Abstract
In order to examine sequence-dependent structural effects in DNA, the ability of alternating purine-pyrimidine fragments to undergo a B-Z transition when cloned in a supercoiled plasmid was determined solely as a function of sequence, with base and nearest-neighbor composition held constant. Sequences of 22 GC and 2 AT base pairs were synthesized such that the AT base pairs varied between contiguous placement and separation by eight GC base pairs. Results show, surprisingly, that the ease of the B-Z transition varies with the position of the two AT base pairs, occurring at lower superhelical densities when AT base pairs are contiguous, and at higher torsional strain when the AT base pairs are moved further apart.
Full text
PDF
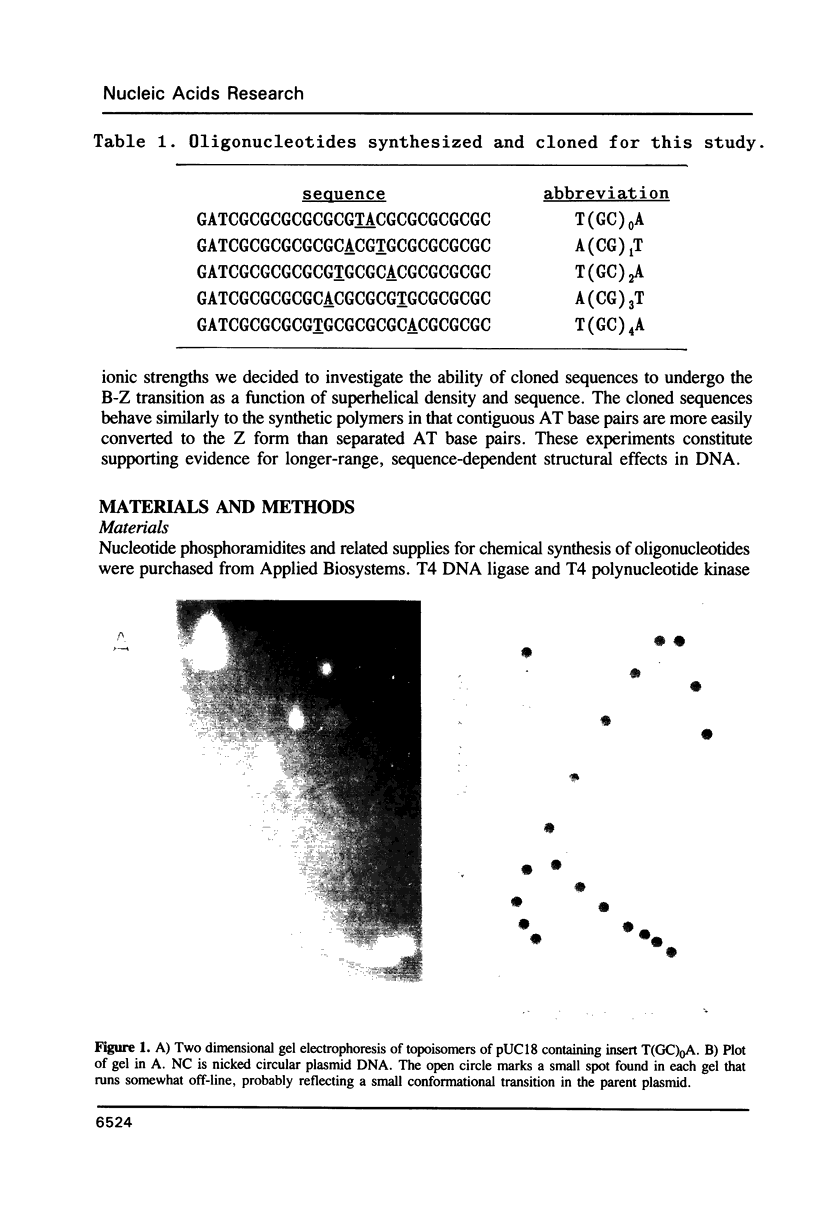
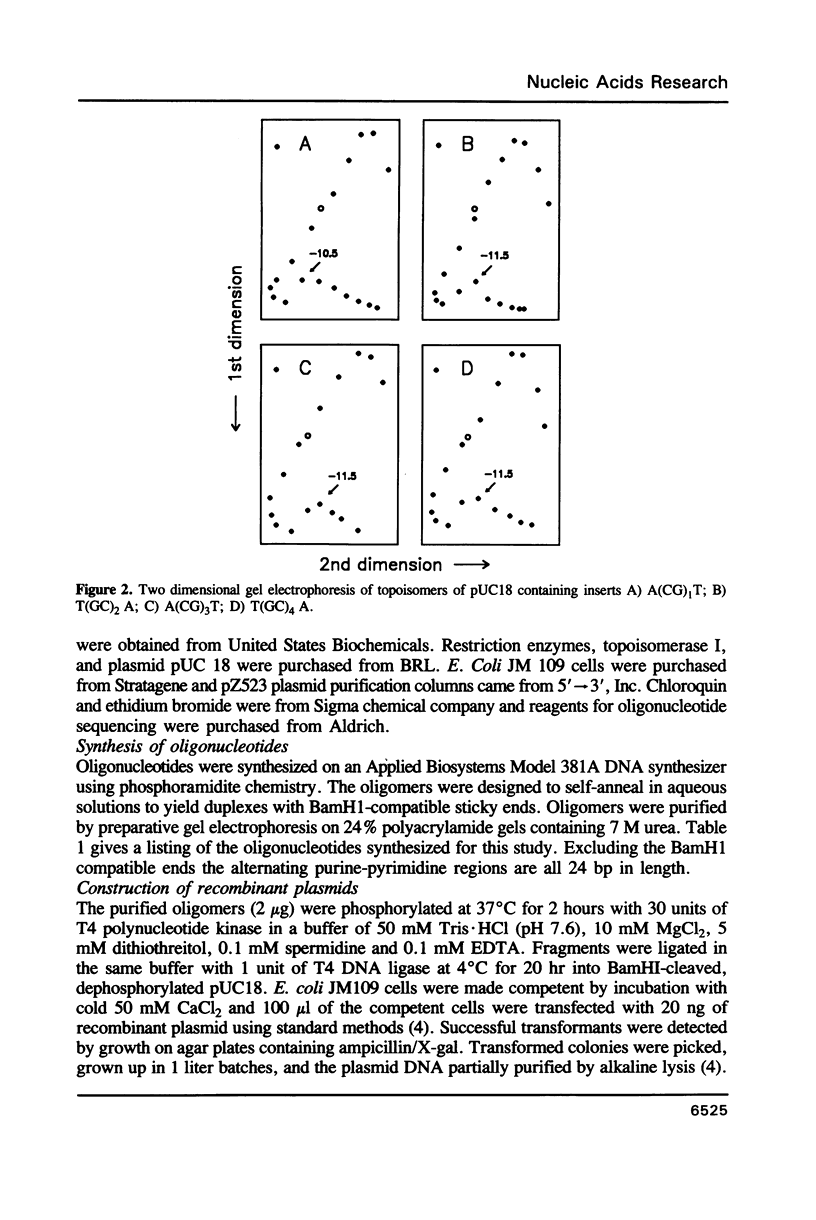

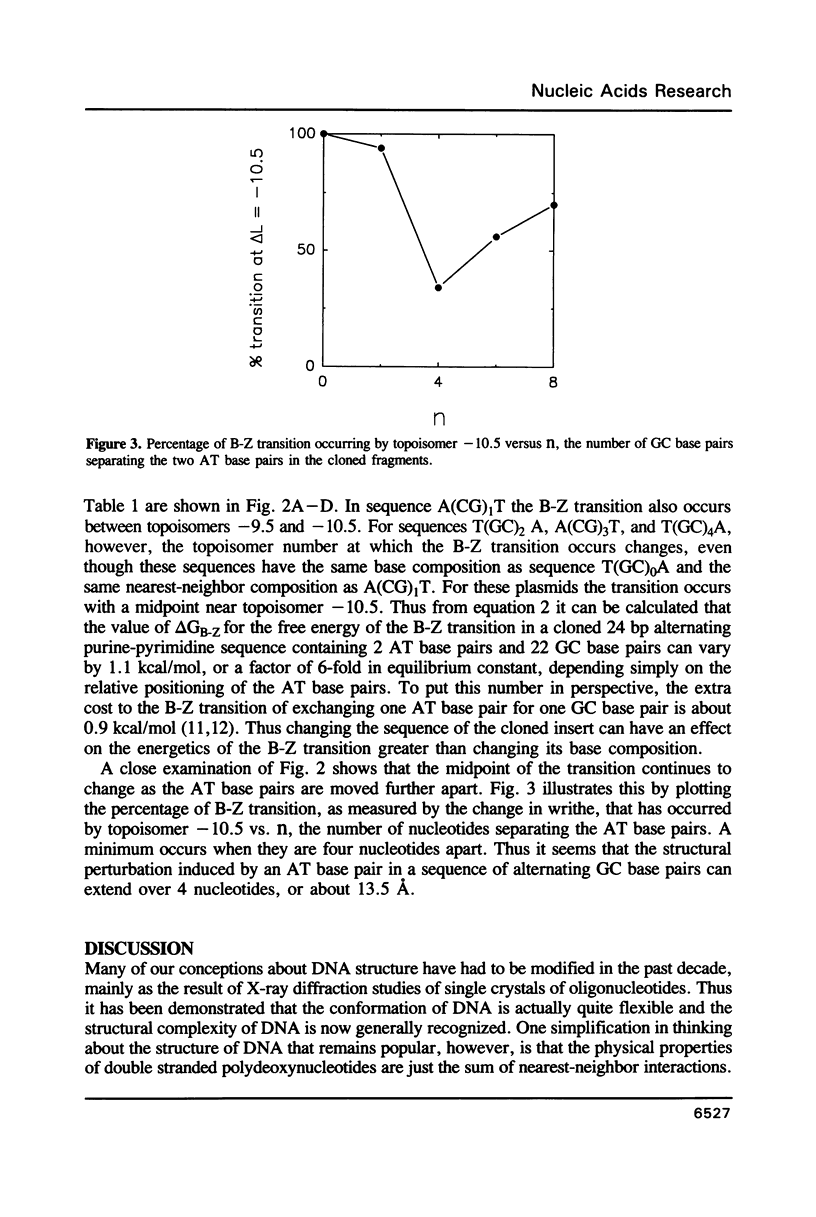


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen F. S., Gray D. M., Roberts G. P., Tinoco I., Jr The ultraviolet circular dichroism of some natural DNAs and an analysis of the spectra for sequence information. Biopolymers. 1972;11(4):853–879. doi: 10.1002/bip.1972.360110410. [DOI] [PubMed] [Google Scholar]
- Depew D. E., Wang J. C. Conformational fluctuations of DNA helix. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4275–4279. doi: 10.1073/pnas.72.11.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison M. J., Feigon J., Kelleher R. J., 3rd, Wang A. H., Habener J. F., Rich A. An assessment of the Z-DNA forming potential of alternating dA-dT stretches in supercoiled plasmids. Biochemistry. 1986 Jun 17;25(12):3648–3655. doi: 10.1021/bi00360a026. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Hamilton F. D., Vaughan M. R. The analysis of circular dichroism spectra of natural DNAs using spectral components from synthetic DNAs. Biopolymers. 1978 Jan;17(1):85–106. doi: 10.1002/bip.1978.360170107. [DOI] [PubMed] [Google Scholar]
- Ho P. S., Ellison M. J., Quigley G. J., Rich A. A computer aided thermodynamic approach for predicting the formation of Z-DNA in naturally occurring sequences. EMBO J. 1986 Oct;5(10):2737–2744. doi: 10.1002/j.1460-2075.1986.tb04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopka M. L., Fratini A. V., Drew H. R., Dickerson R. E. Ordered water structure around a B-DNA dodecamer. A quantitative study. J Mol Biol. 1983 Jan 5;163(1):129–146. doi: 10.1016/0022-2836(83)90033-5. [DOI] [PubMed] [Google Scholar]
- Luthman K., Behe M. J. Sequence dependence of DNA structure. The B, Z, and A conformations of polydeoxynucleotides containing repeating units of 6 to 16 base pairs. J Biol Chem. 1988 Oct 25;263(30):15535–15539. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Blaho J. A., Kilpatrick M. W., Wells R. D. Consecutive A X T pairs can adopt a left-handed DNA structure. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5884–5888. doi: 10.1073/pnas.83.16.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson W. K., Flory P. J. Spatial configurations of polynucleotide chains. I. Steric interactions in polyribonucleotides: a virtual bond model. Biopolymers. 1972 Jan;11(1):1–23. doi: 10.1002/bip.1972.360110102. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Shure M., Tang D., Vinograd J., Vosberg H. P. Action of nicking-closing enzyme on supercoiled and nonsupercoiled closed circular DNA: formation of a Boltzmann distribution of topological isomers. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4280–4284. doi: 10.1073/pnas.72.11.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Wells R. D. The facile generation of covalently closed, circular DNAs with defined negative superhelical densities. Anal Biochem. 1982 May 15;122(2):253–257. doi: 10.1016/0003-2697(82)90277-9. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Peck L. J., Becherer K. DNA supercoiling and its effects on DNA structure and function. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):85–91. doi: 10.1101/sqb.1983.047.01.011. [DOI] [PubMed] [Google Scholar]



