Abstract
Background
Lipoprotein lipase (LPL) has a prominent role in the metabolism of triglycerides (TG) and high-density lipoprotein cholesterol (HDL-C) and is a potential interesting target for the development of antiatherogenic treatment. To provide deeper insight into the role of natural variation in this gene, we investigated the association between the LPL S447X variant with lipids and risk of coronary heart disease (CHD) in three independent, prospective studies.
Methods
The S447X variant was genotyped in case-control studies of incident CHD nested within the Nurses’ Health Study (NHS), the Health Professionals Follow-up Study (HPFS), and the Danish Diet, Cancer and Health (DCH) study, totaling 245, 258, and 962 cases, respectively.
Results
S447X-carriers tended to have lower TG and higher HDL-C concentrations than non-carriers. The S447X variant was associated with a lower risk of CHD in the NHS, the association was weaker in the HPFS and not statistically significant in the DCH women and men. The pooled relative risk per minor allele was 0.74 (0.56–1.00). There was a suggestion that the associations of the S447X variant with plasma lipids and CHD-risk were more pronounced in obese individuals in the NHS study, but this finding was not consistent across the studies.
Conclusions
The LPL S447X variant tended to be associated with lower TG and higher HDL-C levels, and lower risk of CHD in all three cohorts. LPL is an attractive target for clinical intervention, but studies are needed to clarify whether greater benefit from this variant may be conferred in some subgroups.
Keywords: Genetic epidemiology, CHD, prospective study, lipoprotein lipase, plasma lipids
In prospective observational studies, plasma triglyceride (TG) levels are directly associated and high density lipoprotein (HDL) cholesterol levels inversely associated with risk of coronary heart disease (CHD).1,2 However, therapeutic manipulation of TG and HDL-C as new relevant drug targets remain elusive. Studies of DNA sequence variants located in genes of major importance to lipid metabolism may provide important knowledge about the role of their encoded proteins in relation to long-term measures of plasma lipids and risk of clinical endpoints.
The lipoprotein lipase (LPL) enzyme has a prominent role in the metabolism of TG and HDL. LPL hydrolyzes TG carried in very low density lipoprotein (VLDL) and chylomicrons and generates excess phospholipids and apolipoproteins that are transferred to HDL.3 Thus, high LPL activity is associated with lower TG and higher HDL-C levels.4 The gene encoding LPL is located on chromosome 8p22, spans close to 30kb and contains 10 exons.5 Most of the identified single nucleotide polymorphisms (SNPs) with functional effects cause loss of enzymatic function and predispose to elevated TG and reduced HDL-C.6 In contrast, the commonly occurring S447X polymorphism in exon 9 is an intriguing exception: the variant allele encodes a prematurely truncated LPL protein that has increased lipolytic activity in vitro and in vivo in mice.7 The S447X variant has consistently been associated with lower TG and higher HDL-C levels in candidate gene association studies,8–14 and its importance was recently emphasized by its identification as one of 18 loci significantly associated with TG and HDL-C concentration among over 350,000 SNPs examined in a combined analysis of over 8800 individuals.15 However, its association with risk of CHD varies considerably between study populations,12–14, 16–21 and although environmental factors, such as smoking,22, 23 alcohol,22 and particularly adiposity,10, 11, 18, 24, 25 may modulate the association of the S447X variant (or the tightly linked HindIII variant)26 with plasma lipids, context-dependent effects of the S447X variant on CHD risk have received little attention. Thus, we aimed to (1) investigate the association between the LPL S447X SNP with plasma lipids and risk of CHD in three independent prospective studies of men and women, and (2) examine the role of this functional variant in subgroups of participants based on smoking habits, alcohol, and adiposity.
Methods
Study populations
The Nurses’ Health Study (NHS) enrolled 121,701 female nurses aged 35 to 55 who returned a mailed questionnaire in 1976 regarding lifestyle and medical history.27 The Health Professionals’ Follow-up Study (HPFS) enrolled 51,529 males aged 40 to 75 who returned a similar questionnaire in 1986.28 Both cohorts are followed via biennial follow-up questionnaires. The Diet, Cancer, and Health (DCH) study was initiated in 1993–1997 when 57,053 Danish born residents, aged 50 to 64 years and free of cancer, participated in a clinical examination and detailed lifestyle survey.29
Endpoint and study designs
Smaller studies nested within these three cohorts were designed. A blood sample was requested from all active participants in 1989–1990 in NHS and 1993–1995 in the HPFS. Samples were returned by 32,826 NHS participants and 18,224 HPFS participants. Nested case-control studies used incident CHD, with non-fatal myocardial infarction (MI) and fatal CHD as the outcome. Cases were identified primarily through review of medical records, as previously described.30 Among participants who provided blood samples and who were without cardiovascular disease or cancer at blood draw, 249 women sustained an incident CHD between blood draw and June 30, 1998, and 266 cases occurred prior to January 31, 2000 in HPFS. Using risk-set sampling,31 controls were selected randomly and matched in a 2:1 ratio on age, smoking, and month of blood return.
In the DCH, all participants provided a non-fasting blood sample at baseline. A case-cohort study was designed using incident CHD (fatal and nonfatal MI) as the outcome. Information on the disease endpoint was obtained by linkage with central Danish registries via the unique identification number assigned to all Danish citizens. Details for the assessment of the hospital records have been published.32 In total, 1084 cases of CHD were identified and validated. For the creation of the case cohort sample, 1800 participants were selected from the entire DCH study at random (for consistency referred to as ‘controls’, although 32 individuals overlap with the CHD case group).
Laboratory analysis
Blood was obtained from mostly fasting participants in the US studies whereas Danes were all non-fasting. Lipids were analyzed in samples stored at −150° C using standard methods. Details on methods used in NHS/HPFS have previously been published.33 The S447X variant was genotyped using Taqman SNP allelic discrimination by means of an ABI 7900HT (Applied Biosystems, Foster City, CA), using rs328. Technicians were blinded to case status of the samples. Controls were included in each run and repeated genotyping of a random 10% subset yielded 100% identical genotypes.
Statistical analysis
Hardy-Weinberg equilibrium among control participants was tested with an exact test and no departures were observed. Multivariable regression analysis with robust variance was used to address the associations of S447X with lipids among the controls. Relative risks (RR) and 95% confidence intervals (CIs) for the association between genotype and CHD were estimated using conditional logistic regression for the US nested case-control data and Cox proportional hazard regression in DCH, using Kalbfleisch and Lawless weights and robust variance suitable for the case-cohort data.34, 35 Sex-specific analyses were conducted in the DCH study to facilitate comparison with the sex-specific HPFS and NHS studies.
Despite little impact on the RR’s, lifestyle covariates were included in the multivariable models because they may account for some of the heterogeneity between study participants. In total, numbers with information available on plasma lipids, genotype, and covariates were: 245 cases, 485 controls in NHS; 258 cases, 515 controls in HPFS; and 2629 (962 cases) in DCH.
To pool the estimates from the three study populations, we used the weighted average of regression estimates using the random-effects model.36
We explored joint effects of S447X and smoking, alcohol, and adiposity, based on prior data suggesting possible interaction in relation to lipids.10, 11, 18, 22–24 Tests for statistical interaction was examined by including the cross-product term between genotype and these factors modeled continuously. Because few participants were homozygous variant carriers, we compared non-carriers (SS447) and carriers (S447X and XX447).
Analyses were performed using SAS 9 (SAS Institute Inc., Cary, NC) and STATA 9.1 (STATA Corp., College Station, TX).
This study was supported by research grants HL35464, CA55075, AA011181, CA87969, CA49449, and HL34594 from the National Institute of Health, Bethesda; M.D, the Danish Ministry of Health, the Research Centre for Environmental Health's Fund; IMAGE, and the Danish Cancer Society (DP00027). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Table 1 shows baseline characteristics of cases and controls in the three study populations.
Table 1.
Characteristics of cases and controls in the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Diet, Cancer and Health study.*
| NHS | HPFS | DCH | ||||||
|---|---|---|---|---|---|---|---|---|
| Women | Men | |||||||
| Variable | Cases (n=245) |
Controls (n=485) |
Cases (n=258) |
Controls (n=515) |
Cases (n=229) |
Controls † (n=792) |
Cases (n=733) |
Controls † (n=907) |
| Age (yrs) | 62 (47; 69) | 62 (48; 68) | 66 (50; 78) | 66 (51; 78) | 60 (52; 64) | 56 (51; 63) | 58 (52; 64) | 56 (51; 63) |
| BMI (kg/m2) | 24.8 (18.4; 36.2) | 23.4 (18.9; 32.0) | 25.7 (20.9; 31.9) | 25.1 (19.5; 31.9) | 26.3 (21.4; 32.8) | 24.6 (21.0; 30.5) | 26.9 (23.3–32.4) | 26.4 (22.6; 31.1) |
| Waist (cm) | 81 (66; 107) | 76 (64; 97) | 99 (86; 118) | 97 (84; 117) | 85 (71; 101) | 80 (69; 96) | 97 (87; 112) | 95 (85; 108) |
| Diabetes | 19.8% | 6.7% | 9.4% | 4.5% | 5.3% | 1.1% | 5.6% | 2.8% |
| Hypercholesterolemia‡ | 53.6% | 39.6% | 49.3% | 40.6% | 17.0% | 5.8% | 12.3% | 9.8% |
| Hypertension | 57.7% | 29.1% | 42.1% | 30.8% | 43.9% | 17.4% | 26.3% | 16.0% |
| Postmenopausal | 86.0% | 84.1% | N/A | N/A | 72.5% | 59% | N/A | N/A |
| Current smoker | 31.4 | 32.0 | 17.1 | 17.7 | 60.6% | 36.4% | 58.6% | 39.0% |
| Alcohol (g/d) | 0.9 (0; 24) | 1.8 (0; 29) | 5.8 (0; 46) | 6.8 (0; 77) | 5.7 (0.7; 33) | 8.5 (1.1; 34) | 17 (2; 61) | 20 (3; 62) |
|
Plasma lipids (mg/dL)§ |
||||||||
| Triglycerides | 136.9 (63.7; 294.8) |
108.3 (50.3; 222.0) |
147.0 (66.8; 302.3) |
112.5 (60.5; 262.0) |
181.4 (87.7; 314.4) |
137.3 (93.6; 182.2) |
209.4 (93.0; 361.4) |
180.1 (83.3; 306.5) |
| Cholesterol | 235.0 (182.8; 289.9) |
223.0 (178.8;274.0) |
215.5 (165.0; 264.0) |
203.0 (163.2; 253.9) |
255.9 (203.4; 311.3) |
234.4 (187.5; 284.2) |
243.8 (193.3; 297.0) |
231.4 (185.6; 280.4) |
| HDL-C | 49.6 (34.8; 70.7) | 58.2 (40.2; 83.4) | 40.8 (29.2; 56.0) | 43.8 (31.9; 61.7) | 62.5 (45.2; 80.4) | 69.8 (50.3; 93.6) | 51.7 (39.1; 66.5) | 56.5 (41.8; 74.6) |
Medians (5th and 95th percentiles) of continuous covariates
Random sample of cohort at baseline, includes 32 participants who became cases during follow-up.
Diagnosed with hypercholesterolemia or reporting to use cholesterol-lowering medication.
Not all plasma lipids available on all subjects. Majority of NHS and HPFS participants were fasting, DCH participants were non-fasting.
To convert HDL and total cholesterol to mmol/L multiply by 0.02586. To convert triglycerides to mmol/L multiply by 0.01129.
LPL S447X and lipids
The S447X minor allele frequency was 0.12 in the US studies and 0.10 in DCH. Among controls, carriers of the S447X variant had lower levels of TG compared with non-carriers. The association was strongest in the NHS and HPFS. S447X carriers also had approximately 10 mg/dL lower TG levels among the DCH participants, albeit not statistically significant (Table 2). The S447X variant was also associated with a slightly higher HDL-C level, which was most evident among DCH men. The TG to HDL-C ratio reflected these associations.
Table 2.
Mean lipid concentrations (95% confidence intervals) of controls in the Nurses’ Health Study (NHS), the Health Professionals Follow-Up Study (HPFS), and the Diet, Cancer and Health study.*
| NHS | HPFS | DCH | ||
|---|---|---|---|---|
| Variable | Controls (n=485) | Controls (n=515) | Women, controls† (n=792) | Men, Controls† (n=907) |
| Triglycerides | ||||
| SS447 | 131.7 (123.6;139.7) | 158.0 (145.5;170.5) | 140.1 (134.2;145.9) | 182.9 (175.8;190.0) |
| S447X carriers | 109.6 (96.8;122.3) | 134.1 (118.8;149.4) | 128.6 (116.8;140.4) | 173.8 (158.6–189.0) |
| P | 0.01 | 0.02 | 0.09 | 0.29 |
| HDL-C | ||||
| SS447 | 59.7 (58.1;61.2) | 45.5 (44.4;46.5) | 69.3 (68.0;70.5) | 55.9 (55.0;56.8) |
| S447X carriers | 62.4 (59.2;65.6) | 46.1 (44.2;48.0) | 71.6 (69.1;74.1) | 58.0 (56.1;60.0) |
| P | 0.14 | 0.58 | 0.10 | 0.05 |
| Triglyceride/HDL-C | ||||
| SS447 | 2.64 (2.40;2.88) | 4.09 (3.63;4.55) | 2.26 (2.13;2.39) | 3.65 (3.47;3.82) |
| S447X carriers | 2.06 (1.69;2.44) | 3.23 (2.79;3.67) | 2.04 (1.78;2.30) | 3.28 (2.91;3.66) |
| P | 0.01 | 0.01 | 0.14 | 0.08 |
Least square means using obtained from robust regression models adjusted for smoking, age, alcohol, body mass index, and menopausal status among women. To convert HDL and total cholesterol to mmol/L multiply by 0.02586. To convert triglycerides to mmol/L multiply by 0.01129.
Majority of NHS and HPFS participants were fasting. Analyses adjusted for fasting status.
LPL genotype and risk of CHD
The S447X variant allele was more frequent among controls than among those who developed CHD. The S447X polymorphism was associated with risk of CHD in a co-dominant fashion in the three studies, although only statistically significant in the NHS (Table 3). In a pooled analysis, the adjusted RR was 0.74 (95% confidence interval, 0.56–1.00) per copy of the minor allele.
Table 3.
Relative risk [RR] and 95% confidence intervals [CI] of CHD according to Lipoprotein Lipase S447X genotype in the Nurses’ Health Study (NHS), the Health Professionals Follow Up Study (HPFS), and the Diet, Cancer and Health (DCH) study.*
| MAF (%)† | N (cases/controls) | Relative risk | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Control | SS | SX | XX | SS | SX | XX | SX/XX | |
| NHS | 7.4 | 12.1 | 211/379 | 33/100 | 1/6 | 1.0 (ref) | 0.57 (0.36–0.90) | 0.31 (0.04–2.82) | 0.56 (0.36–0.87) |
| HPFS | 8.5 | 12.0 | 216/406 | 40/99 | 2/10 | 1.0 (ref) | 0.73 (0.49–1.10) | 0.37 (0.08–1.69) | 0.70 (0.47–1.04) |
| DCH women | 8.1 | 10.5 | 193/638 | 35/142 | 1/12 | 1.0 (ref) | 1.02 (0.65–1.61) | 0.38 (0.05–2.98) | 0.97 (0.62–1.52) |
| DCH men | 8.9 | 9.2 | 606/745 | 123/157 | 4/5 | 1.0 (ref) | 0.89 (0.67–1.18) | 1.14 (0.29–4.45) | 0.89 (0.67–1.18) |
| Pooled‡ | 1.0 (ref) | 0.77 (0.58–1.03) | 0.53 (0.29–0.97) | 0.74 (0.56–1.00) | |||||
Conditional logistic regression models were run in NHS and HPFS data (stratified by matching factors). Cox proportional hazard regression models in DCH. All models adjusted for age, smoking, alcohol intake, body mass index, and menopausal status among women.
Minor allele frequency
Meta-analysis using random effects. P for tests of between study heterogeneity: SX: 0.7; XX: 0.13; dominant (SX/XX): 0.12.
Because use of lipid-lowering drugs, especially among cases, may obscure our findings, we repeated our analyses in samples restricted to participants who did not report use of such drugs. Although a sizable number of the cases were excluded, we found that the associations between the S447X variant and risk of CHD were stronger in all three studies (NHS: 0.55, 0.34–0.90; HPFS: 0.65, 0.44–0.96; DCH women: 0.88, 0.56–1.30; DHC men: 0.83, 0.62–1.11). The pooled estimate after these exclusions was 0.75 (0.62–0.90).
Exploratory subgroup analyses
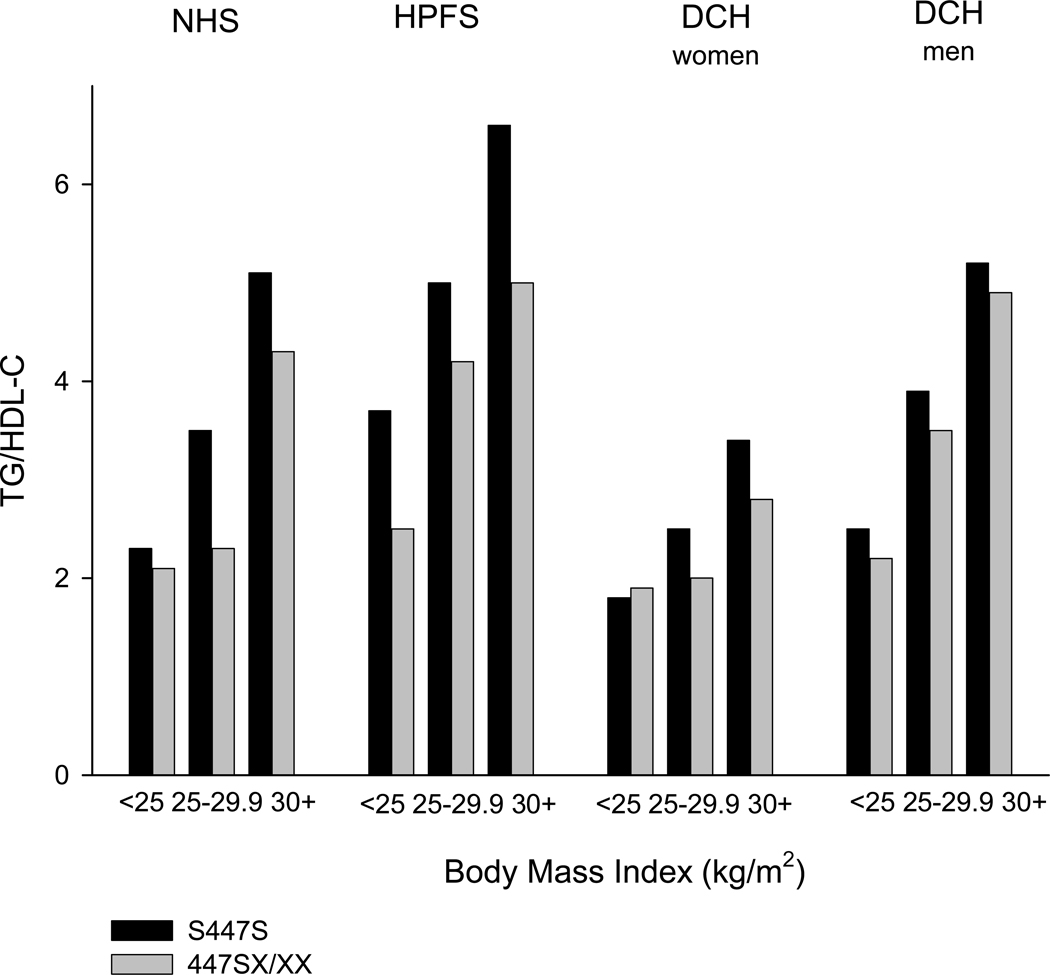
We did not observe any modification of the association between S447X genotype and plasma lipids, by smoking, alcohol, or sex (data not shown). However, there was some evidence that the S447X variant had the most pronounced association with TG and HDL-C concentrations among the overweight and obese individuals. The mean TG to HDL-C ratio is shown according to S447X genotype and BMI in figure 1. In the NHS and DCH women, differences in the TG to HDL-C ratio were largest among the overweight and obese individuals, whereas differences between genotype groups were smaller in magnitude across BMI groups in the HPFS and DCH men. Similar results were observed when waist circumference was used (data not shown). However, tests of interaction between the S447X variant and BMI or waist circumference were not statistically significant in any of the studies.
Figure.
Mean triglyceride to HDL-C ratio according to body mass index and S447X genotype in the NHS, HPFS and DCH (separated for women and men) study populations.
Black bar: SS447 homozygotes. Light grey bar: 447X carriers (SX and XX).
The suggestive evidence for a stronger effect of the S447X variant among the overweight and obese women was also reflected in analyses of CHD risk in the NHS. The S447X variant was not associated with CHD among NHS participants who were normal weight (p=0.5), but the association was stronger in the overweight (p=0.02) and strongest in the obese (p<0.01) (p for interaction= 0.01; data not shown). An analysis using waist circumference showed a similar pattern (p for interaction=0.01). Although the S447X variant had a stronger association with CHD among obese HPFS men, the confidence intervals were broad and the interaction was not statistically significant. There was no interaction between BMI or waist circumference on risk of CHD in the Danish population.
Discussion
In three independent studies, we found that carriers of the LPL S447X variant tended to have lower TG and higher HDL-C levels compared to SS447 homozygotes. Concomitantly, the risk of CHD was lower among carriers.
The association between the S447X variant and elevated TG and lower HDL-C has been shown in several studies,8–14 and this association is supported by animal data showing that the S447X variant produces a LPL protein with higher lipolytic function.7 Although epidemiologic data on the risk of CHD associated with the S447X variant have not been completely consistent,12–14, 16–21 the lower risk of CHD we observed among S447X carriers was recently supported by a comprehensive candidate-gene association study of 142 loci where only two variants that are tightly linked with the S447X SNP were consistently associated with coronary artery disease in two case-control studies.37
Although low-density lipoprotein cholesterol has been the main therapeutic target for high cardiovascular risk patients, renewed attention has focused on the potential risk reduction that may be achieved by HDL-C manipulation.38 However, trials of promising HDL-C-increasing drugs such as CETP inhibitors have been consistently negative to date.39, 40 Given this, other targets have become more attractive, and indeed clinical trials targeting LPL with specific activators (NO-1886) or with gene therapy using the S447X variant are planned.41, 42 Studies of genetic variants may provide important insight into the expected effects of pharmacological manipulation of HDL's complex metabolism. For example, studies of genetic CETP variants showed several of the adverse cardiovascular effects – including hypertension - that were ultimately seen in clinical trials, and indeed these genetic studies were rather mixed with respect to risk of CHD despite positive effects on HDL-C levels.43–45 Thus, we believe it crucial to examine the S447X variant in LPL in well-designed prospective studies of healthy population samples prior to use of LPL activators in large-scale trials. In addition, exploratory subgroup analyses may give hints to the most appropriate study population for trials of LPL activators on the basis of maximal sensitivity to its potential benefits. Such information may prove critical for the design and success of clinical trials.
We found some evidence that the association of S447X with lipid concentrations and CHD risk might be greatest among overweight and obese individuals. Differences in TG and HDL-C concentrations between carriers and non-carriers of the S447X variant, and the closely linked HindIII SNP, have previously been reported to be greater in participants characterized by central or general obesity.10, 11, 24, 25 However, there has been relatively little research on risk of clinical endpoints in potentially susceptible subgroups of the general population. An increased efflux of non-esterified fatty acids from the adipose tissue to the liver among obese individuals and the accompanying increase in the secretion of VLDL may be one of the main forces driving the atherosclerotic effects of obesity. Thus, it is possible that the S447X variant could be of greater importance among individuals where the normal lipid transport system is under stress to maintain normal lipid levels.
One of the strengths of this analysis was the ability to address the role of the same variant in three independent studies of generally healthy populations. However, the populations were derived from different countries with some marked differences in lifestyle and levels of cardiovascular risk factors. A slightly higher baseline risk in the DCH study could dilute the relative risk of CHD associated with S447X. It remains possible that other differences between study populations could explain a greater influence of this LPL variant in the US cohorts.
Our study is limited by the lack of a direct measure of LPL activity. However, the functional effects of the S447X variant are well studied and higher LPL activity in carriers has been reported in other studies.6 In addition, the S447X variant is located in the C-terminal region of the protein that is responsible for the binding between endothelial cell surface receptors and lipoproteins.46, 47 Thus it is possible this variant may play a role in the non-catalytic bridging function of the LPL protein.5
The low number of cases in the three studies may have limited our power to detect interaction effects. Because we examined multiple subgroups, the potential interaction with BMI should be interpreted with caution. Larger studies are needed to fully elucidate whether specific environmental factors modify the significance of the LPL SNP.
In conclusion, our data provide some support for associations of the S447X SNP in the LPL gene with lower TG levels, small elevations in HDL-C levels, and a modestly reduced risk of CHD. This makes LPL an attractive target for drug developments, although further insights into the molecular mechanism behind the associations are needed.
Acknowledgements
We thank Hardeep Ranu and Pati Soule from the DF/HCC Genotyping Core and Anne-Karin Jensen for genotyping and data management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 2.Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 3.Preiss-Landl K, Zimmermann R, Hammerle G, Zechner R. Lipoprotein lipase: the regulation of tissue specific expression and its role in lipid and energy metabolism. Curr Opin Lipidol. 2002;13:471–481. doi: 10.1097/00041433-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rip J, Nierman MC, Wareham NJ, et al. Serum lipoprotein lipase concentration and risk for future coronary artery disease: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol. 2006;26:637–642. doi: 10.1161/01.ATV.0000201038.47949.56. [DOI] [PubMed] [Google Scholar]
- 5.Murthy V, Julien P, Gagne C. Molecular pathobiology of the human lipoprotein lipase gene. Pharmacol Ther. 1996;70:101–135. doi: 10.1016/0163-7258(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Rip J, Nierman MC, Ross CJ, et al. Lipoprotein lipase S447X: a naturally occurring gain-of-function mutation. Arterioscler Thromb Vasc Biol. 2006;26:1236–1245. doi: 10.1161/01.ATV.0000219283.10832.43. [DOI] [PubMed] [Google Scholar]
- 7.Ross CJ, Liu G, Kuivenhoven JA, et al. Complete rescue of lipoprotein lipase-deficient mice by somatic gene transfer of the naturally occurring LPLS447X beneficial mutation. Arterioscler Thromb Vasc Biol. 2005;25:2143–2150. doi: 10.1161/01.ATV.0000176971.27302.b0. [DOI] [PubMed] [Google Scholar]
- 8.Groenemeijer BE, Hallman MD, Reymer PW, et al. Genetic variant showing a positive interaction with beta-blocking agents with a beneficial influence on lipoprotein lipase activity, HDL cholesterol, and triglyceride levels in coronary artery disease patients. The Ser447-stop substitution in the lipoprotein lipase gene. REGRESS Study Group. Circulation. 1997;95:2628–2635. doi: 10.1161/01.cir.95.12.2628. [DOI] [PubMed] [Google Scholar]
- 9.Wittrup HH, Tybjaerg-Hansen A, Steffensen R, et al. Mutations in the lipoprotein lipase gene associated with ischemic heart disease in men. The Copenhagen city heart study. Arterioscler Thromb Vasc Biol. 1999;19:1535–1540. doi: 10.1161/01.atv.19.6.1535. [DOI] [PubMed] [Google Scholar]
- 10.Garenc C, Perusse L, Gagnon J, et al. Linkage and association studies of the lipoprotein lipase gene with postheparin plasma lipase activities, body fat, and plasma lipid and lipoprotein concentrations: the HERITAGE Family Study. Metabolism. 2000;49:432–439. doi: 10.1016/s0026-0495(00)80004-9. [DOI] [PubMed] [Google Scholar]
- 11.Huang AQ, Hu YH, Zhan SY, et al. Lipoprotein lipase gene S447X polymorphism modulates the relation between central obesity and serum lipids, a twin study. Int J Obes (Lond) 2006;30:1693–1701. doi: 10.1038/sj.ijo.0803332. [DOI] [PubMed] [Google Scholar]
- 12.Gagne SE, Larson MG, Pimstone SN, et al. A common truncation variant of lipoprotein lipase (Ser447X) confers protection against coronary heart disease: the Framingham Offspring Study. Clin Genet. 1999;55:450–454. doi: 10.1034/j.1399-0004.1999.550609.x. [DOI] [PubMed] [Google Scholar]
- 13.Jemaa R, Fumeron F, Poirier O, et al. Lipoprotein lipase gene polymorphisms: associations with myocardial infarction and lipoprotein levels, the ECTIM study. Etude Cas Temoin sur l'Infarctus du Myocarde. J Lipid Res. 1995;36:2141–2146. [PubMed] [Google Scholar]
- 14.Sing K, Ballantyne CM, Ferlic L, et al. Lipoprotein lipase gene mutations, plasma lipid levels, progression/regression of coronary atherosclerosis, response to therapy, and future clinical events. Lipoproteins and Coronary Atherosclerosis Study. Atherosclerosis. 1999;144:435–442. doi: 10.1016/s0021-9150(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 15.Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet. 2008;40:189–197. doi: 10.1038/ng.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wittrup HH, Nordestgaard BG, Steffensen R, et al. Effect of gender on phenotypic expression of the S447X mutation in LPL: the Copenhagen City Heart Study. Atherosclerosis. 2002;165:119–126. doi: 10.1016/s0021-9150(02)00183-1. [DOI] [PubMed] [Google Scholar]
- 17.Clee SM, Loubser O, Collins J, et al. The LPL S447X cSNP is associated with decreased blood pressure and plasma triglycerides, and reduced risk of coronary artery disease. Clin Genet. 2001;60:293–300. doi: 10.1034/j.1399-0004.2001.600407.x. [DOI] [PubMed] [Google Scholar]
- 18.Arca M, Campagna F, Montali A, et al. The common mutations in the lipoprotein lipase gene in Italy: effects on plasma lipids and angiographically assessed coronary atherosclerosis. Clin Genet. 2000;58:369–374. doi: 10.1034/j.1399-0004.2000.580507.x. [DOI] [PubMed] [Google Scholar]
- 19.Brousseau ME, Goldkamp AL, Collins D, et al. Polymorphisms in the gene encoding lipoprotein lipase in men with low HDL-C and coronary heart disease: the Veterans Affairs HDL Intervention Trial. J Lipid Res. 2004;45:1885–1891. doi: 10.1194/jlr.M400152-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Srinivasan SR, Elkasabany A, et al. Influence of lipoprotein lipase serine 447 stop polymorphism on tracking of triglycerides and HDL cholesterol from childhood to adulthood and familial risk of coronary artery disease: the Bogalusa heart study. Atherosclerosis. 2001;159:367–373. doi: 10.1016/s0021-9150(01)00508-1. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Ruiz-Narvaez E, Niu T, et al. Genetic variants of the lipoprotein lipase gene and myocardial infarction in the Central Valley of Costa Rica. J Lipid Res. 2004;45:2106–2109. doi: 10.1194/jlr.M400202-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Tan CS, Chia KS, Tan CE, et al. The lipoprotein lipase S447X polymorphism and plasma lipids: interactions with APOE polymorphisms, smoking, and alcohol consumption. J Lipid Res. 2004;45:1132–1139. doi: 10.1194/jlr.M400016-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Komurcu-Bayrak E, Onat A, Poda M, et al. The S447X variant of lipoprotein lipase gene is associated with metabolic syndrome and lipid levels among Turks. Clin Chim Acta. 2007;383:110–115. doi: 10.1016/j.cca.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Senti M, Bosch M, Aubo C, et al. Relationship of abdominal adiposity and dyslipemic status in women with a common mutation in the lipoprotein lipase gene. The REGICOR investigators. Atherosclerosis. 2000;150:135–141. doi: 10.1016/s0021-9150(99)00355-x. [DOI] [PubMed] [Google Scholar]
- 25.Vohl MC, Lamarche B, Moorjani S, et al. The lipoprotein lipase HindIII polymorphism modulates plasma triglyceride levels in visceral obesity. Arterioscler Thromb Vasc Biol. 1995;15:714–720. doi: 10.1161/01.atv.15.5.714. [DOI] [PubMed] [Google Scholar]
- 26.Humphries SE, Nicaud V, Margalef J, et al. Lipoprotein lipase gene variation is associated with a paternal history of premature coronary artery disease and fasting and postprandial plasma triglycerides: the European Atherosclerosis Research Study (EARS) Arterioscler Thromb Vasc Biol. 1998;18:526–534. doi: 10.1161/01.atv.18.4.526. [DOI] [PubMed] [Google Scholar]
- 27.Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6:49–62. doi: 10.1089/jwh.1997.6.49. [DOI] [PubMed] [Google Scholar]
- 28.Rimm EB, Giovannucci EL, Stampfer MJ, et al. Reproducibility and validity of a expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 29.Tjonneland A, Olsen A, Boll K, et al. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007;35:432–441. doi: 10.1080/14034940601047986. [DOI] [PubMed] [Google Scholar]
- 30.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 31.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika. 1978;65:153–158. [Google Scholar]
- 32.Jensen MK, Chiuve SE, Rimm EB, et al. Obesity, behavioral lifestyle factors, and risk of acute coronary events. Circulation. 2008;117:3062–3069. doi: 10.1161/CIRCULATIONAHA.107.759951. [DOI] [PubMed] [Google Scholar]
- 33.Pai JK, Curhan GC, Cannuscio CC, et al. Stability of novel plasma markers associated with cardiovascular disease: processing within 36 hours of specimen collection. Clin Chem. 2002;48:1781–1784. [PubMed] [Google Scholar]
- 34.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–12. [Google Scholar]
- 35.Petersen L, Sorensen TI, Andersen PK. Comparison of case-cohort estimators based on data on premature death of adult adoptees. Stat Med. 2003;22:3795–3803. doi: 10.1002/sim.1672. [DOI] [PubMed] [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 37.Samani NJ, Erdmann J, Hall AS, et al. Genomewide association analysis of coronary artery disease. N Engl J Med. 2007;357:443–453. doi: 10.1056/NEJMoa072366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Superko HR, King S., III Lipid management to reduce cardiovascular risk: a new strategy is required. Circulation. 2008;117:560–568. doi: 10.1161/CIRCULATIONAHA.106.667428. [DOI] [PubMed] [Google Scholar]
- 39.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 40.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 41.Tsutsumi K. Lipoprotein lipase and atherosclerosis. Curr Vasc Pharmacol. 2003;1:11–17. doi: 10.2174/1570161033386673. [DOI] [PubMed] [Google Scholar]
- 42.Rip J, Nierman MC, Sierts JA, Petersen W, et al. Gene therapy for lipoprotein lipase deficiency: working toward clinical application. Hum Gene Ther. 2005;16:1276–1286. doi: 10.1089/hum.2005.16.1276. [DOI] [PubMed] [Google Scholar]
- 43.Borggreve SE, Hillege HL, Wolffenbuttel BH, et al. An increased coronary risk is paradoxically associated with common cholesteryl ester transfer protein gene variations that relate to higher high-density lipoprotein cholesterol: a population-based study. J Clin Endocrinol Metab. 2006;91:3382–3388. doi: 10.1210/jc.2005-2322. [DOI] [PubMed] [Google Scholar]
- 44.Boekholdt SM, Sacks FM, Jukema JW, et al. Cholesteryl ester transfer protein TaqIB variant, high-density lipoprotein cholesterol levels, cardiovascular risk, and efficacy of pravastatin treatment: individual patient meta-analysis of 13,677 subjects. Circulation. 2005;111:278–287. doi: 10.1161/01.CIR.0000153341.46271.40. [DOI] [PubMed] [Google Scholar]
- 45.Keavney B, Palmer A, Parish S, et al. Lipid-related genes and myocardial infarction in 4685 cases and 3460 controls: discrepancies between genotype, blood lipid concentrations, and coronary disease risk. Int J Epidemiol. 2004;33:1002–1013. doi: 10.1093/ije/dyh275. [DOI] [PubMed] [Google Scholar]
- 46.Merkel M, Kako Y, Radner H, et al. Catalytically inactive lipoprotein lipase expression in muscle of transgenic mice increases very low density lipoprotein uptake: direct evidence that lipoprotein lipase bridging occurs in vivo. Proc Natl Acad Sci U S A. 1998;95:13841–13846. doi: 10.1073/pnas.95.23.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clee SM, Bissada N, Miao F, et al. Plasma and vessel wall lipoprotein lipase have different roles in atherosclerosis. J Lipid Res. 2000;41:521–531. [PubMed] [Google Scholar]