Abstract
Background
Gemcitabine (Gem) has limited clinical benefits in pancreatic ductal adenocarcinoma (PDAC). Sunitinib (Su) is a novel, multi-target receptor tyrosine kinase inhibitor that has antitumour activities. This study tested the benefits of combined gemcitabine and sunitinib in PDAC.
Methods
Cell viability and protein expression were evaluated by WST-1 assay and Western blotting. Tumour growth and survival experiments were performed in murine xenografts.
Results
In PDAC cells, Gem, Su and Su + Gem, respectively, caused 28%, 22% and 48% inhibition in proliferation at 100 nM. In endothelial cells, Gem, Su and Su + Gem, respectively, caused 49%, 32% and 72% inhibition in proliferation. In fibroblasts, Gem, Su and Su + Gem, respectively, caused 65%, 14% and 79% inhibition in proliferation. Su increased apoptosis, as evidenced by the cleavage of caspase-3 and PARP-1 proteins. Net tumour growth compared with controls in the Gem, Su and Su + Gem groups was 57%, 6% and 1%, respectively. Intratumoral proliferative activity was reduced by 33%, 82% and 75% in the Gem, Su and Su + Gem groups, respectively, compared with controls. Median survival in the control, Su, Gem and Su + Gem groups was 16, 21, 24 and 30 days, respectively (P = 0.007).
Conclusions
These findings support a combination approach using multi-target antiangiogenic agents such as sunitinib with standard gemcitabine therapy in the treatment of PDAC.
Keywords: pancreatic cancer, sunitinib, gemcitabine, combination therapy
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive human malignancies and is characterized by early tissue invasion and metastasis. Gemcitabine (Gem), a cytotoxic chemotherapeutic based on a pyrimidine analogue, is currently the standard systemic treatment for PDAC, although its clinical benefit remains marginal.1 The failure of conventional chemotherapy in PDAC patients emphasizes the urgent requirement for novel and more effective therapeutic strategies.
Angiogenesis plays a critical role in the progression of primary and metastatic solid tumours, including PDAC. Several antiangiogenic agents have been studied in experimental PDAC models, including anti-vascular endothelial growth factor (VEGF) agents like bevacizumab, matrix metalloproteinase inhibitors (marimastat) and cyclooxygenase-2 inhibitors (celecoxib), with limited survival benefit both when used alone and in combination with chemotherapy.2–4 The epidermal growth factor receptor inhibitor erlotinib has recently been approved for PDAC treatment in combination with gemcitabine, based on a small but significant improvement in overall survival.5
Sunitinib (Su) is a multi-target inhibitor of several receptor tyrosine kinases (RTKs) relevant to tumour angiogenesis, including VEGF receptor (types 1, 2, 3) and platelet-derived growth factor (PDGF) receptors (α and β).6,7 Sunitinib is currently approved for the treatment of advanced renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumours.8,9 The antitumour effects of sunitinib have been partly attributed to its ability to inhibit tumour angiogenesis.10 Sunitinib has also been shown to directly inhibit the survival and proliferation of a variety of cancer cells.6,7,11–13 In human PDAC, VEGF receptors and PDGF receptors are overexpressed and have been correlated with poor prognosis.14–16 Recently, sunitinib has been shown to sensitize pancreatic cancer to the cytotoxic effects of ionizing radiation17 and, when administered in combination with metronomic gemcitabine, improved survival in an orthotopic model of pancreatic cancer.18 The present study examined the in vitro effects of sunitinib and gemcitabine on endothelial cell, PDAC cell and fibroblast proliferation and apoptosis. The in vivo antitumour effects of a combination therapy with sunitinib and gemcitabine in murine pancreatic cancer xenograft models were also evaluated.
Materials and methods
Cell culture and reagents
The human pancreatic cancer cell line AsPC-1 and the human fibroblast cell line WI-38 were grown in RPMI 1640 and DMEM (Dulbecco's modified Eagle's medium), respectively (Sigma Chemical Co., St Louis, MO, USA), supplemented with 10% foetal bovine serum and 100 U/ml penicillin/streptomycin solution. Primary human umbilical vein endothelial cells (HUVECs) were grown in EndoGRO-LS medium containing endothelial cell growth supplements (Millipore Corp., Billerica, MA, USA). Gemcitabine was purchased from Eli Lilly Corporation (Indianapolis, IN, USA) and sunitinib was purchased from LC Laboratories, Inc. (Woburn, MA, USA).
Cell proliferation assay
In vitro cell proliferation assays were performed using a colorimetric WST-1 reagent (Roche Applied Science, Indianapolis, IN, USA) as previously described.19 This assay is based on the ability of viable cells to cleave the sulphonated tetrazolium salt WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulphonate) by mitochondrial dehydrogenases. The cells were placed in a 96-well plate and treated with gemcitabine and sunitinib, either alone or in combination. After 72 h incubation, 10 µl of WST-1 reagent was added to each well, and absorbance at 450 nm was measured after 2 h using a microplate reader.
Western blot analysis
A sub-confluent monolayer of cells was treated with gemcitabine (10 µM) or sunitinib (10 µM) for 16 h. Cells were lysed and equal amounts of protein were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). After blocking for 1 h in blocking solution [5% milk in TBS-T (Tris-buffered saline containing Tween-20)], the membranes were incubated overnight at 4 °C with the following antibodies: cleaved poly (ADP-ribose) polymerase-1 (PARP-1); cleaved caspase-3 (Cell Signaling Technology, Inc., Beverly, MA, USA) or α-tubulin (Sigma Chemical Co.). The membranes were then incubated with HRP (horseradish peroxidase)-conjugated secondary antibodies (Pierce Biotechnology, Inc., Rockford, IL, USA) for 1 h at room temperature. Specific bands were detected using the enhanced chemiluminescence reagent (ECL) (PerkinElmer Life Sciences, Inc., Boston, MA, USA) on autoradiographic film and quantitated by densitometry.
Tumour implantation and in vivo tumour growth experiment
Athymic female nude mice (aged 4–8 weeks) were used for in vivo tumour growth studies in a subcutaneous xenograft model. Human pancreatic cancer AsPC-1 cells (0.75 × 106) were injected subcutaneously in each mouse. After 14 days the animals were randomly grouped (n = 6–8 per group) and treated intraperitoneally with phosphate-buffered saline (PBS) (control), sunitinib (40 mg/kg for 1st week, then 20 mg/kg for 2nd week, in 100 µl PBS, five times weekly) and gemcitabine (100 mg/kg in 100 µl PBS, twice weekly), either alone or in combination for 2 weeks. Doses of gemcitabine and sunitinib were determined according to the effective and maximum tolerable doses reported in previous studies.20,21 Tumour size in all mice was measured twice weekly by calliper. Tumour volume was estimated using the formula [V = πh(h2+ 3 ∞2)/6], where V = volume, h = height and ∞ = (length + width)/2 as previously described.22 Net growth in tumour size was calculated by subtracting tumour volume on the first day of treatment from that on the last day. After completion of therapy, all animals were killed and the tumours were excised, weighed, dissected and fixed in 4% paraformaldehyde for histological or immunohistochemical analysis. The intratumoral proliferative index (in percent) was determined using a 1 : 200 dilution of Ki67 antibody (Abcam, Inc., Cambridge, MA, USA) and counting the number of Ki67-positive cells vs. total cells per high-power field (HPF), averaged from five HPFs within viable tumour areas as previously described.23 Intratumoral apoptosis was measured by TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling) staining as previously described.24
Animal survival study
In vivo animal survival studies were performed using SCID-NOD (severe combined immune deficiency, non-obese diabetic) mice aged 6–8 weeks as previously reported.24,25 Briefly, female SCID mice were injected intraperitoneally with 0.75 × 106 AsPC-1 cells in 100 µl serum-free RPMI 1640 medium. Fourteen days after tumour cell injection, the animals were randomly grouped (n = 6–8 per group) and treated intraperitoneally with PBS (control), gemcitabine (100 mg/kg in 100 µl PBS, twice weekly) or sunitinib (40 mg/kg for 1st week, then 20 mg/kg for 2nd week, in 100 µl PBS, five times weekly), either alone or in combination for 2 weeks. There were no apparent signs of toxicity at these doses. Animals were killed when they became moribund. Survival was evaluated from the first day of treatment until death.
Statistical analysis
Statistical significance was analysed with the two-tailed Student's t-test using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA, USA). In vitro cell proliferation assay results are expressed as the mean ± standard deviation. In in vivo studies, statistical analysis was performed using an analysis of variance (anova) for multiple group comparisons and Student's t-test for individual group comparisons. In survival studies, statistical differences were analysed with StatView for Macintosh Version 5.0.1 (SAS, Inc., Cary, NC, USA) by non-parametric survival statistics and log-rank testing. P-values of <0.05 were considered to represent statistically significant group differences.
Results
Effects of gemcitabine and sunitinib on cell proliferation
Within WST-1 in vitro assays on AsPC-1 cells, gemcitabine and sunitinib inhibited cell proliferation in a dose-dependent manner, with 45% and 92% inhibition observed at 10 µM, respectively. Combinations of gemcitabine and sunitinib had additive effects that were more obvious at lower concentrations. At a concentration of 100 nM, rates of inhibition of proliferation in the Gem, Su and Su + Gem groups were 28%, 22% and 48%, respectively. In HUVECs, gemcitabine and sunitinib had greater effects inhibiting proliferation, with 95% and 88% inhibition at 10 µM, respectively. The combination of gemcitabine and sunitinib had significant additive effects, as indicated by the inhibition in proliferation in the Gem, Su and Su + Gem groups at 100 nM of 49%, 32% and 72%, respectively. In WI-38 cells, inhibition in cell proliferation at the concentration of 10 µM in the Gem and Su groups was 95% and 87%, respectively. In this cell line gemcitabine was very effective even at lower concentrations, whereas a proliferation-inhibiting response to sunitinib was observed only at higher concentrations. In WI-38 cells, Gem, Su and Su + Gem resulted in inhibition of 65%, 14% and 79%, respectively, at 100 nM concentration (Fig. 1).
Figure 1.
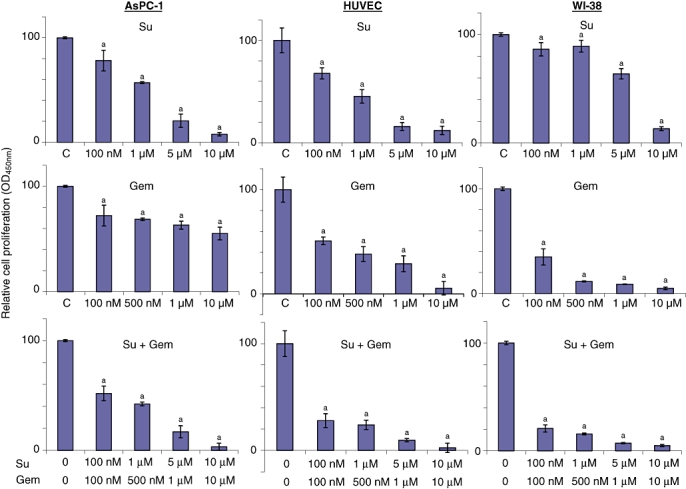
Effects of gemcitabine (Gem) and sunitinib (Su) on in vitro cell proliferation. Cells were plated on 96-well plates and treated with phosphate-buffered saline [PBS, control (C)], Su (100 nM, 1 µM, 5 µM or 10 µM) or Gem (100 nM, 500 nM, 1 µM or 10 µM), either alone or in combination. After 72 h, 10 µl WST-1 reagent was added to each well and incubated for a further 2 h. The resulting number of viable cells was calculated by measuring the absorbance of colour produced in each well. Data represent the mean ± standard deviation of quadruplicate determinants. aP-values of < 0.03 compared with controls
Effects of gemcitabine and sunitinib on apoptosis-related proteins
Expression of protein related to an induction in cellular apoptosis was evaluated to determine whether the loss of cell viability might occur by this mechanism. Western blot analysis revealed that in all three cell lines tested (AsPC-1, HUVECs and WI-38), sunitinib, either alone or in combination with gemcitabine, caused a significant increase in expression of cleaved caspase-3 and cleaved PARP-1 proteins, indicating the induction of caspase-dependent apoptosis. No detectable levels of cleaved caspase-3 or cleaved PARP-1 proteins were observed in gemcitabine treatment alone (Fig. 2).
Figure 2.

Effects of gemcitabine (Gem) and sunitinib (Su) treatment on cleavage of caspase-3 and PARP-1. A cell monolayer was treated with Gem (10 µM) or Su (10 µM) for 16 h. Western blot analysis showed the cellular expression of cleaved caspase-3 (18 kda), full-length PARP-1 (116 kDa), cleaved PARP-1 (89 kDa), and loading control α-tubulin (55 kDa). The intensity of bands was quantitated by densitometry and is represented in the bar graph after normalizing values with α-tubulin expression. Data represent results of two independent experiments in identical conditions
Effects of gemcitabine and sunitinib on local tumour growth
Sunitinib alone was quite effective in inhibiting local tumour growth in subcutaneous murine PDAC xenografts (Fig. 3). Gemcitabine alone displayed a moderate inhibitory effect on local tumour growth, but the combination of Su + Gem was more effective than either monotherapy. The relative net tumour growth compared with control referenced at 100% in the Gem, Su and Su + Gem groups was 57%, 6% and 1%, respectively. Tumour weight at the time of experiment termination was highly correlated with tumour volume (r2 = 0.875).
Figure 3.

Effects of gemcitabine (Gem) and sunitinib (Su) treatment on local tumour growth. AsPC-1 cells (0.75 × 106) were injected subcutaneously into nude mice. At 14 days post-injection, mice were treated with phosphate-buffered saline (PBS, control), Gem or Su, either alone or in combination, for 2 weeks. (A) Tumour size was measured twice weekly with callipers. (B) Net tumour growth was calculated by subtracting tumour volume on the first day of treatment from that on the last day. Data represent mean ± standard deviation values (n = 6–8 mice per group)
Effects of gemcitabine and sunitinib on intratumoral proliferative and apoptotic activity
Analysis of intratumoral proliferative activity within tumour tissue sections showed a significant reduction in Ki67-positive cells in sunitinib-treated cells consistent with a decrease in the frequency of dividing cells. Gemcitabine alone caused a small decrease in Ki67-positive cells. The Su + Gem combination was effective, but did not enhance intratumoral proliferation inhibition beyond levels achieved by sunitinib alone (Fig. 4). The Gem, Su and Su + Gem groups showed reductions in the proliferative index of 33%, 82% and 75%, respectively, of that in controls. Increases in TUNEL-positive cells were observed in the Gem and Su groups. The apoptotic indices (TUNEL-positive cells/total cells per HPF) in the control, Gem, Su and Su + Gem groups were 0.13 ± 0.03, 0.21 ± 0.06, 0.47 ± 0.05 and 0.54 ± 0.01, respectively (P < 0.00002 vs. control).
Figure 4.
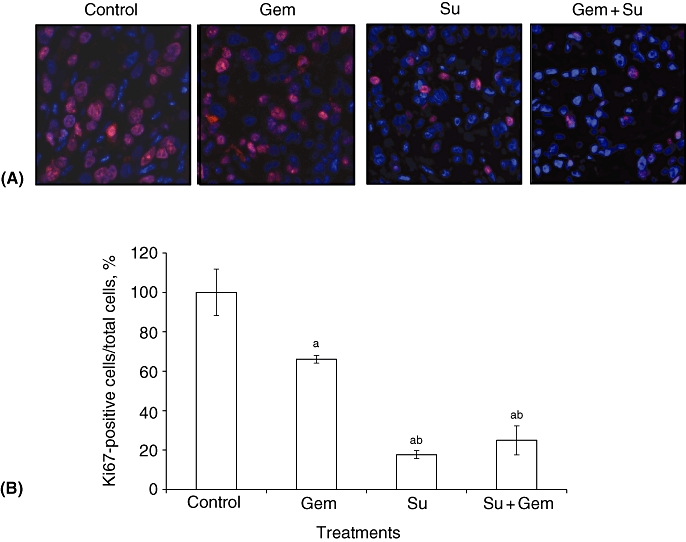
Effects of gemcitabine (Gem) and sunitinib (Su) treatment on intratumoral proliferative activity. AsPC-1 cells (0.75 × 106) were injected subcutaneously into nude mice. At 14 days post-injection, mice were treated with phosphate-buffered saline (PBS, control), Gem or Su for 2 weeks. (A) Tumour tissue sections were immunostained with Ki67 nuclear antigen antibody and photographed. (B) Ki67-positive cells were counted per high-power field and the mean counts of five fields were calculated. Data represent mean ± standard deviation values. Significant differences at P < 0.05 compared with the acontrol and bGem groups
Effects of gemcitabine and sunitinib on animal survival
Animal survival studies performed in AsPC-1 PDAC murine xenografts in SCID-NOD mice resulted in a median survival of 16 days in the control group. This increased modestly after sunitinib (median: 21 days) and gemcitabine (median: 24 days), but the Su + Gem combination led to a significant further increase in survival (median: 30 days; P = 0.007) (Fig. 5).
Figure 5.
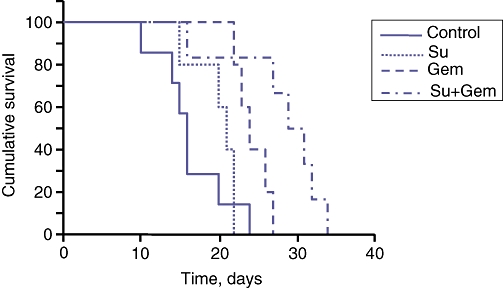
Effects of gemcitabine (Gem) and sunitinib (Su) treatment on overall mouse survival. AsPC-1 cells (0.75 × 106) were injected intraperitoneally into SCID mice. At 14 days post-injection, treatment was started with Gem (100 mg/kg twice weekly) or Su (40 mg/kg for 1st week, then 20 mg/kg for 2nd week) for 2 weeks. Curves represent survival time from the first day of treatment until death. The survival difference between the Su + Gem treatment group and the control group was significant at P = 0.007
Discussion
Early metastasis, late-stage diagnosis, high local recurrence risk and generalized resistance to conventional chemotherapy continue to impact survival in pancreatic cancer. Gemcitabine represents the standard cytotoxic agent used in patients with this disease. However, gemcitabine mediates only limited benefit, both when administered alone and in combination with other chemotherapy agents26–28 or antiangiogenic agents.2–4,29 Tumour growth is dependent on the complex interaction between multiple components, including epithelial tumour cells, endothelial cells and fibroblasts that are exposed to a milieu of growth factors and cytokines that probably stimulate multiple signalling pathways including those that are not inhibited by the therapy administered. Combining conventional cytotoxic drugs with novel targeted agents that specifically interfere with key operational pathways responsible for PDAC progression has recently gained much attention in the effort to identify a novel, more effective and less cytotoxic approach to treating PDAC.
Sunitinib is a multi-target RTK inhibitor with antiangiogenic and antitumour activity.6,7,30 Sunitinib inhibits VEGF receptors, PDGF receptors, as well as c-KIT, FLT-3 and RET receptors, all of which can play an important role in tumour progression.10 The broader target spectrum of sunitinib renders it particularly interesting as an agent in combination therapy, as narrowly defined tyrosine kinase inhibitors (TKIs) have so far lacked greater clinical activity. In the present study, the combined effects of gemcitabine and sunitinib were evaluated in vitro and in vivo. Pancreatic cancer cell lines display marked heterogeneity towards gemcitabine.31 As PDAC is clinically characterized as very aggressive and highly resistant to chemotherapy, AsPC-1 cells were used in the present study because they are only modestly responsive to gemcitabine in vitro and display a reliable in vivo tumorigenicity. Sunitinib not only significantly inhibited AsPC-1 cell proliferation, but also inhibited the proliferation of endothelial cells and fibroblasts. Modest additive effects were observed for sunitinib administered in combination with gemcitabine.
These findings indicate that sunitinib affects tumour cells, endothelial cells and fibroblasts, which supports the proposal that the antitumour activity of sunitinib may rely on its direct effects on tumour cells as well as on stromal components including the tumour vasculature.6,7,10–12 The antitumour activity of sunitinib has been shown to be accompanied by induction in apoptosis32 and the present findings show that sunitinib administered either alone or in combination with gemcitabine induced cleaved caspase-3 and cleaved PARP-1 protein, thereby indicating induction in apoptosis. In accordance with these in vitro effects, sunitinib alone resulted in a significant reduction in local tumour growth accompanied by a reduction in intratumoral proliferative activity, indicating that sunitinib obviously has a direct effect on tumour cells in vivo. Gemcitabine alone also inhibited net tumour growth and intratumoral proliferative activity and the combination of Su + Gem was effective, but not significantly more than sunitinib alone. A recently published report showed that a 1-week treatment with Su + Gem elicited greater antitumour effects than either agent administered alone in Pan02 murine xenografts.33 The present study selected a longer therapy interval of 2 weeks, which might lead to an extended progression-free control. The studies were further extended to observe the effects of the combination of these agents on actual animal survival. Although the exact mechanism or optimal combination ability with additional targeting agents is not yet known, these findings may provide a foundation for future analyses. The question of whether this combination approach will beneficially affect clinical PDAC therapy remains open because these results are based on a single cell line study in a xenograft setting and clinical tumours are expected to represent a wide spectrum of genetic heterogeneity that may limit the presence of the susceptibility parameters necessary to mediate this combination benefit. Defining these markers and validating them clinically will therefore be an important future undertaking.
Taken together, the results of the present study show that a combination of gemcitabine and sunitinib can have additive effects in vitro and in vivo in an experimental pancreatic cancer model. These findings support the rationale for combining gemcitabine with multi-target antiangiogenic agents in the search for a novel and more effective therapy for PDAC.
Conflicts of interest
None declared.
References
- 1.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 2.Ko AH, Dito E, Schillinger B, Venook AP, Xu Z, Bergsland EK, et al. A phase II study evaluating bevacizumab in combination with fixed-dose rate gemcitabine and low-dose cisplatin for metastatic pancreatic cancer: is an anti-VEGF strategy still applicable? Invest New Drugs. 2008;26:463–471. doi: 10.1007/s10637-008-9127-2. [DOI] [PubMed] [Google Scholar]
- 3.Dragovich T, Burris H, III, Loehrer P, Von Hoff DD, Chow S, Stratton S, et al. Gemcitabine plus celecoxib in patients with advanced or metastatic pancreatic adenocarcinoma: results of a phase II trial. Am J Clin Oncol. 2008;31:157–162. doi: 10.1097/COC.0b013e31815878c9. [DOI] [PubMed] [Google Scholar]
- 4.Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomized study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 6.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumour activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 7.Abrams TJ, Murray LJ, Pesenti E, Holway VW, Colombo T, Lee LB, et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with ‘standard of care’ therapeutic agents for the treatment of breast cancer. Mol Cancer Ther. 2003;2:1011–1021. [PubMed] [Google Scholar]
- 8.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 10.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25:884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 11.Ikezoe T, Nishioka C, Tasaka T, Yang Y, Komatsu N, Togitani K, et al. The antitumour effects of sunitinib (formerly SU11248) against a variety of human haematologic malignancies: enhancement of growth inhibition via inhibition of mammalian target of rapamycin signalling. Mol Cancer Ther. 2006;5:2522–2530. doi: 10.1158/1535-7163.MCT-06-0071. [DOI] [PubMed] [Google Scholar]
- 12.Seandel M, Shia J, Linkov I, Maki RG, Antonescu CR, Dupont J. The activity of sunitinib against gastrointestinal stromal tumour seems to be distinct from its antiangiogenic effects. Clin Cancer Res. 2006;12(20 Pt 1):6203–6204. doi: 10.1158/1078-0432.CCR-06-1292. [DOI] [PubMed] [Google Scholar]
- 13.Prenen H, Cools J, Mentens N, Folens C, Sciot R, Schoffski P, et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumour mutants refractory to imatinib mesylate. Clin Cancer Res. 2006;12:2622–2627. doi: 10.1158/1078-0432.CCR-05-2275. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto K, Hosotani R, Wada M, Lee JU, Koshiba T, Miyamoto Y, et al. Expression of two angiogenic factors, vascular endothelial growth factor and platelet-derived endothelial cell growth factor in human pancreatic cancer, and its relationship to angiogenesis. Eur J Cancer. 1998;34:1439–1447. doi: 10.1016/s0959-8049(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 15.Korc M. Pancreatic cancer-associated stroma production. Am J Surg. 2007;194(4) Suppl.:84–86. doi: 10.1016/j.amjsurg.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang YT, Chang MC, Wei SC, Tien YW, Hsu C, Liang PC, et al. Serum vascular endothelial growth factor/soluble vascular endothelial growth factor receptor 1 ratio is an independent prognostic marker in pancreatic cancer. Pancreas. 2008;37:145–150. doi: 10.1097/MPA.0b013e318164548a. [DOI] [PubMed] [Google Scholar]
- 17.Cuneo KC, Geng L, Fu A, Orton D, Hallahan DE, Chakravarthy AB. SU11248 (sunitinib) sensitizes pancreatic cancer to the cytotoxic effects of ionizing radiation. Int J Radiat Oncol Biol Phys. 2008;71:873–879. doi: 10.1016/j.ijrobp.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 18.Tran Cao HS, Bouvet M, Kaushal S, Keleman A, Romney E, Kim G, et al. Metronomic gemcitabine in combination with sunitinib inhibits multisite metastasis and increases survival in an orthotopic model of pancreatic cancer. Mol Cancer Ther. 2010;9:2068–2078. doi: 10.1158/1535-7163.MCT-10-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Awasthi N, Schwarz MA, Verma V, Cappiello C, Schwarz RE. Endothelial monocyte activating polypeptide II interferes with VEGF-induced proangiogenic signalling. Lab Invest. 2009;89:38–46. doi: 10.1038/labinvest.2008.106. [DOI] [PubMed] [Google Scholar]
- 20.Solorzano CC, Hwang R, Baker CH, Bucana CD, Pisters PW, Evans DB, et al. Administration of optimal biological dose and schedule of interferon alpha combined with gemcitabine induces apoptosis in tumour-associated endothelial cells and reduces growth of human pancreatic carcinoma implanted orthotopically in nude mice. Clin Cancer Res. 2003;9:1858–1867. [PubMed] [Google Scholar]
- 21.de Bouard S, Herlin P, Christensen JG, Lemoisson E, Gauduchon P, Raymond E, et al. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9:412–423. doi: 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz RE, Konduri S, Awasthi N, Cafasso D, Schwarz MA. An antiendothelial combination therapy strategy to increase survival in experimental pancreatic cancer. Surgery. 2009;146:241–249. doi: 10.1016/j.surg.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Awasthi N, Schwarz MA, Schwarz RE. Enhancing cytotoxic agent activity in experimental pancreatic cancer through EMAP II combination therapy. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-010-1514-7. doi: 10.1007/s00280-010-1514-7. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz RE, Awasthi N, Konduri S, Cafasso D, Schwarz MA. EMAP II-based antiangiogenic-antiendothelial in vivo combination therapy of pancreatic cancer. Ann Surg Oncol. 2010;17:1442–1452. doi: 10.1245/s10434-009-0879-5. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz RE, McCarty TM, Peralta EA, Diamond DJ, Ellenhorn JD. An orthotopic in vivo model of human pancreatic cancer. Surgery. 1999;126:562–567. [PubMed] [Google Scholar]
- 26.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB., III Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–3275. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 27.Rocha Lima CM, Green MR, Rotche R, Miller WH, Jr, Jeffrey GM, Cisar LA, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumour response rate. J Clin Oncol. 2004;22:3776–3783. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 28.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–3952. doi: 10.1200/JCO.2005.05.1490. [DOI] [PubMed] [Google Scholar]
- 30.O'Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–3605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 31.Akada M, Crnogorac-Jurcevic T, Lattimore S, Mahon P, Lopes R, Sunamura M, et al. Intrinsic chemoresistance to gemcitabine is associated with decreased expression of BNIP3 in pancreatic cancer. Clin Cancer Res. 2005;11:3094–3101. doi: 10.1158/1078-0432.CCR-04-1785. [DOI] [PubMed] [Google Scholar]
- 32.Yang F, Jove V, Xin H, Hedvat M, Van Meter TE, Yu H. Sunitinib induces apoptosis and growth arrest of medulloblastoma tumour cells by inhibiting STAT3 and AKT signalling pathways. Mol Cancer Res. 2010;8:35–45. doi: 10.1158/1541-7786.MCR-09-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casneuf VF, Demetter P, Boterberg T, Delrue L, Peeters M, Van Damme N. Antiangiogenic versus cytotoxic therapeutic approaches in a mouse model of pancreatic cancer: an experimental study with a multitarget tyrosine kinase inhibitor (sunitinib), gemcitabine and radiotherapy. Oncol Rep. 2009;22:105–113. doi: 10.3892/or_00000412. [DOI] [PubMed] [Google Scholar]


