Abstract
Non-invasive and quantitative imaging of tumor angiogenesis is essential for lesion detection, patient stratification, drug development, and personalized anti-cancer therapies. In particular, the right timing is critical for antiangiogenic cancer therapy and non-invasive imaging can help determine whether to start and when to start such treatment. In this inaugural issue of the American Journal of Nuclear Medicine and Molecular Imaging, a peptoid-based positron emission tomography (PET) tracer was reported for imaging of VEGFR expression in a prostate cancer model. This important proof-of-principle study opened the door to a fertile area of research, which holds tremendous potential for various applications in future personalized medicine.
Keywords: Peptoid, cancer, tumor angiogenesis, positron emission tomography (PET), molecular imaging, 64Cu
Introduction
Molecular imaging of tumor angiogenesis has been an extremely dynamic field during the first decade of the 21st century [1]. This phenomenon is partly due to the shift in drug discovery from conventional cytotoxic drugs to molecularly specific agents, in particular anti-angiogenic agents [2]. Conventional anatomical imaging modalities (e.g. computed tomography) are usually inadequate for monitoring the tumor responses to anti-angiogenic therapies, since tumor shrinkage may not occur within a short period of time even when the treatment is effective. Non-invasive imaging of tumor angiogenesis is essential for lesion detection, patient stratification, drug development, and personalized anti-cancer therapies. In particular, imaging can help determine whether to start and when to start anti-angiogenic therapies.
The vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) signaling pathway plays a pivotal role in both normal vasculature development and many disease processes [3]. The angiogenic actions of VEGF are mainly mediated via two endothelium-specific receptor tyrosine kinases, VEGFR-1 (Flt-1/FLT-1) and VEGFR-2 (Flk-1/KDR). VEGFR-1 is important for physiologic and developmental angiogenesis, where its function varies with the stages of development, the states of physiologic/pathologic conditions, as well as the cell types in which it is expressed. VEGFR-2 is the major mediator of the mitogenic, angiogenic, and permeability-enhancing effects of VEGF. In addition to these 2 receptors, VEGFR-3 (Flt-4) mediates lymphangiogenesis in response to VEGF-C and VEGF-D.
Overexpression of VEGF and/or VEGFRs has been implicated as poor prognostic markers in many clinical studies [4]. To date, most VEGFR-targeted imaging agents are based on VEGF-A isoforms such as VEGF121 and VEGF165 [1,5]. For future clinical translation, protein-based tracers are not ideal because of the relatively high cost, many regulatory hurdles, challenges in current Good Manufacturing Practice (cGMP) compliance, just to name a few. Small molecule- or peptide-based positron emission tomography (PET) tracers are advantageous due to ease of synthesis and quality control. However, the Achilles’ heel of peptides is that they are susceptible to degradation by a variety of enzymes hence are not very stable in vivo. Many approaches have been employed to improve the in vivo stability of peptide-based imaging/therapeutic agents, such as cyclization and the use of unnatural amino acids [6].
Peptoids, or poly-N-substituted glycines, are a class of peptidomimetics whose side chains are appended to the nitrogen atoms of the peptide backbone, rather than to the α-carbons [7] (Figure 1). Peptoids are very stable in vivo and are less expensive to synthesize than peptides and proteins, which makes them very attractive for PET tracer development. Surprisingly, very few peptoids have been investigated for imaging applications to date, likely due to a disconnection between chemists, biologists, and/or imaging scientists. In this inaugural issue of the American Journal of Nuclear Medicine and Molecular Imaging (AJNMMI, www.ajnmmi.us), a peptoid-based PET tracer was reported for imaging of VEGFR expression [8], opening the door to a fertile area of future research and development.
Figure 1.
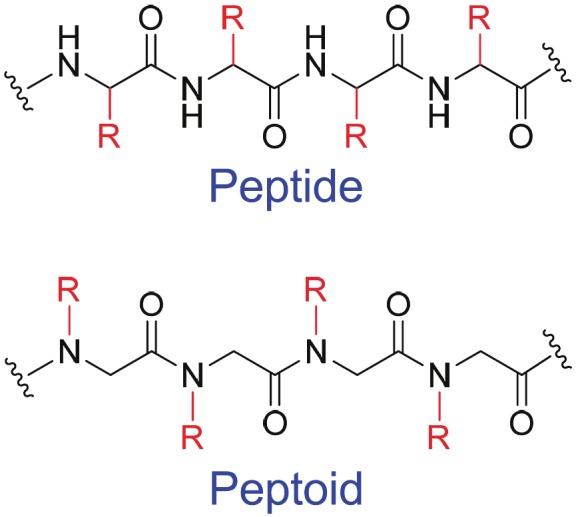
Peptide vs. peptoid.
Since no high affinity peptides have been developed for VEGFR-1/2/3 binding, to date there is no report on imaging VEGFR expression with peptide-based tracers. The VEGFR-2-binding peptoid used in this study was selected through a two-color, cell-based screening [9]. The initially identified peptoids bound to VEGFR-2 with KD values in the micromolar (μM) range, similar to other VEGFR-2-binding peptides reported in the literature. To improve the binding affinity to VEGFR-2, a dimerization approach afforded a peptoid with a Kd of 30 nanomolar (nM), suitable for imaging applications. Although the focus of this study was on VEGFR-2 [8], which is generally accepted to be more functionally important than VEGFR-1 during cancer progression [3,4], this peptoid also binds to VEGFR-1 with similar affinity (KD: 40 nM).
Modification of a targeting ligand for imaging applications should always be performed with utmost caution, since in many cases nonspecific conjugation of chelators (for radiometal labeling) or other synthons (e.g. for 11C/18F-labeling) may affect the target binding affinity/specificity of the ligand. In this report, site-specific conjugation of the chelator was performed on an additional cysteine residue, which did not affect its VEGFR-2 binding affinity as indicated by competitive binding assay. Incorporation of a cysteine residue also opens the door for future 18F-labeling since many thiol-specific synthons have been developed [10,11]. For clinical PET studies, 18F-based tracers are the mainstay [12-15]. Very few 64Cu-based agents have reached clinical development.
The use of a PC-3 prostate cancer model for imaging of tumor angiogenesis is interesting. Anti-angiogenic therapy is a validated approach in several solid tumor types such as lung and colorectal cancer, where survival benefit was achieved when bevacizumab (a humanized anti-VEGF monoclonal antibody) is combined with chemotherapy [16,17]. However, in the CALGB 90401 Phase III trial, bevacizumab in combination with docetaxel/prednisone did not improve the overall survival in metastatic castration-resistant prostate cancer (conference reports). Thus, whether anti-angiogenic therapy is applicable/valid for advanced prostate cancer remains controversial. PET imaging of tumor angiogenesis may play a key role in selecting the right patient population for treatment with the right drug(s) at the right time, as well as monitoring the efficacy/response in a quantitative, non-invasive, and accurate manner in real time.
In vivo stability of 64Cu-chelates has been a hotly debated topic over the last decade. Many chelators have been designed and investigated for 64Cu-labeling [18]. Recently, an elegant study compared the effect of the bifunctional chelator on the biodistribution of a 64Cu-labeled antibody [19]. It was found that differences in the thermodynamic stability of these 64Cu-chelator complexes were not associated with significant differences in tumor uptake of the tracer. However, there were significant differences in tracer concentration in other tissues, in particular those involved in tracer clearance (e.g. liver and spleen). It is now generally agreed that NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) is one of the best chelators for 64Cu-labeling. In this study, a similar macrocyclic chelator DOTA (1,4,7,10-tetraazacyclododecane -1,4,7,10-tetraacetic acid) was used. Unlike antibodies which typically circulate for hours to days in the blood, the short circulation half-lives of peptide/peptoid-based tracers (typically on the order of minutes) make the difference between NOTA- and DOTA-based tracers insignificant.
Now that the proof-of-principle has been demonstrated for PET imaging of VEGFR expression with a peptoid-based tracer [8], much more future investigation is warranted and expected. First, tumor angiogenesis is a dynamic process and the expression level of VEGFR-2 has been demonstrated to vary significantly over time in other tumor models [20,21]. Longitudinal imaging of VEGFR expression in the PC-3 tumor model and other models with different levels of angiogenic activity, as well as monitoring the therapeutic efficacy of anti-angiogenic therapies in these models, should be investigated in future studies. Second, VEGFR-1, VEGFR-2, or VEGFR-3 each plays a different role in physiological/pathological angiogenesis. The fact that the naturally occurring VEGF can bind to multiple VEGFRs significantly complicates the situation in studies that target this critical pathway. Since all VEGF-A isoforms bind to both VEGFR-1 and VEGFR-2, specificity to VEGFR-2 has rarely been achieved in previous studies [22]. Identifying high-affinity (multimeric) peptoids for noninvasive imaging of individual VEGFR subtype will undoubtedly facilitate unraveling the disease mechanisms in cancer progression/intervention, by providing invaluable tools for pre-clinical/clinical evaluation of new antiangiogenic agents or therapies. Third, nanotechnology has great potential for early detection, accurate diagnosis, and personalized treatment of cancer [23,24]. Since most nanoparticles do not extravasate well, vascular proteins (e.g. VEGFR) are ideal targets for cancer nanomedicine. Peptoids with small size, high receptor binding affinity, and superb in vivo stability are ideal ligands for in vivo tumor targeting of these nanoparticles.
The predominant killer in prostate cancer is bone metastasis. The 5-year survival rate is ~100% in patients with localized prostate cancer, which plummets to ~30% when distant metastases are present [25]. Like most other solid malignancies, prostate cancer can metastasize to distant organs such as the liver, lungs, and brain, but it has an unusually high propensity for metastasizing to the bone. In an autopsy study, 80% of the men who had died from prostate cancer possessed bone metastases [26]. Testing this peptoid-based tracer or future generations of VEGFR-2-binding peptoids in bone metastasis models will give more clinically relevant insights in prostate cancer management. Lastly, given the demonstrated success of antiangiogenic therapies in patients of many solid tumor types [16,17,27], VEGFR-binding peptoids will have potential clinical applications in patient selection and theranostics. Clearly, the potential for peptoid-based PET imaging of VEGFR, as demonstrated here [8], is tremendous and we will surely see many more exciting reports in the near future.
References
- 1.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl 2):113S–128S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 5.Cai W, Chen X. Multimodality imaging of vascular endothelial growth factor and vascular endothelial growth factor receptor expression. Front Biosci. 2007;12:4267–4279. doi: 10.2741/2386. [DOI] [PubMed] [Google Scholar]
- 6.Cai W, Niu G, Chen X. Imaging of integrins as biomarkers for tumor angiogenesis. Curr Pharm Des. 2008;14:2943–2973. doi: 10.2174/138161208786404308. [DOI] [PubMed] [Google Scholar]
- 7.Yoo B, Kirshenbaum K. Peptoid architectures: elaboration, actuation, and application. Curr Opin Chem Biol. 2008;12:714–721. doi: 10.1016/j.cbpa.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Hao G, Hajibeigi A, De León-Rodríguez LM, Öz OK, Sun X. Peptoid-based PET imaging of vascular endothelial growth factor receptor (VEGFR) expression. Am J Nucl Med Mol Imaging. 2011;1:65–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Udugamasooriya DG, Dineen SP, Brekken RA, Kodadek T. A peptoid "antibody surrogate" that antagonizes VEGF receptor 2 activity. J Am Chem Soc. 2008;130:5744–5752. doi: 10.1021/ja711193x. [DOI] [PubMed] [Google Scholar]
- 10.Olberg DE, Hjelstuen OK. Labeling strategies of peptides with 18F for positron emission tomography. Curr Top Med Chem. 2010;10:1669–1679. doi: 10.2174/156802610793176747. [DOI] [PubMed] [Google Scholar]
- 11.Cai W, Zhang X, Wu Y, Chen X. A thiol-reactive 18F-labeling agent, N-[2-(4-18F-fluorobenzamido) ethyl] maleimide (18F-FBEM), and the synthesis of RGD peptide-based tracer for PET imaging of avb3 integrin expression. J Nucl Med. 2006;47:1172–1180. [PMC free article] [PubMed] [Google Scholar]
- 12.Vach W, Høilund-Carlsen PF, Fischer BM, Gerke O, Weber W. How to study optimal timing of PET/CT for monitoring of cancer treatment. Am J Nucl Med Mol Imaging. 2011;1:54–62. [PMC free article] [PubMed] [Google Scholar]
- 13.Rakheja R, Ciarallo A, Alabed YZ, Hickeson M. Intravenous administration of diazepam significantly reduces brown fat activity on 18F-FDG PET/CT. Am J Nucl Med Mol Imaging. 2011;1:29–35. [PMC free article] [PubMed] [Google Scholar]
- 14.Eary JF, Hawkins DS, Rodler ET, Conrad EUI. 18F-FDG PET in sarcoma treatment response imaging. Am J Nucl Med Mol Imaging. 2011;1:47–53. [PMC free article] [PubMed] [Google Scholar]
- 15.Iagaru A. 18F-FDG PET/CT: timing for evaluation of response to therapy remains a clinical challenge. Am J Nucl Med Mol Imaging. 2011;1:63–64. [PMC free article] [PubMed] [Google Scholar]
- 16.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 17.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 18.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Coordinating radiometals of copper, gallium, indium, yttrium, and zirconium for PET and SPECT imaging of disease. Chem Rev. 110:2858–2902. doi: 10.1021/cr900325h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dearling JL, Voss SD, Dunning P, Snay E, Fahey F, Smith SV, Huston JS, Meares CF, Treves ST, Packard AB. Imaging cancer using PET--the effect of the bifunctional chelator on the biodistribution of a 64Cu-labeled antibody. Nucl Med Biol. 2011;38:29–38. doi: 10.1016/j.nucmedbio.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, Chen X. PET of vascular endothelial growth factor receptor expression. J Nucl Med. 2006;47:2048–2056. [PubMed] [Google Scholar]
- 21.Chen K, Cai W, Li ZB, Wang H, Chen X. Quantitative PET imaging of VEGF receptor expression. Mol Imaging Biol. 2009;11:15–22. doi: 10.1007/s11307-008-0172-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Cai W, Chen K, Li ZB, Kashefi A, He L, Chen X. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur J Nucl Med Mol Imaging. 2007;34:2001–2010. doi: 10.1007/s00259-007-0524-0. [DOI] [PubMed] [Google Scholar]
- 23.Hong H, Zhang Y, Sun J, Cai W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today. 2009;4:399–413. doi: 10.1016/j.nantod.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai W, Chen X. Nanoplatforms for targeted molecular imaging in living subjects. Small. 2007;3:1840–1854. doi: 10.1002/smll.200700351. [DOI] [PubMed] [Google Scholar]
- 25.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 26.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]


