Abstract
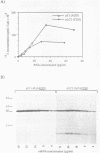
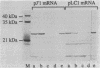
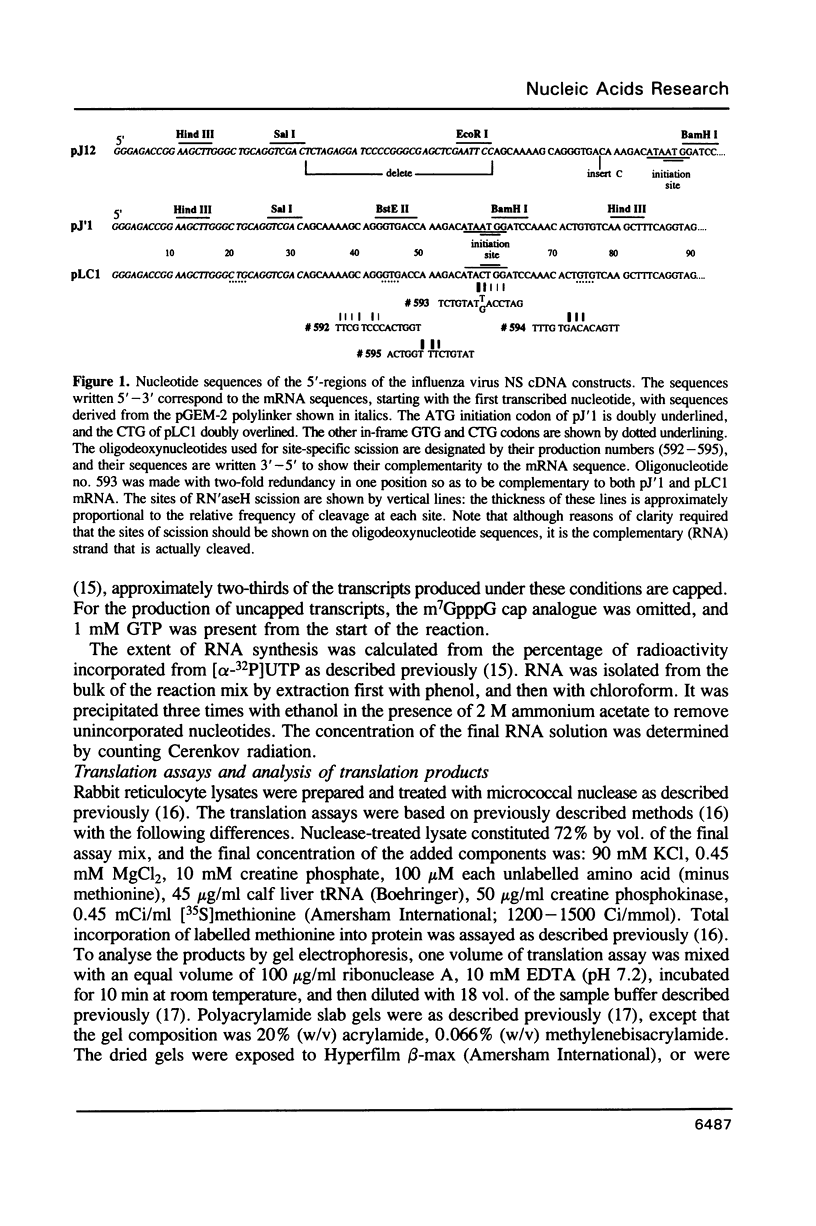
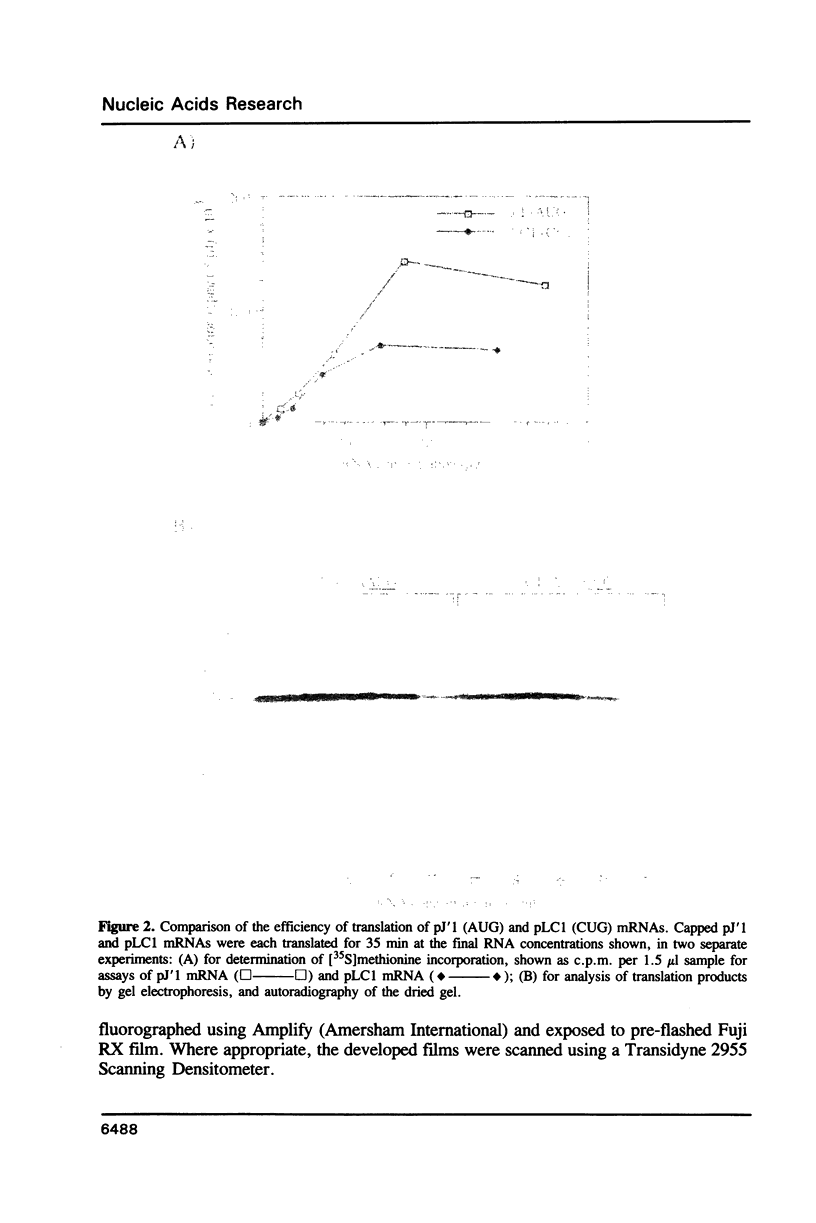
Nucleotide substitutions were made at the initiation codon of an influenza virus NS cDNA clone in a vector carrying the bacteriophage T7 promoter. When capped mRNA transcripts of these constructs were translated in the rabbit reticulocyte lysate, a change in the initiation codon from...AUAAUGG...to...AUACUGG...reduced the in vitro translational efficiency by only 50-60%, and resulted in only a small increase in the yield of short products presumed to be initiated at downstream sites. Synthesis of the full-length product was initiated exclusively at the mutated codon, with negligible use either of in-frame upstream CUG or GUG codons, or of an in-frame downstream GUG codon. We conclude that CUG has the potential to function as an efficient initiation codon in mammalian systems, at least in certain contexts.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becerra S. P., Koczot F., Fabisch P., Rose J. A. Synthesis of adeno-associated virus structural proteins requires both alternative mRNA splicing and alternative initiations from a single transcript. J Virol. 1988 Aug;62(8):2745–2754. doi: 10.1128/jvi.62.8.2745-2754.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. M., Laz T. M., Sherman F. Efficiency of translation initiation by non-AUG codons in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Oct;8(10):4533–4536. doi: 10.1128/mcb.8.10.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran J., Kolakofsky D. Ribosomal initiation from an ACG codon in the Sendai virus P/C mRNA. EMBO J. 1988 Jan;7(1):245–251. doi: 10.1002/j.1460-2075.1988.tb02806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C., Legon S., Hunt T., Jackson R. J. Initiation of protein synthesis: evidence for messenger RNA-independent binding of methionyl-transfer RNA to the 40 S ribosomal subunit. J Mol Biol. 1973 May 25;76(3):379–403. doi: 10.1016/0022-2836(73)90511-1. [DOI] [PubMed] [Google Scholar]
- Dasso M. C., Jackson R. J. On the fidelity of mRNA translation in the nuclease-treated rabbit reticulocyte lysate system. Nucleic Acids Res. 1989 Apr 25;17(8):3129–3144. doi: 10.1093/nar/17.8.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. F., Cigan A. M. Genetic selection for mutations that reduce or abolish ribosomal recognition of the HIS4 translational initiator region. Mol Cell Biol. 1988 Jul;8(7):2955–2963. doi: 10.1128/mcb.8.7.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Translational modulation in vitro of a eukaryotic viral mRNA encoding overlapping genes: ribosome scanning and potential roles of conformational changes in the P/C mRNA of Sendai virus. Biochem Biophys Res Commun. 1985 Aug 30;131(1):91–97. doi: 10.1016/0006-291x(85)91774-7. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Patwardhan S. ACG, the initiator codon for a Sendai virus protein. J Biol Chem. 1988 Jun 25;263(18):8553–8556. [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. A detailed kinetic analysis of the in vitro synthesis and processing of encephalomyocarditis virus products. Virology. 1986 Feb;149(1):114–127. doi: 10.1016/0042-6822(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Hunt T. The use of single-stranded DNA and RNase H to promote quantitative 'hybrid arrest of translation' of mRNA/DNA hybrids in reticulocyte lysate cell-free translations. Nucleic Acids Res. 1986 Aug 26;14(16):6433–6451. doi: 10.1093/nar/14.16.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan S., Gupta K. C. Translation initiation potential of the 5' proximal AUGs of the polycistronic P/C mRNA of Sendai virus. A multipurpose vector for site-specific mutagenesis. J Biol Chem. 1988 Apr 5;263(10):4907–4913. [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at an ACG triplet in mammalian cells. J Biol Chem. 1987 Aug 25;262(24):11847–11851. [PubMed] [Google Scholar]
- Peabody D. S. Translation initiation at non-AUG triplets in mammalian cells. J Biol Chem. 1989 Mar 25;264(9):5031–5035. [PubMed] [Google Scholar]