) in order to increase dietary fat content to 30% kcal, therefore, we tested a diet containing 40% kcal fructose, 10% glucose and 30% kcal fat (MFruc/HF diet).
Thirty six rats were adapted to the environment and then were divided into four weight-matched groups. Each group was offered one of the experimental diets described in Table 1: LFruc/LF, LFruc/HF, MFruc/LF, MFruc/HF. Daily body weights were recorded until the end of the study (49 days), and daily food intakes were recorded for the first 27 days. Because we anticipated that dietary fat would accelerate the onset of leptin resistance in rats fed the MFruc/HF diet we tested leptin responsiveness starting at 7 days on diet. Food intake of the rats was measured 14 hours after an i.p. injection of 2 mg leptin/kg. Body weight was recorded 14, 38 hours after injection. After 7, 10 and 21 days on diet both MFruc/HF and LFruc/LF groups responded to the peripheral injection of leptin. Leptin responsiveness of all groups of rats was tested after 36 and 39 days of the experiment. Rats that were injected with leptin on day 36 were injected with PBS on day 39 and vice versa. At this time measurements of 14 hour food intake and weight change indicated that rats fed the LFruc/HF diet were leptin resistant. Ten days after the last injection (day 49 on diet) the rats were killed for determination of inguinal, epididymal, retroperitoneal and mesenteric fat pad weights, body composition [35], and serum leptin, glucose (Glucose assay kit GAGO20; Sigma-Aldrich, St. Louis, MO), insulin (Rat Insulin RIA kit, MP Biomedicals) and TG.
Table 1.
Composition (g/kg) of diets used in Experiment 4
| LFruc/LF | LFruc/HF | MFruc/LF | MFruc/HF | |
|---|---|---|---|---|
| Casein | 200 | 200 | 200 | 200 |
| Glucose | 100 | 100 | 100 | 100 |
| Fructose | 151 | 151 | 402 | 402 |
| Corn Starch | 414 | 251 | 198 | 0 |
| Maltodextrin-10 | 35 | 0 | 0 | 0 |
| Cellulose | 50 | 50 | 50 | 50 |
| Soybean oil | 25 | 25 | 25 | 25 |
| Lard | 20 | 108 | 20 | 108 |
| Mineral Mix | 10 | 10 | 10 | 10 |
| Vitamin Mix | 10 | 10 | 10 | 10 |
| Energy density (kcal/g) | 3.8 | 4.3 | 3.8 | 4.3 |
| % kcal protein | 20 | 20 | 20 | 20 |
| % kcal fat | 10 | 30 | 10 | 30 |
| % kcal fructose | 15 | 15 | 40 | 40 |
| Research Diets Product# | D02022705 | D02022706 | D02022708 | D02022707 |
Experiment 4: Response to peripheral leptin injections in rats fed Gluc/HF diet
The previous experiment demonstrated that the MFruc/HF diet prevented the development of leptin resistance that was present in LFruc/HF-fed rats. The objective of this study was to test whether this was fructose-specific or whether a similar protective effect would be provided by a different monosaccharide; glucose.
Thirty-two rats were adapted to the environment for 1 week and then were divided into 3 groups. Ten rats were offered the LFruc/HF diet, 11 were offered the MFruc/HF diet, and 11 were offered a diet containing an equivalent amount of glucose in place of the fructose (Gluc/HF: 50% kcal glucose, 30% kcal fat. Research Diets, Inc.) Body weight was recorded twice each week and the rats were tested for leptin responsiveness after 36 and 39 days on diet. On day 36 half of the rats in each group received leptin and half received PBS and on day 39 treatments were reversed. All treatment groups were leptin responsive, therefore the experiment was continued and the rats were tested again after 64 and 67 days, at which time the LFruc/HF rats were leptin resistant.
Four days after the LFruc/HF-fed rats were shown to be leptin resistant (71 days) the rats were food deprived for 4 hours in the morning and then were injected i.p. with either 2.0 mg leptin/kg or PBS. Exactly 20 minutes after injection the rats were anesthetized with ketamine (100 mg/kg) and perfused pericardially for fixation of the brain which was collected and stored in 4% paraformaldehyde at 4°C and then transferred to 25% sucrose, 0.01% sodium azide solution. Using coordinates in the Paxinos Rat Brain Atlas [36], 30 μm serial coronal slices were made through the ARC (Anteroposterior to bregma: −2.12 mm to −4.13 mm) and the medial nucleus of the solitary tract (NTS) (Anteroposterior to bregma: −13.68 mm to −14.30 mm). Every fourth section was used to detect PSTAT3 using the protocol described by Ellacott et al [37] for free floating sections. The sections were mounted and counterstained with cresyl violet.
PSTAT-3 was visualized using a Nikon microscope and images captured using ImagePro Plus Software (Media Cybernetics, Inc., Bethesda, MD). A 27 mm reticule 10×10 1 mm square grid (Nikon Instruments, Melville, NY) was inserted in the microscope and this grid was aligned to a 10×10 grid in Photoshop that was superimposed onto the image of each section. PSTAT-3 positive nuclei were counted for the ARC and the medial NTS.
Experiment 5: Response to central leptin in rats fed MFruc/HF or Gluc/HF diets
The previous experiment suggested that both fructose and glucose prevented the development of peripheral leptin resistance in rats fed a high-fat diet. The objective of this experiment was to determine whether the monosaccharides influenced central leptin responsiveness of rats.
Thirty rats were each fitted with a third ventricle guide cannula and one week after cannula placement was confirmed the rats were divided into three groups and offered one of the diets used in Experiment 4. Body weights were recorded weekly and starting from day 53 daily body weights and food intakes were recorded to adapt the rats to procedures for testing for leptin responsiveness. Central leptin responsiveness was tested after the rats had been on diet for 57 or 60 days. Each rat was tested with leptin and with PBS with the treatments randomized across the 2 days of testing. The procedure was as described for Experiment 1 except that a 1.5 ug dose of leptin was used. On day 65 the rats were killed and inguinal, retroperitoneal and epididymal fat depots were weighed.
Statistical Analysis
Data were analyzed by one or two-way ANOVA. Differences between specific treatment groups were determined by post-hoc Duncans Multiple Range test if rats were tested in only one condition. If rats were tested with both PBS and leptin, then the response was compared by paired t-test. P<0.05 was considered significant. All statistical comparisons were made using Statistica Software (StatSoft, Inc., Tulsa, OK). Data are expressed as mean ± SEM.
RESULTS
Experiment 1: Response to peripheral and central leptin injections in HFruc-fed rats
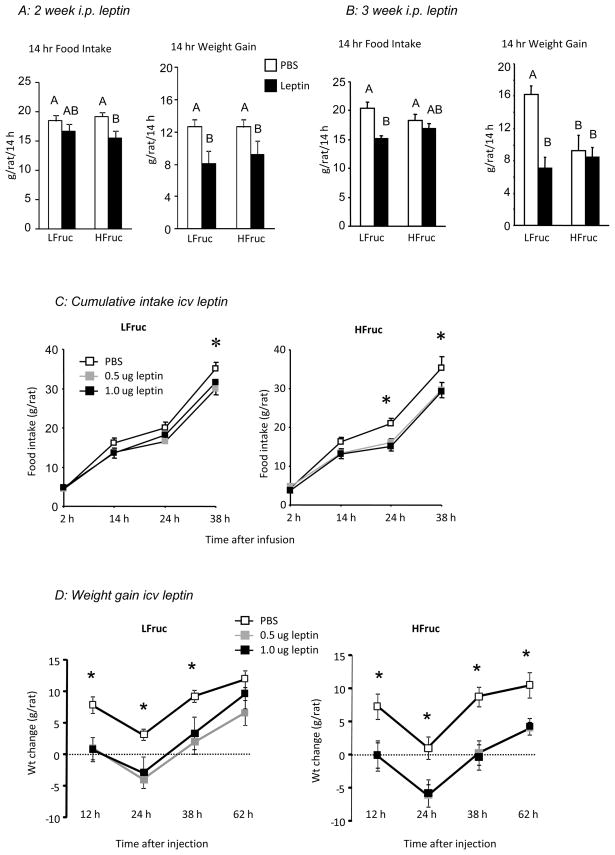
There was no effect of diet on basal food intake of the two groups of rats (LFruc: 22.2 ± 1.1 g/day, HFruc: 22.1 ± 0.9 g/day, n= 11). An i.p. injection of 2.0 mg leptin/kg caused a significant inhibition of food intake in HFruc-fed rats when they had been adapted to diet for 2 weeks (Figure 1A: Diet: NS, Leptin: P<0.02, Interaction: NS), but not when they had been on the diet for 3 weeks (Figure 1B: Diet: NS, Leptin, P<0.002, Interaction: P<0.07). Similarly, the leptin injection inhibited weight gain of HFruc-fed rats at 2 weeks (Figure 1A: Diet: NS, Leptin: P<0.006, Interaction: NS), but not at 3 weeks (Figure 1B: Diet: NS, Leptin, P<0.003, Interaction: P<0.01), indicating the development of peripheral leptin resistance. At 3 weeks, serum leptin measured 2 hours after leptin injection was not different between dietary groups, but was much higher in leptin-injected than PBS-injected rats (PBS-injected: 8 ± 2 ng/mL, leptin-injected: 634 ± 9 ng/mL). At 3 weeks there was no effect of leptin and no effect of diet on post-prandial TG (HFruc: 74 ± 10 mg/dL, LFruc: 61 ± 7 mg/dL). There was no difference in the weight of the rats after 2 weeks on diet (LFruc: 342 ± 5 g, HFruc: 344 ± 4 g), but HFruc-fed rats weighed significantly less than LFruc-fed rats at the start of testing for central leptin responsiveness (392 ± 5 g vs. 411 ± 7 g: P<0.05).
Figure 1.
Food intake and body weight gain of rats in Experiment 1 following an i.p. injection of 2.0 mg/kg leptin after two (Panel A) or three weeks on diet (Panel B). Controls were injected with phosphate buffered saline (PBS). Data for 14 h food intake or weight gain that do not share a common superscript are significantly different at P<0.05. Panel C shows cumulative food intake and Panel D shows weight change following a third ventricle infusion of 0.5 or 1.0 ug leptin. An asterisk indicates a significant (P<0.05) effect of leptin on cumulative food intake or body weight gain.
HFruc-fed rats were at least as responsive to central leptin administration as the LFruc-fed rats (Figure 1C: Diet: NS, Leptin: P<0.03, Time: P<0.001, Leptin × Time: P<0.003). Cumulative food intake 24 hours after infusion was inhibited by both 0.5 and 1.0 ug leptin in HFruc-fed rats, but not Lfruc-fed rats (Diet: NS, Leptin: P<0.007, Int: P< 0.05), whereas intake of both HFruc-fed and LFruc-fed rats was significantly inhibited at 38 hours (Diet: NS, Leptin: P<0.04, Int: NS). Leptin inhibited weight gain in both dietary groups at all time points measured suggesting that leptin stimulated energy expenditure (Figure 1D: Diet: NS, Leptin: P<0.0001, Time: P<0.001, Leptin × Time: P<0.01). The HFruc-fed rats took longer to correct for the inhibition and had not compensated 62 hours after leptin infusion.
Experiment 2: Changes in serum TG and hypothalamic PSTAT3 in rats fed HFruc diet
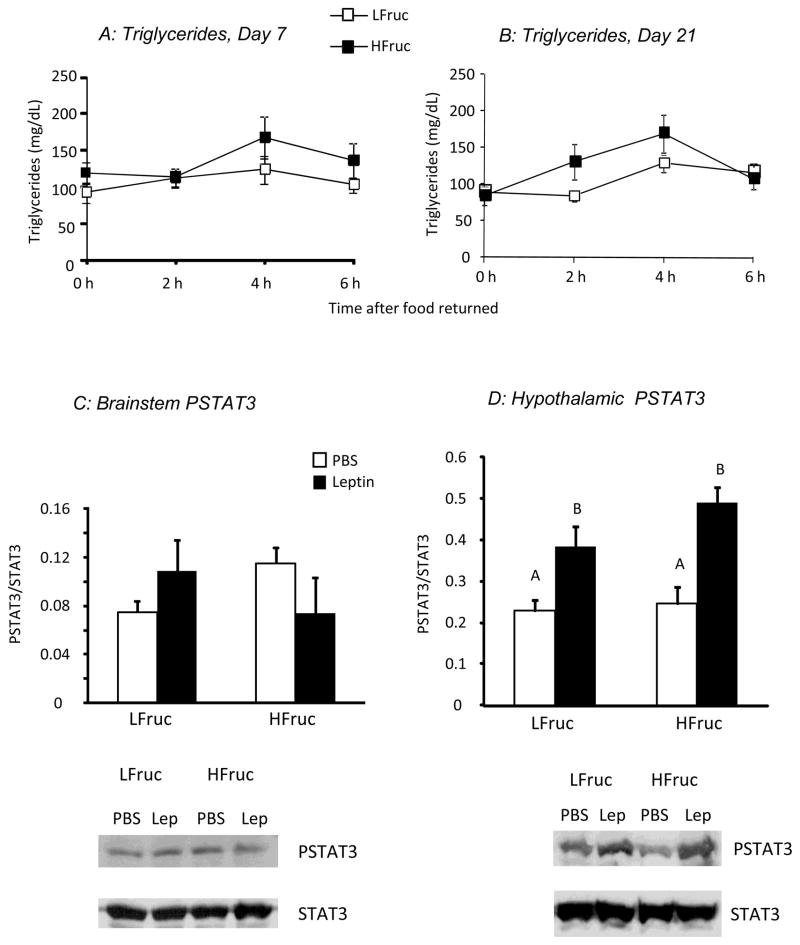
There was no effect of diet on post-prandial serum TG concentrations after either 7 or 21 days on diet (Figure 2A and B: Diet: NS, Time: P<0.001, Interaction: NS). HFruc-fed rats weighed less than LFruc-fed rats after both 7 (390 ± 4 g vs. 408 ± 3 g, P<0.001) and 21 days (419 ± 7 g vs. 439 ± 5 g, P<0.02), but there were no differences in food intake during the 2 hours before blood sampling on day 21 (LFruc: 4.3 ± 0.5 g/2 h, HFruc: 4.5 ± 0.5 g/2 h).
Figure 2.
Top panels show serum TG concentrations measured during the first half of the dark cycle in rats fed LFruc or HFruc diet for 7 (Panel A) or 21 days (Panel B) in Experiment 2. Bottom panels show the ratio of PSTAT3 to total STAT3 in brainstem (Panel C) or hypothalamic (Panel D) tissue collected from rats fed LFruc or HFruc diet for 26 days. Values for hypothalamic tissue that do not share a common superscript are significantly different at P<0.05.
Measurement of hypothalamic and brainstem PSTAT3 in these rats after 26 days on experimental diet showed no effect of diet or leptin injection on PSTAT3 in the brainstem (Figure 2C). By contrast, leptin caused a significant increase in PSTAT3 in hypothalamic tissue of rats from both dietary groups (Figure 2D: Diet: NS, Leptin: P<0.001, Interaction: NS)
Experiment 3: Response to peripheral leptin injections in rats fed MFruc/HF diet
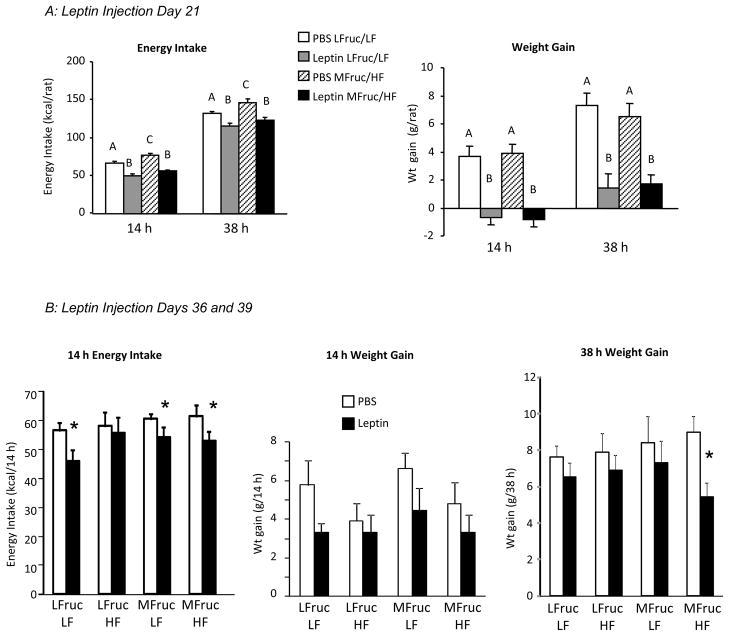
Rats fed the LFruc/HF diet had the highest energy intake and those fed the LFruc/LF diet had the lowest, but all groups gained the same amount of weight during the experimental period (Table 2). Both LFruc/LF and MFruc/HF rats responded to leptin injections on days 7, 14 and 21 by reducing energy intake (Figure 3A: Diet: P<0.05, Leptin: P<0.0001, Interaction: NS) and weight gain (Figure 3A: Diet: NS, Leptin: P<0.0001, Interaction: NS). On days 36 and 39 of the study, there was an overall significant effect of leptin on 14 hour energy intake and weight gain of the rats (Figure 3B: Diet: NS, Leptin: P<0.001, Interaction: NS). Post-hoc comparisons showed that food intake was inhibited in all but the LFruc/HF rats, but inhibition of weight gain was not significant for any specific dietary group. There also was an overall inhibitory effect of leptin on weight gain 38 hours after injection (Figure 3B: Diet: NS, Leptin: P<0.01, Interaction: NS), but post hoc analysis showed a significant effect only in MFruc/HF-fed rats.
Table 2.
Body composition and serum hormones of rats in Experiment 3
| LFruc/LF | LFruc/HF | MFruc/LF | MFruc/HF | |
|---|---|---|---|---|
| Start Wt (g) | 274 ± 3 | 273 ± 3 | 274 ± 2 | 274 ± 2 |
| End Wt (g) | 399 ± 11 | 414 ± 15 | 393 ± 8 | 399 ± 11 |
| Cumulative Intake (kcal/27 d) | 1980 ± 70 A | 2336 ± 92 B | 2124 ± 61 AB | 2154 ± 73 AB |
| Carcass Fat (g) | 22 ± 1 | 26 ± 2 | 23 ± 2 | 22 ± 2 |
| Carcass Lean Body Mass (g) | 360 ± 10 | 387 ± 16 | 353 ± 7 | 361 ± 9 |
| Glucose (mg/dL) | 66 ± 2 | 69 ± 1 | 68 ± 2 | 68 ± 1 |
| Insulin (ng/mL) | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.2 | 1.1 ± 0.1 |
| Total TG (mg/dL) | 118 ± 17 | 96 ± 10 | 86 ± 11 | 113 ± 8 |
| Leptin (ng/mL) | 2.9 ± 0.4A | 4.7 ± 0.8B | 2.9 ± 0.8AB | 2.6 ± 0.3A |
Data are means ± SEM for groups of 9 rats fed experimental diet for 49 days. Values for energy intake or serum leptin that do not share a common superscript are significantly different at P<0.05.
Figure 3.
Energy intake and weight gain of rats in Experiment 3 treated with leptin following 21 days (Panel A) or 36 and 39 days on diet (Panel B). Data are means + SEM for groups of 9 rats. Values in Panel A within a specific time interval that do not share a common superscript are significantly different at P<0.05. In Panel B, asterisks indicate a significant (P<0.05) effect of leptin on 14 hour energy intake or 36 h weight gain of rats within a dietary group.
At the end of the study there were no differences in body composition or fat depot weights of the different groups of rats (Table 2). Serum glucose, insulin and TG were not different, but leptin was higher in LFruc/HF rats than in LFruc/LF or MFruc/HF rats (Table 2).
Experiment 4: Response to peripheral leptin injections in rats fed MFruc/HF diet
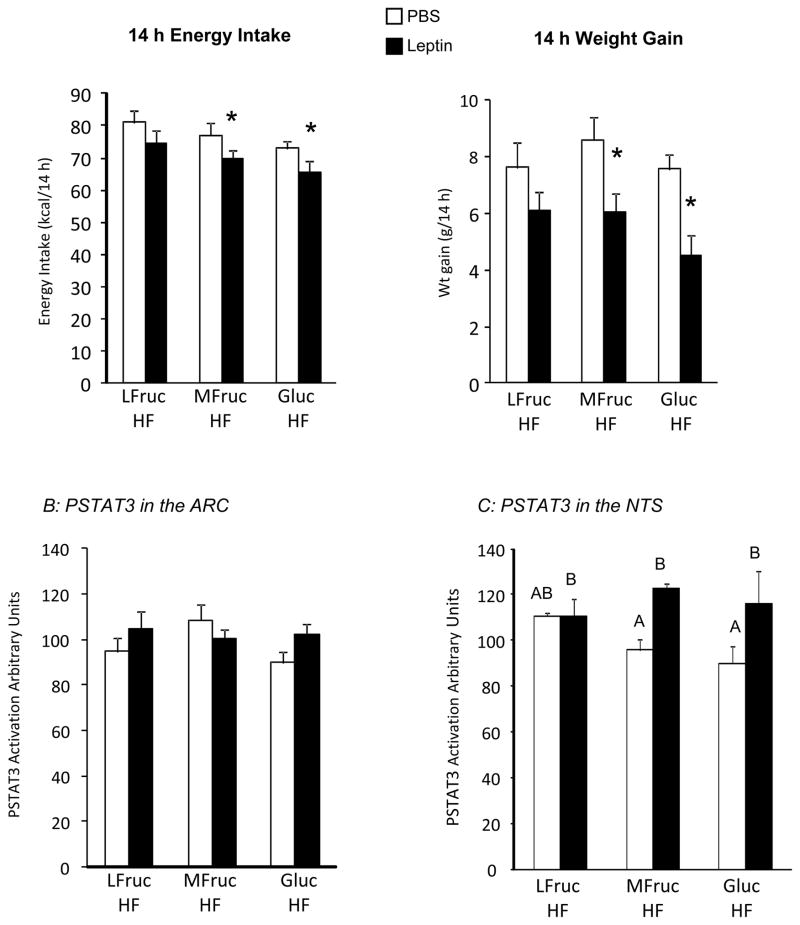
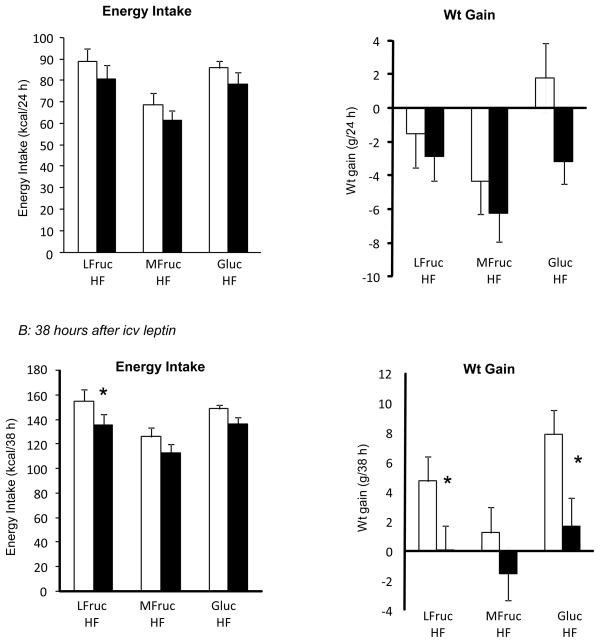
Animals in this study were fed 30% kcal fat diets containing different types of carbohydrate. After 64 days on experimental diet leptin inhibited 14 hour food intake (Figure 4A: Diet: NS, Leptin: P<0.02, Interaction: NS) and post hoc comparisons indicated a significant effect of leptin in MFruc/HF and Gluc/HF rats, but not LFruc/HF animals The inhibitory effect of leptin on 14 hour weight gain (Figure 4A: Diet: NS, Leptin: P<0.01, Interaction: NS) showed the same pattern of response. There was no effect of leptin at later time points.
Figure 4.
Energy intake and weight gain of rats during the first 14 hours following leptin administration after 64 days on diet (Panel A) in Experiment 4. Data are means + SEM for groups of 10 or 11 rats. An asterisk indicates a significant effect of leptin for a specific dietary group. Panel B shows the quantification of PSTAT3 in the ARC and medial NTS 20 minutes after a peripheral injection of leptin. Values for PSTAT3 in the medial NTS that do not share a common superscript are significantly different at P<0.05.
PSTAT-3 was measured as an index of leptin receptor activation in two brain areas. There was no effect of leptin or diet on PSTAT-3 in the ARC (Figure 4B). A peripheral injection of leptin increased PSTAT-3in the medial NTS (Figure 4C: Diet: NS, Leptin: P<0.005, Interaction: P<0.09). Post-hoc comparisons showed a significant effect only in MFruc/HF-fed rats. The levels of PSTAT-3 in tissue from both PBS and leptin treated LFruc/HF rats were the same as those of leptin-treated rats from the MFruc/HF and Gluc/HF groups.
Experiment 5: Response to central leptin in rats fed MFruc/HF or Gluc/HF diets
Central leptin responsiveness was tested in rats that had been consuming MFruc/HF, Gluc/HF or LFruc/HF diets for 57 or 60 days. MFruc/HF rats ate significantly less than LFruc/HF rats and there was a significant overall effect of leptin on cumulative food intake 24 and 38 hours after injection (Diet: P<0.02, Leptin: P< 0.04, Interaction: NS), but the only specific response was inhibition of 38 hours intake of LFruc/HF rats. The inhibitory effect of leptin on weight gain was significant only at 38 hours after injection (Diet: P<0.04, Leptin: P< 0.002, Interaction: NS). Post hoc comparisons indicated that leptin inhibited weight gain in both LFruc/HF and Gluc/HF rats. Data from the 24 and 38 hour time points are shown in Figures 5A and B. At the end of the study the LFruc/HF rats were significantly heavier than the two other groups (LFruc/HF: 453 ± 13g, MFruc/HF: 416 ± 10g, Gluc/HF: 416 ± 10 g) even though there were no differences in the 3 day intakes of the isocaloric diets measured before testing for leptin responsiveness (LFruc/HF: 59 ± 2 g/3 d, MFruc/HF: 55 ± 4 g/3d, Gluc/HF: 59 ± 2 g/3d). Fat depots from LFruc/HF rats were also larger, but the difference was not significant if depot weight was expressed as a percentage of body weight (data not shown).
Figure 5.
Energy intake and weight change of rats 24 hours (Panel A) and 38 hours after a third ventricle injection of 1.5 ug leptin in rats adapted to diet for 57 or 60 days in Experiment 5. An asterisk indicates a significant effect of leptin on energy intake or weight gain of rats within a dietary group.
DISCUSSION
The objective of studies described here was to determine whether a relatively short period of consumption of a high-fructose diet caused leptin resistance in rats and whether increased concentrations of both fat and fructose in the diet would accelerate the onset of leptin resistance. Leptin responsiveness was detected by measuring food intake and weight gain in rats given an acute dose of leptin either as a peripheral injection or as a third ventricle infusion. Shapiro et al [30] recently used the acute response to a peripheral injection of leptin to demonstrate the development of leptin resistance in rats fed a 67% kcal fructose diet for 6 months. The results of the studies described here indicate that the effect of dietary fructose on leptin responsiveness can occur within 4 weeks, but is more complicated than expected. Rats were resistant to peripheral injections of leptin after consuming a high-fructose, low-fat diet (HFruc) for 3 weeks. We did not determine whether the development of leptin resistance in rats fed a low-fat diet could be extended to dietary glucose, or any other monosaccharide. Surprisingly, the results of Experiments 3, 4 and 5 showed that increased concentrations of either fructose or glucose in the diet prevented leptin resistance in rats fed a high-fat diet. This beneficial effect of monosaccharides is in stark contrast to the current belief that dietary fructose has negative effects on metabolism and health [21–22] and was the opposite of the outcome that we had anticipated. More work is needed to determine the threshold amount of fructose required to prevent leptin resistance in high-fat fed rats because the levels included in experiments described here were higher than would be consumed in a normal human diet. Further studies also are needed to determine whether dietary fructose delays the onset of leptin resistance in humans because it is clear from the studies described here and by others [38] that high fructose diets do not necessarily produce the same metabolic disruption in rats as have been reported for human subjects consuming fructose [25–28]. It also is possible that consumption of fructose in liquid form would have a different metabolic impact than when it is consumed as part of a composite dry diet. Some, if not all of the fructose supplement in the human studies was provided in sweetened beverages [25–28] and consumption of sugar sweetened beverages has recently been associated with increased risk for obesity [39] and type 2 diabetes [40].
In Experiment 1 rats fed the HFruc diet were resistant to peripheral injections of leptin, but remained responsive to central injections, implying that leptin was unable to cross the blood brain barrier. Others have shown that high-fructose diets cause hypertriglyceridemia in humans [25–26] and rats [30, 41] and Banks et al [29] reported that intraperitoneal injections of whole milk, but not intralipid, inhibited transport of leptin across the blood brain barrier in anaesthetized mice. Leptin transport appeared to be significantly inhibited when serum TG concentrations reached or exceeded 150 mg/dL [29]. In Experiment 1 described here leptin response was tested in rats that had been fasted for 12 hours, therefore they ate a relatively large meal soon after they were injected, but there was no clear relation between serum TG concentration measured 1 hour after the start of feeding and leptin responsiveness of the rats. In addition, the HFruc-fed rats had serum TG concentrations in the range of 100 mg/dL which would not be expected to have caused any substantial change in the brain/serum leptin ratio [29]. Experiment 2 confirmed that the HFruc diet did not increase serum TG concentrations compared with the LFruc diet. In this study the rats were food deprived for 9 hours during the light period and serum TG concentrations were measured every 2 hours for 6 hours after food was returned. Equal quantities of the two diets were consumed during the first 2 hours that food was returned to the cages and Chong et al [26] reported that there was a significantly greater increase in circulating TG in overnight fasted human subjects 4 to 6 hours after consumption of a high fructose meal compared with a low fructose meal. Therefore, we would have detected a diet-induced difference in serum TG of the rats if it had existed.
Other have reported that fructose increases serum TG concentration in rats, but this may be dependent upon factors other than the fructose content of the diet. For example, Lindquist et al [42] found an increased energy intake, accelerated weight gain and a doubling of serum TG concentration in rats offered 23% liquid fructose for two weeks. By contrast, Botezelli et al [38] found a small increase in energy intake, no change in rate of weight gain and no change in serum TG of rats offered a dry 66% fructose diet for 13 weeks. Oron-Herman et al [43] reported a substantial increase in serum TG concentration of rats offered a dry 60% fructose diet for 7 weeks, but the fructose rats in this study were compared with controls eating chow, whereas control rats in the Botezelli study were consuming a formulated low fructose diet, similar to the controls in our experiments. Furthermore, Gutman et al [44] have reported that dietary sucrose has a phasic effect on serum TG, with an initial elevation (2 weeks), a normalization (7 weeks) and then later development of hyperlipidemia (17 weeks). In addition, Cohen et al [45] reported that a 70% fructose diet raised TG only in rats that also were susceptible to diabetes. In the study reported by Shapiro et al [30] rats fed a 67% fructose diet were leptin resistant and hypertriglyceridemic compared with their controls. Obvious explanations for the different results from the Shapiro study [30] and those described here are the duration of exposure to the diet and the age of the rats at the time that TG were measured. In addition, the diets were obtained from different manufacturers and it is possible that a dietary component other than fructose was responsible for the different effects on serum TG. Because of the difference in metabolic phenotypes of the rats in the Shapiro study [30] and those described here it also is possible that the mechanistic basis for leptin resistance was different for the two groups of animals. We did not find any evidence of leptin changing post-prandial TG concentrations in the rats, even though others have reported that leptin rapidly inhibits hepatic TG secretion [46] by promoting fatty acid oxidation [47]. Because we measured total TG it is possible that we would have detected a difference if we had specifically measured VLDL-TG.
If leptin transport across the blood brain barrier was the cause of the leptin resistance in HFruc-fed animals, then peripheral injections of leptin would not be expected to activate central leptin receptors. Surprisingly, in Experiment 2 we found that i.p. injections of leptin caused a robust increase in PSTAT3 in the ARC of both LFruc-fed and HFruc-fed rats even though a lower dose of leptin was used to test for PSTAT3 than was used to test for a suppression of food intake. These results contrast with those of Shapiro et al [30] who found reduced levels of hypothalamic PSTAT3 in rats fed high fructose diet for 6.5 month, but Shapiro et al [30] did not determine whether peripheral leptin injections increased PSTAT3 in any of the rats and differences in relative levels of hypothalamic PSTAT3 in the two studies may be explained by duration of exposure to the diet. There are several potential explanations for our observations of increased hypothalamic PSTAT3 in rats that did not respond energetically to i.p. leptin injections. The first is that because we used blocks of tissue to measure PSTAT3 it is possible that leptin increased PSTAT3 in hypothalamic structures other than the ARC and that the ARC was leptin resistant. Consistent with this, Munzberg [48] has reported that the ARC, but not the ventromendial or dorsomedial hypothalamus become leptin resistant in diet induced obese mice. The second is that the leptin resistance resulted from an impairment in the leptin signaling cascade downstream of PSTAT3, such as increased expression of the PSTAT3 inhibitor SOCS3, but if either of these possibilities had been the case, then HFruc-fed rats would not have responded to central leptin injections in Experiment 1. Alternatively, the leptin resistance could be independent of hypothalamic PSTAT3 activation. It is now well established that leptin can influence food intake through reward pathways [5] and at the level of the caudal brainstem [6] as well as the more traditional hypothalamic structures. In addition, Rahmouni et al [49] have suggested that hypothalamic extracellular signal-regulated kinase (ERK) mediates the effects inhibitory of leptin on food intake and weight gain and that blockade of ERK1/2 blocks the stimulatory effects of leptin on sympathetic activation of brown adipose tissue, but does not prevent leptin-induced phosphorylation of STAT3 [49].
In contrast to the results from rats fed a low fat diet, addition of either fructose (MFruc/HF) or glucose (Gluc/HF) to a high fat diet prevented the development of peripheral leptin resistance even though it was not clear that the rats remained centrally responsive to leptin (Figure 5) and a peripheral injection of leptin failed to increase PSTAT3 activity in the ARC. Changes in NTS PSTAT3 activity correlated with responsiveness to peripheral leptin in the MFruc/HF and Gluc/HF rats and implied an important role for the caudal brainstem in mediating the changes in energy balance of these rats. A dominant role of the caudal brainstem also would explain the minimal effect of a third ventricle infusion of leptin on food intake even though the rats responded to peripheral leptin administration. This also would be consistent with a report by Skibicka et al. [6] that leptin infusions into the 4th ventricle inhibit food intake, increase core temperature and cause weight loss in rats through a mechanism that is dependent upon activation of melanocortin receptors. In both the MFruc/HF and Gluc/HF-fed rats non-stimulated levels of NTS PSTAT3 were lower than those of rats fed LFruc/HF diet, which was more consistent with the results from Shapiro et al [30].
At the end of the 6 months on high fructose or no fructose diets the rats in the Shapiro study [30] were offered a very high-fat diet (60% kcal lard) and those that had been fed high-fructose initially ate more high-fat diet and gained significantly more weight than the controls. Body fat did not account for the difference in weight, implying increased lean body mass. In the studies described here we found that rats fed the HFruc diet or the MFruc/HF or Gluc/HF diets weighed less than their controls although body fat expressed as a percent of body weight was not different between the three groups fed HF diet. Others have reported that fructose inhibits adipose tissue lipogenesis in rats [50] and increases obligatory thermogenesis [51] which could contribute to the reduced body mass in our studies. Our animals were not switched to a low monosaccharide diet, but it is clear that including high levels of monosaccharide in a high fat diet attenuated growth of the rats.
We did not attempt to determine the metabolic basis of leptin resistance in HFruc-fed rats, or why diets containing high concentrations of fructose or glucose prevent leptin resistance caused by a high-fat diet. The majority of fructose that is consumed is metabolized by the liver and promotes lipid synthesis, therefore it is possible that the resulting increased availability of fatty acids interferes with the normal metabolic impact of leptin in the periphery. It has been suggested that incomplete oxidation of fatty acids can inhibit phosphorylation of leptin receptor signaling proteins and that this accounts for the development of hepatic leptin resistance in rats offered fructose solution [52]. It is difficult to envision how this mechanism would reverse leptin responsiveness in high-fat fed rats that also would be expected to have increased rates of hepatic fatty acid oxidation.
CONCLUSION
The results of the studies described here demonstrate that very high concentrations of fructose in a low fat diet promote the development of peripheral, but not central leptin resistance. By contrast high levels of fructose or glucose inhibit the development of peripheral leptin resistance of rats consuming a high-fat diet. There does not appear to be an association between leptin responsiveness and serum TG concentrations or body fat mass, suggesting modification of a metabolic process that is essential for the effects of leptin on food intake and weight gain.
Research Highlights.
Rats fed a low fat (10% kcal), very high fructose (60% kcal) diet for three weeks were resistant to peripheral, but not central leptin administration.
The peripheral leptin resistance was not caused by a failure of leptin to cross the blood brain barrier because peripheral leptin increased hypothalamic phosphorylation of STAT3.
Rats fed a low fructose (18% kcal), high fat (30% kcal) diet for 64 days were resistant to peripheral leptin, but leptin resistance was prevented in rats fed a high fat diet containing 40% kcal fructose although percent body fat was not changed.
Replacement of fructose with glucose in the high fat diet also prevented development of leptin resistance.
These results suggest that dietary monosaccharides modify a metabolic process that influences the effects of leptin on food intake and weight gain.
Acknowledgments
The authors thank Hayden Kramer for his assistance with the quantification of PSTAT3. S.J.H. conducted research, analyzed data and wrote the paper. R.B.S.H. designed research, conducted research, analyzed data, wrote paper and had primary responsibility for final content. Both authors read and approved the final manuscript
ROLE OF FUNDING SOURCE
This work was supported by NIH grant DK-53903 and by Georgia Agricultural Experiment Station grant CSREES/GEO00932 awarded to RBSH. Neither institution played any role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 5.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 6.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150:1705–1711. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 8.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 9.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 10.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 11.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 12.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 14.Lin L, Martin R, Schaffhauser AO, York DA. Acute changes in the response to peripheral leptin with alteration in the diet composition. Am J Physiol Regul Integr Comp Physiol. 2001;280:R504–509. doi: 10.1152/ajpregu.2001.280.2.R504. [DOI] [PubMed] [Google Scholar]
- 15.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 16.Munzberg H, Myers MG., Jr Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–570. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 17.Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Molecular Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 18.Bates SH, Myers MG. The role of leptin-->STAT3 signaling in neuroendocrine function: an integrative perspective. J Mol Med. 2004;82:12–20. doi: 10.1007/s00109-003-0494-z. [DOI] [PubMed] [Google Scholar]
- 19.Madiehe AM, Schaffhauser AO, Braymer DH, Bray GA, York DA. Differential expression of leptin receptor in high- and low-fat-fed Osborne-Mendel and S5B/Pl rats. Obes Res. 2000;8:467–474. doi: 10.1038/oby.2000.58. [DOI] [PubMed] [Google Scholar]
- 20.Bray GA. Fructose: should we worry? Int J Obes (Lond) 2008;32 (Suppl 7):S127–131. doi: 10.1038/ijo.2008.248. [DOI] [PubMed] [Google Scholar]
- 21.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 22.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 23.Haley S, Reed J, Lin B-H, Cook A. Sweetner consumption in the United States. Distribution by demographic and product characteristics. United States Department of Agriculture; 2005. [Google Scholar]
- 24.Bizeau ME, Pagliassotti MJ. Hepatic adaptations to sucrose and fructose. Metabolism. 2005;54:1189–1201. doi: 10.1016/j.metabol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Teff KL, Elliott SS, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89:2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 26.Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr. 2007;85:1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- 27.Le KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr. 2006;84:1374–1379. doi: 10.1093/ajcn/84.6.1374. [DOI] [PubMed] [Google Scholar]
- 28.Bantle JP, Raatz SK, Thomas W, Georgopoulos A. Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr. 2000;72:1128–1134. doi: 10.1093/ajcn/72.5.1128. [DOI] [PubMed] [Google Scholar]
- 29.Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro A, Mu W, Roncal C, Cheng KY, Johnson RJ, Scarpace PJ. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1370–1375. doi: 10.1152/ajpregu.00195.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- 32.Harris RB, Mitchell TD, Hebert S. Leptin-induced changes in body composition in high fat-fed mice. Exp Biol Med. 2003;228:24–32. doi: 10.1177/153537020322800103. [DOI] [PubMed] [Google Scholar]
- 33.Wang T, Hartzell DL, Rose BS, Flatt WP, Hulsey MG, Menon NK, Makula RA, Baile CA. Metabolic responses to intracerebroventricular leptin and restricted feeding. Physiol Behav. 1999;65:839–848. doi: 10.1016/s0031-9384(98)00243-1. [DOI] [PubMed] [Google Scholar]
- 34.Kasser TR, Harris RB, Martin RJ. Level of satiety: fatty acid and glucose metabolism in three brain sites associated with feeding. Am J Physiol. 1985;248:R447–452. doi: 10.1152/ajpregu.1985.248.4.R447. [DOI] [PubMed] [Google Scholar]
- 35.Harris RB. Growth measurements in Sprague-Dawley rats fed diets of very low fat concentration. J Nutr. 1991;121:1075–1080. doi: 10.1093/jn/121.7.1075. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C. The rat brain. New York: Academic Press; 1998. [Google Scholar]
- 37.Ellacott KL, Halatchev IG, Cone RD. Characterization of leptin-responsive neurons in the caudal brainstem. Endocrinology. 2006;147:3190–3195. doi: 10.1210/en.2005-0877. [DOI] [PubMed] [Google Scholar]
- 38.Botezelli JD, Mora RF, Dalia RA, Moura LP, Cambri LT, Ghezzi AC, Voltarelli FA, Mello MA. Exercise counteracts fatty liver disease in rats fed on fructose-rich diet. Lipids Health Dis. 2010;9:116. doi: 10.1186/1476-511X-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim S, Zoellner JM, Lee JM, Burt BA, Sandretto AM, Sohn W, Ismail AI, Lepkowski JM. Obesity and sugar-sweetened beverages in African-American preschool children: a longitudinal study. Obesity (Silver Spring) 2009;17:1262–1268. doi: 10.1038/oby.2008.656. [DOI] [PubMed] [Google Scholar]
- 40.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR., Jr Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009;32:688–694. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alzamendi A, Giovambattista A, Raschia A, Madrid V, Gaillard RC, Rebolledo O, Gagliardino JJ, Spinedi E. Fructose-rich diet-induced abdominal adipose tissue endocrine dysfunction in normal male rats. Endocrine. 2009;35:227–232. doi: 10.1007/s12020-008-9143-1. [DOI] [PubMed] [Google Scholar]
- 42.Lindqvist A, Baelemans A, Erlanson-Albertsson C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept. 2008;150:26–32. doi: 10.1016/j.regpep.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 43.Oron-Herman M, Kamari Y, Grossman E, Yeger G, Peleg E, Shabtay Z, Shamiss A, Sharabi Y. Metabolic syndrome: comparison of the two commonly used animal models. Am J Hypertens. 2008;21:1018–1022. doi: 10.1038/ajh.2008.218. [DOI] [PubMed] [Google Scholar]
- 44.Gutman RA, Basilico MZ, Bernal CA, Chicco A, Lombardo YB. Long-term hypertriglyceridemia and glucose intolerance in rats fed chronically an isocaloric sucrose-rich diet. Metabolism. 1987;36:1013–1020. doi: 10.1016/0026-0495(87)90019-9. [DOI] [PubMed] [Google Scholar]
- 45.Cohen AM, Teitelbaum A, Rosenman E. Diabetes induced by a high fructose diet. Metabolism. 1977;26:17–24. doi: 10.1016/0026-0495(77)90123-8. [DOI] [PubMed] [Google Scholar]
- 46.Huang W, Dedousis N, Bandi A, Lopaschuk GD, O’Doherty RM. Liver triglyceride secretion and lipid oxidative metabolism are rapidly altered by leptin in vivo. Endocrinology. 2006;147:1480–1487. doi: 10.1210/en.2005-0731. [DOI] [PubMed] [Google Scholar]
- 47.Huang W, Metlakunta A, Dedousis N, Ortmeyer HK, Stefanovic-Racic M, O’Doherty RM. Leptin augments the acute suppressive effects of insulin on hepatic very low-density lipoprotein production in rats. Endocrinology. 2009;150:2169–2174. doi: 10.1210/en.2008-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 49.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–542. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waterman RA, Romsos DR, Tsai AC, Miller ER, Leveille GA. Effects of dietary carbohydrate source on growth, plasma metabolites and lipogenesis in rats, pigs, and chicks. Proc Soc Exp Biol Med. 1975;150:220–225. doi: 10.3181/00379727-150-39006. [DOI] [PubMed] [Google Scholar]
- 51.Tappy L, Jequier E. Fructose and dietary thermogenesis. Am J Clin Nutr. 1993;58:766S–770S. doi: 10.1093/ajcn/58.5.766S. [DOI] [PubMed] [Google Scholar]
- 52.Vila L, Roglans N, Alegret M, Sanchez RM, Vazquez-Carrera M, Laguna JC. Suppressor of cytokine signaling-3 (SOCS-3) and a deficit of serine/threonine (Ser/Thr) phosphoproteins involved in leptin transduction mediate the effect of fructose on rat liver lipid metabolism. Hepatology. 2008;48:1506–1516. doi: 10.1002/hep.22523. [DOI] [PubMed] [Google Scholar]