Abstract
Background:
Sheehan's syndrome manifests as hypopituitarism following a child birth usually preceded by postpartum hemorrhage. The symptoms range from vague feelings of ill health to symptoms of a full blown panhypopituitarism. A large series of such patients is not described in the literature.
Materials and Methods:
We present the details of ten women with partial Sheehan's syndrome. They presented with post-partum hemorrhage and lactation failure.
Results:
After delivery, seven out of ten patients had regular menstrual cycles indicating preservation of gonadotroph function. Lactotroph, thyrotroph, and somatotroph failure were present in all and corticotrophs preservation was documented in four out of ten patients. The hypophysial magnetic resonance imaging (MRI) confirmed empty sella in all.
Conclusion:
lactotroph, somatotroph and thyrotroph failure are common in patients with Sheehan's syndrome. In addition to known preservation of gonadotroph axis, corticotroph axis may be preserved in some of these patients arguing against the universal treatment of these patients with glucocorticoids.
Keywords: Corticotrophs, empty sella, postpartum hemorrhage, regular cycles, Sheehan's syndrome
INTRODUCTION
Sheehan's syndrome (SS) is the occurrence of hypopituitarism after child birth usually preceded by post partum hemorrhage.[1] Improvement in obstetric care and availability of rapid blood transfusion coincided with a remarkable reduction in the frequency of Sheehan's syndrome in western society; however, it continues to be the most common cause of hypopituitarism in underdeveloped or developing countries.[2–4] Clinical manifestation may vary from non-specific symptoms to an acute emergency like hypocortisol crisis or coma. Selective preservation of some of the anterior pituitary functions has been reported as isolated reports;[5,6] however, a large series of these atypical patients describing their details has not been described. Here we present a series of ten patients documenting the preservation of some of the anterior pituitary functions in them.
MATERIALS AND METHODS
This prospective study was conducted at a tertiary care health hospital in north India over the last four years. Around 60 patients of SS referred to the department of endocrinology or accident and emergency services during three years (2007-2010) were included in the study, the details of ten patients with partial SS are presented. The diagnosis of SS was based on: (a) history of postpartum hemorrhage (PPH) or failure of lactation and/or amenorrhea following last child birth; (b) more than one anterior pituitary hormone deficiency; and (c) empty sella on MR imaging.[3] General physical and systemic examination was done. Height, weight, body mass index, and blood pressure were recorded in each patient. Fasting blood samples were obtained for complete blood count (CBC), liver and kidney functions, electrolytes, and glucose. Hormone estimations included serum thyroid stimulating hormone (TSH), total thyroxine (T4), total tri-iodothyronine (T3), follicle-stimulating hormone (FSH), luteinizing hormone (LH), cortisol, prolactin (PRL), and growth hormone (GH). Serum concentrations of T4, T3, TSH, LH, FSH, PRL, and GH were measured by immuno-radiometric assay (IRMA) using commercially available kits (Siemens Medical solutions, Los Angles USA, CA 90045-6900). Serum cortisol was measured by radio immunoassay (RIA) using commercial available kits (Diasorin Stillwater, Minnesota 55082-0285 USA). Contrast enhanced MRI of pituitary was performed to document empty sella. All the patients were started on replacement with appropriate dose of levo-thyroxine. Prednisolone was given to those patients whose basal cortisol was less than 15 μg/dl. After replacement for 3 months, prednisolone was stopped for 48 hours and insulin tolerance test (ITT) was performed after admission in the hospital. An informed consent was obtained from the subjects and study was approved by institutional ethical committee. Results were expressed as mean±SD.
RESULTS
Mean age of the patients was 34.5±6.58 years with a mean parity of 2.6±1.17 (ranged 1-4 deliveries). Mean duration of the last delivery was 6.30±3.97 years (range 2-10 years). PPH was seen in all except one (90%) patient who despite a home delivery did not remember about it, one patient had PPH despite a cesarean section. Seven out of ten patients had received blood transfusion after being shifted to hospital for hypotension. All the patients had lactation failure. Seven out of ten resumed regular menstrual cycles after a period of lactation amenorrhea and one conceived twice. Features of hypothyroidism were seen in all the patients. Table 1 summarizes the details of clinical features, major trophic hormone deficiencies and MR imaging features in these patients. Mean systolic blood pressure was 104.5±14.99 mmHg and diastolic blood pressure was 70±7.5 mmHg. Mean Hb was 10.34±0.25 g/dl, normocytic normochromic type. Mean random glucose was 74± 2.3 mg/dl. Hormonal investigations revealed low serum T4 with inappropriately normal TSH, normal prolactin, and gonadotropins in all the patients. ITT done after achieving euthyroid state revealed subnormal prolactin response and non stimulable GH in all the patients. Five out of ten patients had a normal cortisol response to ITT suggestive of corticotroph preservation [Table 2]. Contrast enhanced MRI revealed evidence of empty sella in all the patients [Figure 1]. All the patients are on substitution therapy in the form of L-thyroxine and prednisolone in those patients with secondary adrenal insufficiency and low dose estrogen progesterone combination in those with secondary hypogonadism and are on regular follow-up.
Table 1.
Summary of clinical and hormonal abnormalities in ten patients

Table 2.
Anterior pituitary and cortisol levels in basal and post stimulated state
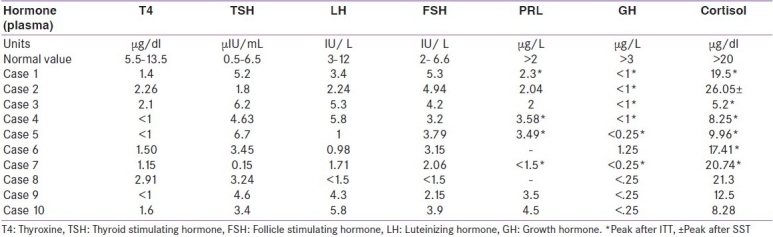
Figure 1.
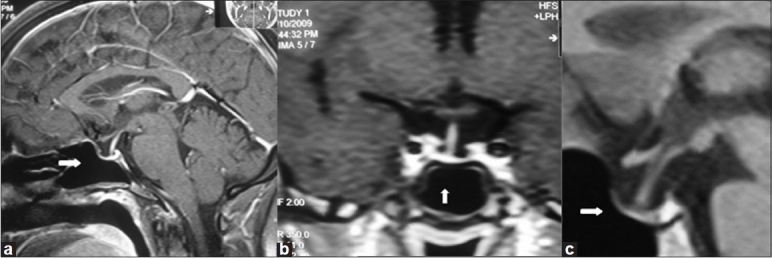
MRI pituitary (a) Sagital view case 1, (b) Coronal view case 3, (c) Sagital view case 4 showing pituitary fossa filled with cerebrospinal fluid and stalk touching the base of pituitary floor (white arrows); features suggestive of empty sella
DISCUSSION
The diagnosis of SS in these patients was based on the basis of PPH, need for blood transfusion followed by trophic hormone deficiency and presence of empty sella on MR imaging. The likely diagnostic criteria for SS have recently been suggested.[3] Pituitary necrosis is believed to be caused by hypotension because of PPH at the time of child birth. Disseminated intravascular coagulation and autoimmunity in addition to pituitary necrosis have been suggested to play a role in development of SS.[1,7,8] Patients with SS exhibit different degrees of hypopituitarism with selective pituitary hormone deficiency in 14 % of patients.[9] In areas where the disorder is common, these patients may come to attention because of so called “non endocrine features”. Anemia and other hematological abnormalities have recently been described in these patients. Anemia of normocytic and normochromic type, leucopenia, and thrombocytopenia in various combinations are common in patients with SS. Replacement with thyroid and glucocorticoids result in complete recovery of abnormalities.[10] Spontaneous pregnancy may occur after the onset of hypopituitarism and preservation/recovery of gonadotroph function has been reported in these patients.[5] It has been reported that pituitary function is relatively preserved for LH, FSH, and TSH but not for prolactin, growth hormone and cortisol.[11] In the present series, all the patients had involvement of somatotroph and lactotroph axis, the likely explanation for uniform involvement of the PRL and GH is the anatomical location of growth hormone and prolactin cells in the lower lateral region of adenohypophysis where they are most susceptible to ischemic damage.[3] Lactotroph preservation is extremely rare in these patients and only two cases have been reported, one before and another after the availability of MRI.[12,13] The interesting observation from the present series is the involvement of thyroid axis in all the patients and preservation of cortisol axis in five of them. In a large study of 86 patients published previously, ITT documented GH deficiency in all and corticotroph deficiency in 85% of patients.[14]
In a series of 15 patients of SS who underwent insulin induced hypoglycemia, only one patient showed normal growth hormone and cortisol response.[15] In an Indian series of 20 patients with typical history of SS, one had normal cortisol response following insulin induced hypoglycemia.[16] Jialal et al., subjected 16 patients of SS to GNRH, TRH, and insulin induced hypoglycemia. All the 10 patients had subnormal growth hormone and prolactin response. Nine patients had impaired cortisol response.[17]
Sert et al., recently reported the clinical experience in 28 patients of SS.[6] All the patients had secondary hypothyroidism, adrenocortical failure, hypogonadotrophic hypogonadism, and GH deficiency. In another recently published study, all the 20 patients had gonadotroph, lactotroph, somatotroph, and thyrotroph deficiency while as 55% of these had normal ACTH stimulation test.[18] There is no anatomical explanation for preservation of cortisol axis in some of these patients but it may have implications on the treatment and some of these patients may be prevented from long term adverse effects of glucocorticoids.
In summary, SS can present with partial involvement of anterior pituitary. In addition to previously documented preservation of gonadal axis, corticotrophs can be preserved in some patients that would prevent these patients from long term undesirable effects of glucocorticoids.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sheehan HL. Postpartum necrosis of anterior pituitary. J Pathol Bacteriol. 1937;45:189–214. [Google Scholar]
- 2.Zargar AH, Singh B, Laway BA, Masoodi SR, Wani AI, Bashir MI. Epidemiological aspects of postpartum pituitary hypofunction (Sheehan's syndrome) Fertil Steril. 2005;84:523–8. doi: 10.1016/j.fertnstert.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Kelestimur F. Sheehan's syndrome. Pituitary. 2003;6:81–8. doi: 10.1023/b:pitu.0000023425.20854.8e. [DOI] [PubMed] [Google Scholar]
- 4.Zargar AH, Laway BA. Inadequate obstetric care and Sheehan's syndrome in young women. Indian J Endocrinol Metab. 2008;12:1–2. [Google Scholar]
- 5.Zargar AH, Masoodi SR, Laway BA, Sofi FA, Wani AI. Pregnancy in Sheehan's syndrome a report of three cases. J Assoc Physicians India. 1998;46:476–8. [PubMed] [Google Scholar]
- 6.Sert M, Tetiker T, Kirim S, Kocak M. Clinical report of 28 patients with Sheehan's syndrome. Endocr J. 2003;50:279–301. doi: 10.1507/endocrj.50.297. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs K. Sheehan's syndrome. Lancet. 2003;361:520–2. doi: 10.1016/S0140-6736(03)12490-7. [DOI] [PubMed] [Google Scholar]
- 8.Goswami R, Kochupillai N, Crock PA, Jaleel A, Gupta N. Pituitary autoimmunity in patients with Sheehan's syndrome. J Clin Endocrinol Metab. 2002;87:4137–41. doi: 10.1210/jc.2001-020242. [DOI] [PubMed] [Google Scholar]
- 9.Dizerega G, Kletzky OA, Mishell DR., Jr Diagnosis of Sheehan's syndrome using a sequential pituitary stimulation test. Am J Obstet Gynecol. 1978;132:348–53. doi: 10.1016/0002-9378(78)90765-2. [DOI] [PubMed] [Google Scholar]
- 10.Laway BA, Mir SA, Bashir MI, Bhat JR, Samoon J, Zargar AH. Prevalence of hematological abnormalities in patients with Sheehan's syndrome - Response to replacement of glucocorticoids and thyroxine. Pituitary. 2011;14:39–43. doi: 10.1007/s11102-010-0255-2. [DOI] [PubMed] [Google Scholar]
- 11.Shahmanesh M, Ali Z, Pourmand M, Nourmand I. Pituitary function test in Sheehan's syndrome. Clin Endocrinol (Oxf) 1980;12:303–11. doi: 10.1111/j.1365-2265.1980.tb02714.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelestimur F. Hyperprolactinemia in a patient with Sheehan's syndrome. South Med J. 1992;85:1008–10. doi: 10.1097/00007611-199210000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Laway BA, Ganie MA, Bashir MI, Kotwal SK, Mir SA, Gojwari T, et al. Sheehan's syndrome with hyperprolactinemia. Turkish J Endocrinol Metab. 2010;14:47–9. [Google Scholar]
- 14.Zargar AH, Masoodi SR, Laway BA, Shah NA, Salahuddin M, Siddiqi M, et al. Clinical Spectrum of Sheehan's syndrome. Ann Saudi Med. 1996;16:338–41. doi: 10.5144/0256-4947.1996.338. [DOI] [PubMed] [Google Scholar]
- 15.Ozbey N, Inanc S, Aral F, Azezli A, Orhan Y, Sencer E, et al. Clinical and laboratory evaluation of 40 patients with Sheehan's syndrome. Isr J Med Sci. 1994;30:826–9. [PubMed] [Google Scholar]
- 16.Dash RJ, Gupta V, Suri S. Sheehan's syndrome: Clinical profile, pituitary hormone responses and computed sellar tomography. Aust N Z J Med. 1993;23:26–31. doi: 10.1111/j.1445-5994.1993.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 17.Jialal I, Naidoo C, Norman RJ, Rajput MC, Omar MA, Joubert SM. Pituitary function in Sheehan' s syndrome. Obstet Gynecol. 1984;63:15–9. [PubMed] [Google Scholar]
- 18.Dokmetas HS, Kilicli F, Korkmaz S, Yonem O. Characteristic features of 20 patients with Sheehan's syndrome. Gynecol Endocrinol. 2006;22:279–83. doi: 10.1080/09513590600630504. [DOI] [PubMed] [Google Scholar]


