Abstract
Background
Recurrence of hepatocellular carcinoma (HCC) after liver transplantation (LT) is rarely curable. However, in view of the advent of new treatments, it is critical that patients at high risk for recurrence are identified.
Methods
Patients undergoing LT for HCC at a single centre between 2002 and 2010 were reviewed and data on clinical parameters and explant pathology were analysed to determine factors associated with HCC recurrence. All necrotic and viable tumour nodules were included in explant staging. All patients underwent LT according to the United Network for Organ Sharing (UNOS) Model for End-stage Liver Disease (MELD) tumour exception policies.
Results
Liver transplantation was performed in 122 patients with HCC during this period. Rates of recurrence-free survival in the entire cohort at 1 year and 3 years were 95% and 89%, respectively. Thirteen patients developed HCC recurrence at a median of 14 months post-LT. In univariate analysis the factors associated with HCC recurrence were bilobar tumours, vascular invasion, and stage exceeding either Milan or University of California San Francisco (UCSF) Criteria. Multivariate analysis showed pathology outside UCSF Criteria was the major predictor of recurrence; when pathology outside UCSF Criteria was found in combination with vascular invasion, the predicted 3-year recurrence-free survival was only 26%.
Conclusions
Explant pathology can be used to predict the risk for recurrent HCC after LT, which may allow for improved adjuvant and management strategies.
Keywords: hepatocellular carcinoma, liver < transplant, indications < transplant, outcomes < transplant
Introduction
Liver transplantation (LT) is widely accepted as an effective treatment for early-stage hepatocellular carcinoma (HCC). In 2002 the United Network for Organ Sharing (UNOS) revised the liver organ allocation process in the USA to include the Model for End-stage Liver Disease (MELD) score, with an important built-in exception which gave an advantage to patients on the waiting list with HCC that met the Milan Criteria.1 In recent years, some UNOS regions have also allowed LT through the MELD exception process for patients with more advanced malignancy, provided they were able to undergo downstaging to bring their tumour burden to within the Milan Criteria.2 The inherent advantage provided by the MELD exception process, along with increasing enthusiasm for LT in more advanced tumours, has been accompanied by an increase in the number of patients with HCC now listed for LT and an increase in the number of transplants performed for HCC. In 2010, 1221 of 5516 (22%) deceased donor LTs in the USA were performed for HCC.3
Patients undergoing LT for HCC face an obvious risk for tumour recurrence. The risk for recurrence, as well as the rate of progression of recurrent tumours, is thought to be accentuated by immunosuppressive medications.4 An important tenet of LT is to balance the risk for tumour recurrence against the value of the organ, taking into consideration the needs and wishes of the cancer patient and the limited number of available donor organs. The Milan Criteria (Table 1) are, in fact, widely accepted as representing a pre-transplant measure of the risk for tumour recurrence because HCC cases that meet these criteria are associated with a low risk for recurrent HCC after LT.5 Other methods can also be used to predict the risk for HCC recurrence after LT, including the presence of vascular invasion in explant tumours, tumour grade and even other numerical staging systems such as the University of California San Francisco (UCSF) Criteria or the Up-to-Seven rule.6 As the transplant environment changes, however, the factors that may predict recurrence need to be continually evaluated to help guide organ allocation and clarify the risks to the patient after LT.
Table 1.
Staging criteria for liver transplantation
| Number of lesions | Milan Criteria | UCSF Standard Criteria | UCSF Expanded Criteria |
|---|---|---|---|
| 1 | ≤5 cm | ≤6.5 cm | ≤8 cm |
| 2 or 3 | ≤3 cm | ≤4.5 cma | ≤5 cma |
| 4 or 5 | N/A | N/A | ≤3 cma |
Maximum total tumour diameter ≤8 cm.
UCSF, University of California San Francisco; N/A, not applicable.
The stratification of patient risk for post-transplant recurrence of HCC may have value beyond simple prognostication. Identification of high-risk groups may allow early conversion to alternative immunosuppression regimens, such as the use of mTOR inhibitors,7 or may allow for appropriate enrolment in trials or protocols of adjuvant therapy, such as the use of the multi-kinase inhibitor sorafenib.8 In the current study, data for a population of patients who underwent LT for HCC over an 8.5-year period were analysed to determine contemporary risk factors for tumour recurrence. Of note, our centre is in UNOS Region 5, which has longer wait times for LT than most UNOS regions.9
Materials and methods
With the approval of the Cedars–Sinai Medical Center's institutional review board, data were obtained from records of all patients who underwent deceased donor LT for HCC at our centre between 1 January 2002 and 1 July 2010. Clinical parameters included patient demographics, time on the wait list after diagnosis of HCC, cancer treatments utilized while on the wait list (bridging treatments), and whether or not the patient underwent downstaging to meet the Milan Criteria, which, in turn, requires formal review by the UNOS Region 5 Regional Review Board. Imaging records were reviewed to determine the most recent radiologic stage prior to LT, as well as the timing and site of any recurrent tumour after LT.
Pathologic staging of liver explants was performed by reviewing pathology reports. All viable and necrotic nodules were considered to represent tumours. The size, number and distribution of tumours (bilobar or unilobar) were noted, as was the presence of microvascular or macrovascular invasion. Based on the size and number of tumours, each explant was staged into one of three categories: (i) within the Milan Criteria; (ii) outside the Milan Criteria but within the expanded UCSF Criteria, and (iii) outside the expanded UCSF Criteria (Table 1). All nodules in the pathologic staging of explants were assessed for two reasons: firstly, some reports from early in the study period did not state whether nodules were necrotic or viable and thus pathologic staging would allow the inclusion of these patients, and, secondly, this type of cumulative staging is more reflective of the multiple sequential bridging treatments patients often receive while on the wait list. Of note, hepatic artery chemoembolization (HACE) was used almost exclusively for bridging during this time period. The type of all-inclusive post-treatment staging used in this study would not be reliable if our centre had used thermal ablative techniques for bridging as this modality creates a zone of necrosis that is larger than the original tumour. With HACE, however, necrotic nodules should be reasonably representative of the original tumour.
Statistical analysis
Direct comparison was performed between patients with and without HCC recurrence using a variety of paired analyses. Clinical and pathologic factors in these groups were compared using Student's t-test (for continuous data), Fisher's exact test (for categorical data) and Wilcoxon rank sum test (for non-parametric ordinal data).
To create a model that would also account for variable time of follow-up, recurrence-free survival was analysed using Kaplan–Meier product limit estimators and Cox proportional hazard models to compute risk in the form of hazard ratios (HRs). Differences in survival times between strata were assessed with the log-rank test. Individual risk factors were considered first in single-variable models using data from all cases. Multiple-variable analysis using forward stepwise selection methods with inclusion/exclusion criteria and a P-value of <0.05 was used to identify the set of factors predictive of disease recurrence. Relevant interaction terms were also considered. The proportional hazards assumption was tested graphically and model diagnostics (Martingale and Shoenfeld residuals) were used to assess model adequacy.
Statistical analysis was performed using sas Version 9.1 (SAS Institute, Inc., Cary, NC, USA). All statistical tests were two-sided and P-values of <0.05 were considered to indicate statistical significance.
Results
The characteristics of the study cohort (n = 122) are presented in Table 2. No patient was lost to follow-up and all patients underwent routine imaging at 3–6-month intervals until 3 years post-LT. The median length of follow-up in the study cohort was 32.7 months (range: 6–96 months). Mean ± standard error (SE) overall survival in the entire group at 1 and 3 years was 92.2 ± 2.5% and 84.8 ± 3.7%, respectively. Disease recurrence was observed in 13 cases (11%) at a median of 13.7 months (range: 5.0–44.8 months). Based on Kaplan–Meier methods, the mean ± SE recurrence-free survival time was 41.1 ± 1.1 months, and 94.6 ± 2.2% and 89.2 ± 3.1% of patients survived without disease progression to 1 and 3 years, respectively.
Table 2.
Characteristics of the study cohort (n = 122)
| Variable | Data |
|---|---|
| Age, years, mean ± SD | 58.3 ± 6.8 |
| Waiting time, months, mean ± SD | 5.0 ± 3.5 |
| Pre-transplant AFP > 25 ng/ml, n (%) | 38a (38%) |
| Pre-transplant bridging therapy, n (%) | 88 (72%) |
| Pathology staging | |
| Within Milan Criteria | 68 (56%) |
| Exceeding Milan Criteria | 54 (44%) |
| Exceeding Milan, within expanded UCSF Criteria | 33 (27%) |
| Exceeding expanded UCSF Criteria | 21 (17%) |
| Max tumour size > 3 cm | 56b (48%) |
| Tumour count > 3 | 25 (20%) |
| Presence of vascular invasion | 19c (18%) |
| Bilobar | |
| On imaging and pathology | 45c (39%) |
| On pathology alone | 33d (31%) |
| Regional Review for Downstaging | 11 (9%) |
| Viable tumour on explant | 60e (73%) |
a–eData available for a101, b117, c106, d105 and e82 cases only.
SD, standard deviation; AFP, alpha-fetoprotein; UCSF, University of California San Francisco.
A direct comparison of patients with and without recurrent HCC is shown in Table 3. Explant pathology factors significantly associated with an increased risk for HCC recurrence after LT included: (i) pathology beyond the Milan Criteria; (ii) pathology beyond the expanded UCSF Criteria; (iii) more than three tumours on explant; (iv) the presence of vascular invasion, and (v) bilobar tumours. None of the clinical or wait list parameters were significantly associated with HCC recurrence. Specifically, higher alpha-fetoprotein (AFP) levels and the need for downstaging to within the Milan Criteria (n = 11) were not associated with higher risk for recurrence.
Table 3.
Comparison of characteristics of patients in the recurrence and non-recurrence groups
| Variable | Recurrence | No recurrence | P-value | |||
|---|---|---|---|---|---|---|
| (n = 13) | (n = 109) | |||||
| Age, years, mean (range) | 58.5 | (51.2–66.6) | 58.3 | (39.3–74.6) | 0.921 | h |
| Waiting time, months (mean, range) | 6.1 | (0.9–17.3) | 4.9 | (0.1–17.0) | 0.238 | h |
| Pre-transplant AFP, ng/ml, mean (range) | 518a | (4–2730) | 239d | (2–11 650) | 0.118 | j |
| Pre-transplant bridging treatment | 10 | 77% | 78 | 72% | 1.000 | i |
| Pathology staging | ||||||
| Within Milan Criteria | 0 | 0% | 68 | 100% | N/A | i |
| Overall exceeding Milan Criteria | 13 | 100% | 41 | 38% | <0.001 | i |
| Exceeding Milan, within expanded UCSF Criteria | 4 | 31% | 29 | 27% | 0.747 | i |
| Exceeding expanded UCSF Criteria | 9 | 69% | 12 | 11% | <0.001 | i |
| Tumour size | ||||||
| Maximal size, cm, mean (range) | 3.7 | (2.2–6.5) | 3.0e | (0.9–6.5) | 0.081 | h |
| Tumour count | ||||||
| Tumour count, mean (range) | 5.2 | (3–9) | 2.0 | (0–9) | <0.001 | h |
| >3 tumours | 12 | 92% | 13 | 12% | <0.001 | i |
| Presence of vascular invasion | 6a | 50% | 13f | 14% | 0.011 | i |
| Bilobar | ||||||
| On imaging and pathology | 8a | 67% | 37e | 35% | 0.057 | i |
| On pathology alone | 7b | 64% | 26f | 35% | 0.034 | i |
| Regional Review for Downstaging | 2 | 15% | 9 | 8% | 0.336 | i |
| Viable | 5c | 100% | 55g | 71% | 0.317 | i |
a–gData available for only a12, b11 and c5 of the recurrence cases or for d89, e104, f94 and g77 of the no-recurrence cases.
h–jP-values computed via hStudent's t-test, iFisher's exact test or jWilcoxon rank sum test.
AFP, alpha-fetoprotein; UCSF, University of California San Francisco.
Significant predictors of disease recurrence were also determined using Cox HR modelling. An analysis of individual variables showed that these same five pathologic criteria were all associated with increased risk for HCC recurrence (Table 4): (i) stage beyond the Milan Criteria; (ii) stage beyond the expanded UCSF Criteria; (iii) more than three tumours; (iv) presence of vascular invasion, and (v) bilobar lesions on pathology. Clinical and wait list parameters, including age at LT, pre-LT AFP, UCSF downstaging and use of pre-LT bridging treatments, were not predictive of time to disease recurrence. Pre-LT waiting time of ≥6 months was marginally associated with a higher risk for recurrence (P = 0.063). Recurrence-free survival of 100% was observed among subjects meeting the Milan Criteria or in whom the tumour was not viable on pathologic examination; therefore statistical analysis of these two variables on recurrence-free survival times is unavailable.
Table 4.
Cox proportional hazard modelling of recurrence-free survival times in the full study cohort
| Variable | Univariate analysis | ||
|---|---|---|---|
| P-value | Hazard ratio | (95% CI) | |
| Age >60 years | 0.438 | 1.54 | (0.52–4.58) |
| Waiting time ≥6 months | 0.063 | 2.91 | (0.94–9.00) |
| Pre-transplant AFP >25 ng/ml | 0.148 | 2.34 | (0.74–7.39) |
| Pre-transplant bridging therapy | 0.451 | 1.65 | (0.45–5.99) |
| Pathology staging | |||
| Exceeds Milan Criteria | 0.991 | Not estimated | |
| Exceeds expanded UCSF Criteria | <0.001 | 13.83 | (4.24–45.12) |
| Exceeds Milan, within expanded UCSF Criteria | 0.728 | 1.23 | (0.38–4.00) |
| Maximal tumour size > 3 cm | 0.110 | 2.62 | (0.81–8.50) |
| Tumour count > 3 | <0.001 | 59.13 | (7.67–456) |
| Presence of vascular invasion | 0.008 | 4.70 | (1.51–14.63) |
| Bilobar | |||
| On imaging and pathology | 0.064 | 3.12 | (0.94–10.37) |
| On pathology alone | 0.028 | 4.00 | (1.17–13.71) |
| Regional Review for Downstaging | 0.337 | 2.10 | (0.46–9.50) |
| Viable tumour on explant | 0.995 | Not estimated | |
95% CI, 95% confidence interval; AFP, alpha-fetoprotein; UCSF, University of California San Francisco.
Of the 68 patients whose disease met the Milan Criteria, none experienced disease recurrence (100% recurrence-free survival was observed). Multivariable Cox proportional hazard modelling of survival times was performed for the 54 patients in whom disease exceeded the Milan Criteria to determine which set of factors is most predictive of disease recurrence. Using forward stepwise selection methods, UCSF Criteria (HR = 8.11, 95% confidence interval [CI] 2.10–1.36; P = 0.002) and presence of vascular invasion of the tumour (HR = 3.28, 95% CI 1.02–10.51; P = 0.046) retained significant association with shorter recurrence-free survival times in patients in whom disease exceeded the Milan Criteria. Table 5 presents the 1- and 3-year disease-free survival rates across the Milan and UCSF Criteria in patients with and without vascular invasion of the tumour. In patients with explant pathology beyond the UCSF Criteria, the group without associated vascular invasion achieved an expected 3-year disease-free survival of 67%, but this dropped to 27% in the presence of associated vascular invasion. This is further evidenced by the Kaplan–Meier disease-free survival curves for all groups (Fig. 1).
Table 5.
Kaplan–Meier recurrence-free survival proportions at 1 and 3 years
| Milan | UCSFa | Vascular invasion | n | 1-year survival | 3-year survival | ||
|---|---|---|---|---|---|---|---|
| Rate | (SE) | Rate | (SE) | ||||
| Within | N/A | N/A | 68 | 100% | (0%) | 100% | (0%) |
| Exceeding | Within | Absent | 23 | 100% | (0%) | 87.5% | (8.3%) |
| Present | 6 | 100% | (0%) | 80.0% | (17.9%) | ||
| Exceeding | Absent | 11 | 80.0% | (12.7%) | 66.7% | (16.1%) | |
| Present | 7 | 53.6% | (20.1%) | 26.8% | (21.4%) | ||
Expanded UCSF Criteria.
UCSF, University of California San Francisco; SE, standard error; N/A, not applicable.
Figure 1.
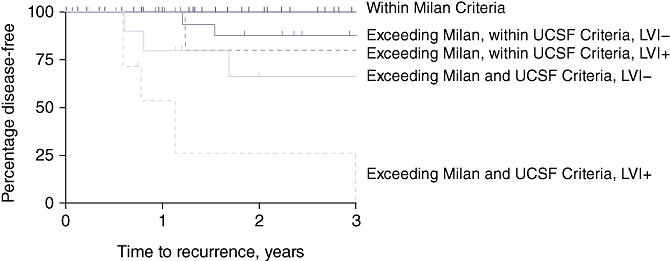
Kaplan–Meier curves for disease-free survival in all groups. LVI, lymphovascular invasion
Discussion
The recurrence of HCC after LT is generally considered an ominous finding. We have demonstrated in this report that several factors, primarily evidenced by explant pathology, can be used to stratify patients into high- and low-risk groups for recurrence (summarized in Table 6). In our series, we saw no recurrence of HCC in patients with explant pathology within the Milan Criteria. Patients with explant pathology beyond the Milan Criteria but within the expanded UCSF Criteria also had a low risk for recurrence, with 3-year disease-free survival of 80–88%. By contrast, patients with pathologic staging outside the expanded UCSF Criteria and with vascular invasion had a risk for recurrence of 73% at 3 years (Table 5). In univariate analysis, several additional pathologic factors, including bilobar tumour distribution, were associated with HCC recurrence, whereas waiting time for LT of ≥6 months after HCC diagnosis was marginally associated with risk for recurrence (Table 4). Wait list variables such as AFP level and the need to downstage the tumour to meet the Milan Criteria were not associated with risk for recurrence, but this may reflect the small sample size. Other studies have demonstrated an increased risk for post-LT recurrence with high pre-transplant AFP levels.10
Table 6.
Cedars–Sinai risk factors for recurrence of hepatocellular carcinoma after liver transplant
| Factor |
|---|
| Pathology outside UCSF Criteriaa |
| Vascular invasiona |
| Pathology outside Milan Criteria |
| More than three tumours |
| Bilobar tumours |
| Waiting time ≥ 6 months |
Significant in both univariate and multivariate analysis.
UCSF, University of California San Francisco.
This study has several obvious limitations. Firstly, in many patients, follow-up time was relatively short and the disease may not have had time to recur. This is accounted for by the time-based Cox proportional modelling, but longer follow-up of the more recent cohort will be helpful. Secondly, we used an all-inclusive staging system which is not commonly used. All viable and necrotic nodules were counted in pathologic staging. Although this type of staging is not standard, we feel that it gives a better picture of the total tumour burden in the liver over time and lessens the error that might be introduced by sampling error in any given necrotic tumour. To further account for this cumulative staging, the expanded UCSF Criteria were used as the break point in staging.11 A final limitation of this study concerns the small size of the sample of recurrent tumours, but the type of detailed information we sought is only available in a single-centre study such as this.
One of the most important objectives of this and similar reports is to help guide the management of the post-LT patient with respect to both monitoring for tumour recurrence and employing preventative strategies. Our data suggest that the patient with explant pathology within the Milan Criteria has a negligible risk for tumour recurrence and thus these patients can be monitored with less stringency post-transplant. This has implications for cost savings and for reducing the patient's exposure to radiation and contrast-enhancing agents. However, this study also suggests that the patient with vascular invasion and either bilobar tumours or an overall explant stage beyond the expanded UCSF Criteria faces an exceedingly high risk for tumour recurrence. These patients should be followed with frequent imaging and AFP monitoring. In addition, high-risk patients should be considered for two additional therapeutic manoeuvres: (i) alteration in immunosuppression, either by using the lowest tolerable calcineurin inhibitor regimen or by transitioning to an mTOR inhibitor, such as sirolimus or everolimus, and (ii) enrolment in protocols or trials aimed at preventing or delaying recurrence, such as by using the multi-kinase inhibitor sorafenib, which has recently been shown to have activity against HCC.12 In our programme, we have now adopted this low risk/high risk stratification in order to determine the surveillance protocol and the ideal immunosuppressive regimen for each individual patient.
One finding in the current study that may raise concern is that 44% of our patients were found to have explant pathology beyond the Milan Criteria, although the protocol for transplant eligibility required the patient to meet radiologic Milan Criteria during the pre-transplant period. This may be partly explained by radiographic understaging and partly by the all-inclusive pathology system we utilized. All patients undergoing LT for HCC according to the MELD exception system are required to undergo imaging every 3 months, the results of which are reported to UNOS. It is important to note, however, that pre-LT radiographic staging includes only viable tumours, which on current magnetic resonance imaging and computed tomography scans are interpreted as tumours with hypervascularity. Treated non-viable tumours are not counted in the pre-transplant staging system. Our post-transplant staging system included both necrotic and viable tumours, and in this way reflects the cumulative effect of repeated treatments over the lifespan of the liver. As demonstrated by the results, this form of all-inclusive explant staging is fairly accurate in predicting risk for recurrent tumour after transplant. These findings also suggest that the risk for recurrent HCC may not be adequately predicted by the snapshot approach currently used by the transplant community, in which transplant candidacy is maintained at each evaluation interval provided the staging of viable tumours is within the Milan Criteria. Equally important in stratifying risk are data on whether the patient requires repeated interventions while on the wait list or whether he or she has a sum of treated tumours that surpass the expanded UCSF Criteria.
The findings of this single-centre report are not unique, but, rather, add to the growing body of literature on the phenotype of patients undergoing deceased donor LT for HCC in the USA, and in particular in UNOS Region 5. This region has one of the longest wait list times for LT in the country, which results in the widespread use of multiple wait list bridging modalities. As in our series, several other larger reports have similarly demonstrated that the risk for HCC recurrence after LT is increased in patients found to have tumours that fall outside the Milan or UCSF Criteria or which involve vascular invasion.13,14 Our data suggest, however, that the only group at prohibitive risk for recurrence is the group with all-inclusive explant pathology beyond the expanded UCSF Criteria and with vascular invasion. Recurrence-free survival at 3 years was only 27% in this group. Patients with less advanced tumour size or number, even in the presence of vascular invasion, achieved survival rates that were quite acceptable. Based on our data, a more reliable pre-LT indicator of vascular invasion would eliminate most recurrences, even in patients with long wait times and in advanced tumour staging groups. The ideal surrogate marker for vascular invasion remains elusive; we propose that, in select patients, direct tissue acquisition may be a useful although perhaps risky way to ensure the best chance of survival and the best use of organs.
In summary, this report provides important information for stratifying post-LT patients into groups with low and high risk for recurrent HCC. Low-risk patients are those with explant staging that falls within the Milan Criteria or with complete tumour necrosis. High-risk patients are those with cumulative tumour staging that exceeds the expanded UCSF Criteria, and this risk is especially high if those patients have vascular invasion on explant. These data indirectly support the continued acceptance of the current process of downstaging patients prior to transplant as long as they meet the expanded UCSF Criteria at the time of treatment, as we saw few recurrences in patients who were formally downstaged and we found that explant pathology within the expanded UCSF Criteria was associated with a low overall risk for recurrence.
Conflicts of interest
None declared.
References
- 1.Organ Procurement and Transplantation Network. Policies. http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_8.pdf. [Accessed 1 July 2011]
- 2.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook P, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 3.Organ Procurement and Transplantation Network. Data available online at. http://optn.transplant.hrsa.gov. [Accessed 1 July 2011]
- 4.Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, et al. Analyses of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl. 2005;11:497–503. doi: 10.1002/lt.20391. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Llovet J, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 7.Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 8.Printz C. Clinical trials of note. Sorafenib as adjuvant treatment in the prevention of disease recurrence in patients with hepatocellular carcinoma (HCC) (STORM) Cancer. 2009;115:4646. doi: 10.1002/cncr.24673. [DOI] [PubMed] [Google Scholar]
- 9.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4 Pt 2):1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 10.Merani A, Majno P, Kneteman NM, Berney T, Morel P, Mentha G, et al. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol. 2011 doi: 10.1016/j.jhep.2010.12.040. Feb 18 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 11.Yao FY, Hirose R, LaBerge JM, Davern TJ, 3rd, Bass NM, Kerlan RK, Jr, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Eng J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Duffy J, Vardanian A, Benjamin E, Watson M, Farmer DG, Ghobrial RM, et al. Liver transplantation criteria for hepatocellular cancer should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:509–511. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onaca N, Klintmalm GB. Liver transplantation for hepatocellular carcinoma: the Baylor experience. J Hepatobiliary Pancreat Sci. 2010;17:574–580. doi: 10.1007/s00534-009-0163-x. [DOI] [PubMed] [Google Scholar]


