Abstract
Objectives
Although lymphatic spread is common in intrahepatic cholangiocarcinoma (ICC), lymphadenectomy is not widely performed as part of operative resection in this disease. The objectives of this study were to assess national trends for lymphadenectomy and its impact on survival in patients with ICC.
Methods
The National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) registry was queried to identify patients with ICC (n = 4893) reported during 1988–2007. Kaplan–Maier and Cox proportional hazards regression were used to analyse survival.
Results
Five-year overall survival (OS) was 5.2%. Lymph node (LN) status was available for 48.9% (n = 2391) of patients. Histologic LN evaluation was performed in 13.5% (n = 658) of patients for a median of two (interquartile range: 1–3) LNs. During the study period, the frequency of histologic LN assessment (P = 0.78) did not change in liver resection patients. In the 733 resected patients, positive vs. negative LN status was associated with worse 5-year OS of 8.4% vs. 25.9%, respectively (hazard ratio = 1.8; P < 0.001).
Conclusions
Nodal status is an important prognostic factor for survival in patients diagnosed with ICC. In the USA, few patients undergo hepatic resection with lymphadenectomy; therefore, the clinical benefit of formal lymphadenectomy in ICC remains unknown.
Keywords: cholangiocarcinoma, resection < cholangiocarcinoma, outcomes < cholangiocarcinoma
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy worldwide.1 The incidence of ICC in the USA has increased over the last two decades by 165%.2–5 Despite advances in hepatic resection techniques and decreased perioperative mortality, 5-year overall survival (OS) in resected ICC patients is only 15–40%.6,7 Surgical resection currently represents the only hope of cure. Because of the rarity of the disease, as well as the limitations of single-institution studies, the optimal treatment strategy for ICC has yet to be defined.
The anatomic classification of ICC includes ‘peripheral’ cholangiocarcinoma and is considered to be distinct from extrahepatic cholangiocarcinoma (ECC), which includes Klatskin (hilar) tumours located at the bile duct bifurcation and more ‘distal’ bile duct cancers. Unfortunately, the lack of a consistent anatomic classification scheme and the frequent inclusion of hilar cholangiocarcinoma and gallbladder cancer have complicated the interpretation of existing studies of surgical interventions in ICC.5,8 This discrepancy was addressed in the 7th edition of the American Joint Committee on Cancer (AJCC) Staging Manual.9,10 The revised tumour–node–metastasis (TNM) staging system proposed by Nathan et al.9 places more emphasis on the extent of invasion than on tumour size. Importantly, lymph node (LN) involvement is a key feature of the AJCC staging scheme.
Despite the known predilection of this malignancy to spread to local LNs, lymphadenectomy is not a widely performed component of surgical resection for ICC in Western medical centres. The rarity of resectable ICC and the inadequacies of the findings of under-powered, single-institution studies have limited the ability to assess the utility of lymphadenectomy. The aim of this study was to utilize the US National Cancer Institute (NCI) Surveillance, Epidemiology and End Results (SEER) database (http://seer.cancer.gov) to characterize national trends in LN evaluation in unresectable and resectable patients with ICC. In addition, the survival of patients with ICC based on LN status was evaluated.
Materials and methods
This is a retrospective Mayo Clinic Institutional Review Board-approved cohort study using the SEER cancer registry database. The NCI SEER programme produces a public-use database representing 17 population-based cancer registries.11 The 1973–2007 SEER database contains cancer-specific data on incidence rates, individual patient and tumour characteristics, treatment and follow-up survival rates. Previous studies of ICC have utilized the SEER database to establish the AJCC staging system, assess the survival benefit of surgical resection and adjuvant radiation therapy, and describe the incidence of ICC over the last three decades.2,9,12–14 The SEER database was accessed remotely using the NCI surveillance research software SEER*Stat Version 6.6.2 (http://seer.cancer.gov/seerstat), following authorization from the NCI in August 2010.
Subject identification
The study group was limited to patients reported during 1988–2007 because details on regional LN sampling or dissection were not collected prior to 1988. The study group was derived from 15 registries within the SEER programme and excluded the Rural Georgia and the Alaska Native Tumor Registries, which each reported fewer than 15 patients diagnosed with ICC during this time interval. Patients in Louisiana during July–December 2005 were not included because of disruption caused by hurricane Katrina. Using the International Classification of Diseases for Oncology (3rd edition, ICD-O-3) topography code for liver (22.0), combined with the histology code for cholangiocarcinoma (8160), and the topography code for intrahepatic bile duct (22.1), combined with histology codes for malignant neoplasm (8000), malignant tumour cells (8001), carcinoma (8010), undifferentiated carcinoma (8020), adenocarcinoma (8140), and cholangiocarcinoma (8160), 6650 patients with ICC were identified.15
Specific exclusion criteria were then applied to ensure the identification of ICC patients. Exclusion criteria included: diagnosis at death or on the birth certificate; hilar and extrahepatic cholangiocarcinoma; gallbladder cholangiocarcinoma; hepatocellular carcinoma; metastatic adenocarcinoma (e.g. pancreatic cancer); treatment with liver transplant, and age of <18 years. Five patients with no evidence of malignancy (T0) on final pathology were excluded. Further exclusion criteria included: diagnosis by clinical diagnosis only without confirmatory tissue diagnosis; diagnosis based on direct visualization without microscopic confirmation; diagnosis based on only positive laboratory tests or markers, and diagnosis by radiographic images only without microscopic confirmation. Using the above selection criteria, 4893 patients were identified with histology of cholangiocarcinoma (n = 4166, 85.1%), bile duct adenocarcinoma (n = 631, 12.9%), bile duct carcinoma (n = 68, 1.4%), malignant neoplasm of the bile duct (n = 18, 0.4%), malignant tumour cells of the bile duct (n = 8, 0.2%), and undifferentiated bile duct carcinoma (n = 2, 0.04%).
Identification of procedural codes
Site-specific surgery codes for ICC used in the SEER database have changed several times over the last 30 years. Factoring changes in surgery codes, site-specific surgery was recoded into more general categories of: no surgical procedure (n = 3303, 67.5%); biopsy only (n = 654, 13.4%); limited surgical therapy (n = 81, 1.7%); wedge resection, segmental resection, simple excision of tumour (n = 279, 5.7%); lobectomy, extended lobectomy, hepatectomy, with or without lymphadenectomy, with or without bile duct resection (n = 454, 9.3%); not otherwise specified (NOS) (n = 87, 1.8%), and missing (n = 35, 0.7%). A subgroup, ‘major liver resection’ (n = 733, 15.0%), consisted of patients treated with wedge resection, segmental resection, simple excision of tumour, lobectomy, extended lobectomy or hepatectomy. Liver biopsy and tumour ablation were not considered major liver resections.
American Joint Committee on Cancer staging
Staging for patients reported during 2004–2007 was reported according to the AJCC Staging Manual, 6th edition.16 In order to establish tumour stage for all study patients, extent of disease (EoD) and collaborative staging (CS) codes provided by SEER were used to derive each patient's overall stage and individual T, N and M classifications according to the AJCC Staging Manual, 7th edition.10 The new AJCC, 7th edition T-classification scheme emphasizes tumour invasion and growth pattern rather than size; as the SEER registry did not code periductal invasion, it was not possible to assign patients with a T4 designation or use T4 to derive stage IVa disease. Therefore, stage IVa disease was defined as any T-stage and evidence of regional LN metastasis.
Lymph node assessment
The SEER database provides a detailed record of regional LN evaluation after 1988, including type of LN evaluation (e.g. aspiration), number of regional nodes examined and number of histologically positive regional nodes. During 1988–2003, specific LN regions were coded; however, during 2004–2007, these data were consolidated into data on regional or distant LNs, which prevents analysis based on the site or location of LNs evaluated. The SEER registry used EoD (1988–2003) and CS (2004–2007) codes to document LN involvement based on clinical and pathology records. These codes were used to define AJCC 7th edition N-stage. Regional LN evaluation and positive or negative status were based on pathology reports only and required histologic confirmation. A subgroup (n = 658, 13.5%) of patients, ‘lymph nodes evaluated’, was defined using the code for ‘regional nodes examined’ provided by SEER. Lymph nodes evaluated by aspiration (n = 15, 0.3%) were included in this subgroup.
Follow-up and survival
The date of last follow-up (collected annually) was defined as the last date the patient was actually seen by a physician or contacted by the hospital registry, or as the date of death. Overall survival time was calculated using the date of death, last date known to be alive or the follow-up cut-off date for the dataset (November 2009). If a patient had died within 1 month of diagnosis, survival time was coded as 0.1 month to avoid a time bias in survival (n = 406, 8.3%). Because estimates of overall and disease-specific survival for the entire study group were similar, only OS is reported.
Statistical analysis
Statistical analyses were performed using sas Version 9.2 (SAS Institute, Inc., Cary, NC, USA). Analyses were performed for the entire cohort (n = 4893), as well as for subgroups of patients who underwent major liver resection (n = 733) and patients in whom LNs were evaluated (n = 658). To verify the recoding schemes and completeness of the dataset, additional subgroup analyses were performed by registry and for time intervals in which SEER made major changes in coding definitions (1988–1997, 1998–2003, 2004–2007). Variables in the analyses included age, sex, race, diagnosis year, registry, histology, grade, AJCC 7th edition stage, TNM classification, tumour size, number of regional LNs evaluated, number of positive regional LNs, radiation treatment, cancer-directed surgery (as defined by SEER), and site-specific surgery.
Descriptive statistics are reported for all study variables as the median with interquartile range (IQR) or the count with percentage, as appropriate. The association of metastatic disease documentation (M0, M1, unknown) and LN status (N0, N1, unknown) was assessed using the chi-squared test. Linear regression was used to assess the association between diagnosis year and the number of LNs evaluated. Univariate and multivariable survival analyses were performed using Cox proportional hazards regression. Survival was assessed in three patient cohorts comprising: all patients; the subset of patients who underwent evaluation of at least one LN, and the subset of patients who underwent major liver resection. Multivariable models were developed based on a univariate overall P-value of <0.05 and missing data of <10% (Table 1). The backward selection method was used to identify variables independently associated with survival. Those variables considered for the subgroup multivariable models were age, diagnosis year, radiation, AJCC 7th edition TNM classification, and major liver resection. Proportional hazards assumptions were investigated. Univariate logistic regression was used to identify factors associated with undergoing LN evaluation. All tests with a P-value of <0.05 were accepted as indicating statistical significance.
Table 1.
Intrahepatic cholangiocarcinoma patient characteristics (n = 4893)
| Variable | Missing, n (%) | |
|---|---|---|
| Male sex, n (%) | 2539 (51.9) | 0 (0) |
| White race, n (%) | 3936 (80.6) | 9 (0.2) |
| Age, years, median (IQR) | 68 (57–76) | 0 (0) |
| Diagnosis year, n (%) | 0 (0) | |
| 1988–1997 | 1265 (25.8) | |
| 1998–2003 | 1913 (39.1) | |
| 2004–2007 | 1715 (35.1) | |
| Grade, n (%) | 2778 (56.8) | |
| Well differentiated | 308 (14.6) | |
| Moderately differentiated | 863 (40.8) | |
| Poorly differentiated | 890 (42.1) | |
| Undifferentiated | 54 (2.6) | |
| Tumour size, cm, median (IQR) | 6.0 (3.8–9.0) | 2865 (58.6) |
| AJCC 7th edn stage, n (%) | 901 (18.4) | |
| Stage I | 939 (23.5) | |
| Stage II | 142 (3.6) | |
| Stage III | 712 (17.8) | |
| Stage IV | 2199 (55.1) | |
| T-stage, n (%) | 1788 (36.5) | |
| T1 | 1053 (33.9) | |
| T2a | 171 (5.5) | |
| T2b | 877 (28.2) | |
| T3 | 1004 (32.3) | |
| T4 | a | |
| N-stage (N0), n (%) | 1788 (74.8) | 2503 (51.1) |
| M-stage (M0), n (%) | 2632 (64.2) | 792 (16.2) |
| Histologic regional LN evaluation, n (%) | 658 (13.5) | 185 (3.8) |
| Radiation therapy, n (%) | 767 (16.0) | 89 (1.8) |
| Major liver resection, n (%) | 733 (15.1) | 35 (0.7) |
The AJCC Staging Manual, 7th edition, defines T4 as tumour with periductal invasion. Cases in the SEER registry did not document periductal invasion and therefore no T4 cases were recorded.
IQR, interquartile range; AJCC, American Joint Committee on Cancer; LN, lymph node.
Results
Patient characteristics
Of the 6650 patients identified in the SEER registry for 1988–2007, 4893 (73.6%) were eligible for inclusion in this study. Patient and tumour characteristics are outlined in Table 1.
Lymph node evaluation
Clinical LN status (N0 and N1) was available for 2391 (48.9%) patients. Major liver resection was performed in 733 (15.0%) patients, of whom 361 (49.3%) patients underwent histologic LN evaluation. Histologic status (positive or negative) of regional LNs was documented in 658 (13.5%) patients. Regional LNs were not evaluated in 4050 (82.8%) patients and LN evaluation data were missing in 185 (3.8%) patients.
Evidence of distant disease was significantly associated with documentation of N-stage (P < 0.001). N-stage was documented in 1798 of 2632 (68.3%) patients with no evidence of metastatic disease (M0) and 556 of 1470 (37.8%) patients with metastatic disease (M1).
Of the 658 patients in whom LN evaluation was conducted, the number of nodes evaluated was documented in 529 (80.4%) cases. The number of LNs examined changed over time (P < 0.001) from a median of one LN (IQR: 1–3) in 1988–1997 to two LNs (IQR: 1–4) in 2004–2007. In 15 (2.3%) patients, LNs were evaluated by fine needle aspiration alone. Data on nodal status were missing for one (0.2%) patient. The median number of LNs examined was two (IQR: 1–3) in the entire cohort, two (IQR: 1–4) in liver resection patients, and one (IQR: 1–2) in non-liver resection patients. One LN was evaluated in 248 (46.9%) patients, two LNs in 91 (17.2%) patients, five LNs in 27 (5.1%) and over 10 LNs in only 32 (6.1%) patients.
Positive LNs were confirmed in 317 patients (48.2% of those evaluated) at a median of one positive node (IQR: 1–2). Of patients for whom the number of positive nodes was documented (n = 244), 163 (66.8%) had one positive node, 40 (16.4%) had two positive nodes, 28 (11.5%) had three or four positive nodes, 10 (4.1%) had five to nine positive nodes and only three (1.2%) had 10 or more positive nodes.
Factors associated with LN evaluation
Factors associated with performing LN evaluation were assessed with univariate logistic regression (Table 2). Histologic LN status was documented in 19.1% of patients with M0 disease and 10.0% of M1 patients. A patient with M1 status was 0.5 times as likely as an M0 patient to undergo LN evaluation (P < 0.001). Among all patients, the median age of those who underwent regional LN evaluation was significantly lower than that of those who did not (62 years vs. 69 years; odds ratio [OR] = 0.7 per 10 years; P < 0.001). Increased tumour size was associated with decreased odds of LN evaluation, but this difference was not clinically significant (OR = 0.97 per 1 cm; P = 0.014). Later year of diagnosis was associated with decreased odds of LN evaluation, but this difference was also not clinically significant (OR = 0.98 per 1 year; P = 0.014). At least one LN was evaluated in 188 (15.5%) patients during 1988–1997, 241 (13.1%) patients during 1998–2003, and 229 (13.8%) patients during 2004–2007.
Table 2.
Univariate analysis of factors associated with lymph node evaluation in patients with intrahepatic cholangiocarcinoma
| All patients (n = 4893) | Major liver resection (n = 733) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Agea | 0.71 | (0.67–0.76) | <0.001 | 0.71 | (0.63–0.80) | <0.001 |
| White race | 1.31 | (1.05–1.64) | 0.018 | 1.28 | (0.88–1.88) | 0.197 |
| Female | 1.05 | (0.89–1.24) | 0.572 | 0.88 | (0.66–1.18) | 0.390 |
| Tumour size | 0.97 | (0.94–0.99) | 0.014 | 1.00 | (0.96–1.05) | 0.987 |
| AJCC 7th edition M1 | 0.48 | (0.39–0.58) | <0.001 | 0.87 | (0.53–1.43) | 0.587 |
| Diagnosis yearb | 0.98 | (0.96–1.00) | 0.014 | 1.00 | (0.97–1.04) | 0.780 |
| Major liver resection | 12.68 | (10.50–15.31) | <0.001 | – | – | – |
Odds ratio per 10-year increment.
Modelled as a continuous variable.
OR, odds ratio; 95% CI, 95% confidence interval; AJCC, American Joint Committee on Cancer.
Significant variation in the percentages of patients undergoing regional LN evaluation was noted between registries, with the highest observed percentage (24.8%) in the Atlanta registry and the lowest (10.9%) in the Connecticut registry (P = 0.003). Los Angeles was chosen as the reference registry as it contributed the highest number of patients. Most registries had an OR close to 1.0 (similar odds of having LNs evaluated). Registries in which the odds of LN evaluations were significantly increased were those in Atlanta (OR = 2.3, 95% confidence interval [CI] 1.5–3.5) and Kentucky (OR = 1.7, 95% CI 1.1–2.7).
In patients treated with liver resection, age was associated with LN assessment (OR = 0.7 per 10 years; P < 0.001). Tumour size (P = 0.987), year of diagnosis (P = 0.780) and reporting registry (P = 0.734) were not associated with performance of a lymphadenectomy in patients treated with major liver resection.
Survival analysis
Overall 5-year survival was 5.2% and disease-specific 5-year survival was 7.5% for all study patients. Clinical characteristics potentially associated with survival in all patients and in the subgroups of LN evaluation patients and major liver resection patients are summarized in Table 3.
Table 3.
Univariate survival analysis for all patients and subgroups
| All patients (n = 4893) | Major liver resection (n = 733) | Lymph nodes evaluated (n = 658) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Agea | 1.2 | 1.1–1.2 | <0.001 | 1.1 | 1.0–1.2 | 0.022 | 1.1 | 1.0–1.2 | 0.001 |
| Male sex | 1.1 | 1.0–1.2 | 0.002 | 1.1 | 0.9–1.3 | 0.550 | 1.1 | 1.0–1.3 | 0.176 |
| Diagnosis yearb | 1.0 | 1.0–1.0 | <0.001 | 1.0 | 0.9–1.0 | <0.001 | 1.0 | 0.9–1.0 | <0.001 |
| Non-White race | 1.1 | 1.0–1.1 | 0.253 | 1.0 | 0.8–1.2 | 0.818 | 1.2 | 1.0–1.5 | 0.084 |
| Grade | |||||||||
| Well differentiated | 1.0 | – | Ref | 1.0 | – | Ref | 1.0 | – | Ref |
| Moderately differentiated | 1.1 | 1.0–1.3 | 0.100 | 1.3 | 1.0–1.9 | 0.087 | 1.3 | 1.0–1.9 | 0.101 |
| Poorly differentiated | 1.6 | 1.4–1.8 | <0.001 | 1.9 | 1.3–2.6 | <0.001 | 2.0 | 1.4–2.8 | <0.001 |
| Undifferentiated | 2.1 | 1.5–2.8 | <0.001 | 2.4 | 1.1–5.1 | 0.023 | 2.4 | 1.0–5.2 | 0.037 |
| Regional lymph node status | |||||||||
| Negative | 1.0 | – | Ref | 1.0 | – | Ref | 1.0 | – | Ref |
| Positive | 1.8 | 1.5–2.1 | <0.001 | 1.8 | 1.4–2.4 | <0.001 | 1.9 | 1.6–2.3 | <0.001 |
| Unknown | 2.3 | 2.1–2.7 | <0.001 | 1.1 | 0.9–1.3 | 0.433 | – | – | – |
| Radiation therapy | 0.7 | 0.7–0.8 | <0.001 | 1.1 | 0.9–1.4 | 0.253 | 0.9 | 0.7–1.0 | 0.115 |
| Major liver resection | 0.3 | 0.3–0.4 | <0.001 | – | – | – | 0.4 | 0.3–0.5 | <0.001 |
| Tumour size (cm) | 1.0 | 1.0–1.0 | 0.004 | 1.0 | 1.0–1.1 | 0.018 | 1.0 | 1.0–1.1 | 0.015 |
| AJCC 7th edition stage | |||||||||
| Stage I | 1.0 | – | Ref | 1.0 | – | Ref | 1.0 | – | Ref |
| Stage II | 0.9 | 0.7–1.1 | 0.169 | 1.7 | 1.2–2.5 | 0.005 | 1.4 | 0.8–2.4 | 0.296 |
| Stage III | 1.6 | 1.4–1.7 | <0.001 | 1.9 | 1.4–2.5 | <0.001 | 1.5 | 1.0–2.3 | 0.046 |
| Stage IVa | 1.3 | 1.2–1.5 | <0.001 | 2.7 | 2.1–3.4 | <0.001 | 2.5 | 1.9–3.3 | <0.001 |
| Stage IVb | 2.4 | 2.2–2.7 | <0.001 | 4.3 | 3.0–6.1 | <0.001 | 5.5 | 4.0–7.6 | <0.001 |
| AJCC 7th edition T-stagec | |||||||||
| T1 | 1.0 | – | Ref | 1.0 | – | Ref | 1.0 | – | Ref |
| T2a | 0.9 | 0.7–1.0 | 0.091 | 1.7 | 1.2–2.3 | 0.004 | 1.3 | 0.9–2.1 | 0.167 |
| T2b | 1.5 | 1.4–1.7 | <0.001 | 2.0 | 1.5–2.7 | <0.001 | 1.8 | 1.4–2.5 | <0.001 |
| T3 | 1.4 | 1.3–1.5 | <0.001 | 2.6 | 2.1–3.3 | <0.001 | 2.2 | 1.7–2.8 | <0.001 |
| AJCC 7th edition N-stage | |||||||||
| N0 | 1.0 | – | Ref | 1.0 | – | Ref | 1.0 | – | Ref |
| N1 | 1.3 | 1.2–1.4 | <0.001 | 2.0 | 1.6–2.5 | <0.001 | 1.9 | 1.6–2.3 | <0.001 |
| Unknown N-stage | 1.7 | 1.6–1.8 | <0.001 | 1.6 | 1.3–2.0 | <0.001 | – | – | – |
| AJCC 7th edition M-stage | |||||||||
| M0 | 1.0 | – | Ref | 1.0 | – | Ref | 1.0 | – | Ref |
| M1 | 2.0 | 1.8–2.1 | <0.001 | 2.5 | 1.9–3.3 | <0.001 | 2.6 | 2.2–3.2 | <0.001 |
Age in 10-year increments.
Modelled as a continuous variable.
The AJCC Staging Manual, 7th edition, defines T4 as tumour with periductal invasion. Cases in the SEER registry do not document periductal invasion and therefore no T4 cases were recorded.
95% CI, 95% confidence interval; HR, hazard ratio; AJCC, American Joint Committee on Cancer.
Both AJCC 7th edition clinical N-stage (Fig. 1A) and histologic LN status (Fig. 2A) were associated with survival for all ICC patients. Five-year OS was worse in N1 patients compared with N0 patients, at 2.3% vs. 9.8%, respectively (N1 hazard ratio [HR] = 1.3; P < 0.001). Five-year OS was worse in histologically node-positive patients compared with node-negative patients, at 3.7% vs. 19.3%, respectively (node-positive HR = 1.8; P < 0.001). In patients with unknown clinical N-stage, 5-year OS was 2.8%. Five-year OS in patients with unknown histologic LN status was 4.1%. After controlling for age, race, sex, SEER registry and history of major liver resection, performance of a histologic LN evaluation was not an independent predictor of survival (P = 0.138).
Figure 1.
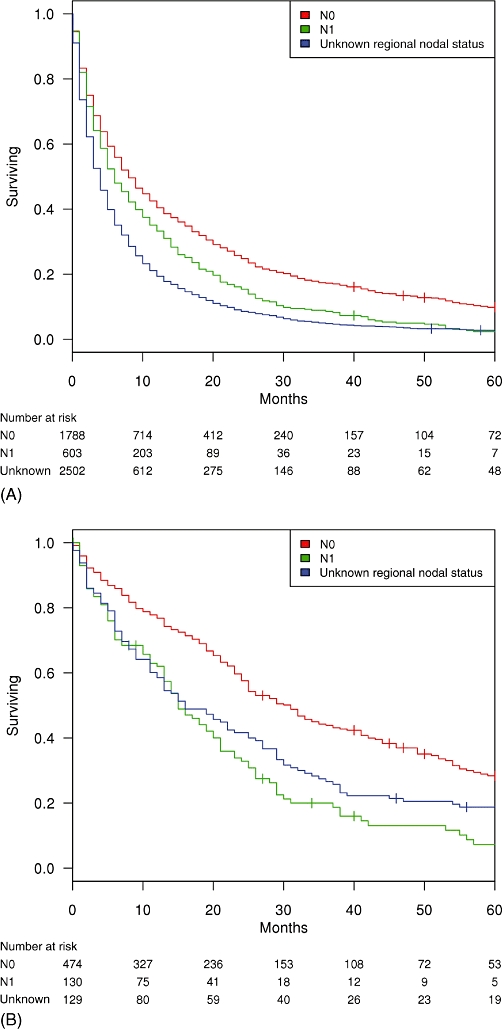
Kaplan–Meier survival by clinical N-stage in (A) all patients with intrahepatic cholangiocarcinoma (ICC; n = 4893) and (B) patients with ICC treated with major liver resection (n = 733)
Figure 2.
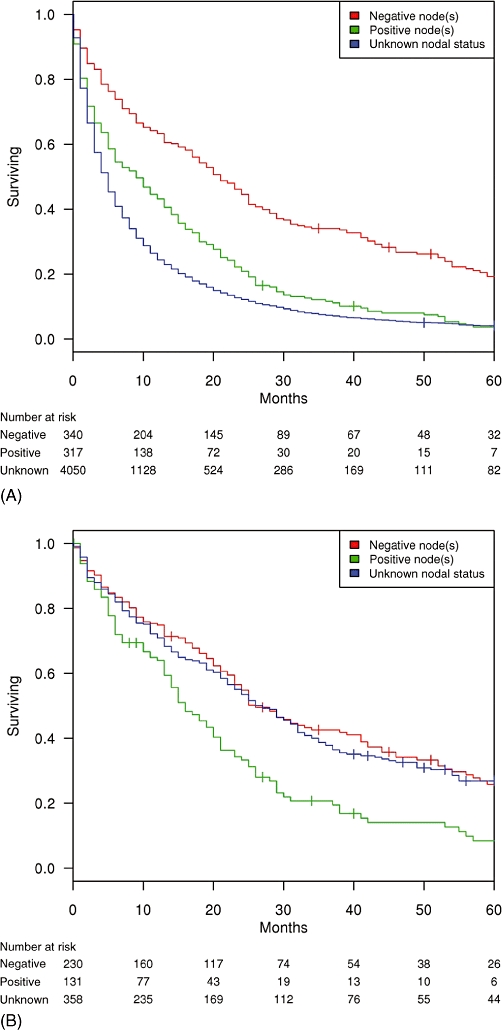
Kaplan–Meier survival curves by histologic lymph node status in (A) all patients with intrahepatic cholangiocarcinoma (ICC; n = 4707; missing data lymph node status data, n = 186) and (B) patients with ICC treated with major liver resection (n = 719; missing lymph node status data, n = 14)
In the 733 resected patients, N-stage (Fig. 1B) and positive LN status (Fig. 2B) were associated with survival. Five-year OS in resected patients was 23.0%. In this subgroup, 5-year OS was worse in N1 patients compared with N0 patients, at 7.3% vs. 28.4%, respectively (N1 HR = 2.0; P < 0.001). Five-year OS was worse in histologically node-positive patients compared with node-negative patients, at 8.4% vs. 25.9%, respectively (node-positive HR = 1.8; P < 0.001). Five-year OS was similar in node-negative patients and patients with unknown regional node status (25.9% vs. 26.8%; P = 0.433).
In a subgroup analysis of 658 ICC patients in whom histologic regional LN evaluation was performed, 5-year OS was 11.7%. Positive LN status resulted in a 5-year OS of 3.7% compared with 19.3% in node-negative patients (node-positive HR = 1.9; P < 0.001) (Fig. 3).
Figure 3.
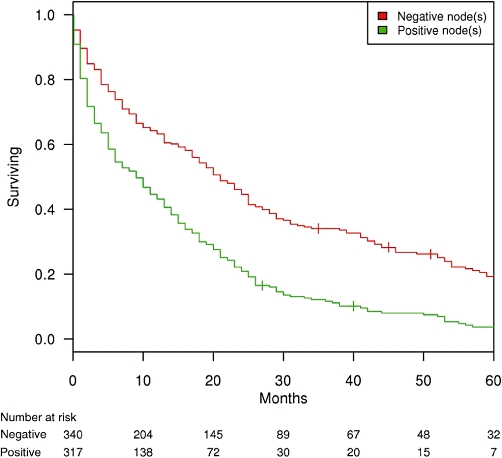
Kaplan–Meier survival curves for intrahepatic cholangiocarcinoma patients in whom at least one regional lymph node (LN) was evaluated, by LN status (n = 657)
Given the significant association between LN status and survival, the impact of the number of positive LNs on survival in patients who underwent major liver resection was evaluated. For each additional positive LN, the risk for death increased slightly (HR = 1.2 per positive LN, 95% CI 1.1–1.4; P < 0.001). Because evaluations of more than two LNs were uncommon, further analyses of survival based on the extent of LN dissection or LN ratio were not performed.
Multivariable Cox regression survival analyses were performed to evaluate the significance of regional LN metastasis for survival in ICC patients treated with major liver resection and patients who underwent histologic LN evaluation. Separate models with N-stage and histologic LN status (positive, negative, unknown) were developed, given their collinearity. Entry variables into the multivariable model included age, diagnosis year, liver resection, T-stage and M-stage. For patients treated with major liver resection, both N1 stage (HR = 1.7, 95% CI 1.3–2.2; P < 0.001) and histologically positive regional LN status (HR = 1.6, 95% CI 1.2–2.1; P < 0.001) were independently associated with worse survival. In patients in whom at least one LN was evaluated, both N1 stage (HR = 1.6, 95% CI 1.3–2.0; P < 0.001) and positive regional LN status (HR = 1.6, 95% CI 1.3–2.0; P < 0.001) were also independently associated with worse survival.
Discussion
Surgical resection is currently the only means of attempting a cure in patients with ICC. For resected patients, numerous studies have demonstrated that metastatic disease to regional LNs is associated with survival in ICC.4,9,17–19 Similarly, studies in pancreatic, gastric and colon cancer have demonstrated the importance of LN evaluation to survival.20–22 Despite this knowledge, formal evaluation of regional LNs in ICC is not widely practised in the USA and no standard approach to LN evaluation in patients diagnosed with ICC exists.
Using the SEER registry, a population-based study of national trends in LN evaluation in patients with ICC during 1988–2007 was conducted. Unlike previous reports on lymphadenectomy and ICC, the importance of LN status in resected and unresected patients was investigated. N-stage was known in 48.9% of patients in the study and histologic regional LN staging was available in only 13.5%. Of patients treated with major liver resection, just 49.3% underwent histologic evaluation of regional LNs and this was typically limited to one or two nodes. Lymph node evaluation was found to be less likely in older patients and in those with metastatic disease. The number of LNs evaluated and the percentage of patients in whom LN evaluation was performed did not increase over the study period. For patients treated with major liver resection, no differences were identified between registries.
In Western countries, few studies have investigated the impact of LN status on the survival of patients with ICC. In 2001, Weber et al. reported the Memorial Sloan Kettering Cancer Center (MSKCC) experience with surgical resection in 33 patients diagnosed with ICC.23 Three-year survival was 25% in LN-positive and 59% in node-negative patients. In this study, routine LN evaluation was advocated to prevent local recurrence. In an updated analysis of patients treated at MSKCC, Endo et al.4 reported that LN metastasis remained an important predictor of local recurrence and worse survival in resected patients; however, the average number of nodes sampled in this study was only 1.9, which is similar to the number reported in patients in the SEER registry. Madariaga et al. reported worse survival in node-positive ICC patients at the University of Pittsburgh.24 In this series of 34 patients, routine lymphadenectomy was performed, but the number of LNs removed was not reported. In an Italian study by Ercolani et al., 40% (four of 10) of patients with ICC had LN metastases at the time of lymphadenectomy.25 Tamandl et al., reporting a study of 46 patients from Austria, stated that the number of LNs retrieved did not predict survival, but an LN ratio > 0.2 did predict worse survival (HR = 9.8, 95% CI 1.5–43.4; P = 0.016).17 In this study, a median of six LNs per patient were retrieved using routine lymphadenectomy.17
Studies in Japan, Korea and China have provided varying perspectives on the clinical benefit of lymphadenectomy in ICC. In 2001, Shimada et al., reporting from Japan, compared survival between no-lymphadenectomy (n = 8) and lymphadenectomy (n = 41) patients and found no survival difference.19 In the same year, Tsuji et al. reported that 62% (24 of 39) of patients who underwent formal lymphadenectomy had positive LNs.26 Only 8% of the node-positive patients (two of 24) survived beyond 3 years. Nakagawa et al., in an analysis of 30 patients treated with hepatic resection and formal lymphadenectomy, reported that an increasing number of positive nodes was associated with worse survival.27 In 2009, Shimada et al. reported a series of 29 patients in which node-negative patients who underwent LN dissection achieved survival similar to that of patients who did not undergo lymphadenectomy (P = 0.807).28 In this study, lymphadenectomy was not standardized and was based on clinical suspicion for positive nodes. In a large series of 429 patients with ICC from China, positive LN status independently predicted worse survival (HR 1.54, 95% CI 1.0–2.4; P = 0.048).29 Other reports from Asia have confirmed that positive LNs predict worse survival, but lymphadenectomy was not routine in any of these studies and was typically based on surgeon preference or clinical suspicion.18,29–32
Previous reports utilizing SEER data have confirmed worse survival in node-positive cholangiocarcinoma.9,33 In a study of gallbladder, ECC and intrapancreatic/ampullary bile duct cancers, Schwarz and Smith determined that the number of positive nodes and total number of LNs examined were independent predictors of survival.33 However, the number of LNs evaluated (median = 4, range: 1–39) was higher than the number evaluated in this study of ICC and 22% of the ECC patients had 10 or more LNs evaluated.33 In a manuscript which outlined the current AJCC 7th edition staging system for ICC, Nathan et al. reported worse survival in LN-positive ICC patients treated with resection.9 In a small subgroup analysis of 54 N1 M0 patients, patients with three or more positive LNs had poorer survival than patients with only one or two positive nodes (HR 3.21, 95% CI 1.23–8.37).9 As we observed earlier, the findings of Nathan et al.9 were confirmed, but specific cut-point analyses were not performed given the low number of LNs sampled, as well as potential study selection and treatment biases. The inclusion of unresected patients in the current study reinforces the association of regional nodal status and worse survival in all patients diagnosed with ICC.
The main limitations of the current study result from the lack of detailed clinical data in the SEER registry. The SEER database is a population-based registry that does not collect data on preoperative evaluation, treatment rationale, treatment complications or comorbidities. Although detailed information is available on the number and status of regional LNs, this study was unable to determine why so few patients underwent the removal and histologic evaluation of more than two LNs. We presume this is a result of surgeon bias. Although adjuvant therapies have not been demonstrated to impact survival in ICC patients, the SEER registry does not include data on chemotherapy in its database and this, therefore, could not be factored into the survival analyses. Finally, the SEER registry has been criticized for substantial missing data.34 The unavailability of data did complicate the multivariable analyses. Specifically, 56.8% (n = 2778) and 58.6% (n = 2865) of data on tumour grade and size, respectively, were missing. Previous studies of ICC have utilized imputation methods to overcome problems associated with missing data, but, given the known and unknown treatment biases associated with lymphadenectomy, imputation was not appropriate.14 The SEER registry maintains a high standard in quality and clearly outlines coding algorithms even in patients with poor documentation. The substantial number of patients for whom LN status is unknown is unlikely to represent an artefact of poor quality in the SEER registry, but, rather, a true reflection of physician biases towards staging and treatment in ICC.
Conclusions
Analysis of SEER registry data for the period 1988–2007 indicates that nodal status remains an important prognostic factor in the survival of patients diagnosed with ICC. Despite undergoing major liver resection, few patients will undergo the evaluation of more than one or two LNs. As a result of these limitations to data on LN status, the clinical benefit of formal lymphadenectomy in ICC remains unknown. This evidence emphasizes the need to perform lymphadenectomy as a routine part of surgical resection in patients with ICC.
Acknowledgments
Funding for this study was provided by the Mayo Clinic, Rochester, MN, USA. KMR-L was funded by the National Center for Research Resources (NCRR; http://www.ncrr.nih.gov) (grant no. 1 UL1 RR024150), a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research (http://nihroadmap.nih.gov). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
Conflicts of interest
None declared.
References
- 1.Gatto M, Bragazzi MC, Semeraro R, Napoli C, Gentile R, Torrice A, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis. 2010;42:253–260. doi: 10.1016/j.dld.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 3.Shaib Y. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case–control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 4.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 5.Hammill CW, Wong LL. Intrahepatic cholangiocarcinoma: a malignancy of increasing importance. J Am Coll Surg. 2008;207:594–603. doi: 10.1016/j.jamcollsurg.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208:134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Gibbs JF. Recent advances in the management of primary hepatic tumours refinement of surgical techniques and effect on outcome. J Surg Oncol. 2010;101:745–754. doi: 10.1002/jso.21506. [DOI] [PubMed] [Google Scholar]
- 8.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case–control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan H, Aloia TA, Vauthey J-N, Abdalla EK, Zhu AX, Schulick RD, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14–22. doi: 10.1245/s10434-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 10.Vauthey JN, Pawlik TM, Abdalla EK, Aloia TA, Boudreaux CS, Chun YS, et al. Intrahepatic bile ducts. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. American Joint Committee on Cancer Cancer Staging Manual. 7th edn. New York, NY: Springer; 2010. pp. 201–205. [Google Scholar]
- 11.Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Institutes of Health; 2010. http://seer.cancer.gov/csr/1975_2007/. [Accessed 1 August 2010.] [Google Scholar]
- 12.Nathan H, Pawlik TM, Wolfgang CL, Ma C, Cameron JL, Schulick RD. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointestin Surg. 2007;11:1488–1496. doi: 10.1007/s11605-007-0282-0. discussion 1496–1497. [DOI] [PubMed] [Google Scholar]
- 13.Tan JCC, Coburn NG, Baxter NN, Kiss A, Law CHL. Surgical management of intrahepatic cholangiocarcinoma – a population-based study. Ann Surg Oncol. 2008;15:600–608. doi: 10.1245/s10434-007-9627-x. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara ET, Mitra N, Guo M, Metz JM. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1495–1501. doi: 10.1016/j.ijrobp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Percy C. SEER site/histology validation list: liver. In: Shanmugaratnam K, Whelan S, Parkin D, Jack A, Fritz A, Sobin L, editors. International Classification of Diseases for Oncology. 3rd edn. Geneva: World Health Organization; 2000. pp. 89–90. [Google Scholar]
- 16.Green FL, American Joint Committee on Cancer, American Cancer Society . Liver (including intrahepatic bile ducts) In: Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al., editors. AJCC Staging Handbook. 6th edn. New York: Springer; 2002. pp. 131–138. [Google Scholar]
- 17.Tamandl D, Kaczirek K, Gruenberger B, Koelblinger C, Maresch J, Jakesz R, et al. Lymph node ratio after curative surgery for intrahepatic cholangiocarcinoma. Br J Surg. 2009;96:919–925. doi: 10.1002/bjs.6654. [DOI] [PubMed] [Google Scholar]
- 18.Choi S-B, Kim K-S, Choi J-Y, Park S-W, Choi J-S, Lee W-J, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–3056. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 19.Shimada M, Yamashita Y, Aishima S, Shirabe K, Takenaka K, Sugimachi K. Value of lymph node dissection during resection of intrahepatic cholangiocarcinoma. Br J Surg. 2001;88:1463–1466. doi: 10.1046/j.0007-1323.2001.01879.x. [DOI] [PubMed] [Google Scholar]
- 20.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 21.Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 22.Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 23.Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. doi: 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- 24.Madariaga JR, Iwatsuki S, Todo S, Lee RG, Irish W, Starzl TE. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg. 1998;227:70–79. doi: 10.1097/00000658-199801000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ercolani G, Grazi GL, Ravaioli M, Grigioni WF, Cescon M, Gardini A, et al. The role of lymphadenectomy for liver tumours: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202–209. doi: 10.1097/01.sla.0000109154.00020.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuji T, Hiraoka T, Kanemitsu K, Takamori H, Tanabe D, Tashiro S. Lymphatic spreading pattern of intrahepatic cholangiocarcinoma. Surgery. 2001;129:401–407. doi: 10.1067/msy.2001.111873. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728–733. doi: 10.1007/s00268-005-7761-9. [DOI] [PubMed] [Google Scholar]
- 28.Shimada K, Sano T, Nara S, Esaki M, Sakamoto Y, Kosuge T, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery. 2009;145:411–416. doi: 10.1016/j.surg.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Shen W-F. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol. 2009;15:5976–5982. doi: 10.3748/wjg.15.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li S-Q, Liang L-J, Hua Y-P, Peng B-G, He Q, M-D L, et al. Longterm outcome and prognostic factors of intrahepatic cholangiocarcinoma. Chin Med J. 2009;122:2286–2291. [PubMed] [Google Scholar]
- 31.Cho SY, Park S-J, Kim SH, Han S-S, Kim Y-K, Lee K-W, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17:1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- 32.Uchiyama K, Yamamoto M, Yamaue H, Ariizumi S-I, Aoki T, Kokudo N, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18:443–452. doi: 10.1007/s00534-010-0349-2. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz R, Smith D. Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on US population data. J Gastrointestin Surg. 2007;11:158–165. doi: 10.1007/s11605-006-0018-6. [DOI] [PubMed] [Google Scholar]
- 34.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15:415–423. doi: 10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]


