Abstract
Turner syndrome (TS) is a genetic disorder affecting females, resulting from the complete or partial absence of an X chromosome. The cognitive profile of TS shows relative strengths in the verbal domain and weaknesses in the procedural domain, including working memory. Neuroimaging studies have identified differences in the morphology of the parietal lobes, and white matter pathways linking frontal and parietal regions, as well as abnormal activation in dorsal frontal and parietal regions. Taken together these findings suggest that abnormal functional connectivity between frontal and parietal regions may be related to working memory impairments in TS, a hypothesis we tested in the present study. We scanned TS and typically developing participants with functional magnetic resonance imaging while they performed visuospatial and phonological working memory tasks. We generated a seed region in parietal cortex based on structural differences in TS and found that functional connectivity with dorsal frontal regions was reduced during working memory in TS. Finally, we found that connectivity was correlated with task performance in TS. These findings suggest that structural brain abnormalities in TS affect not only regional activity but also the functional interactions between regions and that this has important consequences for behavior.
Keywords: fMRI, functional connectivity, parietal cortex, working memory, turner syndrome
Introduction
Turner syndrome (TS) is a genetic disorder affecting females, resulting from the complete or partial absence of an X chromosome and occurs in approximately 1 in 1500–2000 live female births (Lippe 1991; Stochholm et al. 2006). Individuals with TS tend to have relative difficulties in visuospatial (Rovet and Netley 1980), arithmetic (Molko et al. 2003; Kesler et al. 2006), motor (Nijhuis-van der Sanden et al. 2003), social (McCauley et al. 1987; Mazzola et al. 2006), and executive function skills (Waber 1979; Pennington et al. 1985; Bender et al. 1993; Murphy et al. 1994). Despite this range of cognitive difficulties, language skills are typically in the normal range (Murphy 2009), and one hallmark of TS is a large discrepancy between verbal and performance IQ (Garron 1977; Murphy et al. 1993; Rovet 1993; Ross, Roeltgen, et al. 2000; Ross, Zinn, et al. 2000). Therefore, as a relatively common genetic disorder with a well-characterized behavioral phenotype, TS offers a unique opportunity to study the effects of X-chromosome genes on female cognitive and brain development.
Cognitive deficits in TS have been most prominently identified in tests of performance IQ, that is, tasks requiring visuomotor and visuospatial skills, perceptual organization, and fluid reasoning (for a recent review, see Hong et al. 2009). In particular, visuospatial working memory has been cited as a core deficit in TS (Buchanan et al. 1998). Working memory impairments in TS have been linked with regional activation differences relative to typically developing (TD) participants. Haberecht et al. (2001) found that as memory load increased, TS subjects showed reduced activity in inferior and middle frontal gyri (IFG, MFG), the caudate, and the supramarginal gyrus (SMG) in the parietal lobe relative to TD participants, suggesting abnormal frontostriatal and frontoparietal circuits in TS. Hart et al. (2006) used a slow event-related design to compare visuospatial and verbal working memory across encoding, maintenance, and retrieval phases; they performed region of interest analyses on IFG, MFG, intraparietal sulcus (IPS), and inferior temporal gyrus (ITG) and found that TS participants had significantly reduced activity in frontal and parietal regions, during the maintenance phase of the visuospatial working memory task, relative to TD participants. Functional interactions between frontal and parietal regions have been shown to be important for maintenance of information in working memory (Honey et al. 2002). However, it remains unknown whether weakened frontoparietal interactions are related to working memory impairments in TS.
In addition, functional imaging studies have found that during visuospatial (Kesler et al. 2004), executive function (Haberecht et al. 2001; Tamm et al. 2003; Hart et al. 2006), and arithmetic tasks (Molko et al. 2003; Kesler et al. 2006), TS subjects show abnormal activation profiles in frontal and parietal cortices and subcortical regions, such as the caudate. In parallel, morphometric studies have found abnormalities in bilateral parietal lobes and parietooccipital regions (Murphy et al. 1993; Reiss et al. 1995; Molko et al. 2003; Brown et al. 2004), as well as increased volume of superior temporal gyrus (Kesler et al. 2003), amygdala, and orbitofrontal cortex (Good et al. 2003). White matter studies in TS have found structural differences in the superior temporal sulci, orbitofrontal cortex, right IPS, and reduced fractional anisotropy in fibers connecting anterior to posterior temporal lobes (Molko et al. 2004). Holzapfel et al. (2006) identified a potential locus for abnormal frontoparietal interactions, by showing reduced fractional anisotropy in parietooccipital areas, extending along the superior longitudinal fasciculus to the deep white matter of the frontal lobes.
In the typical population, frontal and parietal cortices are engaged by a wide range of attentionally demanding tasks and are consistently identified as nodes in a “frontoparietal attention” or task-positive network (TPN) (Fox et al. 2005; Toro et al. 2008). Activity in this network is anticorrelated with networks more active during rest (e.g., the default mode network or DMN) (Fox et al. 2005). Working memory tasks reliably engage regions of the TPN, including dorsolateral prefrontal cortex (DLPFC), IFG, and posterior parietal cortex (for a review, see Wager and Smith 2003). Functional interactions within both the TPN (Honey et al. 2002) and the DMN (Hampson et al. 2006) have been shown to be important for effective maintenance of information in working memory.
In TS, while frontal and parietal regions show abnormal activation profiles (Haberecht et al. 2001; Hart et al. 2006), and frontoparietal structural connections are weakened (Holzapfel et al. 2006), no study to our knowledge has investigated functional interactions between these regions during cognitive processing in TS. In the present study, we scanned TS and TD participants with structural and functional magnetic resonance imaging (sMRI and fMRI) while they performed N-back working memory tasks in visuospatial and phonological modalities and under varying load (1-back and 2-back). We used a voxel-wise volumetric analysis to define a potential locus for functional connectivity differences. We hypothesized that in TS, structurally abnormal parietal regions might show weakened white matter connections with frontal regions, which would be reflected by weakened frontoparietal functional connectivity. Additionally, we hypothesized that reduced connectivity would predict working memory performance. To test this, we performed an additional analysis on a larger cohort of TS participants who performed only the visuospatial working memory task, in which we correlated performance with measures of activation, connectivity and structure.
Materials and Methods
Participants
Participants in this study ranged from 7 to 13 years of age and were recruited as part of a longitudinal study on brain development in TS. TS girls were recruited from chapters of the TS Society of America and the TS Society of Canada and from university- and community-based pediatric endocrinologists. TD participants were recruited as siblings of TS participants and locally through parent organizations and local advertisements in the Palo Alto, CA, area. Thirty-seven TS and 18 TD participants, aged 7–13, performed the visuospatial working memory task and 26 TS and 17 TD participants performed the phonological working memory task. Participants who performed below 75% on the control condition during the visuospatial working memory task, or whose scans were contaminated with excessive motion or scanner artifact, were excluded from our analysis. Two TS participants were eliminated for benign structural abnormalities near the cerebellum, which impeded normalization to the group template. We included 14 TS (10 45,X (TS-monosomic) and 4 with a mosaic karyotype (TS-mosaic)) and 12 TD participants, in our analysis combining visuospatial and phonological tasks. Mean age for TS participants was 10.6 (standard deviation [SD] 1.6) and for TD participants 10.6 (SD 1.5). In order to correlate measures of connectivity with behavior, we performed a second analysis on a larger group of participants who performed only the visuospatial working memory task (this group included the participants in the first analysis); this second cohort included 30 TS (20 TS-monosomic; 10 TS-mosaic) and 15 TD participants. In this second cohort, the mean age for TS participants was 10.7 (SD 2.2) and for TD participants 10.5 (SD 1.6). Among the 20 TS-monosomic participants included in this study, 6 had an X chromosome of paternal origin. Around the age of puberty, girls with TS typically begin estrogen therapy (Bondy 2007); in our sample, 3 TS girls were confirmed to have started estrogen therapy, and information was not collected from 4 participants.
Study Protocol
Study participation required 2 days of testing and MRI scans at Stanford University. Participants from outside the San Francisco Bay area were flown in and accommodations were arranged at a local hotel. All TS participants underwent a standard karyotype assessment to confirm diagnosis. Prior to MRI scanning, all participants underwent a “mock scan session” in an MRI simulator, to familiarize themselves with the scanner environment, and to rehearse staying still during scanning. Study participants underwent 2 scan sessions, each up to 1.5 h, on consecutive days. On the first day, sMRI and DTI scans were collected, as well as functional tasks, presented in pseudorandom order. On the second day, participants performed additional functional tasks and repeated any scans that were inadequately collected on the first day. Among the functional tasks were the visuospatial and phonological working memory tasks described here, in addition to other tasks not reported here. Pubertal status was not explicitly assessed in either the TS or the TD groups. In addition to fMRI and sMRI scans, participants received a cognitive and neuropsychological test battery. Informed consent was obtained from a family member and informed assent was obtained from all participants. The human subjects committee at Stanford University School of Medicine approved the protocols used in this study.
Cognitive and Neuropsychological Assessments
In order to compare our participant groups on standardized measures of working memory and visuospatial processing, we administered the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) (Wechsler 2003) and the developmental neuropsychological assessment (NEPSY) (Korkman et al. 1998) to all study participants. In addition to full-scale IQ (FSIQ) (Wechsler 2003), we report index scores from the WISC-IV for the verbal comprehension, perceptual reasoning, working memory, and processing speed indexes. We also report the visuospatial domain score from the NEPSY. Scores from neurocognitive tests were compared between groups using independent samples t-tests.
MRI Data Acquisition
MRI was performed on a 3.0 T GE whole-body scanner (GE Healthcare Systems). High-resolution structural scans were acquired using a spoiled GRASS sequence (124 slices, 1-mm2 in-plane and 1.5-mm through-plane resolution, flip angle = 15°, field of view [FOV] = 22 cm), facilitating subsequent localization and coregistration of functional data. A T2*-sensitive gradient echo spiral-in/out pulse sequence (Glover and Law 2001) was used for functional imaging (time repetition [TR] = 2000 ms, time echo = 30 ms, flip angle = 80°, matrix 64 × 64, FOV = 22 cm). Thirty oblique axial slices were obtained parallel to the AC–PC with 4-mm slice thickness, 1-mm skip. A high-order shimming procedure was used to reduce B0 heterogeneity prior to the functional scans (Kim et al. 2002).
fMRI Tasks
During fMRI tasks, visual stimuli were controlled using ePrime software on a PC and presented to participants in the scanner using a projector positioned at the front of the room; the image reflected off a mirror attached to the fMRI head coil. The order of visuospatial and phonological working memory tasks was randomly assigned to each participant. All participants learned the tasks and practiced outside the scanner to ensure that they understood the task instructions.
Visuospatial Working Memory
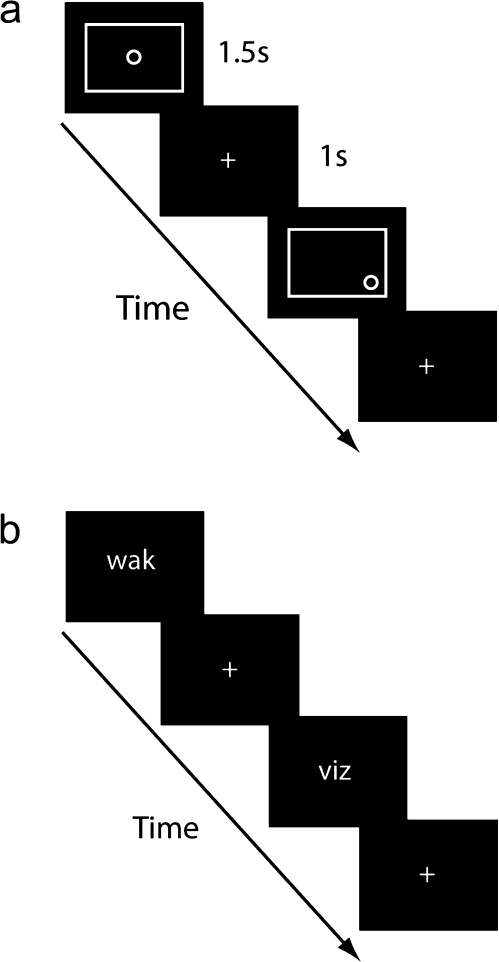
During the visuospatial working memory task (Fig. 1a), a large white rectangle appeared, centered, on the screen. On each trial a small “O” appeared in 1 of 9 positions within the rectangle: 4 corners, middle of the 4 edges, or in the center. The O remained on the screen for 1.5 s, followed by a fixation cross for 1 s. During the 1-back blocks (1B), participants responded by pressing a button with their index finger if the O appeared in the same position on 2 consecutive trials and during 2-back blocks (2B), if the O appeared in the same position 2 trials previously. During control blocks (C), subjects pressed the button any time the O appeared in the center of the rectangle. Blocks consisted of an instruction screen presented for 2 s, followed by 14 trials, approximately 25% of which were targets, for a duration of 35 s. Blocks were presented, including 30 s rest blocks (R), in the following order: R-1B-C-1B-C-1B-C-R-2B-C-2B-C-2B-C-R.
Figure 1.
Task. Subjects performed visuospatial (a) and phonological (b) working memory tasks in the scanner. Both tasks consisted of rest (R), 1-back (1B), 2-back (2B), and match-control (C) blocks, in the following order (R-1B-C-1B-C-1B-C-R-2B-C-2B-C-2B-C-R). Each block consisted of 14 trials, approximately 25% of which were targets. Subjects responded according to the block, which was cued by an instruction screen presented for 2 s at the start of each block. (a) During the visuospatial working memory task, on each trial subjects saw the stimulus (a white circle inside a white rectangle, on a black background) for 1.5 s, followed by a fixation cross for 1 s. The participants pressed the button if the cue was in the center (C), same position as the previous trial (1B), or same position as 2 trials previously (2B). (b) During the phonological working memory task, on each trial participants saw a 3-letter nonword syllable at the center of the screen for 1.5 s, followed by a fixation cross for 1 s. During the match-control block, 2 syllables were presented, one above the other. The participants pressed the button if the 2 syllables rhymed (C), rhymed with the syllable shown on the previous trial (1B), or rhymed with the syllable shown 2 trials previously (2B).
Phonological Working Memory
During the phonological working memory task (Fig. 1b), 3-letter nonword syllables were presented at the center of the screen (e.g., wak, suz, vit). The syllable remained on the screen for 1.5 s, followed by a fixation cross for 1 s. During the 1-back blocks (1B), subjects were asked to judge whether the syllable currently presented rhymed with the previously presented symbol; rhyming could be determined based on the last 2 letters of the syllable. During the 2-back blocks (2B), subjects were asked to respond if the currently presented syllable rhymed with the syllable presented 2 trials previously. During the control blocks (C), 2 syllables were presented at the same time, and subjects were asked to judge whether they rhymed. Timing and block order were identical to the visuospatial task.
Behavioral Data Analysis
Accuracy measures from the working memory tasks were entered into a repeated measures analysis of variance (ANOVA), with modality (visuospatial vs. phonological) and working memory load as within subject factors, diagnosis as a between subjects factor, and age as a centered covariate.
Voxel-Based Morphometry Analysis
Studies of brain structure in TS have consistently identified differences in parietal lobe morphology. As Molko et al. (2003) identified a prominent difference in the morphology of the right IPS (rIPS), we performed a voxel-based morphometry (VBM) analysis in order to identify a locus of structural differences in the rIPS in our group of TS participants. This analysis included the 30 TS and 15 TD participants for whom usable anatomical and functional MRI data were available. Images were processed using Statistical Parametric Mapping software (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) software in Matlab (Mathworks). Each image was manually aligned onto the axis of the anterior and posterior commissures. Images were initially segmented into gray matter, white matter and cerebrospinal fluid using unified segmentation (New Segment) as implemented in SPM8 (Ashburner and Friston 2005). A custom gray matter template was then generated for the entire group using the Diffeomorphic Anatomical Registration Through Lie Algebra toolbox (Ashburner 2007; DARTEL, Wellcome Department of Imaging Neuroscience, University College London; www.fil.ion.ucl.ac.uk/spm). Images were then warped into Montreal Neurological Institute (MNI) space, with modulation to preserve local concentrations and resliced to 1.5 mm cubic voxels. Finally, images were spatially smoothed using an 8 mm full-width at half-maximum (FWHM) Gaussian kernel.
We tested for group differences in local gray matter volume by entering processed gray matter images into a 2-sample t-test, including total gray matter as a covariate of no interest. Inferences were drawn at a cluster-level threshold family wise error (FWE) corrected for multiple comparisons (P < 0.05) over the whole brain, with a height threshold of P < 0.001 uncorrected. This analysis identified several significant gray matter volume differences (see Results and Table 2), including bilaterally along the IPS. We used the MarsBaR toolbox (Brett et al. 2002) to generate a volume of interest (VOI) from the rIPS (at a height threshold of P < 0.001 uncorrected; this cluster also survived our cluster-level threshold), in which the TS group showed reduced volume relative to the TD group; this VOI was used for subsequent functional connectivity analyses.
Table 2.
Group differences in regional volumes
| Region | BA | X | Y | Z | Equiv Z | Cluster size |
| TD > TS | ||||||
| Cuneus | 31 | −6 | −76 | 24 | 6.15 | 3687 |
| Left middle occipital gyrus | 19 | 36 | −82 | 0 | 5.37 | 328 |
| Left IPS | 7/40 | −32 | −63 | 46 | 5.21 | 4705 |
| Right IPS | 40 | 30 | −45 | 43 | 4.69 | 806 |
| Right lingual gyrus | 18 | 22 | −81 | −8 | 4.33 | 313 |
| TS > TD | ||||||
| Right hippocampus | 33 | −4 | −29 | 5.94 | 1384 | |
| Left hippocampus | −38 | −21 | −17 | 5.43 | 699 | |
| Left posterior insula | 13 | −38 | −15 | −9 | 4.87 | 348 |
| Left cerebellar tonsil | −12 | −55 | −47 | 4.7 | 558 | |
| Right cerebellar tonsil | 10 | −57 | −42 | 4.65 | 408 | |
Note: Clusters surviving an FWE-corrected threshold of P < 0.05 over the whole brain, with a height threshold of P < 0.001.
fMRI Preprocessing
Functional images were preprocessed using SPM8 software. Images were corrected for slice timing, realigned to the third scan in each functional series, coregistered to the corresponding structural scan, and resliced to 1.5 mm cubic voxels. We used ArtRepair software (http://cibsr.stanford.edu/tools/ArtRepair/ArtRepair.htm) to improve signal quality losses due to subject motion and data artifacts (Mazaika et al. 2009). Image time series were smoothed with a 4 mm FWHM Gaussian kernel, detrended using a high-pass filter, and then corrected for interpolation errors introduced during realignment, using trigonometric form adjustment similar to Grootoonk et al. (2000). Artifacts indicated by rapid scan-to-scan motion greater than 0.5 mm/TR or global signal intensity fluctuations greater than 1.5% were repaired using interpolation between the nearest unrepaired scans. Participants were removed from further analysis if more than 25% of scans required repairs. Flow fields from the DARTEL template generation were used to normalize the functional images to MNI space. Finally, the functional scans were smoothed with a 7 mm FWHM Gaussian.
fMRI Analysis
For each subject, we constructed a general linear model with regressors for the 1-back, 2-back, and 2 control conditions (though stimuli in the 2 control conditions were identical, we modeled blocks interleaved with 1-back or 2-back separately), for both modalities, motion parameters estimated during realignment, and a constant for each session. The task regressors were modeled as 35 s boxcar functions convolved with the hemodynamic response function, and a 128 s high-pass filter was applied. For each subject, we calculated separate contrasts for 1-back versus control and 2-back versus control, within the visuospatial and phonological modalities, resulting in 4 contrast images per subject. We then entered these contrast images into a factorial model at the second level, with task as a within subjects factor (4 levels), diagnosis as a between subjects factor, and a factor to account for repeated measures within subjects. Group contrasts were calculated to compare effects of diagnosis on working memory overall and interactions between diagnosis and working memory load. In a second model, we also included accuracy as a covariate, to control for effects of performance on regional activity. Inferences were drawn at a cluster-level threshold FWE corrected for multiple comparisons (P < 0.05) over the whole brain, or VOI as specified, with a height threshold of P < 0.001 uncorrected.
Functional Connectivity Analysis
We assessed whether interregional interactions contributed to working memory difficulties in TS, by testing whether functional connectivity between parietal and frontal regions was weaker during working memory tasks, in TS participants relative to TD. Specifically, we were interested in assessing the impact of abnormal parietal lobe morphology (Molko et al. 2003; Brown et al. 2004) on frontoparietal interactions. The VBM analysis we performed confirmed reduced gray matter volume in our TS cohort relative to controls, bilaterally along the IPS. We identified a cluster in the rIPS in the TD > TS VBM contrast, at a height threshold of P < 0.001 and used this as our seed region in a psychophysiological interaction (PPI) analysis (Friston et al. 1997). For this analysis, we constructed a general linear model for each subject, which included both working memory tasks modeled as separate sessions. Within each session (visuospatial and phonological), we modeled the main effects of the time course from the seed VOI, the 4 conditions (1B, 1C, 2B, 2C), and the product of the deconvolved time courses and the condition vectors (Gitelman et al. 2003), which were then convolved with the hemodynamic response function. Models also included motion parameters estimated during realignment and session-specific constants. We generated t-contrast images comparing 1B and 2B working memory conditions to their respective controls and entered these into a factorial model at the second level. This model included task as a within subjects factor (V-1B, V-2B, P-1B, P-2B), diagnosis as a between subjects factor, and a factor to account for repeated measures within subjects; in a second model, we also included accuracy as a covariate. Given the previously observed profile of structural differences in parietal regions and activation differences in frontal and parietal regions, we were specifically interested in testing whether frontal regions showing group differences in activation also showed differences in interaction with parietal regions. Accordingly, we used the Automated Anatomical Labeling (AAL) (Tzourio-Mazoyer et al. 2002) interface to define regions of interest in bilateral superior and middle frontal gyrus (SFG and MFG) and bilateral inferior parietal lobule (IPL).
Brain Behavior Correlations
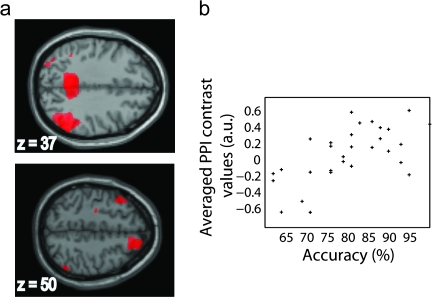
We ran similar Statistical Parametric Mapping (SPM) and PPI analyses as those described above, in a larger cohort of subjects who performed only the visuospatial task (TD = 15, TS = 30). We used this larger cohort to test correlations between behavioral performance as measured by accuracy and measures of activity, connectivity, and structure. In particular, we were interested in assessing whether connectivity explains variance in behavior, over and above measures of regional activation. We chose to look at accuracy measures in the more challenging 2-back condition, where group differences were more prominent and test for dependence on regional activation, connectivity, and structure. To do this, we began by asking which regions were significantly correlated with accuracy in the structural (VBM), main fMRI, and PPI analyses. Structural images and (2B-C) contrast images from the main SPM and PPI analyses were entered into separate multiple regression models, which included age and 2-back accuracy, as well as a constant as regressors. From each model, we generated masks consisting of clusters significantly correlated with accuracy, thresholded at P < 0.05 with a minimum extent of 15 voxels (slices from one of the masks are shown as an example in Fig. 5a). We generated 2 masks per modality: one consisting of clusters positively correlated with accuracy and one consisting of clusters negatively correlated with accuracy. For each participant, we averaged structural or (2B-C) contrast images within these masks; example averaged values are shown in Figure 5b. We reasoned that the resulting averaged (volumetric or contrast) values provided a summary measure for each participant of the volume (VBM), activation (ACT), or connectivity (PPI) that significantly correlated with accuracy. Next, we tested whether these summary measures explained variation in accuracy, by entering the averaged values into a stepwise multiple linear regression which also included age, in the following order: age, ACT(+), ACT(−), PPI(+), PPI(−), VBM(+), VBM(−).
Figure 5.
Example mask and averaged contrast values. (a) Masks were generated from clusters showing a significant correlation with accuracy at P < 0.05 with a minimum extent of 15 voxels. Shown are slices from the mask generated for the PPI 2B-C contrast. (b) Averaged contrast values extracted from the mask shown in a, plotted against 2-back accuracy.
Results
Cognitive and Neuropsychological Assessments
Scores on cognitive and neuropsychological assessments were compared between diagnoses, using the entire participant group (TS-monosomic = 20; TS-mosaic = 10; TD = 15) (Table 1). For FSIQ, the TD group was significantly higher than the TS-mosaic group (t23 = 4.2, P < 0.001) and the TS-mosaic group was significantly higher than the TS-monosomic group (t27 = 2.5, P < 0.05). This pattern (TD > TS-mosaic, P < 0.05 one-tailed and TS-mosaic > TS-monosomic, P < 0.05 one-tailed) was repeated in the perceptual reasoning (WISC perceptual reasoning index; t23 = 3.9; t28 = 2.7) and working memory (WISC working memory index; t23 = 1.74; t28 = 3.4) scores. For the verbal comprehension (WISC verbal comprehension index [VCI]) and the processing speed indices (WISC processing speed index [PSI]), the TD group was significantly higher than the TS-mosaic (VCI: t23 = 2.4, P < 0.05; PSI: t23 = 4, P < 0.001) and TS-monosomic (VCI: t33 = 3.9, P < 0.001; PSI: t32 = 6.0, P < 0.001) groups, but the TS-mosaic group was not significantly higher than the TS-monosomic (VCI: t27 = 1.5, P = 0.14; PSI: t27 = 1.4, P = 0.19). For the NEPSY visuospatial test, we had data from only one mosaic TS participant, however, the TS-monosomic group was significantly lower than the TD group (NEPSY visuospatial; t33 = 4.7, P < 0.001). Broadly, these data indicate that the TS-monosomic group shows impairments in visuospatial skills and working memory, as expected, but unexpectedly, also fares significantly worse than TDs in the verbal domain. As expected, the TS-mosaic group also shows significant impairments relative to the TD group, but on several measures was less impaired than the TS-monosomic group.
Table 1.
Cognitive and neuropsychological test scores
| FSIQ | WISC-IV VCI | WISC-IV PRI | WISC-IV WMI | WISC-IV PSI | NEPSY VS | |
| TD | 121.13 (9.3; N = 15) | 120.8 (13.5; N = 15) | 119.73 (9.8; N = 15) | 107.13 (9.8; N = 15) | 113.93 (11.1; N = 15) | 102.2 (14.0; N = 15) |
| TS all | 91.8 (16.7; N = 29) | 101.23 (17.8; N = 30) | 90.7 (16.3; N = 30) | 86.4 (16.0; N = 30) | 89.2 (14.5; N = 29) | 80.5 (12.9; N = 21) |
| TS mosaic | 101.7 (14.0; N = 10) | 108.1 (11.8; N = 10) | 100.9 (14.2; N = 10) | 98.4 (15.4; N = 10) | 94.1 (13.5; N = 10) | 80.0 (N = 1) |
| TS nonmosaic | 86.6 (15.9; N = 19) | 97.8 (19.5; N = 20) | 85.6 (15.0; N = 20) | 80.5 (12.9; N = 20) | 86.6 (14.7; N = 19) | 80.6 (13.2; N = 20) |
Note: Mean (SD; sample size); WISC-IV PRI—WISC-IV perceptual reasoning index; WISC-IV WMI—WISC-IV working memory index; and NEPSY VS—NEPSY visuospatial index.
VBM Results
We compared regional gray matter volumes between groups for all participants (TS = 30; TD = 15). The VBM analysis confirmed that our group of TS participants showed significant volumetric reductions bilaterally along the IPS (peaks at [−36 −63 46] and [30 −45 43]), as well as in several occipital regions (Fig. 2a and Table 2), relative to TDs. We were interested in assessing how this structural difference might relate to abnormal functional connectivity in TS. To this end, we defined a VOI in the rIPS, using the TD > TS contrast thresholded at P < 0.001, which was used for subsequent functional connectivity analyses (size = 806 voxels). This cluster also survived our FWE cluster-level threshold.
Figure 2.
Group differences in structure and working memory activation. (a) VBM results showing volumetric reductions in TS relative to TDs bilaterally along the IPS (Table 3). (b) Regions more engaged in the TD group relative to the TS group during working memory tasks included the right SMG (lower panel; whole-brain corrected), and left MFG, and right SFG (upper panel; corrected for small volume). (c) Regions of significant volumetric reductions (red) and working memory activation reductions (yellow) in TS relative to TDs overlaid on a canonical structural image. Statistical images thresholded at P < 0.001 uncorrected for display purposes.
As the cognitive deficits were less severe in the TS-mosaic than the TS-monosomic group, we repeated this analysis using only the TS-monosomic group (TD = 15; TS-monosomic = 20). We found that results were qualitatively similar to the analysis on the combined group. Specifically, the TS-monosomic group showed significantly reduced volumes relative to the TD group in cuneus ([−4 −76 25], Z = 5.02, size = 2611), paracentral ([−8 −42 70], Z = 4.56, size = 1919), and left IPS ([−32 −64 48], Z = 4.1, size = 764). The rIPS cluster ([30 −51 43], Z = 4.94, size = 471) did not survive our cluster-level threshold. The TS-monosomic group showed significantly enlarged volumes relative to TDs in bilateral hippocampal ([34 −22 −24], Z = 5.49, size = 3367; [−38 −18 12], Z = 5.01, size = 1920) and cerebellar regions ([−15 −96 −33], Z = 4.01, size = 728; [−6 −60 −48], Z = 3.82, size = 1010).
Analysis 1: Visuospatial and Phonological Working Memory Tasks
Working Memory Performance
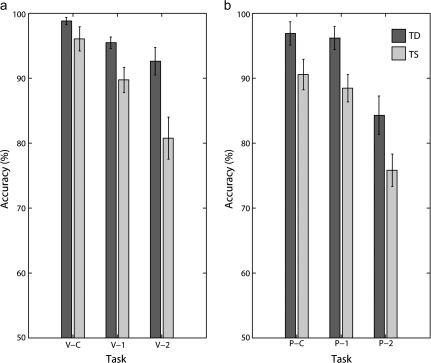
A repeated measures ANOVA showed that accuracy was significantly affected by diagnosis (TD/TS) (F1,23 = 13.8, P < 0.05), modality (visuospatial vs. phonological) (F1,23 = 7.04, P < 0.05), and memory load (F2,46 = 81.1, P < 0.001) (Table 3 and Fig. 3). These results indicate that girls with TS performed worse overall, participants in both groups were less accurate with increasing load, and participants were less accurate on the phonological task. The load × modality interaction was significant (F2,46 = 3.7, P < 0.05), indicating that as the task became more difficult, accuracy decreased more in the phonological modality. The diagnosis × load interaction (F2,46 = 6.7, P < 0.05) was significant, indicating that as the task became more difficult, accuracy decreased more in the TS group. The load × age interaction (F2,46 = 4.7, P < 0.05) was also significant, indicating that as the task became more difficult, younger participants were less accurate. The load × modality × diagnosis interaction was not significant, nor was the modality × diagnosis interaction, indicating that TS participants did not find the visuospatial task to be especially difficult, as we might have expected. This is likely due in part to the asymmetric difficulty between the visuospatial and the phonological tasks, as both TD and TS participants were less accurate on the phonological tasks.
Table 3.
Accuracy on working memory tasks (%)
| TD | TS | ||||
| Mean | SD | Mean | SD | ||
| Group 1 | |||||
| Phonological | CTRL | 96 | 7.1 | 91 | 8 |
| 1B | 97 | 5.6 | 87 | 6.9 | |
| 2B | 85 | 10 | 76 | 9.8 | |
| Visuospatial | CTRL | 99 | 1.7 | 96 | 6.7 |
| 1B | 94 | 5.1 | 90 | 8.2 | |
| 2B | 93 | 6.7 | 80 | 11.5 | |
| Group 2 | |||||
| Visuospatial | CTRL | 98 | 2.5 | 95 | 8.4 |
| 1B | 93 | 5.6 | 89 | 7.5 | |
| 2B | 90 | 8.8 | 80 | 10.5 | |
Figure 3.
Accuracy. (a) Accuracy during the visuospatial task, for control (V-C), 1-back (V-1B), and 2-back (V-2B) conditions. (b) Accuracy during the phonological task, for control (P-C), 1-back (P-1B), and 2-back (P-2B) conditions.
fMRI Results
In the TD group, we estimated a contrast to identify regions that increased in activity during working memory, relative to the control task, across both modalities. A network of regions that are typically involved in working memory tasks was identified, with peaks in bilateral IPL (peaks at [−50 −45 45] and [48 −42 45]), a large cluster that included bilateral MFG, supplementary motor area (SMA) and bilateral anterior insula (Supplementary Table S1). Next, we investigated group differences in working memory activity by estimating a TD > TS contrast over the 4 working memory conditions (V-1B, V-2B, P-1B, P-2B). This analysis showed reduced activation in TS relative to TDs in the right SMG ([48 −42 39], Z = 4.8, size = 1367) at our whole-brain corrected threshold (Fig. 2b). Additionally, we investigated differences in activation in our a priori VOIs in left IPL and bilateral MFG and SFG, using small-volume correction. This identified additional significant clusters in left IPL at [−56 −49 43] (Z = 3.77, size = 196), left MFG at [−27 68 12] (Z = 3.84, size = 173), and right SFG at [16 63 24] (Z = 3.93, size = 194) which showed reduced activation in TS. We did not find any regions which were more engaged in the TS group relative to TD, nor did any regions show significant group × load interactions.
In Figure 2c, we overlay the significant activations for both the structural and the functional analyses, showing that while there is some overlap, the peak functional differences are actually adjacent and lateral to, rather than superimposed on, the structural differences.
We verified the impact of behavioral performance, as measured by accuracy, on the group differences described above, by running a second model including accuracy as a covariate. For the group comparison TD > TS, the right SMG ([48 −43 49], Z = 4.37, size = 826) remained significant at a height threshold of P < 0.001, though the spatial extent was reduced, and no longer met our cluster-level whole-brain corrected threshold. Small volume correction yielded significant clusters in the right SFG at [16 62 22] (Z = 3.86, size = 195) and [20 36 58] (Z = 3.56, size = 209) and in the right IPL at [50 −43 40] (Z = 4.25, size = 240). These results indicate that while overall performance accounts for some of the variance in activation, group differences in regional activation nonetheless persist.
Although the modality × diagnosis interaction did not reach significance in the behavioral data, we tested for regions in the brain which showed this interaction. We found that right MFG ([33 47 27], Z = 5, size = 2331) and cingulate ([9 21 36, Z = 4.5, size = 2360], Z = 4.55, size = 2360) increased more from visuospatial to phonological in the TD group, and that a region of right occipital cortex decreased more from visuospatial to phonological in the TD group ([36 −69 −12], Z = 4.9, size = 3155).
We also repeated this analysis comparing the TD group to only the TS-monosomic participants (TD = 12, TS = 10). We obtained qualitatively similar results, namely the TD > TS contrast showed significant clusters in right SMG ([58 −45 50], Z = 4.37, size = 68), and SMA ([−14 21 55], Z = 3.85, size = 56), as well as clusters surviving small-volume correction in left MFG ([−48 21 40], Z = 4.34, size = 28), right MFG ([34 41 25], Z = 3.94, size = 19), left SFG ([21 24 60, Z = 4.04, size = 24]), and right SFG ([−38 58 20], Z = 3.85, size = 13). There were no significant activations in the TS > TD contrast.
Functional Connectivity Results
A PPI analysis was used to explore group differences in functional connectivity with the rIPS seed during working memory. Looking first at the TD group across the 4 task conditions, we found that connectivity increased during working memory relative to the control task in precuneus ([−6 −51 42], Z = Inf, size = 9181), bilateral inferior parietal lobule ([45 −54 37], Z = 5.61, size = 4144; [−42 −54 34], Z = 4.79, size = 2900), and left SFG ([−27 29 48], Z = 5.85, size = 1907). The TD > TS contrast showed significantly greater connectivity in the TD group in the right IPL ([60 −43 34], Z = 4.15, size = 1132) at our whole-brain corrected threshold. We used small volume correction to test for significant differences in our a priori VOIs in MFG, SFG, and IPL. We identified significant clusters in left MFG at [−36 30 46] (Z = 3.82, size = 251) and left IPL ([−63 −36 45], Z = 3.48, size = 21, P = 0.07), where the TS group showed reduced connectivity (Fig. 4a). The TS group showed significantly greater connectivity in a large cluster that included bilateral caudate, thalamus, and anterior cingulate (peaks at [34 8 30], Z = 5.22; [0 21 4], Z = 5.19; [10 −31 15], Z = 4.77; size = 11341). When including accuracy as a covariate in this model, in the TD > TS contrast, we found a significant peak in the precuneus ([−9 −49 42], Z = 3.89, size = 1031) at a whole-brain corrected threshold, and in left MFG ([−32 27 48], Z = 4.09, size = 320), left IPL ([−34 −49 54], Z = 3.34, size = 48), and right IPL ([30 −43 58], Z = 3.71, size = 64) small-volume corrected in our a priori VOIs. No regions survived the TS > TD contrast when controlling for accuracy.
Figure 4.
PPI analysis. (a) Group differences in interactions with the rIPS seed region, during working memory tasks across both modalities. The TD group showed significantly greater connectivity in the right IPL (whole-brain corrected), and in left MFG, and left IPL (corrected for small volume). (b) Group differences in interactions with the rIPS seed region, during the visuospatial working memory task. The TD > TS contrast showed a significant difference in right IPL at our whole-brain corrected threshold, and in left MFG, left SFG, right MFG, and right SFG (corrected for small volume). Statistical images thresholded at P < 0.001 uncorrected for display purposes.
We repeated this analysis in the TS-monosomic group alone (TD = 12; TS = 10) and again found qualitatively similar results to those obtained from the combined TS-monosomic/mosaic group. The TD > TS contrast showed significantly greater connectivity in the right IPL ([63 −30 49], Z = 4.57, size = 1613). Small-volume correction identified a significant difference in left MFG ([−39 30 49], Z = 3.9, size = 365).
Analysis 2: Brain Behavior Correlations during Visuospatial Working Memory
Having identified a group difference in functional connectivity with the right IPL, we were interested in assessing whether this tells us more about the mechanisms underlying working memory difficulties in TS, relative to local differences in activity that have been identified in previous studies. Accordingly, we looked at a larger cohort of subjects who performed only the visuospatial working memory task, which included 30 TS and 15 TD participants. In this larger group of TS participants, we had greater statistical power (relative to the smaller sample of participants who completed both visuospatial and phonological tasks) to identify significant correlations between behavior and measures of activity, structure and connectivity.
Working Memory Performance
Similar to the combined modality analysis on accuracy (Table 3), we found main effects of load (F2,84 = 47.8, P < 0.05), diagnosis (F1,42 =10.5, P < 0.05) and age (F1,42 = 10.6, P < 0.05), and significant interactions between load and age (F2,84 = 4.8, P < 0.05), and load and diagnosis (F2,84 = 4.5, P < 0.05). These results indicate that girls with TS performed significantly worse on this task overall and were increasingly impaired as the task became more difficult. As we included a larger group of TS-mosaic participants in this analysis, we also compared accuracy scores for the TS-monosomic versus TS-mosaic groups. The TS-monosomic group was significantly less accurate than the TD group in the 1-back (t33 = 2.4, P < 0.05) and 2-back conditions (t33 = 3.4, P < 0.005), but the TS-mosaic group was not significantly different from the TS-monosomic group in either working memory condition (1-back: t28 = 1.4, P = 0.2; 2-back: t28 = 1.3, P = 0.2).
fMRI Results
Looking first at the TD group, we found significantly increased activation during working memory relative to control conditions in many of the same regions described in the combined modality analysis, including bilateral anterior insula, bilateral IPL, and bilateral MFG (Supplementary Table S2). The TD > TS contrast did not show any significant activations at our whole-brain corrected threshold. Small-volume correction in our a priori VOIs identified significant differences in left IPL ([−54 −43 51], Z = 3.55, size = 115), right IPL ([36 −34 45], Z = 3.69, size = 35), and left MFG ([−44 17 36], Z = 3.72, size = 180). The TS > TD contrast did not show any significant activations.
Functional Connectivity Results
A contrast testing for increased connectivity in the TD group in the visuospatial working memory tasks, relative to the control tasks, showed significant activations in precuneus ([−6 −42 49], Z = 6.25, size = 7424) and left SFG ([−22 32 43], Z = 4.24, size = 1910). The TD > TS contrast showed a significant difference in right IPL ([52 −28 60], Z = 4.12, size = 1440) at our whole-brain corrected threshold. In our a priori VOIs, we found significant differences in left MFG ([−32 39 48], Z = 3.52, size = 238), left SFG ([−34 51 34], Z = 4.21, size = 134), right MFG ([50 0 46], Z = 3.63, size = 170), and right SFG ([28 53 34], Z = 3.87, size = 184) (Fig. 4b).
Brain–Behavior Correlations
We assessed whether functional connectivity was relevant for explaining behavioral variability in the TS group. We chose to look at accuracy measures in the more challenging 2-back condition (where group differences were more prominent) in relation to measures of structure, activity, and connectivity. Correlating 2B accuracy with 2B-C contrast maps from the main SPM and PPI analyses did not yield any significant clusters at our whole-brain corrected threshold; correlation with gray matter images showed one significant cluster in postcentral gyrus ([57 −22 21], Z = 4.8, n = 1690). We used a reduced threshold of P < 0.05 uncorrected (minimum extent 15 voxels) to generate maps of clusters that were positively and negatively correlated with accuracy. This yielded 24(+) and 35(−) clusters from the main SPM model, 40(+) and 22(−) clusters from the PPI model, and 21(+) and 5(−) clusters from the structural model. Slices from the mask generated for PPI(+) are shown in Figure 5a. For each participant, we averaged structural, and activation, and connectivity (2B-C) contrast images over their respective maps. Averaged values for PPI(+) are shown in Figure 5b; correlations between accuracy and predictors are shown in Table 4. Predictors were entered into a stepwise linear regression in the following order: age, ACT(+), ACT(−), PPI(+), PPI(−), VBM(+), VBM(−). The final model included the following predictors: age (β = 0.466), ACT(+) (β = 0.176), ACT(−) (β = −0.235), PPI(+) (β = 0.430), and PPI(−) (β = −0.421) (standardized coefficients, all at P < 0.05). This indicates that measures of connectivity provide information relevant to explaining behavioral variability, over and above measures of activation alone in individuals with TS.
Table 4.
Correlations between predictors and accuracy
| Age | 0.603** |
| VBM(+) | 0.516** |
| VBM(−) | −0.179 |
| ACT(+) | 0.445* |
| ACT(−) | −0.648** |
| PPI(+) | 0.621** |
| PPI(−) | −0.278 |
**Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed).
Discussion
In this study, we investigated whether differences in functional connectivity between parietal and frontal regions were related to working memory difficulties in TS. We replicated previous findings of reduced parietal lobe volume in our group of participants. We used the parietal volumetric difference to define a seed region for functional connectivity analysis and found that functional connectivity between this seed region and dorsal frontal regions was reduced during working memory in girls with TS relative to TD girls. Connectivity with the parietal seed region was predictive of performance in girls with TS, underlining the importance of functional interactions for working memory, and pointing to a mechanism by which structural abnormalities in TS might affect cognitive performance.
Previous fMRI studies of cognitive function in TS have found activation differences in parietal and frontal brain regions during performance of visuospatial (Kesler et al. 2004), mathematical (Kesler et al. 2006), and working memory tasks (Haberecht et al. 2001; Hart et al. 2006). In parallel, structural imaging studies have identified differences in parietal lobe morphology (Molko et al. 2003) and frontoparietal white matter connectivity (Holzapfel et al. 2006). As well, cortical thickness in bilateral DLPFC has been found to correlate with cortical thickness in the left IPS in TS (Raznahan et al. 2010). However, to our knowledge, this is the first analysis of functional connectivity suggesting that abnormal functional interactions contribute to performance differences in TS. Several studies have pointed to functional interactions as being important for working memory in healthy subjects (Honey et al. 2002; Hampson et al. 2006), and related to performance differences in aging subjects (Grady et al. 2009), individuals with schizophrenia (Meyer-Lindenberg et al. 2001; Schlösser et al. 2003; Hashimoto et al. 2010), and individuals with autism (Koshino et al. 2005). Taken together, these studies suggest that interregional interactions are critical for online maintenance of information, as required by working memory tasks, and that reduced interregional interactions may be a common cause of impaired performance.
Here, we have presented correlative evidence that functional connectivity is important for working memory in TS. Specifically, we found that a measure of connectivity was predictive of performance in TS, over and above variance explained by age and regional activations. However, this effect was tested on a relatively small sample size and should be replicated in a larger group. Furthermore, we cannot know based on this experiment whether reduced functional connectivity has a causal effect on working memory difficulties in TS. It is also plausible that because the 2-back working memory tasks are challenging, the TS girls become less engaged and that is reflected in measures of neural activity. Participants in this analysis were excluded if they stopped responding; however, it is difficult to quantify engagement on a target detection task that requires relatively infrequent responding. An interesting topic for future study will be to test whether structural connectivity places limitations on performance or functional connectivity.
In addition to difficulties with visuospatial skills and executive function, girls with TS have well documented difficulties in the social domain. These include difficulties with face and affect recognition (Romans et al. 1998; Lawrence et al. 2003; Mazzola et al. 2006), as well as “theory of mind” or mental state attribution (Lawrence et al. 2007). Structural differences have been found in TS in regions involved in social processing, such as amygdala (Good et al. 2003), superior temporal gyrus (Kesler et al. 2003), and orbitofrontal cortex (Good et al. 2003), suggesting that the social processing deficit may be parallel and relatively independent from performance IQ deficits. However, problems with working memory may also contribute to abnormal social behaviors in TS. In particular, mental state attribution likely relies on attention and working memory. Executive function deficits including difficulties with attention (Romans et al. 1998; Ross, Roeltgen, et al. 2000; Ross, Zinn, et al. 2000; Lagrou et al. 2006) and response inhibition (Kirk et al. 2005), likely contribute to impaired social competence in TS (Lagrou et al. 2006) and may be mediated by a similar reduction in connectivity with frontal regions.
Working memory activity in frontal and parietal regions has been found to increase from adolescence to adulthood (Olesen et al. 2007). A recent study using computational modeling to investigate how developmental changes affect neural activity during working memory maintenance showed that the strength of frontoparietal connections predicted observed changes in neural activity with development better than other hypothesized structural developmental changes (Edin et al. 2007). This computational result is consistent with differences in delay period activity in TS demonstrated by Hart et al. (2006). Our finding of reduced functional connectivity and previous findings of reduced anatomical connectivity (Holzapfel et al. 2006) are also consistent with the assertion that reduced connectivity relates to reduced working memory capacity in TS. The study presented here was set up as a block design, making it difficult to discern whether connectivity differences were related to encoding, maintenance, retrieval, or the active inhibition necessary during N-back working memory tasks. In future studies, it will be interesting to discern whether delay period connectivity specifically is reduced in TS, perhaps using a technique with higher temporal resolution, such as magnetoencephalography or electroencephalography.
In general, girls with TS have difficulties with visuospatial stimuli, relative to verbal. Few studies have investigated the neural basis for this difference in the context of working memory. In the present study, we did not find a group × modality interaction in accuracy, however, participants overall were less accurate on the phonological task relative to the visuospatial, perhaps indicating that the overall difficulty of this task overwhelmed modality-specific group differences. We also note that our participant group showed impairments with verbal comprehension as well as performance IQ measures. Hart et al. (2006) found significant group × modality interactions in delay period activity in the IPS, MFG, and IFG—these regions increased more from phonological to visuospatial in the TD group. However, in both the Hart study and the present study, participants overall were more accurate in one modality than the other, indicating that the tasks were not well matched for difficulty. A challenge for future studies will be to design behavioral tasks such that the structure remains similar between modalities and the difficulty level more closely equated.
Most girls with TS, particularly from X monosomy, do not spontaneously begin normal pubertal development, and puberty is usually induced with estrogen supplementation around the age of typical pubertal onset; the participants in our study were mostly prepubertal. Studies of the effects of estrogen repletion have shown behavioral changes in motor and nonverbal processing speed (Ross et al. 1998), and some evidence for effects on memory (Ross, Roeltgen, et al. 2000; Ross, Zinn, et al. 2000). Nonetheless, a comprehensive longitudinal study of brain development following estrogen repletion in TS has yet to be performed.
A better understanding of the neural mechanisms leading to working memory impairments in TS could lead to the development of targeted interventions. Intensive training on working memory tasks has been shown to improve performance (Olesen et al. 2004; Takeuchi et al. 2010), modify brain activity (Olesen et al. 2004), and modify brain structure (Takeuchi et al. 2010). Takeuchi et al. (2010) found significant changes in fractional anisotropy near the IPS following a 2-month program of intensive working memory training. However, it remains to be seen whether intensive behavioral training can improve working memory performance in TS, and it will be especially interesting to determine whether training can override what appears to be a genetically influenced predisposition for weakened frontoparietal connectivity. As the frontoparietal attention network (Fox et al. 2005; Toro et al. 2008) is important for attentionally demanding cognitive processing, targeting connectivity in this network could potentially ameliorate executive function deficits in TS more broadly.
In conclusion, we have demonstrated that girls with TS show reduced frontoparietal connectivity during working memory tasks, and that a measure of this connectivity correlates with behavior in TS participants. While previous studies have identified differences in regional structure and activations in TS, the findings presented here suggest that reduced functional interactions may be a mechanism by which structural differences affect cognitive processing. Reduced functional connectivity in the frontoparietal attention network may be a common mechanism for broad executive function and attention difficulties in TS; this work emphasizes the importance of frontoparietal interactions for supporting attentionally demanding tasks. TS offers a unique opportunity to study hormonal and genetic influences on brain development in females, and this work suggests that structural brain differences emerging early in development can affect the dynamics of interregional communication, with pernicious effects on cognitive function.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by grants from the National Institute of Child Health and Human Development (HD049653); National Institute of Mental Health (MH050047); Chain of Love Foundation and Genentech; and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award. D.H. is supported by a T32-MH19908 training grant and an APIRE/Lilly Psychiatric Research Fellowship.
Acknowledgments
We gratefully acknowledge the assistance of Kristen Sheau, Yeana Park, Matthew Marzelli and Rianne Hastie. We would also like to thank all of the families who generously participated in this study and the TS Society of USA for assistance with recruitment. Conflict of Interest : None declared.
References
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bender BG, Linden MG, Robinson A. Neuropsychological impairment in 42 adolescents with sex chromosome abnormalities. Am J Med Genet. 1993;48:169–173. doi: 10.1002/ajmg.1320480312. [DOI] [PubMed] [Google Scholar]
- Bondy CA Turner Syndrome Study Group. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J. Presented at the 8th International Conference on Functional Mapping of the Human Brain; 2002 June 2--6. Sendai (Japan): 2002. Region of interest analysis using an SPM toolbox [abstract] [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Reiss AL. A volumetric study of parietal lobe subregions in Turner syndrome. Dev Med Child Neurol. 2004;46:607–609. doi: 10.1017/s0012162204001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan L, Pavlovic J, Rovet J. A reexamination of the visuospatial deficit in Turner syndrome. Dev Neuropsychol. 1998;14:341–367. [Google Scholar]
- Edin F, Macoveanu J, Olesen P, Tegnér J, Klingberg T. Stronger synaptic connectivity as a mechanism behind development of working memory-related brain activity during childhood. J Cogn Neurosci. 2007;19:750–760. doi: 10.1162/jocn.2007.19.5.750. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Garron DC. Intelligence among persons with Turner's syndrome. Behav Genet. 1977;7:105–127. doi: 10.1007/BF01066000. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Good CD, Lawrence K, Thomas NS, Price CJ, Ashburner J, Friston KJ, Frackowiak RS, Oreland L, Skuse DH. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 2003;126:2431–2446. doi: 10.1093/brain/awg242. [DOI] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JA, Churchill N, McIntosh AR. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2009;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootoonk S, Hutton C, Ashburner J, Howseman AM, Josephs O, Rees G, Friston KJ, Turner R. Characterization and correction of interpolation effects in the realignment of fMRI time series. Neuroimage. 2000;11:49–57. doi: 10.1006/nimg.1999.0515. [DOI] [PubMed] [Google Scholar]
- Haberecht MF, Menon V, Warsofsky IS, White CD, Dyer-Friedman J, Glover GH, Neely EK, Reiss AL. Functional neuroanatomy of visuo-spatial working memory in Turner syndrome. Hum Brain Mapp. 2001;14:96–107. doi: 10.1002/hbm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SJ, Davenport ML, Hooper SR, Belger A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 2006;129:1125–1136. doi: 10.1093/brain/awl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Lee K, Preus A, McCarley RW, Wible CG. An fMRI study of functional abnormalities in the verbal working memory system and the relationship to clinical symptoms in chronic schizophrenia. Cereb Cortex. 2010;20:46–60. doi: 10.1093/cercor/bhp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel M, Barnea-Goraly N, Eckert MA, Kesler SR, Reiss AL. Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in Turner syndrome. J Neurosci. 2006;26:7007–7013. doi: 10.1523/JNEUROSCI.1764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Fu CH, Kim J, Brammer MJ, Croudace TJ, Suckling J, Pich EM, Williams SC, Bullmore ET. Effects of verbal working memory load on corticocortical connectivity modeled by path analysis of functional magnetic resonance imaging data. Neuroimage. 2002;17:573–582. [PubMed] [Google Scholar]
- Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Dev Disabilities Res Rev. 2009;15:270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, Reiss AL. Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biol Psychiatry. 2003;54:636–646. doi: 10.1016/s0006-3223(03)00289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Haberecht MF, Menon V, Warsofsky IS, Dyer-Friedman J, Neely EK, Reiss AL. Functional neuroanatomy of spatial orientation processing in Turner syndrome. Cereb Cortex. 2004;14:174–180. doi: 10.1093/cercor/bhg116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Menon V, Reiss AL. Neuro-functional differences associated with arithmetic processing in Turner syndrome. Cereb Cortex. 2006;16:849–856. doi: 10.1093/cercor/bhj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magn Reson Med. 2002;48:715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Kirk JW, Mazzocco MMM, Kover ST. Assessing executive dysfunction in girls with fragile X or Turner syndrome using the contingency naming test (CNT) Dev Neuropsychol. 2005;28:755–777. doi: 10.1207/s15326942dn2803_2. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NESPY: a developmental neuropsychological assessment. San Antonio (TX): The Psychological Corporation; 1998. [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Lagrou K, Froidecoeur C, Verlinde F, Craen M, De Schepper J, Francois I, Massa G. Psychosocial functioning, self-perception and body image and their auxologic correlates in growth hormone and oestrogen treated young adult women with Turner syndrome. Horm Res. 2006;66:277–284. doi: 10.1159/000095547. [DOI] [PubMed] [Google Scholar]
- Lawrence K, Jones A, Oreland L, Spektor D, Mandy W, Campbell R, Skuse D. The development of mental state attributions in women with X-monosomy, and the role of monoamine oxidase B in the sociocognitive phenotype. Cognition. 2007;102:84–100. doi: 10.1016/j.cognition.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lawrence K, Kuntsi J, Coleman M, Campbell R, Skuse D. Face and emotion recognition deficits in Turner syndrome: a possible role for X-linked genes in amygdala development. Neuropsychology. 2003;17:39–49. [PubMed] [Google Scholar]
- Lippe B. Turner syndrome. Endocrinol Metab Clin North Am. 1991;20:121–152. [PubMed] [Google Scholar]
- Mazaika P, Hoeft F, Glover G, Reiss A. Human Brain Mapping Conference. 2009. Methods and software for fMRI analysis for clinical subjects. June 11–15; Florence, Italy. [Google Scholar]
- Mazzola F, Seigal A, MacAskill A, Corden B, Lawrence K, Skuse DH. Eye tracking and fear recognition deficits in Turner syndrome. Soc Neurosci. 2006;1:259–269. doi: 10.1080/17470910600989912. [DOI] [PubMed] [Google Scholar]
- McCauley E, Kay T, Ito J, Treder R. The Turner syndrome—cognitive deficits, affective discrimination, and behavior problems. Child Dev. 1987;58:464–473. [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Rivière D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40:847–858. doi: 10.1016/s0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, LeBihan D, Cohen L, Dehaene S. Brain anatomy in Turner syndrome: evidence for impaired social and spatial-numerical networks. Cereb Cortex. 2004;14:840–850. doi: 10.1093/cercor/bhh042. [DOI] [PubMed] [Google Scholar]
- Murphy DG, Allen G, Haxby JV, Largay KA, Daly E, White BJ, Powell CM, Schapiro MB. The effects of sex steroids, and the X chromosome, on female brain function: a study of the neuropsychology of adult Turner syndrome. Neuropsychologia. 1994;32:1309–1323. doi: 10.1016/0028-3932(94)00065-4. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, Daly E, Haxby JV, Allen G, White BJ, McIntosh AR, Powell CM, Horwitz B, Rapoport SI. X-chromosome effects on female brain: a magnetic resonance imaging study of Turner's syndrome. Lancet. 1993;342:1197–1200. doi: 10.1016/0140-6736(93)92184-u. [DOI] [PubMed] [Google Scholar]
- Murphy MM. Language and literacy in Turner syndrome. Top Lang Disord. 2009;29:187–194. doi: 10.1097/TLD.0b013e3181a72044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhuis-van der Sanden MW, Eling PA, Otten BJ. A review of neuropsychological and motor studies in Turner syndrome. Neurosci Biobehav Rev. 2003;27:329–338. doi: 10.1016/s0149-7634(03)00062-9. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Macoveanu J, Tegnér J, Klingberg T. Brain activity related to working memory and distraction in children and adults. Cereb Cortex. 2007;17:1047–1054. doi: 10.1093/cercor/bhl014. [DOI] [PubMed] [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Heaton RK, Karzmark P, Pendleton MG, Lehman R, Shucard DW. The neuropsychological phenotype in Turner syndrome. Cortex. 1985;21:391–404. doi: 10.1016/s0010-9452(85)80004-6. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, Skuse DH, Ross J, Lerch JP, Giedd JN, et al. Cortical anatomy in human X monosomy. Neuroimage. 2010;49:2915–2923. doi: 10.1016/j.neuroimage.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Mazzocco MM, Greenlaw R, Freund LS, Ross JL. Neurodevelopmental effects of X monosomy: a volumetric imaging study. Ann Neurol. 1995;38:731–738. doi: 10.1002/ana.410380507. [DOI] [PubMed] [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL. Transition to young adulthood in Ullrich-Turner syndrome: neurodevelopmental changes. Am J Med Genet. 1998;79:140–147. [PubMed] [Google Scholar]
- Ross J, Zinn A, McCauley E. Neurodevelopmental and psychosocial aspects of Turner syndrome. Ment Retard Dev Disabil Res Rev. 2000;6:135–141. doi: 10.1002/1098-2779(2000)6:2<135::AID-MRDD8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Ross JL, Roeltgen D, Feuillan P, Kushner H, Cutler GB. Effects of estrogen on nonverbal processing speed and motor function in girls with Turner's syndrome. J Clin Endocrinol Metab. 1998;83:3198–3204. doi: 10.1210/jcem.83.9.5087. [DOI] [PubMed] [Google Scholar]
- Ross JL, Roeltgen D, Feuillan P, Kushner H, Cutler GB. Use of estrogen in young girls with Turner syndrome: effects on memory. Neurology. 2000;54:164–170. doi: 10.1212/wnl.54.1.164. [DOI] [PubMed] [Google Scholar]
- Rovet J, Netley C. The mental rotation task performance of Turner syndrome subjects. Behav Genet. 1980;10:437–443. doi: 10.1007/BF01073648. [DOI] [PubMed] [Google Scholar]
- Rovet JF. The psychoeducational characteristics of children with Turner syndrome. J Learn Disabil. 1993;26:333–341. doi: 10.1177/002221949302600506. [DOI] [PubMed] [Google Scholar]
- Schlösser R, Gesierich T, Kaufmann B, Vucurevic G, Hunsche S, Gawehn J, Stoeter P. Altered effective connectivity during working memory performance in schizophrenia: a study with fMRI and structural equation modeling. Neuroimage. 2003;19:751–763. doi: 10.1016/s1053-8119(03)00106-x. [DOI] [PubMed] [Google Scholar]
- Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897–3902. doi: 10.1210/jc.2006-0558. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R. Training of working memory impacts structural connectivity. J Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Abnormal prefrontal cortex function during response inhibition in Turner syndrome: functional magnetic resonance imaging evidence. Biol Psychiatry. 2003;53:107–111. doi: 10.1016/s0006-3223(02)01488-9. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Waber DP. Neuropsychological aspects of Turner's syndrome. Dev Med Child Neurol. 1979;21:58–70. doi: 10.1111/j.1469-8749.1979.tb01581.x. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. 2003. Technical and interactive manual. San Antonio (TX): The Psychological Corporation. [Google Scholar]