Abstract
Multiple myeloma is incurable with standard therapies but is susceptible to a T-cell-mediated graft versus myeloma effect after allogeneic stem cell transplantation. We sought to identify myeloma-specific antigens that might be used for T-cell immunotherapy of myeloma. MAGE-C1 (CT-7) is a cancer-testis antigen that is expressed by tumor cells in >70% of myeloma patients and elicits a humoral response in up to 93% of patients with CT-7+ myeloma. No CD8+ T-cell epitopes have been described for CT-7, so we used a combination of reverse immunology and immunization of HLA-A2 transgenic mice with a novel cell-based vaccine to identify three immunogenic epitopes of CT-7 that are recognized by human CD8+ T-cells. CT-7-specific T-cells recognizing two of these peptides are able to recognize myeloma cells as well as CT-7 gene-transduced tumor cells, demonstrating that these epitopes are naturally processed and presented by tumor cells. This is the first report of the identification of immunogenic CD8+ T-cell epitopes of MAGE-C1 (CT-7), which is the most commonly expressed cancer-testis antigen found in myeloma, and these epitopes may be promising candidate targets for vaccination or T-cell therapy of myeloma or other CT-7+ malignancies.
Keywords: MAGE-C1, CT-7, Multiple myeloma, Cancer immunology, Immunotherapy
Introduction
Multiple myeloma is a clonal B-cell malignancy characterized by the accumulation of plasma cells in the bone marrow, leading to bone destruction and marrow failure. Myeloma accounts for >10% of hematologic malignancies and is incurable with conventional therapies. Survival can be prolonged with autologous stem cell transplantation (SCT) and other therapy combinations [1–5], but the disease recurs in almost all patients. Myeloma is susceptible to a graft versus myeloma (GVM) effect mediated by donor T-cells following allogeneic SCT [6, 7]. Direct evidence for a GVM effect comes from treatment of myeloma patients with persistent disease after allogeneic SCT, where the use of donor lymphocyte infusions (DLI) induces a remission in up to 50% of patients [8]. Unfortunately, allogeneic SCT and DLI are associated with graft versus host disease and infections related to immunosuppression, and approaches that could harness T-cell-mediated killing of myeloma without damaging normal tissues are needed.
Recent studies have demonstrated that T-cells from myeloma patients are unable to directly respond to autologous tumor, suggesting a tumor-induced state of unresponsiveness to myeloma antigens [9–11]. However, upon in vitro restimulation using myeloma-loaded or peptide-loaded dendritic cells (DCs), myeloma-reactive cytotoxic T-cells can be generated from patients’ blood and marrow [9, 12–15]. Interestingly, patients with monoclonal gammopathy of undetermined significance (MGUS), which is a pre-myeloma clonal plasma cell disorder, often exhibit both a T-cell and humoral response against autologous bone marrow plasma cells [16, 17]. Furthermore, a T-cell response against peptides derived from the Sox 2 protein has been shown to correlate with a decreased risk of progression to myeloma [18]. These observations suggest that tolerance of specific host immunity occurs during the progression from MGUS to myeloma but may be reversible with adequate conditions for T-cell activation.
Tumor-specific T-cell therapy is being utilized in humans with increasing success, particularly in melanoma, where many tumor antigens have been defined [19, 20]. An obstacle to applying this approach to myeloma is the paucity of well-defined myeloma antigens. MAGE-C1 (CT-7), a member of the cancer-testis family of proteins, is an attractive potential target, because it is expressed in 70–86% of myeloma tumor samples [21–23]. Like other cancer-testis antigens, CT-7 is not expressed in normal adult tissues other than germline cells [22, 23]. Recently, others have shown that 93% of myeloma patients with tumor cells expressing CT-7 have a detectable humoral immune response against various portions of the CT-7 protein [24], and T-cells from myeloma patients are capable of recognizing CT-7, but their target peptides presented by MHC are unknown [25].
To identify potential CT-7 epitopes for CD8+ T-cells, we stimulated T-cells from healthy normal donors in vitro with autologous dendritic cells pulsed with 13 CT-7 peptides predicted to bind to HLA-A2 and immunized HLA-A2 transgenic mice with T-cells transduced with the CT-7 gene. We identified CD8+ T-cell responses against 3 HLA-A2-restricted CT-7 peptides and show that 2 of these (p1083 and p959) are processed and presented by myeloma cells, CT-7-transduced tumor cells, and HLA-A2 transgenic mouse T-cells transduced with CT-7. The CT-7 p1083 and p959 peptides are the first CD8+ T-cell epitopes of CT-7 to be reported and may be useful targets for T-cell therapy or vaccination of myeloma patients.
Materials and methods
Peripheral blood samples
Peripheral blood mononuclear cells (PBMC) were obtained by leukapheresis or phlebotomy from HLA-A2+ healthy volunteers who gave written informed consent on protocols approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center. Donors were screened for HLA-A2 expression by flow cytometry of PBMC using an HLA-A2-specific monoclonal antibody (mAb; BB7.2), and HLA-A*0201 expression was confirmed by molecular typing in the Clinical Immunogenetics Laboratory at the Seattle Cancer Care Alliance (Seattle, WA).
Patient samples
We obtained an aliquot of bone marrow and blood from 11 patients with symptomatic Stage II–III multiple myeloma according to a protocol approved by the Institutional Review Board of Fred Hutchinson Cancer Research Center. All subjects gave written informed consent. Mononuclear cells were isolated from bone marrow and blood samples by density gradient centrifugation.
Peptide synthesis and HLA A2 binding
CT-7 peptides that are predicted to bind to HLA-A2 were identified using 2 computer algorithms for epitope prediction: “BIMAS” (http://www.bimas.dcrt.nih.gov/molbio/hla_bind) and “SYFPEITHI” (http://www.syfpeithi.de/). The CT-7 peptides (n = 13) that ranked highest for binding were synthesized (NMI peptides, Reutlingen Germany) (Table 1). Control peptides included Influenza MP58–66 (GILGFVFTL) and CMVpp65495–503 (NLVPMVATV), as well as peptides derived from B-cell maturation antigen (BCMA). Binding to HLA-A2 was confirmed using a TAP-deficient T2 cell binding assay to measure cell surface stabilization of HLA-A2 after overnight incubation with peptide in serum-free medium [26].
Table 1.
Prediction and screening of HLA-A2 restricted CT-7 peptides
| CT-7 peptidesa | Amino acid sequence | BIMAS score | SYFPEITHI score | Mean fluorescence (% increase)b | Responses identifiedc |
|---|---|---|---|---|---|
| p1087–1095 | FLAMLKNTV | 320 | 27 | 193 | No |
| p1013–1022 | YASEEVIWDV | 314 | 22 | 26 | No |
| p1046–1054 | KVWVQEHYL | 299 | 17 | 37 | No |
| p1083–1091 | KVVEFLAML | 234 | 24 | 135 | 3/6 |
| p1001–1009 | ILILSIIFI | 224 | 26 | 14 | No |
| p959–968 | ILFGISLREV | 201 | 31 | 239 | 6/6 |
| p1018–1026 | VIWDVLSGI | 191 | 24 | 131 | No |
| p884–892 | TLLESDSLT | 113 | 18 | 24 | No |
| p998–1006 | RLLILILSI | 89 | 28 | 5 | 2/6 |
| p535–543 | SLPEWEDSL | 43 | 26 | 52 | No |
| p907–915 | TLDEKVDEL | 40 | 29 | 27 | No |
| p1089–1097 | AMLKNTVPI | 30 | 24 | 67 | No |
| p999–1007 | LLILILSII | 17 | 26 | 6 | No |
aThe protein sequence of CT-7 was scanned using epitope prediction algorithms (BIMAS, http://www.bimas.dcrt.nih.gov/molbio/hla_bind; SYFPEITHI, http://www.syfpeithi.bmi-heidelberg.com) to identify nonamer and decamer peptides predicted to bind HLA-A2
bFluorescence is expressed as percentage increase of HLA-A2 mean fluorescence by flow cytometry of T2 cells in the presence of peptide versus the level on empty T2 cells
cResponses were determined by screening CD8+ T-cell cultures from 6 healthy donors for specific lysis of T2 cells pulsed with CT-7 peptide and confirmed by IFN-γ Elispot assay
Cell lines
Human myeloma cell lines U266 (HLA-A2+CT-7+) and H929 (HLA-A2negCT-7+) were obtained from ATCC (Manassas, VA), and L-363 (HLA-A2+CT7neg/low) was kindly provided by Dr. Kenneth Anderson and Dr. C. Mitsiades (Dana Farber, Boston, MA). These cell lines were grown in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Hyclone, Logan, UT). Phoenix E and Phoenix Galv cells (gifts from the Nolan lab, Stanford, San Francisco, CA) were used to package retroviruses for transduction of mouse and human cells, respectively. SW480 colon carcinoma cells (HLA-A2+CT7neg) were obtained from ATCC and transduced with CT-7/LZRS and BCMA/LZRS retroviral vectors to make SW480/CT-7 and SW480/BMCA, respectively. (LZRS is a retroviral vector developed by the Nolan lab, Stanford, CA, and the constructs are described in the gene-modified T-cell section of methods below). SW480 cells and packaging cells were maintained as adherent monolayers in DMEM supplemented with 10% fetal bovine serum.
Flow cytometry and antibodies
Expression of HLA-A2 was determined using BB7.2-FITC (BD Biosciences). Intracellular CT-7 expression was examined following fixation and permeabilization of cells with Becton–Dickinson (BD) Fix/Perm solution (BD Biosciences) according to the manufacturer’s directions, followed by staining with anti-CT-7 mAb (CT7-33, Dako, Carpinteria, CA) and a PE-conjugated F(ab′)2 goat anti-mouse secondary antibody (Invitrogen). After washing thoroughly, myeloma bone marrow samples were counter-stained with mouse mAb-FITC against disease-specific intracellular kappa or lambda light chains (Invitrogen) to facilitate gating on monoclonal tumor cells. HLA-A2/PE Pentamers folded with CT-7 p959 and p1083 (Pro-Immune, Bradenton, FL) were used with anti-CD8-FITC (Invitrogen) to stain T-cells. Flow cytometry was performed using a BD FACScalibur and analyzed using WinMDI software.
Peptide stimulation of CD8+ T-cells
Dendritic cells were derived from PBMC by culture of adherent monocytes for 2–5 days in AIM-V medium (Invitrogen) supplemented with 800 units/mL granulocyte–macrophage colony-stimulating factor (GM-CSF) and 1,000 units/mL interleukin (IL)-4 (R&D Systems, Minneapolis, MN) and then matured for 24–48 h with a cocktail of cytokines as described [27]. Mature dendritic cells (DCs) were pulsed for 4 h in serum-free medium with synthetic peptides, each at the concentration of 20 μg/ml, washed, and used as stimulator cells. CD8+ T lymphocytes were enriched from PBMC to >95% purity by positive selection using Miltenyi microbeads (Miltenyi Biotec, Auburn, CA). CD8+ T-cells were co-cultured at a ratio of 10–20:1 with peptide-pulsed autologous DCs in 24-well plates in the presence of 10 ng/ml IL-12 in CTL media (CTLM), which consisted of RPMI 1640 supplemented with 25 mmol/l HEPES, 4 mmol/l l-glutamine, 100 U/ml penicillin (Sigma), 100 μg/ml streptomycin (Sigma), 25 μmol/l 2-mercaptoethanol (Sigma), and 10% heat-inactivated human serum (FHCRC). Cultures were re-stimulated with γ-irradiated (35 Gy) peptide-pulsed dendritic cells under similar conditions, except IL-12 was replaced by IL-7 (5 ng/ml) and IL-15 (1 ng/ml) in the second stimulation cycle, and IL-15 was then added at 1 ng/ml every 3–4 days. In some experiments, T-cells (3–8 × 104/well) were co-cultured with peptide-pulsed DCs in 96-well plates and 10 ng/ml IL-15 was added on day 7 without a second peptide stimulation. An aliquot of each well was screened on day 12 (for cultures in 96-well plates) or day 14 (for cultures in 24-well plates) for lysis of peptide-pulsed T2 cells in a 6-h 51Cr release assay. Aliquots from wells with specific lytic activity were expanded as described [28].
Cytotoxicity assay
Target cells were labeled with 51Cr and peptide(s) for 2 h at 37°C, washed 3 times, and then added directly to 96-well plates in duplicate or triplicate wells with effectors at various E:T ratios. After a 6 h incubation at 37°C, supernatants were counted for 51Cr release and the percentage of specific lysis determined by the formula: 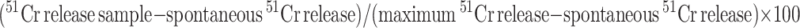 .
.
Interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISPOT)
ELISPOT assays were used to quantify antigen-specific IFN-γ-producing human and mouse T-cells as described [26, 29]. CD8+ T-cells or immunized mouse splenocytes were added to wells along with peptides at 10 μg/ml or with 1–5 × 104 of the indicated stimulator cells at 37°C for 16–24 h. Spot-forming cells were counted using a BioReader 3000 optical reader (ImmunoBioSys, The Colony, TX) and are expressed as spot-forming cells per well (SFC/well). A positive test well was pre-defined as containing at least 10 spots more than control wells and at least twice as many spots as control wells containing T-cells and no peptide.
Mice
HLA-A2 transgenic HHD II mice (referred to as HHD), in which the H-2Db and mouse β2m genes have been disrupted by homologous recombination and a transgene has been inserted for HLA-A-0201 α1–α 2, H-2Db α3-transmembrane and intracytoplasmic domains, were a kind gift of Dr. F. Lemonnier (Pasteur Institute, France) [30], and breeders were kindly provided by Dr. Don Diamond (City of Hope, Duarte, CA). Mice were bred at our animal facility under an approved institutional protocol.
Generation of gene-modified T-cells (T-APC)
The cDNA for the coding regions of CT-7 and BCMA were cloned from mRNA isolated from the U266 myeloma cell line using RNEasy (Quiagen) and the following primers: (CT-7 Forward 5′-TGTGACGAGGATCGTCTCAGGTC-3′) (CT-7 Reverse 5′-GTGCGACTCATGTGCATAAACTAGGATT-3′) (BCMA Forward 5′-TTACTTGTCCTTCCAGGCTGTTCT-3′) (BCMA Reverse 5′-CATGAAACCAAGGAAGTTTCTACC-3′). Each cDNA was inserted into the pcDNA3.1 TOPO vector (Invitrogen) and then subcloned into the LZRS retroviral vector and designated CT-7/LZRS or BCMA/LZRS. Phoenix E and Phoenix Galv packaging cells were transfected using lipofectamine LTX (Invitrogen) for generation of retroviruses. Transduction with polyethylene glycol (PEG)-precipitated retroviral supernatant was performed after activation of human T-cells for 3 days with anti-CD3 mAb and 50 units/mL IL-2, or after activation of HHD mouse splenocytes for 48 h with 2.5 μg/ml Concanavalin A (ConA) and 100 units/ml IL-2. Polybrene (4–8 μg/ml) was added during transduction, and the cells were centrifuged for 2 h in the plate followed by overnight incubation before washing. After 3 days, the T-cells were stained for expression of BCMA or CT-7.
Vaccination of HLA-A2 transgenic (HHD) mice
Gene-modified T-cells (T-APC) with at least 15% expression of CT-7 or BCMA were injected (1 × 107 per mouse) via lateral tail vein. Vaccinations were repeated after 14 days. Other groups of mice were immunized subcutaneously in the flank twice at a 14-day interval with 100 μg of one of the 3 CT-7 peptides (p959, p998, or p1083) or with 100 μg of a control viral peptide (CMVpp65NLVPMVATV or influenza MPGILGFVFTL). The peptides were mixed with hepatitis B core helper peptide (140 μg) and emulsified 1:1 in incomplete Freund’s adjuvant (IFA; Difco Laboratories, Detroit, MI) in a final injection volume of 200 μl. Splenocytes were isolated 8–10 days after the second vaccination and assayed by direct ex vivo IFN-γ Elispot for responses to CT-7 peptides. In some experiments, an aliquot of the splenocytes was also in vitro stimulated (IVS) with peptide-pulsed 30 Gy irradiated splenocytes for 5–7 days before testing by IFN-γ Elispot. Media for culture of murine T-cells was the same as CTLM except it contained 12.5 mM HEPES buffer and 10% FBS (Hyclone) instead of human serum.
Statistical analysis
We used GraphPad Prism 5 for data handling and analysis. Graphs display averages with error bars indicating standard error of the mean. Student’s t-test was used when comparing only 2 groups of data. Statistical significance was set at P < 0.05.
Results
Expression of MAGE-C1 (CT-7) by myeloma cells
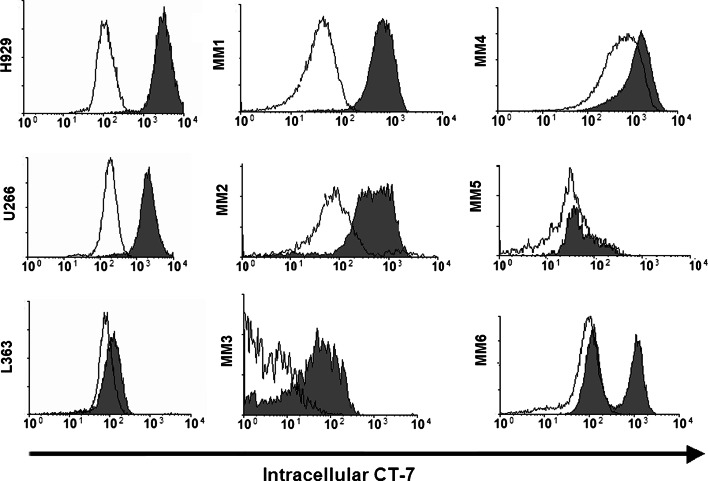
Prior studies using immunohistochemistry and reverse transcriptase PCR to detect expression of CT-7 protein and RNA, respectively, showed that 70–86% of cases of multiple myeloma express CT-7 [21, 23, 31, 32]. Flow cytometry should be superior to these methods for determining the homogeneity of CT-7 expression within a population of myeloma cells and for comparing the level of expression between patients. We permeabilized human myeloma cell lines (H929, U266, and L363), and stained the cells with an anti-CT-7 mAb (gray histograms) or isotype control mAb (open histograms) followed by a PE-conjugated goat anti-mouse F(ab′)2 secondary antibody. H929 and U266 homogeneously expressed CT-7, whereas L363 expressed minimal to undetectable levels (Fig. 1). We then examined CT-7 expression in primary myeloma cells present in aliquots of bone marrow obtained from untreated patients with Stage II–III multiple myeloma (MM). Each sample (MM 1 through MM11) was counterstained with a mAb against intracellular kappa or lambda light chains to facilitate gating on monoclonal myeloma tumor cells, and analysis was performed on the myeloma cell fraction. The CT-7 expression by myeloma cells from 6 representative patient samples is shown (Fig. 1, MM1–MM6). Of note, background isotype control staining of primary myeloma cells was often higher than for cultured cells and different for each patient (open histograms). Although there was no detectable expression of CT-7 by flow cytometry in 4 of the 11 patient samples (MM5 and 3 others not shown), 7 of the 11 primary myeloma patient samples (64%) expressed CT-7 by flow cytometry. Expression was homogeneous in some of these (e.g., MM1, MM2) and heterogeneous or bimodal in others (e.g., MM3, MM6). In some cases, there was a CT-7 low or negative subset of cells, which could represent either heterogeneous expression of CT-7 in the tumor cell sub-population, different levels of expression with changes in cell cycle, or the presence of contaminating normal plasma cells or B cells that expressed the same light chain isotype. The latter is unlikely since counterstaining for CD19 to gate out normal B cells and normal plasma cells did not eliminate the CT-7-low/negative population, and no bone marrow cells other than the light chain restricted myeloma cells expressed CT-7 (data not shown).
Fig. 1.
Expression of CT-7 by myeloma cells. Myeloma cell lines (H929, U266, and L363) and bone marrow samples from 11 patients with stage II–III multiple myeloma (MM) were stained for intracellular CT-7 expression (gray histograms) or with an isotype control mAb (open histograms) following fixation/permeabilization. Analysis of the patient samples was limited to only myeloma cells by gating on appropriately sized cells counterstained for intracellular disease-specific kappa or lambda light chains, and data are shown for 6 samples representative of the different staining patterns seen. Seven of 11 patient samples expressed CT-7 (as represented by patients MM1, 2, 3, 4, and 6), while no detectable expression was seen in 4 patients (as represented by MM5). Expression was homogeneous in some samples (e.g., MM1 and MM2) and was heterogeneous (MM3) or even bimodal (MM6) in other samples. Of the 5 MM patient samples not shown, 2 had staining similar to MM3 and MM4 and the other 3 were not above background staining. The nontumor bone marrow cells did not express CT-7 (not shown)
Identification of CT-7 peptides recognized by CD8+ cytotoxic T-cells
The observation that myeloma cells in the majority of patients with advanced disease express CT-7 and that this expression can be homogeneous suggests that CT-7 may be a suitable target for immunotherapy [15, 33, 34]. No CT-7 epitopes recognized by CD8+ T-cells have been previously reported; therefore, we used epitope prediction algorithms to identify nonamer and decamer peptides within the 1142 amino acid CT-7 sequence that were predicted to bind HLA-A2 and synthesized 13 peptides with the highest binding scores to test for immunogenicity (Table 1).
We examined the immunogenicity of CT-7 peptides in healthy donors in this study, because the numbers of T-cells and dendritic cells that can be isolated from myeloma patients is limiting, and T-cell responses might be more difficult to detect in patients due to tumor-induced or corticosteroid-induced T-cell defects [11, 35]. CD8+ T-cells were purified from six healthy donors, and aliquots were stimulated with autologous monocyte-derived mature dendritic cells pulsed with CT-7 peptides as antigen-presenting cells (APC). After 12–14 days, T-cells from individual wells were tested to identify T-cells that specifically lysed T2 cells pulsed with CT-7 peptide(s) but not control or unpulsed T2 cells. Wells with specific cytolytic activity were screened further by IFN-γ Elispot assay to confirm peptide specificity before expansion. Cytotoxic T-cell responses specific for three of the 13 novel CT-7 peptides (CT-7959–968, CT-71083–1091, and CT-7998–1006) were detected and confirmed by Elispot in at least 2 donors each, and all 6 donors responded to at least one of these three peptides (Table 1). The responses against CT-7959–968 (p959) and CT-71083–1091 (p1083) were reproducible upon repeat testing of a second aliquot of T-cells from the same donors.
CT-7 p1083 and p959 are processed and presented by CT-7+ myeloma cells and CT-7 gene-transduced tumor cells
To determine whether any of the 3 CT-7 peptides that elicited CD8+ T-cell responses in vitro were presented by HLA-A2 on myeloma cells, we expanded wells containing peptide-reactive T-cells using OKT3 mAb, interleukin-2 (IL-2), and allogeneic feeder cells. T-cells expanded from the two cultures that had exhibited CT-7998–1006 (p998) specificity no longer exhibited a detectable response against CT-7 p998, likely due to overgrowth of irrelevant T-cells. However, T-cells specific for CT-71083–1091 (p1083) and CT-7959–968 (p959) were expanded successfully, retained specificity, and were evaluated further.
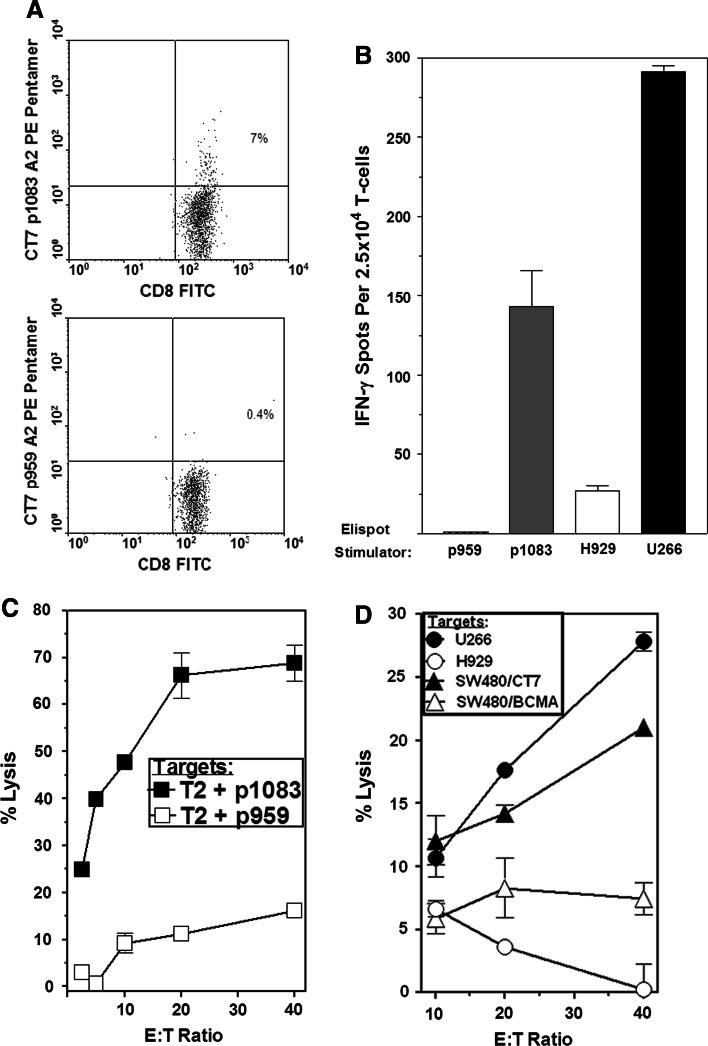
Pentamer staining and IFN-γ Elispot of the p1083-reactive T-cell line demonstrated the presence of a moderately high frequency of p1083-specific CD8+ T-cells after expansion (Fig. 2a, b). The p1083-specific T-cells produced IFN-γ in response to stimulation with p1083 and to HLA-A2+CT-7+ U266 myeloma cells, but did not recognize the control HLA-A2(−)CT-7+ H929 myeloma cell line (Fig. 2b). The ability of HLA-A2+ tumor cells to present endogenous p1083 was substantiated by 51Cr release assays, which showed that the p1083-specific T-cells lysed p1083-pulsed T2 cells, U266 myeloma cells, and HLA-A2+ SW480 tumor cells retrovirally transduced to express CT-7 (SW480/CT-7), and they did not lyse control peptide-pulsed T2 cells, HLA-A2(−) H929 cells, or BCMA-transduced SW480 tumor cells (SW480/BCMA) (Fig. 2c, d).
Fig. 2.
CT-7 p1083-specific T-cells recognize HLA-A2+CT-7+ myeloma and CT-7 gene-transduced tumor cells. T-cells were stimulated with p1083-pulsed dendritic cells and then nonspecifically expanded with anti-CD3 (see “Materials and methods”). a Staining of expanded CT-7 p1083-specific CD8+ T-cell line with an HLA-A2/p1083 pentamer (top panel) and a control HLA-A2/p959 pentamer (bottom panel). b p1083-specific T-cells produce IFN-γ in response to HLA-A2+CT-7+ U266 myeloma cells but not control HLA-A2(−) H929 cells. c Lysis of 51Cr-labeled p1083-pulsed and control T2 cells by p1083-specific T-cells. d Lysis of 51Cr-labeled HLA-A2+CT-7+ U266, CT-7 gene-transduced SW480 tumor cells (SW480/CT7, >90% CT-7+ by flow cytometry), HLA-A2+CT-7(−) SW480/BCMA cells, and HLA-A2(−) H929 cells by p1083-specific T-cells
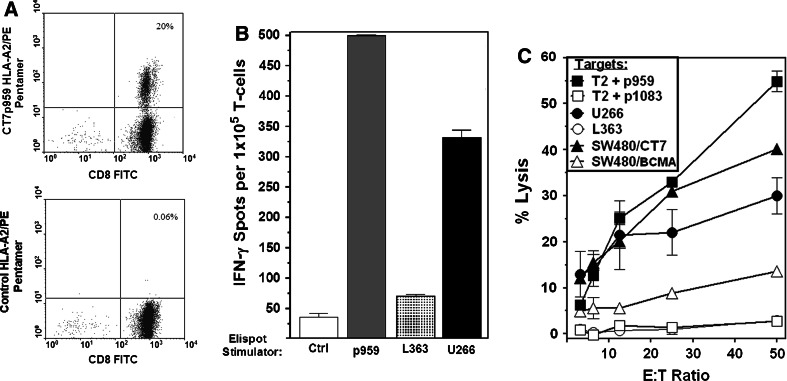
We used a similar approach to determine whether CT-7 p959 was processed and presented by myeloma cells. Pentamer staining and IFN-γ Elispot assays showed a high frequency of p959-specific T-cells in the expanded cultures (Fig. 3a, b), and these T-cells efficiently lysed p959-pulsed T2 cells, U266 myeloma cells, and SW480/CT-7 tumor cells but not control SW480/BCMA cells or the HLA-A2+CT-7negative/low myeloma cell line L363 (Fig. 3c). IFN-γ Elispot assays also showed similar recognition of U266 myeloma but not L363 (Fig. 3b). A separate experiment showed lack of reactivity against the HLA-A2-negative myeloma cell line H929 (data not shown).
Fig. 3.
CT-7 p959-specific T-cells recognize HLA-A2+CT-7+ myeloma cells and CT-7 gene-transduced tumor cells. Healthy donor T-cells were expanded (see “Materials and methods”) from a p959-stimulated microculture that showed p959 peptide specificity in screening assays. a Staining of 959-specific T-cells with an HLA-A2/p959 pentamer and a control HLA-A2/BCMAp64 pentamer. b IFN-γ production by p959-specific T-cells stimulated with p959 and HLA-A2+CT-7+ U266 cells and with a control peptide and CT-7negative/low L363 myeloma cells. c Lysis of 51Cr-labeled target cells by p959-specific T-cells
Vaccination of HLA-A2 transgenic mice with CT-7 gene-modified vaccine cells induces T-cell responses to CT-7 p1083 and CT-7 p959
The ability to isolate CT-7-specific T-cells from healthy individuals indicates that tolerance to CT-7 is not complete. However, the frequency of T-cells recognizing the two CT-7 peptides that were presented by myeloma cells was estimated to be approximately 1 in 3–15 × 106 peripheral blood CD8+ T-cells, determined from the proportion of wells that were positive by Elispot after peptide stimulation under these culture conditions. Thus, to utilize CT-7 as a target for immunotherapy, it may be necessary to vaccinate patients to expand the rare CT-7 reactive T-cells in vivo. We have been developing a cell-based vaccine based on expression of the CT-7 protein in autologous T-cells (T-APC). The rationale for using T-cells as APC is based on the rapid induction of T-cell immune responses to transgene products in clinical trials in which gene-modified T-cells were administered to immunocompetent patients [36–40]. Mouse studies of gene-modified T-cells have recently shown that the antigens appear to be chiefly cross-presented to endogenous CTL by dendritic cells that phagocytose the infused T-cells and become activated [41]. We chose to use this “problem” of gene therapy to our advantage by infusing T-APC as a “Trojan horse” for carrying the cancer antigen of interest to dendritic cells in vivo.
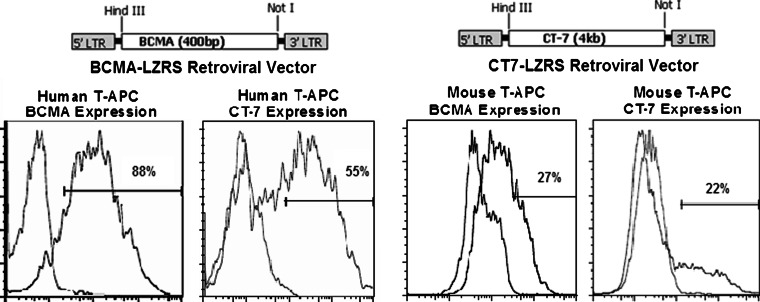
We tested T-APC vaccination against CT-7 in HLA-A2 transgenic (HHD) mice, which express human HLA-A2 and lack all mouse class I MHC molecules (and therefore present only HLA-A2-restricted peptides to CD8+ T-cells). To generate gene-expressing T-APC, we constructed retroviral vectors encoding CT-7 (CT7-LZRS) and BCMA as a control (BCMA-LZRS) and transduced activated T-cells from HHD mice and humans (Fig. 4). Expression of surface BCMA and intracellular CT-7 in transduced T-APC cells was evaluated by flow cytometry 3–5 days after transduction. The transduction efficiency of HHD T-cells was inferior to that of human T-cells and typically resulted in the expression of CT-7 or BCMA in 15–30% of T-cells, compared to 50–95% expression in human T-cells (Fig. 4).
Fig. 4.
Retroviral vectors encoding CT-7 and BCMA enable expression of these antigens in T-cells from humans and HLA-A2 transgenic mice. The cDNAs for the CT-7 and BCMA open reading frames were cloned into the LZRS retroviral vector (top). The vectors were packaged in Phoenix Galv and Phoenix E cells for transduction of human and mouse cells, respectively. Expression was confirmed by flow cytometry for surface BCMA or intracellular CT-7. A representative example of the expression of BCMA and CT-7 after transduction of human and mouse T-cells with each of the vectors is shown (bottom panels)
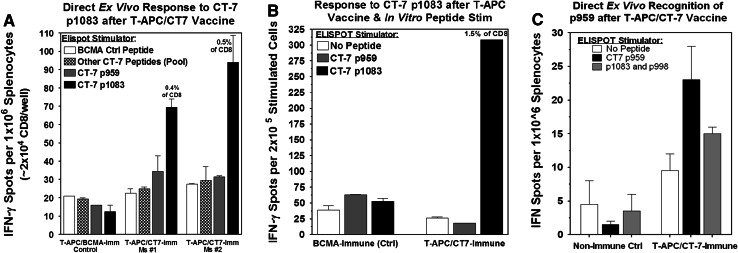
The gene-modified HHD T-APC were administered intravenously in a dose of 1 × 107 cells on days 0 and 14. Eight to ten days after the second vaccination, splenocytes were isolated and tested directly ex vivo for recognition of CT-7 peptides by IFN-γ Elispot. Six of 6 mice inoculated with T-APC/CT-7 exhibited T-cell responses to at least one CT-7 peptide (either p1083 or p959), while none of 4 control mice inoculated with T-APC/BCMA exhibited T-cell responses to CT-7 peptides (Fig. 5). In five of the six mice vaccinated with T-APC/CT-7, approximately 0.4–0.5% of the CD8+ T-cells responded to CT-7 p1083 by direct ex vivo Elispot (Fig. 5a). CT-7 p1083-specific T-cells could also be expanded/enriched to ~1.5% of CD8+ T-cells by a single round of in vitro peptide stimulation with p1083-pulsed splenocytes, and this was not a primary in vitro response since splenocytes from control mice did not react with p1083 after in vitro stimulation (Fig. 5b). One of the 6 mice immunized with T-APC/CT-7 (the only mouse without a detectable response to p1083) had a detectable T-cell response against CT-7 p959 (~0.1% of CD8 T-cells, Fig. 5c). Responses were not detected against any of the other 11 CT-7 peptides predicted to bind to HLA A2 in any of the mice (Fig. 5a). The induction of T-cell responses to CT-7 p1083 and CT-7 p959 after vaccination with T-APC expressing CT-7 protein confirms the ability of these peptides to be endogenously processed and presented to T-cells and suggests these two epitopes may be immunodominant epitopes for presentation by HLA-A2.
Fig. 5.
Vaccination with CT-7 gene-modified T-APC induces T-cell responses against immunodominant CT-7 peptides p1083 and p959. Six mice were immunized by i.v. injection of CT-7-transduced T-APC (T-APC/CT-7) or control BCMA-transduced T-APC (T-APC/BCMA). Eight to ten days after the second immunization, splenocytes were tested directly ex vivo by IFN-γ Elispot against the CT-7 peptides p959 and p1083 alone, and the 11 other peptides from Table 1 combined in a pool. a T-cell responses to p1083 detected by IFN-γ Elispot in 2 representative mice that responded to p1083. b IFN-γ Elispot of splenocytes from CT-7 and BCMA immunized mice after one in vitro stimulation with p1083 peptide. Cultures were analyzed 7 days after stimulation. c One of 6 mice immunized with T-APC/CT-7 (the only mouse without a significant response to p1083) had a low level but detectable response directed against CT-7 peptide p959 (P = 0.05 compared to no peptide control)
Vaccination of HLA-A2 transgenic mice with CT-7 peptides
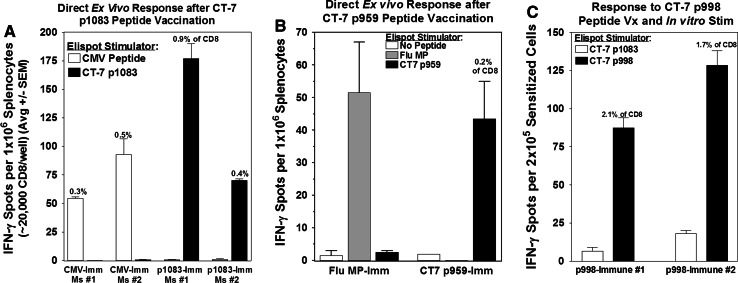
The immunogenicity of the three most promising candidate CT-7 peptides (p1083, p959, and p998) was further examined in HHD mice by vaccination of cohorts of mice subcutaneously twice at a 2-week interval with one of the three CT-7 peptides or with a control viral peptide (CMVpp65NLVPMVATV or influenza MPGILGFVFTL) mixed with a hepatitis B core helper peptide in incomplete Freund’s adjuvant [42]. Splenocytes were isolated and assayed by ex vivo IFN-γ Elispot 8–10 days after the second vaccination. The frequency of CD8+ T-cells responding to CT-7 p1083 was 0.4–0.9% in 4 of 4 mice, which was similar in magnitude to the T-cell response elicited by CMV pp65 vaccination in control mice (Fig. 6a), and notably similar to the p1083-specific T-cell response elicited after vaccination with T-APC/CT-7 (Fig. 5a).
Fig. 6.
CT-7 p1083, p959, and p998 are immunogenic in A2 transgenic mice. Cohorts of HHD mice were immunized with one of three CT-7 peptides (p1083, p959, or p998), or a control viral peptide, by subcutaneous injection of 100 μg peptide emulsified with 140 μg hepatitis B virus helper peptide in incomplete Freund’s adjuvant. a Direct ex vivo Elispot assay of CT-7 p1083-specific and CMV pp65-specific T-cells in immunized mice. b Direct ex vivo Elispot assay of CT-7 p959-specific and influenza MP-specific T-cells in immunized mice (data are shown for one representative experiment). c CT-7 p998 is weakly immunogenic, and T-cell responses were not detectable directly ex vivo (not shown), but were detectable following a single in vitro stimulation with p998-pulsed splenocytes
Two of 4 mice immunized with CT-7 p959 developed a specific T-cell response that was demonstrable directly ex vivo at a frequency of ~0.2% of CD8+ T-cells, which was comparable to the anti-viral response in control mice immunized with influenza MP peptide (Fig. 6b). Immunization with the CT-7 p998 peptide did not elicit a T-cell response detectable directly ex vivo, but after in vitro stimulation of p998-immunized T-cells with p998-pulsed splenocytes, 1.7–2.1% of the CD8+ T-cells responded in the IFN-γ Elispot assay, confirming that a low level response was primed by vaccination in 2 of 2 mice (Fig. 6c). Thus, all three of the CT-7 peptides that we found to elicit T-cell responses in healthy HLA-A2-positive donors were also immunogenic in HLA-A2 transgenic mice.
Discussion
Therapeutic options for multiple myeloma have improved with the development of immunomodulators and proteosome inhibitors, but the majority of patients are not cured. Myeloma is susceptible to a T-cell-mediated GVM effect after allogeneic stem cell transplantation, and autologous T-cell responses to myeloma cells have been detected in patients, suggesting that immunotherapy, either by vaccination or adoptive T-cell therapy, could be used in combination with or as an alternative to current therapies. A major impediment for the development of specific immunotherapy is the lack of defined target antigens recognized by T-cells. The studies presented here focused on identifying epitopes in a candidate myeloma antigen CT-7 that are presented by HLA-A2 and recognized by CD8+ T-cells.
In this study, we evaluated the presence of CT-7-specific T-cells in healthy HLA-A2-positive donors, because the T-cell repertoire in myeloma patients may be impaired by prior cytotoxic therapy or tumor-induced immune dysregulation. T-cells specific for three different CT-7 peptides (p1083, p959, and p998) that were predicted by computer algorithms to bind to HLA-A2 were detected in healthy donors. T-cells specific for p959 and p1083 were isolated from 6/6 and 3/6 healthy donors, respectively, and could be identified by pentamer staining, IFN-γ ELISPOT assay, and killing of human myeloma cell lines and CT-7 cDNA-transduced tumor cells. These data demonstrate that the CT-7 epitopes p959 and p1083 are derived by processing of intact CT-7 protein and presented in tumor cell lines that express HLA-A2. We were unable to evaluate T-cell recognition of primary myeloma samples with the described T-cell lines due to either low numbers of T-cells remaining after the assays presented, low numbers of myeloma cells obtained from the bone marrow samples available at the time, or lack of HLA-A2 expression on available myeloma samples, but future studies will address this important issue.
Some T-cell lines derived by peptide stimulation were not able to recognize myeloma cells or CT-7 transfectants (data not shown), likely due to the outgrowth of T-cells with low avidity [43]. However, these data show that at least in healthy donors, tolerance to CT-7 is incomplete, and the repertoire does contain T-cells with sufficient avidity to recognize CT-7 expressing tumors. Vaccination with cell-based vaccines that express the CT-7 gene should preferentially expand T-cells of high avidity and avoid the activation of low avidity T-cells [43, 44].
The immunogenicity of CT-7 p1083 and p959 was confirmed by immunizing HLA-A2 transgenic (HHD) mice. Vaccination of HHD mice with syngeneic T-cells transduced with the CT-7 gene (T-APC/CT-7) elicited p1083-specific T-cells in 5/6 mice and p959-specific T-cells in 1/6 mice. Responses were not detected to any of the other 11 CT-7 peptides that were predicted to bind to HLA-A2, suggesting that the p1083 and p959 epitopes are immunodominant for presentation by HLA-A2. Vaccination with peptide-based vaccines elicited responses to p1083 and p959 that were similar in magnitude to those seen against cytomegalovirus and influenza peptides known to be presented by HLA A2, and these studies also confirmed the weakly immunogenic nature of p998, which elicited a T-cell response that was only detectable after in vitro peptide stimulation of T-cells from vaccinated mice.
This is the first report of the identification of immunogenic epitopes of CT-7 recognized by cytotoxic T-cells and suggests that CT-7, which is the most commonly expressed cancer-testis antigen in myeloma, should be further pursued as a target for immunotherapy. These results have implications for the majority of myeloma patients since CT-7 is expressed in 70–86% of myeloma cases [32]. Others have shown that the level and frequency of CT-7 expression in myeloma cells increases with increasing tumor burden, adverse prognostic features, and advanced stage [21, 23, 31, 45, 46], and that CT-7 expression may be important for myeloma cell survival [47]. Thus, targeting CT-7 with immunotherapy might provide an option for patients with even the worst prognosis. Furthermore, by targeting the CT-7+ subpopulation of myeloma cells even in those patients with heterogenous expression, we may be able to halt the aggressive phase of disease and convert the myeloma back to a more indolent state, even if the T-cells do not eradicate all myeloma cells.
Another recently published study described the detection of spontaneous CD4+ T-cell responses to CT-7 in 3 out of 26 (11.5%) patients with melanoma (known to be more immunogenic than myeloma) [48]. This has been the only report of T-cell epitopes from CT-7, but they did not identify CD8+ T-cell responses, and their epitopes were not overlapping with our currently described CD8 epitopes.
Studies to determine whether CT-7-specific T-cells can be isolated and expanded from the blood of myeloma patients for adoptive therapy are in progress. We have recently tested limited numbers of peripheral blood CD8+ T-cells (2–5 × 106 per patient) from 3 untreated patients with CT7+HLA-A2+ myeloma for recognition of p959, p998, and p1083 after in vitro stimulation with autologous peptide-pulsed dendritic cells. Although no responses were seen by Elispot assay (data not shown), it is noted that a major limitation of using frozen blood samples from myeloma patients is that the numbers of both dendritic cells and T-cells recovered for testing are extremely limiting with much fewer cells to work with compared to our leukapheresis products from healthy donors. Since the precursor frequency of CT-7-reactive T-cells in healthy donors in our study was on the order of only 1 in 3–15 × 106 CD8+ T-cells (and myeloma patient dendritic cells are also known to be less functional than healthy dendritic cells), these studies could have easily missed a response even if the patient’s T-cell repertoire had a low frequency of cells capable of responding to a CT-7 vaccine in vivo. Therefore, future studies will use leukapheresis products from myeloma patients for better T-cell sampling. It is also likely that it will be difficult to isolate these cells from patients who have already received standard treatment with cytotoxic or corticosteroid therapy, so pre-treatment cells will be critical and will be compared with post-treatment samples.
Even if CT-7-specific T-cells cannot be isolated from myeloma patients, our data suggest two alternative strategies for obtaining T-cells that target CT-7. First, the T-cell receptor genes could be isolated from high avidity CT-7-specific T-cells, such as the ones we have derived from normal healthy donors or from HHD mice and introduced into patient T-cells to confer specificity to myeloma [49]. Second, vaccination with CT-7 gene-modified T-APC might be used as a “Trojan horse” for delivering the antigen and eliciting a T-cell response. This strategy may be useful for vaccinating patients since it involves the use of the whole gene/protein without requiring knowledge of peptide epitopes, and it does not restrict vaccination to patients with particular HLA types. This strategy may also be useful for the identification of immunogenic epitopes from other candidate myeloma antigens, which may be important to use as targets in combination with CT-7 in order to limit outgrowth of antigen loss variants that may occur if a single tumor-associated antigen is targeted [50, 51].
In summary, we have identified 3 immunogenic HLA-A2-restricted CT-7 peptides, and 2 of these appear to be immunodominant and naturally presented by myeloma cells, CT-7 gene-transduced tumor cells, and CT-7 gene-transduced T-cells used as a vaccine in HLA-A2 transgenic mice. These findings may be useful to help facilitate further studies of CT-7 immunotherapy in myeloma patients, where either vaccines or infusions of T-cells targeting CT-7 may offer a way to improve the outcome of a disease that is currently incurable.
Acknowledgments
This work was supported by a Multiple Myeloma Research Foundation Fellow Award (LDA), an ASCO Young Investigator Award (LDA), an NIH K12 Career Development Award (LDA), an American Cancer Society Institutional Research Grant (LDA), and NIH grants CA18029 and 114536 (SRR).
Conflict of interest
No conflicts of interest to disclose.
References
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, Monconduit M, Hulin C, Caillot D, Bouabdallah R, Voillat L, Sotto JJ, Grosbois B, Bataille R. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 3.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, Brown J, Drayson MT, Selby PJ. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 4.Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, Renaud M, Harousseau JL, Guillerm G, Chaleteix C, Dib M, Voillat L, Maisonneuve H, Troncy J, Dorvaux V, Monconduit M, Martin C, Casassus P, Jaubert J, Jardel H, Doyen C, Kolb B, Anglaret B, Grosbois B, Yakoub-Agha I, Mathiot C, Avet-Loiseau H. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99-06): a randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 5.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 6.Bensinger WI, Buckner CD, Anasetti C, Clift R, Storb R, Barnett T, Chauncey T, Shulman H, Appelbaum FR. Allogeneic marrow transplantation for multiple myeloma: an analysis of risk factors on outcome. Blood. 1996;88:2787–2793. [PubMed] [Google Scholar]
- 7.Bruno B, Rotta M, Patriarca F, Mordini N, Allione B, Carnevale-Schianca F, Giaccone L, Sorasio R, Omede P, Baldi I, Bringhen S, Massaia M, Aglietta M, Levis A, Gallamini A, Fanin R, Palumbo A, Storb R, Ciccone G, Boccadoro M. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 8.Lokhorst HM, Wu K, Verdonck LF, Laterveer LL, van de Donk NW, van Oers MH, Cornelissen JJ, Schattenberg AV. The occurrence of graft-versus-host disease is the major predictive factor for response to donor lymphocyte infusions in multiple myeloma. Blood. 2004;103:4362–4364. doi: 10.1182/blood-2003-11-3862. [DOI] [PubMed] [Google Scholar]
- 9.Dhodapkar MV, Krasovsky J, Olson K. T cells from the tumor microenvironment of patients with progressive myeloma can generate strong, tumor-specific cytolytic responses to autologous, tumor-loaded dendritic cells. Proc Natl Acad Sci USA. 2002;99:13009–13013. doi: 10.1073/pnas.202491499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massaia M, Dianzani U, Bianchi A, Camponi A, Boccadoro M, Pileri A. Defective generation of alloreactive cytotoxic T lymphocytes (CTL) in human monoclonal gammopathies. Clin Exp Immunol. 1988;73:214–218. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, Ho PJ, Hart D, Joshua D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001;98:2992–2998. doi: 10.1182/blood.V98.10.2992. [DOI] [PubMed] [Google Scholar]
- 12.Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J Exp Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noonan K, Matsui W, Serafini P, Carbley R, Tan G, Khalili J, Bonyhadi M, Levitsky H, Whartenby K, Borrello I. Activated marrow-infiltrating lymphocytes effectively target plasma cells and their clonogenic precursors. Cancer Res. 2005;65:2026–2034. doi: 10.1158/0008-5472.CAN-04-3337. [DOI] [PubMed] [Google Scholar]
- 14.Goodyear OC, Pratt G, McLarnon A, Cook M, Piper K, Moss P. Differential pattern of CD4+ and CD8+ T-cell immunity to MAGE-A1/A2/A3 in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma. Blood. 2008;112:3362–3372. doi: 10.1182/blood-2008-04-149393. [DOI] [PubMed] [Google Scholar]
- 15.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, Schilling G, Faltz C, Wolschke C, Dierlamm J, Ritter G, Eiermann T, Hossfeld DK, Zander AR, Jungbluth AA, Old LJ, Bokemeyer C, Kroger N. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109:1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 16.Blotta S, Tassone P, Prabhala RH, Tagliaferri P, Cervi D, Amin S, Jakubikova J, Tai YT, Podar K, Mitsiades CS, Zullo A, Franco B, Anderson KC, Munshi NC. Identification of novel antigens with induced immune response in monoclonal gammopathy of undetermined significance. Blood. 2009;114:3276–3284. doi: 10.1182/blood-2009-04-219436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med. 2003;198:1753–1757. doi: 10.1084/jem.20031030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spisek R, Kukreja A, Chen LC, Matthews P, Mazumder A, Vesole D, Jagannath S, Zebroski HA, Simpson AJ, Ritter G, Durie B, Crowley J, Shaughnessy JD, Jr, Scanlan MJ, Gure AO, Barlogie B, Dhodapkar MV. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhodapkar MV, Osman K, Teruya-Feldstein J, Filippa D, Hedvat CV, Iversen K, Kolb D, Geller MD, Hassoun H, Kewalramani T, Comenzo RL, Coplan K, Chen YT, Jungbluth AA. Expression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of disease. Cancer Immunol. 2003;3:9. [PubMed] [Google Scholar]
- 22.Jungbluth AA, Chen YT, Busam KJ, Coplan K, Kolb D, Iversen K, Williamson B, Van Landeghem FK, Stockert E, Old LJ. CT7 (MAGE-C1) antigen expression in normal and neoplastic tissues. Int J Cancer. 2002;99:839–845. doi: 10.1002/ijc.10416. [DOI] [PubMed] [Google Scholar]
- 23.Jungbluth AA, Ely S, DiLiberto M, Niesvizky R, Williamson B, Frosina D, Chen YT, Bhardwaj N, Chen-Kiang S, Old LJ, Cho HJ. The cancer-testis antigens CT7 (MAGE-C1) and MAGE-A3/6 are commonly expressed in multiple myeloma and correlate with plasma-cell proliferation. Blood. 2005;106:167–174. doi: 10.1182/blood-2004-12-4931. [DOI] [PubMed] [Google Scholar]
- 24.Curioni-Fontecedro A, Knights AJ, Tinguely M, Nuber N, Schneider C, Thomson CW, von Boehmer L, Bossart W, Pahlich S, Gehring H, Moch H, Renner C, Knuth A, Zippelius A. MAGE-C1/CT7 is the dominant cancer-testis antigen targeted by humoral immune responses in patients with multiple myeloma. Leukemia. 2008;22:1646–1648. doi: 10.1038/leu.2008.43. [DOI] [PubMed] [Google Scholar]
- 25.Lendvai N, Gnjatic S, Ritter E, Mangone M, Austin W, Reyner K, Jayabalan D, Niesvizky R, Jagannath S, Bhardwaj N, Chen-Kiang S, Old LJ, Cho HJ. Cellular immune responses against CT7 (MAGE-C1) and humoral responses against other cancer-testis antigens in multiple myeloma patients. Cancer Immun. 2010;10:4. [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Epler J, Salazar LG, Riddell SR. Recognition of breast cancer cells by CD8+ cytotoxic T-cell clones specific for NY-BR-1. Cancer Res. 2006;66:6826–6833. doi: 10.1158/0008-5472.CAN-05-3529. [DOI] [PubMed] [Google Scholar]
- 27.Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/S0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 28.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310:40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 29.Scheibenbogen C, Lee KH, Mayer S, Stevanovic S, Moebius U, Herr W, Rammensee HG, Keilholz U. A sensitive ELISPOT assay for detection of CD8+ T lymphocytes specific for HLA class I-binding peptide epitopes derived from influenza proteins in the blood of healthy donors and melanoma patients. Clin Cancer Res. 1997;3:221–226. [PubMed] [Google Scholar]
- 30.Firat H, Garcia-Pons F, Tourdot S, Pascolo S, Scardino A, Garcia Z, Michel ML, Jack RW, Jung G, Kosmatopoulos K, Mateo L, Suhrbier A, Lemonnier FA, Langlade-Demoyen P. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur J Immunol. 1999;29:3112–3121. doi: 10.1002/(SICI)1521-4141(199910)29:10<3112::AID-IMMU3112>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Tinguely M, Jenni B, Knights A, Lopes B, Korol D, Rousson V, Curioni Fontecedro A, Cogliatti SB, Bittermann AG, Schmid U, Dommann-Scherrer C, Maurer R, Renner C, Probst-Hensch NM, Moch H, Knuth A, Zippelius A. MAGE-C1/CT-7 expression in plasma cell myeloma: sub-cellular localization impacts on clinical outcome. Cancer Sci. 2008;99:720–725. doi: 10.1111/j.1349-7006.2008.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Baren N, Brasseur F, Godelaine D, Hames G, Ferrant A, Lehmann F, Andre M, Ravoet C, Doyen C, Spagnoli GC, Bakkus M, Thielemans K, Boon T. Genes encoding tumor-specific antigens are expressed in human myeloma cells. Blood. 1999;94:1156–1164. [PubMed] [Google Scholar]
- 33.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065X.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 34.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS, Manukian G, Xu Y, Roman RA, Terzulli SL, Heywood M, Pogoriler E, Ritter G, Old LJ, Allison JP, Wolchok JD. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prabhala RH, Neri P, Bae JE, Tassone P, Shammas MA, Allam CK, Daley JF, Chauhan D, Blanchard E, Thatte HS, Anderson KC, Munshi NC. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107:301–304. doi: 10.1182/blood-2005-08-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, Lupton SD, Overell RW, Reynolds TC, Corey L, Greenberg PD. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 37.Berger C, Flowers ME, Warren EH, Riddell SR. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muul LM, Candotti F. Immune responses to gene-modified T cells. Curr Gene Ther. 2007;7:361–368. doi: 10.2174/156652307782151489. [DOI] [PubMed] [Google Scholar]
- 39.Mercier-Letondal P, Deschamps M, Sauce D, Certoux JM, Milpied N, Lioure B, Cahn JY, Deconinck E, Ferrand C, Tiberghien P, Robinet E. Early immune response against retrovirally transduced herpes simplex virus thymidine kinase-expressing gene-modified T cells confused with a T cell-depleted marrow graft: an altered immune response? Hum Gene Ther. 2008;19:937–950. doi: 10.1089/hum.2007.156. [DOI] [PubMed] [Google Scholar]
- 40.Fontana R, Bregni M, Cipponi A, Raccosta L, Rainelli C, Maggioni D, Lunghi F, Ciceri F, Mukenge S, Doglioni C, Colau D, Coulie PG, Bordignon C, Traversari C, Russo V. Peripheral blood lymphocytes genetically modified to express the self/tumor antigen MAGE-A3 induce antitumor immune responses in cancer patients. Blood. 2009;113:1651–1660. doi: 10.1182/blood-2008-07-168666. [DOI] [PubMed] [Google Scholar]
- 41.Russo V, Cipponi A, Raccosta L, Rainelli C, Fontana R, Maggioni D, Lunghi F, Mukenge S, Ciceri F, Bregni M, Bordignon C, Traversari C. Lymphocytes genetically modified to express tumor antigens target DCs in vivo and induce antitumor immunity. J Clin Invest. 2007;117:3087–3096. doi: 10.1172/JCI30605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snyder JT, Belyakov IM, Dzutsev A, Lemonnier F, Berzofsky JA. Protection against lethal vaccinia virus challenge in HLA-A2 transgenic mice by immunization with a single CD8(+) T-cell peptide epitope of vaccinia and variola viruses. J Virol. 2004;78:7052–7060. doi: 10.1128/JVI.78.13.7052-7060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atanackovic D, Luetkens T, Hildebrandt Y, Arfsten J, Bartels K, Horn C, Stahl T, Cao Y, Zander AR, Bokemeyer C, Kroger N. Longitudinal analysis and prognostic effect of cancer-testis antigen expression in multiple myeloma. Clin Cancer Res. 2009;15:1343–1352. doi: 10.1158/1078-0432.CCR-08-0989. [DOI] [PubMed] [Google Scholar]
- 46.Andrade VC, Vettore AL, Felix RS, Almeida MS, Carvalho F, Oliveira JS, Chauffaille ML, Andriolo A, Caballero OL, Zago MA, Colleoni GW. Prognostic impact of cancer/testis antigen expression in advanced stage multiple myeloma patients. Cancer Immunol. 2008;8:2. [PMC free article] [PubMed] [Google Scholar]
- 47.Atanackovic D, Hildebrandt Y, Jadczak A, Luetkens T, Meyer S, Bartels K, Cao YR, Zander AR, Bokemeyer C, Kroeger N. Expression of cancer-testis antigens MAGE-C1/CT7 and MAGE-A3 Is central to the survival of myeloma cells and their resistance to chemotherapy. Blood. 2008;112:1256–1257. [Google Scholar]
- 48.Nuber N, Curioni-Fontecedro A, Matter C, Soldini D, Tiercy JM, von Boehmer L, Moch H, Dummer R, Knuth A, van den Broek M. Fine analysis of spontaneous MAGE-C1/CT7-specific immunity in melanoma patients. Proc Natl Acad Sci USA. 2010;107:15187–15192. doi: 10.1073/pnas.1002155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roszkowski JJ, Lyons GE, Kast WM, Yee C, Van Besien K, Nishimura MI. Simultaneous generation of CD8+ and CD4+ melanoma-reactive T cells by retroviral-mediated transfer of a single T-cell receptor. Cancer Res. 2005;65:1570–1576. doi: 10.1158/0008-5472.CAN-04-2076. [DOI] [PubMed] [Google Scholar]
- 50.Anderson LD, Jr, Mori S, Mann S, Savary CA, Mullen CA. Pretransplant tumor antigen-specific immunization of allogeneic bone marrow transplant donors enhances graft-versus-tumor activity without exacerbation of graft-versus-host disease. Cancer Res. 2000;60:5797–5802. [PubMed] [Google Scholar]
- 51.Sanchez-Perez L, Kottke T, Diaz RM, Ahmed A, Thompson J, Chong H, Melcher A, Holmen S, Daniels G, Vile RG. Potent selection of antigen loss variants of B16 melanoma following inflammatory killing of melanocytes in vivo. Cancer Res. 2005;65:2009–2017. doi: 10.1158/0008-5472.CAN-04-3216. [DOI] [PubMed] [Google Scholar]